Thymoquinone Protective Effect Against Mercury-Induced Reproductive Derangement in Rats: In Vivo and In Silico Investigation
Abstract
1. Introduction
2. Methods
2.1. Sample Size Determination and Animal Welfare
2.2. Chemicals
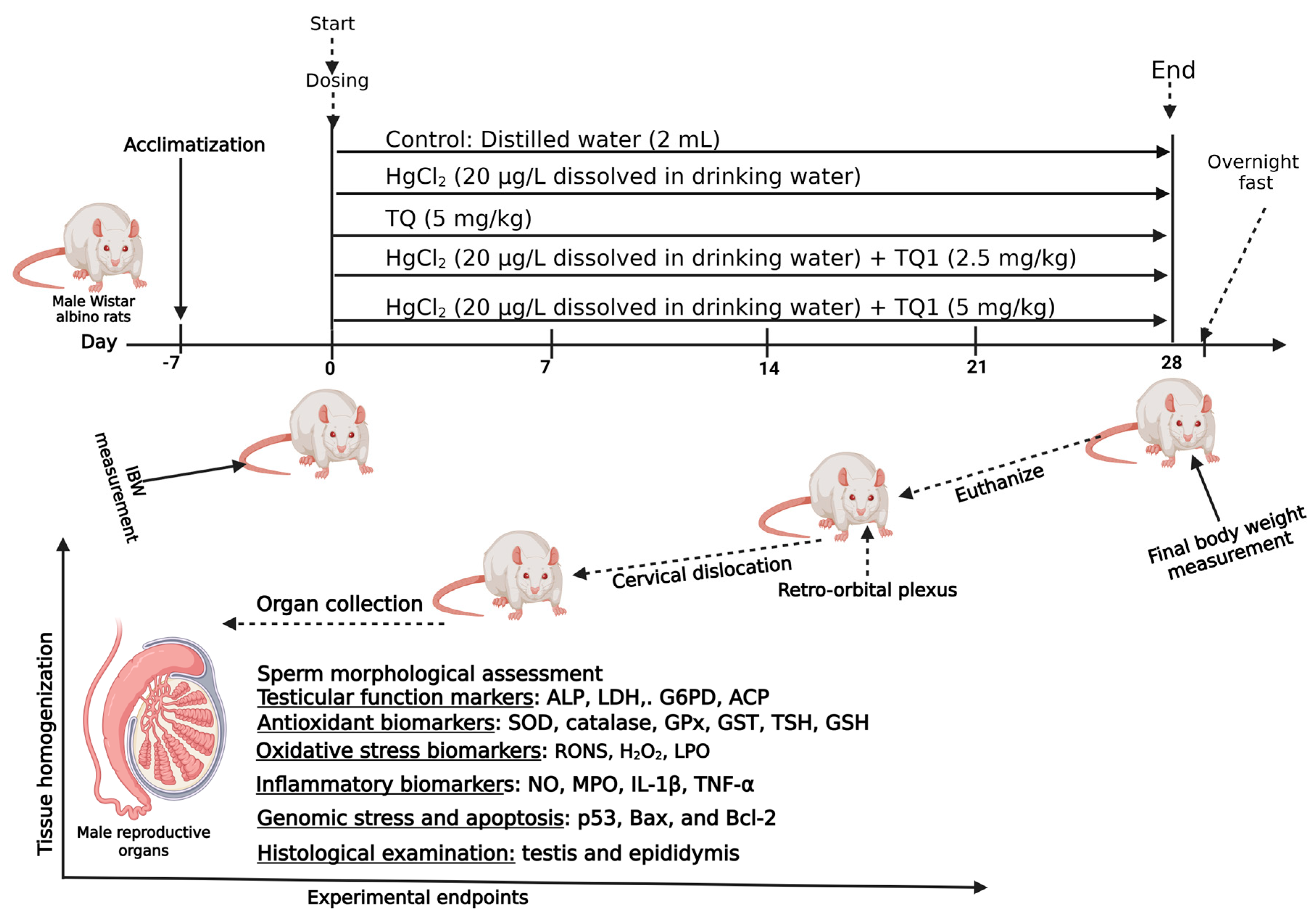
2.3. Experimental Protocol
2.4. Study Conclusion and Euthanasia
2.5. Determination of Spermatozoa Motility
2.6. Assessment of Epididymal Sperm Count
2.7. Assessment of Sperm Morphological Abnormalities and Viability Assay
2.8. Assessment of Reproductive Hormone Levels
2.9. Assessment of Testicular Enzyme Function
2.10. Evaluation of Testes, Epididymides, and Hypothalamus Antioxidant Biomarker Status
2.11. Assessment of RONS and LPO Concentrations and XO Activity in the Testes, Epididymides, and Hypothalamus of Rats
2.12. Assessment of Pro-Inflammatory Markers in Rat Testes, Epididymides, and Hypothalamus
2.13. Evaluation of Apoptosis Biomarkers
2.14. Histopathological Examination of the Testes, Epididymides, and Hypothalamus
2.15. Molecular Docking Method
2.16. Statistical Analysis
3. Results
3.1. The Effect of TQ on the Body Weight and Organosomatic Index of Experimental Animals Treated with HgCl2
3.2. Effect of TQ on Sperm Functional Parameters and Morphological Abnormalities in HgCl2-Treated Rats
3.3. TQ Improved HgCl2-Induced Alteration in Reproductive Hormones in the Serum of Treated Rats
3.4. TQ Co-Administration Increased the Activities of Testicular Enzymes in Experimental Animals
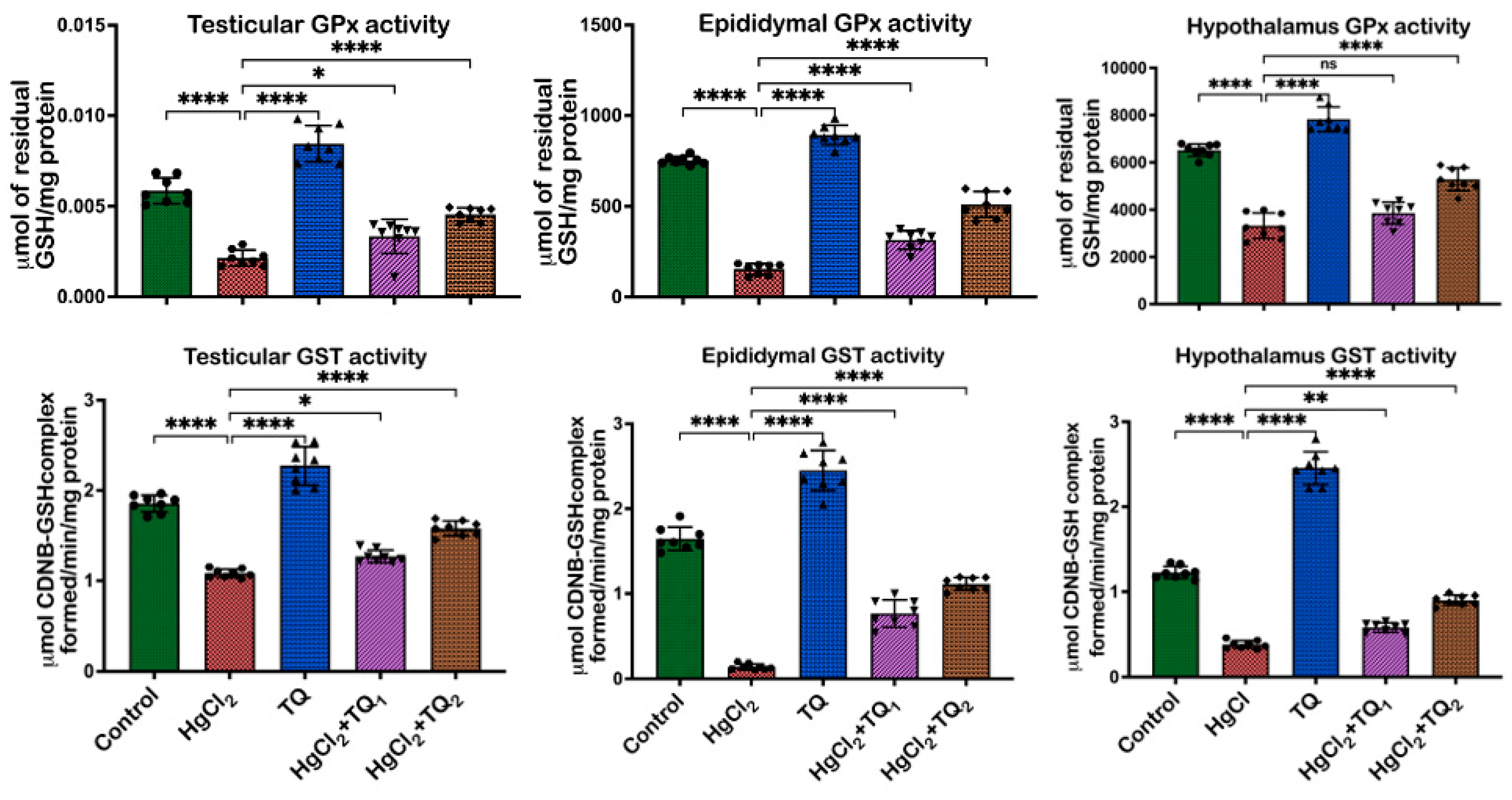
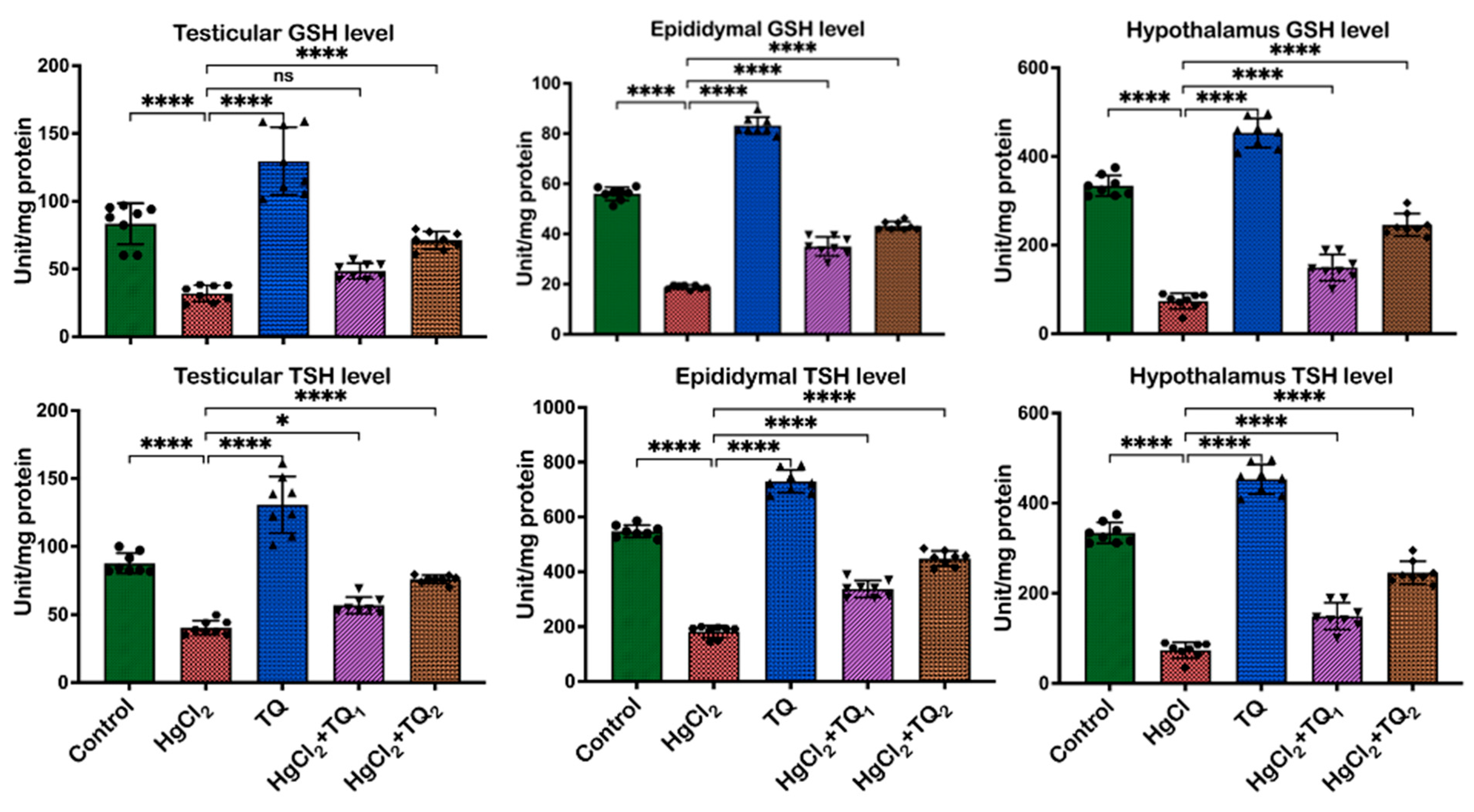
3.5. TQ Co-Treatment Restored the Antioxidant Status of Rats Treated with HgCl2
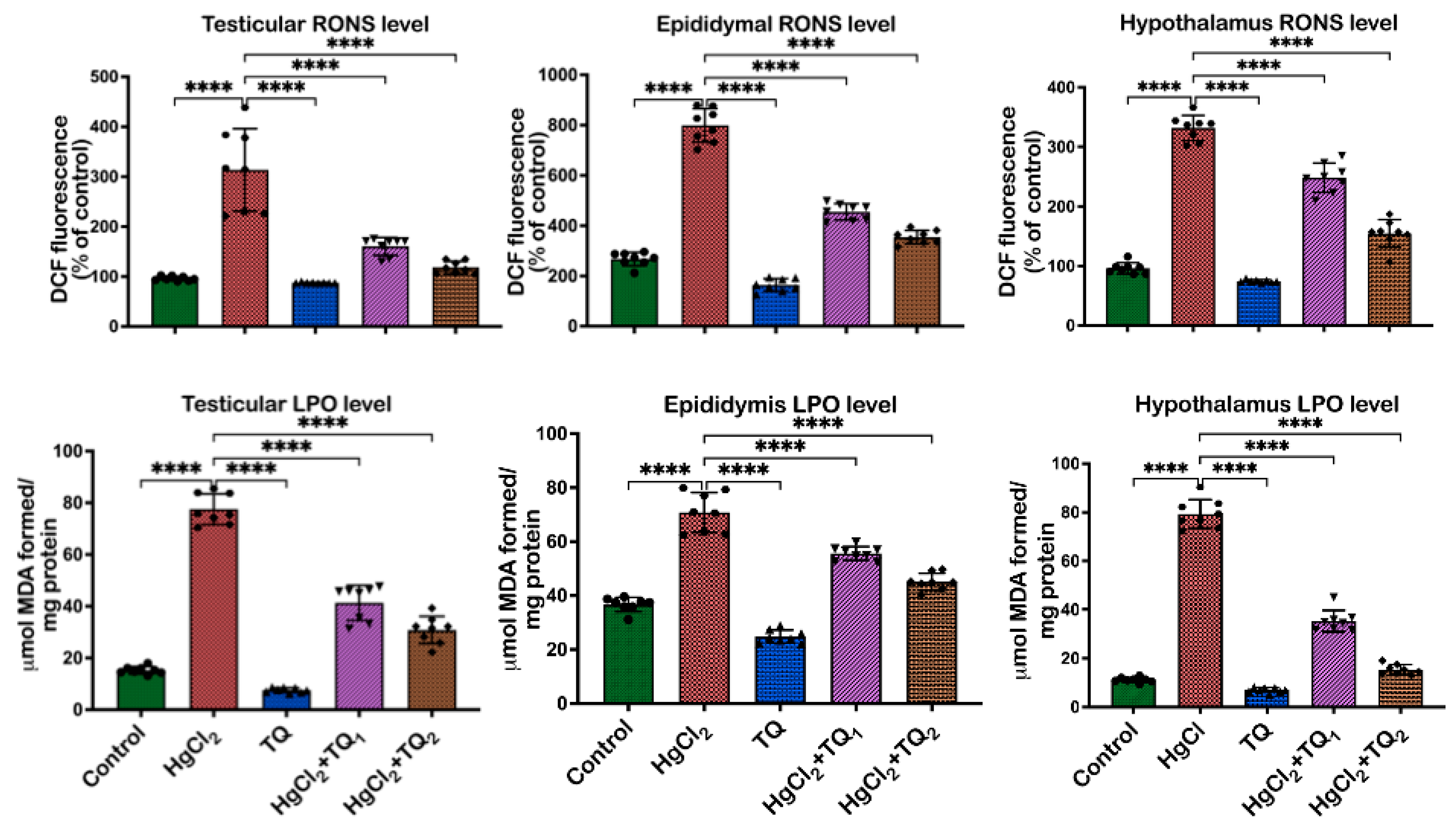
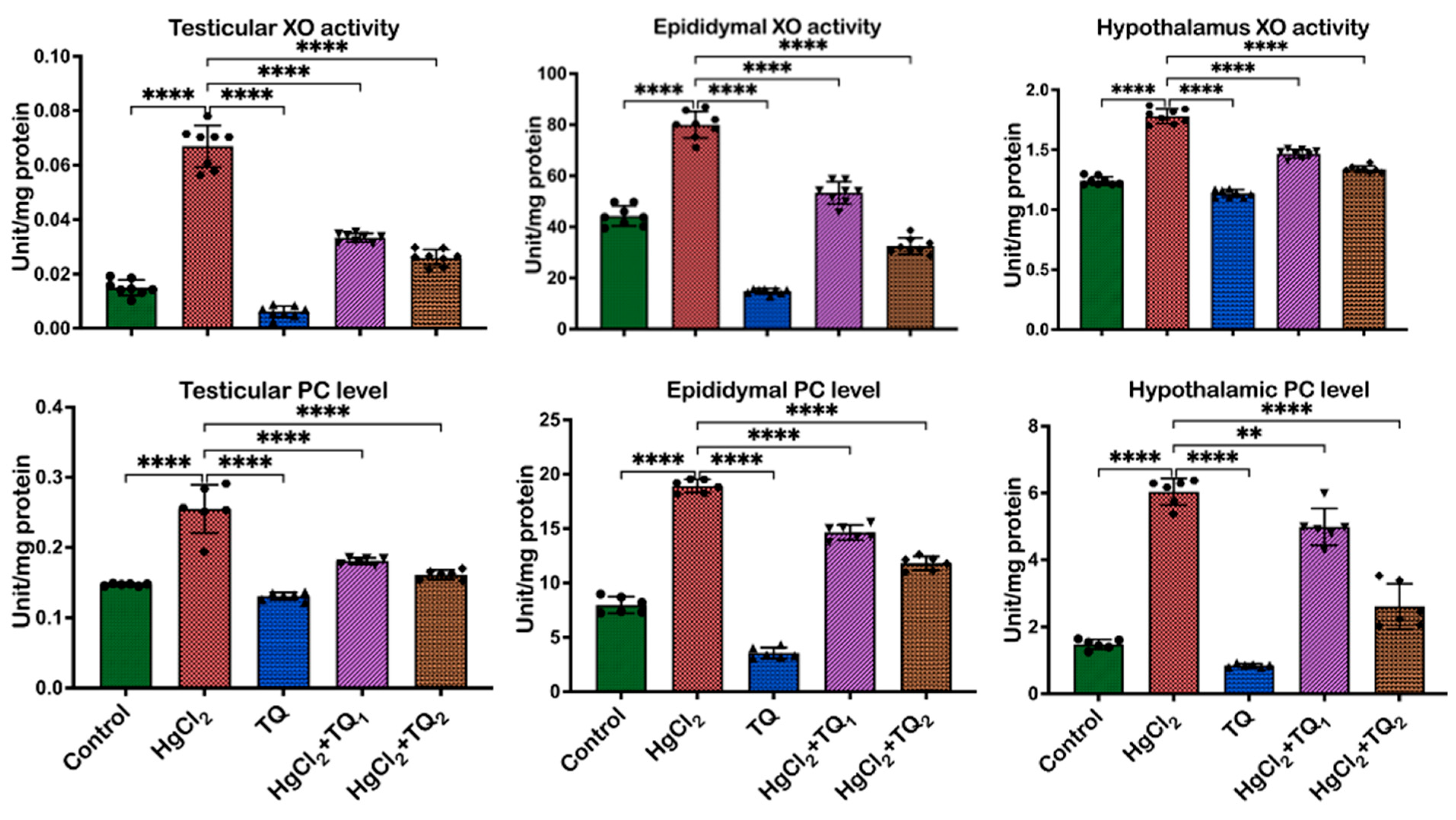
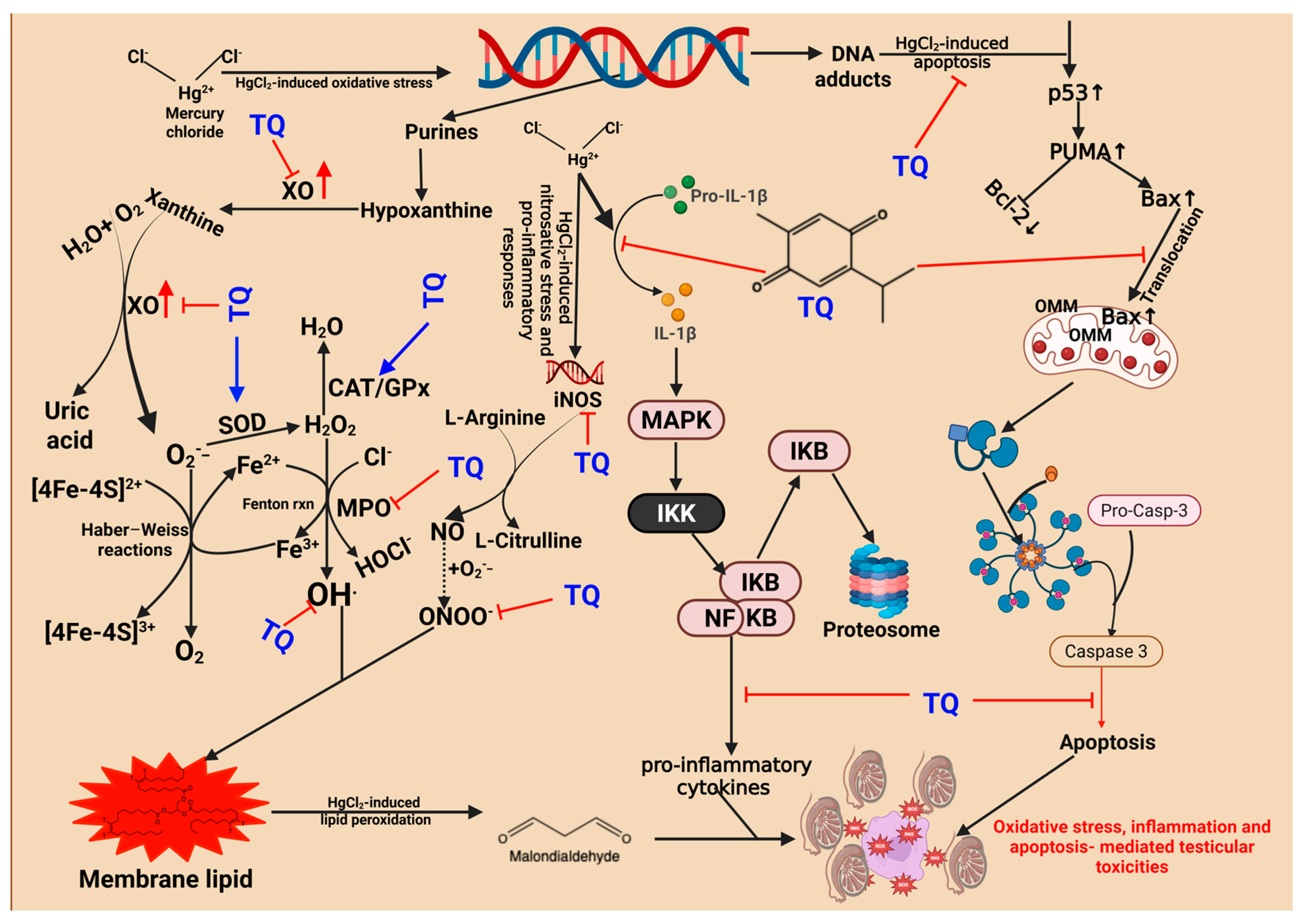
3.6. TQ Co-Treatment Attenuated HgCl2-Induced Oxidative Stress in the Reproductive Tissues of Rats
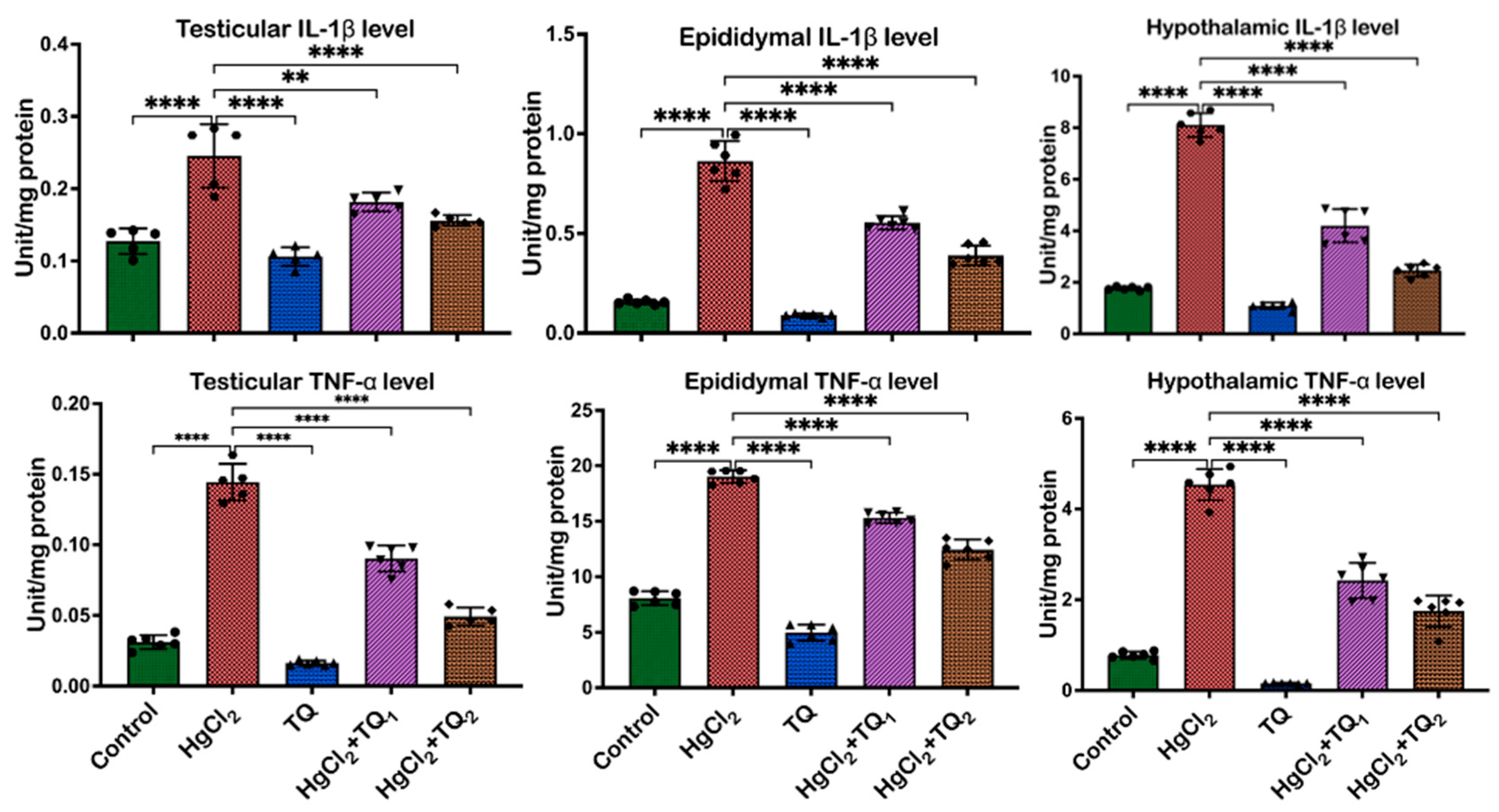
3.7. TQ Co-Administration Reversed Inflammation Caused by HgCl2 Exposure in the Testes, Epididymis, and Hypothalamus of Rats
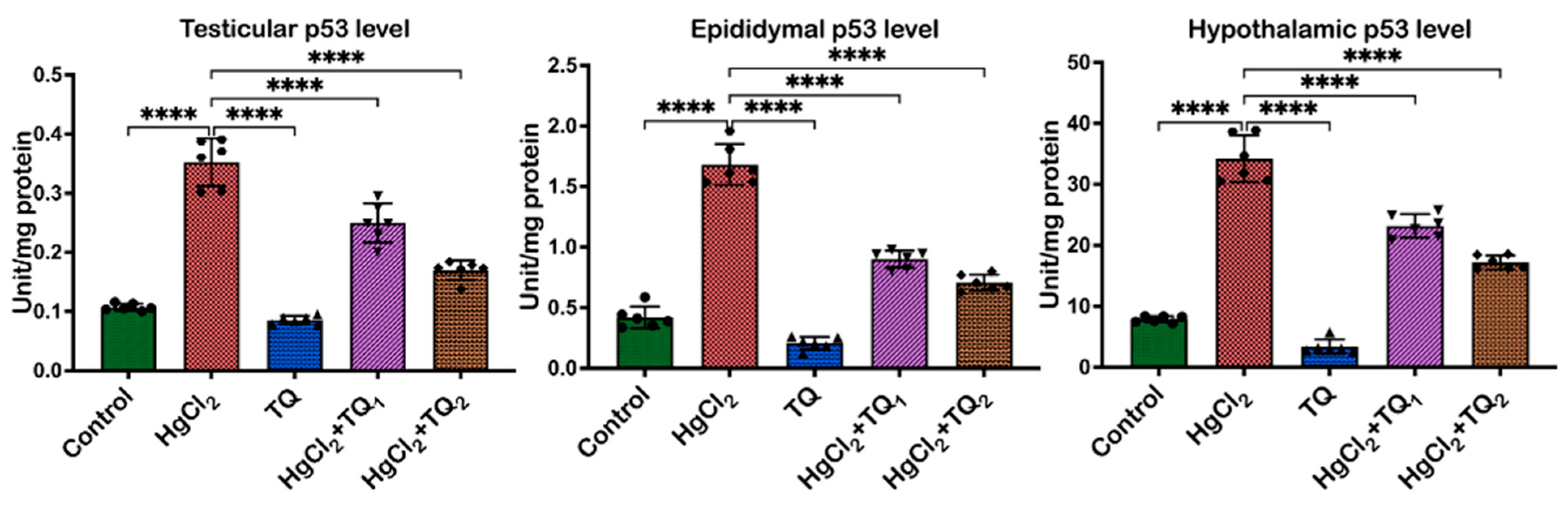
3.8. TQ Co-Treatment Assuaged Apoptosis and Cellular Damage in Experimental Rats Treated with HgCl2
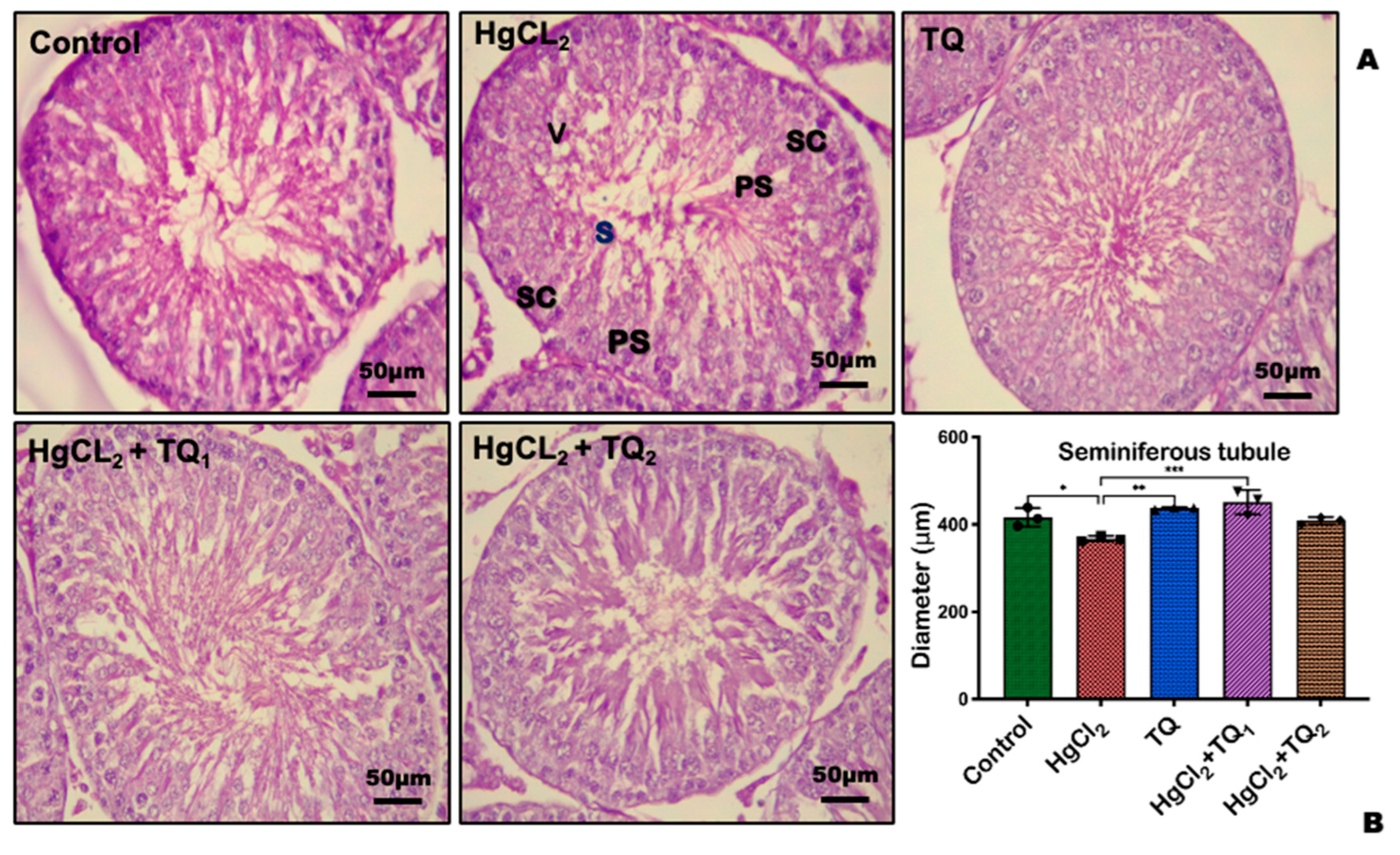
3.9. TQ Reduced HgCl2-Induced Testicular and Epididymal Histomorphometry Alteration
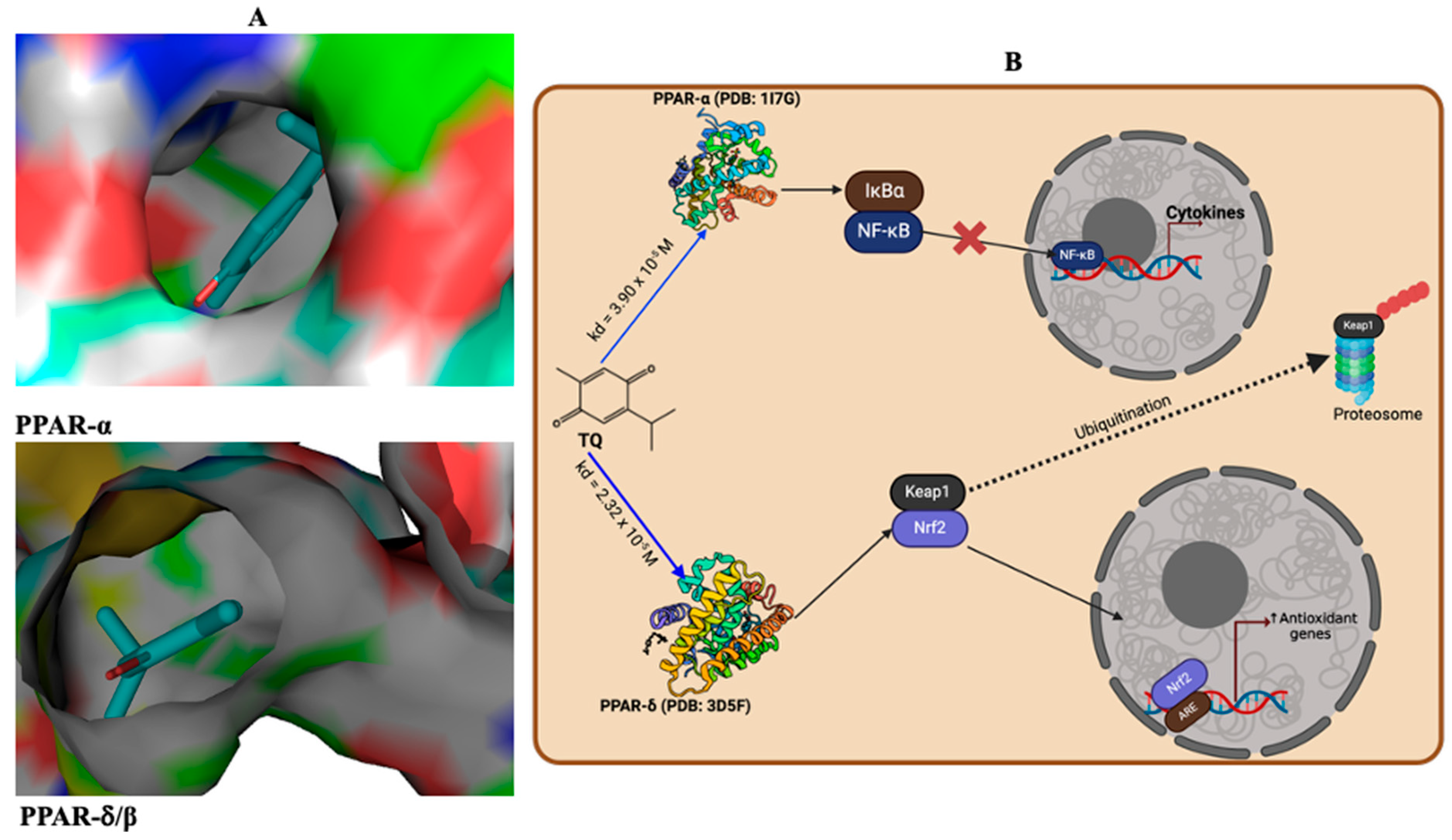
3.10. Antioxidant and Anti-Inflammatory Effects Are Promoted Through the Interaction of TQ and PPAR-α or PPAR δ/β Signalling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martinez, C.S.; Torres, J.G.; Peçanha, F.M.; Anselmo-Franci, J.A.; Vassallo, D.V.; Salaices, M.; Alonso, M.J.; Wiggers, G.A. Reproductive dysfunction after mercury exposure at low levels: Evidence for a role of glutathione peroxidase (GPx) 1 and GPx4 in male rats. Reprod. Fertil. Dev. 2017, 29, 1803–1812. [Google Scholar] [CrossRef]
- Jahan, S.; Azad, T.; Ayub, A.; Ullah, A.; Afsar, T.; Almajwal, A.; Razak, S. Ameliorating potency of Chenopodium album Linn. and vitamin C against mercuric chloride-induced oxidative stress in testes of Sprague Dawley rats. Environ. Health Prev. Med. 2019, 24, 62. [Google Scholar] [CrossRef]
