Zebrafish Embryo Developmental Toxicity Assay (ZEDTA) for Regulatory Testing—Protocol Optimization and Historical Control Data
Abstract
1. Introduction
2. Materials and Methods
2.1. General Study Design
2.2. Optimization of Study Protocol
- Use of solvent (0.5% v/v DMSO vs. blank exposure medium). In the experiments in which the effects of DMSO were tested, a volume of the blank medium was spiked with DMSO (Merck, Darmstadt, Germany) at a loading of 5 mL per liter medium and subsequently distributed to test vessels. In total, 1081 zebrafish embryos/larvae were tested in a blank control (47 plates) and 241 embryos/larvae with a solvent control (12 plates), with different combinations of the remaining parameters in valid experiments.
- Temperature (26 vs. 28 °C). In total, 842 embryos/larvae (38 plates) were tested at 26 °C and 480 embryos/larvae (21 plates) at 28 °C, with different combinations of the remaining parameters in valid experiments.
- Renewal periods (static vs. semi-static). For the semi-static exposure, during the renewal of test solutions, the old medium was removed using a Pasteur pipette either every 24 h or once after 48 h of exposure. Each time a small volume of medium was left to prevent exposure of the embryo/larvae to air. Fresh medium, pre-heated to the desired temperature, was added with a pipette. Care was taken not to touch the embryo/larvae. In total, 930 embryos/larvae were exposed in a 24 h semi-static design (42 plates), 48 embryos/larvae in a 48 h semi-static design (7 plates), and 231 embryos/larvae in a static design (10 plates), with different combinations of the remaining parameters in valid experiments.
- Exposure vessels (24-well vs. 96-well plates). Twenty-four embryos/larvae were exposed in 24-well plates; each well contained 2.0 mL of medium. Twenty embryos/larvae were exposed in 96-well plates; each well contained 0.2 mL of medium. One embryo/larva was exposed per well. In total, 1143 embryos/larvae were tested on 49 “24-well” plates and 179 embryos/larvae on 10 “96-well plates”, with different combinations of the remaining parameters in valid experiments.
2.2.1. Growth
2.2.2. Data Evaluation
- Survival (%) was calculated as a percentage of the number of larvae surviving the 96 h exposure period divided by the initial number of eggs.
- Effects on development as assessed with the extended GMS were based on the most frequent score (mode) on a given time point per plate.
- Teratogenicity:
- -
- Individual malformations: For each plate, the percentage of larvae showing a given malformation was calculated.
- -
- Total score: For each organism, a teratogenic score, i.e., a sum of all malformations, was calculated (integer 0–7; 0: no malformations; 7: seven malformations). Next, the average total score per plate was calculated.
2.3. Historical Control Data Obtained with the Optimized Protocol
- For each experiment, twenty-four embryos in the blastula phase were exposed to the exposure medium on a 24-well plate.
- Four embryos (last column of the well plate) served as internal control and were scored only for survival but not for development and teratogenicity.
- Exposure temperature was 26 ± 1.0 °C, and the medium was renewed once after 48 h.
3. Results
3.1. Optimization of Study Protocol
3.1.1. Mortality
3.1.2. Development
3.1.3. Growth
3.1.4. Malformations
3.2. Control Data of Optimized Protocol
3.2.1. Mortality
3.2.2. Development
3.2.3. Malformations
4. Discussion
- An incubation temperature of 26 °C.
- A 24-well plate (2 mL per well) combined with self-adhesive film as a test chamber.
- Static, semi-static, and flow-through systems were discussed as exposure methods, but an optimal method was not defined.
- In total, 0.1% v/v DMSO was considered safe for use, but it was noted that the most optimal would be to perform the assay without any solvent.
4.1. Historical Control Data Derived from the Optimized ZEDTA Protocol [20]
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Glossary
| Egg | Fertilized egg including embryo, perivitelline space and chorion. |
| Embryo | Organism before hatching. |
| Larvae | Organism after hatching. |
| Static exposure | No renewal of exposure medium during the entire exposure period |
| Semi-static exposure | Renewal of exposure medium at defined intervals. |
| Malformation | A structural defect in the body due to abnormal embryonic or larval development. |
| Dead embryo (coagulated) | Macroscopic appearance on dark underground is white, and microscopic appearance is brownish, hampering the transparency of the embryo. This is accompanied by lack of heartbeat when observed at 48 h post-fertilization and later. |
| Dead larvae | Immobility and/or absence of respiratory movement and/or absence of a heartbeat and/or white opaque coloration of the central nervous system and/or lack of reaction to mechanical stimulus. |
References
- OECD. Test No. 414: Prenatal Developmental Toxicity Study; OECD: Paris, France, 2018. [Google Scholar] [CrossRef]
- Series 870—Health Effects Test Guidelines|US EPA. Available online: https://www.epa.gov/test-guidelines-pesticides-and-toxic-substances/series-870-health-effects-test-guidelines (accessed on 20 February 2025).