- Badary, O.A.; Hamza, M.S.; Tikamdas, R. Thymoquinone: A Promising Natural Compound with Potential Benefits for COVID-19 Prevention and Cure. Drug Des. Devel Ther. 2021, 15, 1819–1833. [Google Scholar] [CrossRef]
- Kandemir, F.M.; Caglayan, C.; Aksu, E.H.; Yildirim, S.; Kucukler, S.; Gur, C.; Eser, G. Protective effect of rutin on mercuric chloride-induced reproductive damage in male rats. Andrologia 2020, 52, e13524. [Google Scholar] [CrossRef] [PubMed]
- Simsek, H.; Gur, C.; Kucukler, S.; Ileriturk, M.; Akaras, N.; Oz, M.; Kandemir, F.M. Carvacrol Reduces Mercuric Chloride-Induced Testicular Toxicity by Regulating Oxidative Stress, Inflammation, Apoptosis, Autophagy, and Histopathological Changes. Biol. Trace Elem. Res. 2024, 202, 4605–4617. [Google Scholar] [CrossRef] [PubMed]
- Albasher, G.; Alkahtani, S.; Alarifi, S. Berberine mitigates oxidative damage associated with testicular impairment following mercury chloride intoxication. J. Food Biochem. 2020, 44, e13385. [Google Scholar] [CrossRef] [PubMed]
- hai, M.K.P.; Binesh, A.; Shanmugam, S.A.; Venkatachalam, K. Effects of mercury chloride on antioxidant and inflammatory cytokines in zebrafish embryos. J. Biochem. Mol. Toxicol. 2024, 38, e23589. [Google Scholar]
- Almeer, R.S.; Albasher, G.; Kassab, R.B.; Ibrahim, S.R.; Alotibi, F.; Alarifi, S.; Ali, D.; Alkahtani, S.; Abdel Moneim, A.E. Ziziphus spina-christi leaf extract attenuates mercury chloride-induced testicular dysfunction in rats. Environ. Sci. Pollut. Res. Int. 2020, 27, 3401–3412. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef]
- Moradi Maryamneghari, S.; Shokri-Asl, V.; Abdolmaleki, A.; Jalili, C. Genetic, biochemical and histopathological evaluations of thymoquinone on male reproduction system damaged by paclitaxel in Wistar rats. Andrologia 2021, 53, e14192. [Google Scholar] [CrossRef]
- Asaduzzaman Khan, M.; Tania, M.; Fu, S.; Fu, J. Thymoquinone, as an anticancer molecule: From basic research to clinical investigation. Oncotarget 2017, 8, 51907–51919. [Google Scholar] [CrossRef] [PubMed]
- Hassan, E.; El-Neweshy, M.; Hassan, M.; Noreldin, A. Thymoquinone attenuates testicular and spermotoxicity following subchronic lead exposure in male rats: Possible mechanisms are involved. Life Sci. 2019, 230, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Owumi, S.; Otunla, M.; Akindipe, P.; Oluwawibe, B.; Babalola, J.O.; Chimezie, J.; Arunsi, U.; Owoeye, O.; Oyelere, A.K. Thymoquinone modulates oxidative stress and inflammation, correcting mercury-induced HO-1/NRF/Trx pathway disruption in experimental rat hepatorenal system: An in vivo and in silico study. Biometals 2025, 38, 1179–1202. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, M.; Fukuhara, K. Antioxidant and Prooxidant Effects of Thymoquinone and Its Hydroquinone Metabolite. Biol. Pharm. Bull. 2022, 45, 1389–1393. [Google Scholar] [CrossRef]
- Algaidi, S.A.; Faddladdeen, K.A.; Alrefaei, G.I.; Qahl, S.H.; Albadawi, E.A.; HM, A.L.; Ayuob, N.N. Thymoquinone protects the testes of hypothyroid rats by suppressing pro-inflammatory cytokines and oxidative stress and promoting SIRT1 testicular expression. Front. Pharmacol. 2022, 13, 1040857. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Owumi, S.E.; Kazeem, A.I.; Wu, B.; Ishokare, L.O.; Arunsi, U.O.; Oyelere, A.K. Apigeninidin-rich Sorghum bicolor (L. Moench) extracts suppress A549 cells proliferation and ameliorate toxicity of aflatoxin B1-mediated liver and kidney derangement in rats. Sci. Rep. 2022, 12, 7438. [Google Scholar] [CrossRef]
- Boujbiha, M.A.; Hamden, K.; Guermazi, F.; Bouslama, A.; Omezzine, A.; Kammoun, A.; El Feki, A. Testicular toxicity in mercuric chloride treated rats: Association with oxidative stress. Reprod. Toxicol. 2009, 28, 81–89. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Zemjanis, R. Collection and evaluation of semen. Diagn. Ther. Tech. Anim. Reprod. 1970, 2, 467–523. [Google Scholar]
- WHO. WHO Laboratory Manual for the Examination and Processing of Human Semen. 2010. Fifth Edition. Available online: https://www.who.int/publications/i/item/9789240030787 (accessed on 22 June 2021).
- Wells, M.E.; Awa, O.A. New technique for assessing acrosomal characteristics of spermatozoa. J. Dairy. Sci. 1970, 53, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Vanha-Perttula, T.; Nikkanen, V. Acid phosphatases of the rat testis in experimental conditions. Acta Endocrinol. 1973, 72, 376–390. [Google Scholar] [CrossRef] [PubMed]
- Malymy, M.; Horecker, B. Methods in Enzymology; Academy Press: New York, NY, USA, 1966; Volume IX. [Google Scholar]
- Wolf, B.H.; Weening, R.S.; Schutgens, R.B.; van Noorden, C.J.; Vogels, I.M.; Nagelkerke, N.J. Detection of glucose-6-phosphate dehydrogenase deficiency in erythrocytes: A spectrophotometric assay and a fluorescent spot test compared with a cytochemical method. Clin. Chim. Acta 1987, 168, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Vassault, A. Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Verlag Chemic: Weinheim, Germany, 1983; pp. 118–125. [Google Scholar]
- Owumi, S.E.; Otunla, M.T.; Arunsi, U.O.; Oyelere, A.K. Apigeninidin-enriched Sorghum bicolor (L. Moench) extracts alleviate Aflatoxin B(1)-induced dysregulation of male rat hypothalamic-reproductive axis. Exp. Biol. Med. 2022, 247, 1301–1316. [Google Scholar] [CrossRef]
- Wong, J.J.H.; Hsieh, D.P. Mutagenicity of aflatoxins related to their metabolism and carcinogenic potential. Proc. Natl. Acad. Sci. USA 1976, 73, 2241–2244. [Google Scholar] [CrossRef]
- Clairborne, A. Catalase activity. In Handbook of Methods for Oxygen Radical Research; Greewald, A.R., Ed.; CRC Press: Boca Raton, FL, USA, 1995. [Google Scholar]
- Beutler, E.; Duron, O.; Kelly, B.M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar]
- Jollow, D.J.; Mitchell, J.R.; Zampaglione, N.; Gillette, J.R. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 1974, 11, 151–169. [Google Scholar] [CrossRef]
- Owumi, S.E.; Akinwunmi, A.O.; Nwozo, S.O.; Arunsi, U.O.; Oyelere, A.K. Aflatoxin B1-induced dysfunction in male rats’ reproductive indices were abated by Sorghum bicolor (L. Moench) hydrophobic fraction. Reprod. Toxicol. 2023, 120, 108425. [Google Scholar] [CrossRef]
- Owumi, S.E.; Arunsi, U.O.; Otunla, M.T.; Oluwasuji, I.O. Exposure to lead and dietary furan intake aggravates hypothalamus-pituitary-testicular axis toxicity in chronic experimental rats. J. Biomed. Res. 2022, 37, 100–114. [Google Scholar] [CrossRef]
- Bergmeyer, H.I.; Gawehn, K.; Grassl, M. Methods of Enzymatic Analysis, 2nd ed.; Academic Press Inc.: New York, NY, USA, 1974; Volume 1. [Google Scholar]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Trush, M.A.; Egner, P.A.; Kensler, T.W. Myeloperoxidase as a biomarker of skin irritation and inflammation. Food Chem. Toxicol. 1994, 32, 143–147. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Gamble, M. Theory and Practise of Histological Techniques, 6th ed.; Churchill Livingstone Elsevier: Philadelphia, PA, USA, 2008; pp. 83–134. [Google Scholar]
- Tian, P.; Yang, Z.; Qu, C.; Qi, X.; Zhu, L.; Hao, G.; Zhang, Y. Exploration of tissue fixation methods suitable for digital pathological studies of the testis. Eur. J. Med. Res. 2024, 29, 319. [Google Scholar] [CrossRef] [PubMed]
- Meng, E.C.; Goddard, T.D.; Pettersen, E.F.; Couch, G.S.; Pearson, Z.J.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Tools for structure building and analysis. Protein Sci. 2023, 32, e4792. [Google Scholar] [CrossRef] [PubMed]
- Henriques, M.C.; Loureiro, S.; Fardilha, M.; Herdeiro, M.T. Exposure to mercury and human reproductive health: A systematic review. Reprod. Toxicol. 2019, 85, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Lazic, S.E.; Semenova, E.; Williams, D.P. Determining organ weight toxicity with Bayesian causal models: Improving on the analysis of relative organ weights. Sci. Rep. 2020, 10, 6625. [Google Scholar] [CrossRef]
- Shalan, M.G. Amelioration of mercuric chloride-induced physiologic and histopathologic alterations in rats using vitamin E and zinc chloride supplement. Heliyon 2022, 8, e12036. [Google Scholar] [CrossRef]
- Tanga, B.M.; Qamar, A.Y.; Raza, S.; Bang, S.; Fang, X.; Yoon, K.; Cho, J. Semen evaluation: Methodological advancements in sperm quality-specific fertility assessment—A review. Anim. Biosci. 2021, 34, 1253–1270. [Google Scholar] [CrossRef]
- Dabbagh Rezaeiyeh, R.; Mehrara, A.; Mohammad Ali Pour, A.; Fallahi, J.; Forouhari, S. Impact of Various Parameters as Predictors of The Success Rate of In Vitro Fertilization. Int. J. Fertil. Steril. 2022, 16, 76–84. [Google Scholar]
- Altunkaynak, M.E.; Akgul, N.; Yahyazadeh, A.; Altunkaynak, B.Z.; Turkmen, A.P.; Akgul, H.M.; Aksak, S.; Unal, B. A stereological and histopathological study of the effects of exposure of male rat testes to mercury vapor. Biotech. Histochem. 2015, 90, 529–534. [Google Scholar] [CrossRef]
- Moretti, E.; Signorini, C.; Noto, D.; Corsaro, R.; Collodel, G. The relevance of sperm morphology in male infertility. Front. Reprod. Health 2022, 4, 945351. [Google Scholar] [CrossRef]
- Kushawaha, B.; Yadav, R.; Garg, S.K.; Pelosi, E. The impact of mercury exposure on male reproduction: Mechanistic insights. J. Trace Elem. Med. Biol. 2025, 87, 127598. [Google Scholar] [CrossRef] [PubMed]
- Homma-Takeda, S.; Kugenuma, Y.; Iwamuro, T.; Kumagai, Y.; Shimojo, N. Impairment of spermatogenesis in rats by methylmercury: Involvement of stage- and cell- specific germ cell apoptosis. Toxicology 2001, 169, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Sokwala, S. Chapter 2—Reproductive endocrine physiology. In Subfertility; Rehman, R., Sheikh, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 39–64. [Google Scholar]
- Sadiq, N.M.; Tadi, P. Physiology, Pituitary Hormones. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2024. [Google Scholar]
- Sampada, M.; David, M. Mercuric chloride induced reproductive toxicity associated with oxidative damage in male Wistar albino rat, Rattus norvegicus. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 398, 7273–7299. [Google Scholar] [CrossRef] [PubMed]
- Petersenn, S.; Fleseriu, M.; Casanueva, F.F.; Giustina, A.; Biermasz, N.; Biller, B.M.K.; Bronstein, M.; Chanson, P.; Fukuoka, H.; Gadelha, M.; et al. Diagnosis and management of prolactin-secreting pituitary adenomas: A Pituitary Society international Consensus Statement. Nat. Rev. Endocrinol. 2023, 19, 722–740. [Google Scholar] [CrossRef]
- Sekaran, S.; Vimalraj, S.; Thangavelu, L. The Physiological and Pathological Role of Tissue Nonspecific Alkaline Phosphatase beyond Mineralization. Biomolecules 2021, 11, 1564. [Google Scholar] [CrossRef]
- Rotimi, D.E.; Iyobhebhe, M.; Oluwayemi, E.T.; Olajide, O.P.; Akinsanola, B.A.; Evbuomwan, I.O.; Asaleye, R.M.; Ojo, O.A. Energy metabolism and spermatogenesis. Heliyon 2024, 10, e38591. [Google Scholar] [CrossRef]
- Chen, P.H.; Tjong, W.Y.; Yang, H.C.; Liu, H.Y.; Stern, A.; Chiu, D.T. Glucose-6-Phosphate Dehydrogenase, Redox Homeostasis and Embryogenesis. Int. J. Mol. Sci. 2022, 23, 2017. [Google Scholar] [CrossRef]
- Monageng, E.; Offor, U.; Takalani, N.B.; Mohlala, K.; Opuwari, C.S. A Review on the Impact of Oxidative Stress and Medicinal Plants on Leydig Cells. Antioxidants 2023, 12, 1559. [Google Scholar] [CrossRef]
- Narayanankutty, A.; Job, J.T.; Narayanankutty, V. Glutathione, an Antioxidant Tripeptide: Dual Roles in Carcinogenesis and Chemoprevention. Curr. Protein Pept. Sci. 2019, 20, 907–917. [Google Scholar] [CrossRef]
- Laborde, E. Glutathione transferases as mediators of signaling pathways involved in cell proliferation and cell death. Cell Death Differ. 2010, 17, 1373–1380. [Google Scholar] [CrossRef]
- Pardillo-Diaz, R.; Perez-Garcia, P.; Castro, C.; Nunez-Abades, P.; Carrascal, L. Oxidative Stress as a Potential Mechanism Underlying Membrane Hyperexcitability in Neurodegenerative Diseases. Antioxidants 2022, 11, 1511. [Google Scholar] [CrossRef] [PubMed]
- Battelli, M.G.; Polito, L.; Bortolotti, M.; Bolognesi, A. Xanthine Oxidoreductase-Derived Reactive Species: Physiological and Pathological Effects. Oxid. Med. Cell Longev. 2016, 2016, 3527579. [Google Scholar] [CrossRef] [PubMed]
- Mabrouk, A.; Ben Cheikh, H. Thymoquinone supplementation reverses lead-induced oxidative stress in adult rat testes. Gen. Physiol. Biophys. 2015, 34, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Vona, R.; Pallotta, L.; Cappelletti, M.; Severi, C.; Matarrese, P. The Impact of Oxidative Stress in Human Pathology: Focus on Gastrointestinal Disorders. Antioxidants 2021, 10, 201. [Google Scholar] [CrossRef]
- Khan, A.Q.; Rashid, K.; AlAmodi, A.A.; Agha, M.V.; Akhtar, S.; Hakeem, I.; Raza, S.S.; Uddin, S. Reactive oxygen species (ROS) in cancer pathogenesis and therapy: An update on the role of ROS in anticancer action of benzophenanthridine alkaloids. Biomed. Pharmacother. 2021, 143, 112142. [Google Scholar] [CrossRef]
- Soliman, A.M.; Barreda, D.R. Acute Inflammation in Tissue Healing. Int. J. Mol. Sci. 2023, 24, 641. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Sheng, J.A.; Bales, N.J.; Myers, S.A.; Bautista, A.I.; Roueinfar, M.; Hale, T.M.; Handa, R.J. The Hypothalamic-Pituitary-Adrenal Axis: Development, Programming Actions of Hormones, and Maternal-Fetal Interactions. Front. Behav. Neurosci. 2020, 14, 601939. [Google Scholar] [CrossRef]
- Fouad, A.A.; Jresat, I. Thymoquinone therapy abrogates toxic effect of cadmium on rat testes. Andrologia 2015, 47, 417–426. [Google Scholar] [CrossRef]
- Leong, X.F.; Choy, K.W.; Alias, A. Anti-Inflammatory Effects of Thymoquinone in Atherosclerosis: A Mini Review. Front. Pharmacol. 2021, 12, 758929. [Google Scholar] [CrossRef]
- Singh, V.; Khurana, A.; Navik, U.; Allawadhi, P.; Bharani, K.K.; Weiskirchen, R. Apoptosis and Pharmacological Therapies for Targeting Thereof for Cancer Therapeutics. Sci. 2022, 4, 15. [Google Scholar] [CrossRef]
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of apoptosis in health and disease: The balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193. [Google Scholar] [CrossRef] [PubMed]
- Edlich, F. BCL-2 proteins and apoptosis: Recent insights and unknowns. Biochem. Biophys. Res. Commun. 2018, 500, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Vaddavalli, P.L.; Schumacher, B. The p53 network: Cellular and systemic DNA damage responses in cancer and aging. Trends Genet. 2022, 38, 598–612. [Google Scholar] [CrossRef]
- Shen, J.; Wang, Q.; Mao, Y.; Gao, W.; Duan, S. Targeting the p53 signaling pathway in cancers: Molecular mechanisms and clinical studies. MedComm 2023, 4, e288. [Google Scholar] [CrossRef]
- Sheikhbahaei, F.; Khazaei, M.; Rabzia, A.; Mansouri, K.; Ghanbari, A. Protective Effects of Thymoquinone against Methotrexate-Induced Germ Cell Apoptosis in Male Mice. Int. J. Fertil. Steril. 2016, 9, 541–547. [Google Scholar]
- Erboga, M.; Aktas, C.; Kurt, O.; Uygur, R.; Caglar, V.; Turan, B.C.; Topcu, B.; Fidanol Erboga, Z.; Gurel, A.; Ozen, O.A. Protective effects of thymoquinone on experimental testicular ischaemia-reperfusion injury: An apoptotic, proliferative and biochemical study. Andrologia 2016, 48, 222–230. [Google Scholar] [CrossRef]
- Homayoonfal, M.; Asemi, Z.; Yousefi, B. Potential anticancer properties and mechanisms of thymoquinone in osteosarcoma and bone metastasis. Cell. Mol. Biol. Lett. 2022, 27, 21. [Google Scholar] [CrossRef]
- Adinew, G.M.; Messeha, S.S.; Taka, E.; Badisa, R.B.; Antonie, L.M.; Soliman, K.F.A. Thymoquinone Alterations of the Apoptotic Gene Expressions and Cell Cycle Arrest in Genetically Distinct Triple-Negative Breast Cancer Cells. Nutrients 2022, 14, 2120. [Google Scholar] [CrossRef]
- Kalender, S.; Uzun, F.G.; Demir, F.; Uzunhisarcikli, M.; Aslanturk, A. Mercuric chloride-induced testicular toxicity in rats and the protective role of sodium selenite and vitamin E. Food Chem. Toxicol. 2013, 55, 456–462. [Google Scholar] [CrossRef]
- El-Desoky, G.E.; Bashandy, S.A.; Alhazza, I.M.; Al-Othman, Z.A.; Aboul-Soud, M.A.; Yusuf, K. Improvement of mercuric chloride-induced testis injuries and sperm quality deteriorations by Spirulina platensis in rats. PLoS ONE 2013, 8, e59177. [Google Scholar] [CrossRef]
- Owumi, S.; Chimezie, J.; Otunla, M.; Oluwawibe, B.; Agbarogi, H.; Anifowose, M.; Arunsi, U.; Owoeye, O. Prepubertal Repeated Berberine Supplementation Enhances Cerebrocerebellar Functions by Modulating Neurochemical and Behavioural Changes in Wistar Rats. J. Mol. Neurosci. 2024, 74, 72. [Google Scholar] [CrossRef]
- Lee, T.W.; Bai, K.J.; Lee, T.I.; Chao, T.F.; Kao, Y.H.; Chen, Y.J. PPARs modulate cardiac metabolism and mitochondrial function in diabetes. J. Biomed. Sci. 2017, 24, 5. [Google Scholar] [CrossRef]
- Savkur, R.S.; Miller, A.R. Investigational PPAR-gamma agonists for the treatment of Type 2 diabetes. Expert. Opin. Investig. Drugs 2006, 15, 763–778. [Google Scholar] [CrossRef]
- Okada-Iwabu, M.; Yamauchi, T.; Iwabu, M.; Honma, T.; Hamagami, K.; Matsuda, K.; Yamaguchi, M.; Tanabe, H.; Kimura-Someya, T.; Shirouzu, M.; et al. A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature 2013, 503, 493–499. [Google Scholar] [CrossRef]
- Billin, A.N. PPAR-beta/delta agonists for Type 2 diabetes and dyslipidemia: An adopted orphan still looking for a home. Expert. Opin. Investig. Drugs 2008, 17, 1465–1471. [Google Scholar] [CrossRef]






| Control | HgCl2 | TQ | HgCl2 + TQ1 | HgCl2 + TQ2 | |
|---|---|---|---|---|---|
| * Total Rats Per Grouping | (8) | (8) | (8) | (8) | (8) |
| Initial body weight (g) | 162.20 ± 10.72 | 156.70 ± 7.45 | 166.70 ± 15.26 | 162.00 ± 9.62 | 161.60 ± 13.99 |
| Final body weight (g) | 219.00 ± 8.08 | 209.40 ± 9.64 * | 225.10 ± 22.78 | 227.10 ± 14.60 | 238.40 ± 13.10 # |
| Body weight gain (g) | 60.86 ± 8.07 | 57.00 ± 12.45 | 61.29 ± 14.20 ns | 65.25 ± 10.26 #,* | 72.63 ± 16.18 #,* |
| Testes weight (g) | 2.50 ± 0.12 | 2.32 ± 0.07 | 2.52 ± 0.13 ns | 2.63 ± 0.17 ns | 2.73 ± 0.22 ns |
| Relative testes weight (%) | 1.14 ± 0.18 | 1.11 ± 0.08 | 1.12 ± 0.13 ns | 1.16 ± 0.09 ns | 1.15 ± 0.10 ns |
| Epididymides weight (g) | 0.29 ± 0.01 | 0.29 ± 0.01 | 0.35 ± 0.04 ns | 0.30 ± 0.03 ns | 0.34 ± 0.05 ns |
| Relative Epididymides weight (%) | 0.13 ± 0.01 | 0.14 ± 0.01 | 0.16 ± 0.02 ns | 0.13 ± 0.01 ns | 0.14 ± 0.01 ns |
| Hypothalamus Weight (g) | 0.09 ± 0.01 | 0.07 ± 0.03 | 0.05 ± 0.01 ns | 0.08 ± 0.02 ns | 0.07 ± 0.02 ns |
| Relative Hypothalamus Weight (%) | 0.04 ± 0.01 | 0.03 ± 0.01 | 0.02 ± 0.01 ns | 0.04 ± 0.01 ns | 0.03 ± 0.01 ns |
| Control | HgCl2 | TQ | HgCl2 + TQ1 | HgCl2 + TQ2 | |
|---|---|---|---|---|---|
| * Total Rats Per Grouping | (8) | (8) | (8) | (8) | (8) |
| Sperm Functional Analysis | |||||
| Motility | 90.00 ± 4.62 | 75.00 ± 5.34 **** | 72.50 ± 4.62 | 67.50 ± 4.62 * | 65.00 ± 5.34 ** |
| Viability | 96.50 ± 1.60 | 96.13 ± 1.55 | 96.50 ± 1.60 | 96.50 ± 1.64 | 94.88 ± 4.25 |
| Sperm Volume | 5.16 ± 0.05 | 5.17 ± 0.04 | 5.17 ± 0.04 | 5.18 ± 0.03 | 5.18 ± 0.05 |
| Epididymal Sperm Count | 132.40 ± 9.89 | 117.00 ± 11.10 * | 113.00 ± 8.55 | 101.90 ± 8.25 * | 98.50 ± 10.61 ** |
| Sperm Abnormalities | |||||
| Abnormality of the Head (%) | 2.08 ± 0.27 | 2.14 ± 0.13 | 2.15 ± 0.30 | 2.23 ± 0.31 | 2.17 ± 0.53 |
| Abnormality of the Midpiece (%) | 4.21 ± 0.38 | 4.65 ± 0.25 | 4.67 ± 0.35 | 4.86 ± 0.38 | 5.14 ± 0.25 |
| Abnormality of the Tail (%) | 5.23 ± 0.45 | 5.61 ± 0.53 | 5.56 ± 0.56 | 5.88 ± 0.93 | 6.25 ± 0.48 |
| Total Abnormality (%) | 11.52 ± 0.44 | 12.41 ± 0.63 | 12.38 ± 0.63 | 12.98 ± 1.28 | 13.56 ± 1.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Owumi, S.; Otunla, M.; Akindipe, P.; Arunsi, U.; Babalola, J.O.; Irozuru, C.E.; Altayyar, A.; Oluwawibe, B.; Owoeye, O.; Oyelere, A.K. Thymoquinone Protective Effect Against Mercury-Induced Reproductive Derangement in Rats: In Vivo and In Silico Investigation. Toxics 2025, 13, 896. https://doi.org/10.3390/toxics13100896
Owumi S, Otunla M, Akindipe P, Arunsi U, Babalola JO, Irozuru CE, Altayyar A, Oluwawibe B, Owoeye O, Oyelere AK. Thymoquinone Protective Effect Against Mercury-Induced Reproductive Derangement in Rats: In Vivo and In Silico Investigation. Toxics. 2025; 13(10):896. https://doi.org/10.3390/toxics13100896
Chicago/Turabian StyleOwumi, Solomon, Moses Otunla, Pelumi Akindipe, Uche Arunsi, Jesutosin O. Babalola, Chioma E. Irozuru, Ahmad Altayyar, Bayode Oluwawibe, Olatunde Owoeye, and Adegboyega K. Oyelere. 2025. "Thymoquinone Protective Effect Against Mercury-Induced Reproductive Derangement in Rats: In Vivo and In Silico Investigation" Toxics 13, no. 10: 896. https://doi.org/10.3390/toxics13100896
APA StyleOwumi, S., Otunla, M., Akindipe, P., Arunsi, U., Babalola, J. O., Irozuru, C. E., Altayyar, A., Oluwawibe, B., Owoeye, O., & Oyelere, A. K. (2025). Thymoquinone Protective Effect Against Mercury-Induced Reproductive Derangement in Rats: In Vivo and In Silico Investigation. Toxics, 13(10), 896. https://doi.org/10.3390/toxics13100896