- EMEA ICH Topic S 5 (R3) Detection of Reproductive and Developmental Toxicity for Human Pharmaceuticals. Available online: https://www.ema.europa.eu/en/ich-s5-r3-guideline-detection-reproductive-developmental-toxicity-human-pharmaceuticals-scientific-guideline (accessed on 18 August 2021).
- Panzica-Kelly, J.M.; Zhang, C.X.; Augustine-Rauch, K.A. Optimization and Performance Assessment of the Chorion-off[Dechorinated] Zebrafish Developmental Toxicity Assay. Toxicol. Sci. 2015, 146, 127–134. [Google Scholar] [CrossRef]
- Dach, K.; Yaghoobi, B.; Schmuck, M.R.; Carty, D.R.; Morales, K.M.; Lein, P.J. Teratological and Behavioral Screening of the National Toxicology Program 91-Compound Library in Zebrafish (Danio rerio). Toxicol Sci. 2019, 167, 77–91. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Spence, R.; Gerlach, G.; Lawrence, C.; Smith, C. The Behaviour and Ecology of the Zebrafish, Danio Rerio. Biol. Rev. Camb. Philos. Soc. 2008, 83, 13–34. [Google Scholar] [CrossRef] [PubMed]
- European Parliament Directive 2010/63/EU—On the Protection of Animals Used for Scientific Purposes. Off. J. Eur. Union 2010, 33–79. Available online: http://data.europa.eu/eli/dir/2010/63/oj (accessed on 6 October 2025).
- Strähle, U.; Scholz, S.; Geisler, R.; Greiner, P.; Hollert, H.; Rastegar, S.; Schumacher, A.; Selderslaghs, I.; Weiss, C.; Witters, H.; et al. Zebrafish Embryos as an Alternative to Animal Experiments—A Commentary on the Definition of the Onset of Protected Life Stages in Animal Welfare Regulations. Reprod. Toxicol. 2012, 33, 128–132. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of Embryonic Development of the Zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 236: Fish Embryo Acute Toxicity (FET) Test. In OECD Guidelines for the Testing of Chemicals; OECD: Paris, France, 2013; ISBN 9789264203709. [Google Scholar]
- Gustafson, A.L.; Stedman, D.B.; Ball, J.; Hillegass, J.M.; Flood, A.; Zhang, C.X.; Panzica-Kelly, J.; Cao, J.; Coburn, A.; Enright, B.P.; et al. Inter-Laboratory Assessment of a Harmonized Zebrafish Developmental Toxicology Assay—Progress Report on Phase I. Reprod. Toxicol. 2012, 33, 155–164. [Google Scholar] [CrossRef]
- Panzica-Kelly, J.M.; Zhang, C.X.; Danberry, T.L.; Flood, A.; DeLan, J.W.; Brannen, K.C.; Augustine-Rauch, K.A. Morphological Score Assignment Guidelines for the Dechorionated Zebrafish Teratogenicity Assay. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2010, 89, 382–395. [Google Scholar] [CrossRef]
- Brannen, K.C.; Panzica-Kelly, J.M.; Danberry, T.L.; Augustine-Rauch, K.A. Development of a Zebrafish Embryo Teratogenicity Assay and Quantitative Prediction Model. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2010, 89, 66–77. [Google Scholar] [CrossRef]
- Panzica-Kelly, J.M.; Zhang, C.X.; Augustine-Rauch, K. Zebrafish embryo developmental toxicology assay. Methods Mol. Biol. 2012, 889, 25–50. [Google Scholar] [CrossRef]
- Beekhuijzen, M.; de Koning, C.; Flores-Guillén, M.-E.; de Vries-Buitenweg, S.; Tobor-Kaplon, M.; van de Waart, B.; Emmen, H. From cutting edge to guideline: A first step in harmonization of the zebrafish embryotoxicity test (ZET) by describing the most optimal test conditions and morphology scoring system. Reprod. Toxicol. 2015, 56, 64–76. [Google Scholar] [CrossRef]
- Mori, K.; Aoki, Y.; Hayashi, M.; Sugimoto, W.; Ono, M.; Umekita, S.; Niino, T.; Ebata, T.; Mikashima, F.; Maki, K.; et al. Variation and classification of chemically-induced zebrafish malformations for the ICH S5 (R3) guideline: An atlas for zebrafish teratogenesis. J. Toxicol. Sci. 2025, 50, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Vargesson, N. Zebrafish. In Manual of Animal Technology; Barnett, S.W., Ed.; Wiley-Blackwell: Oxford, UK, 2007; pp. 78–84. [Google Scholar]
- Beasley, A.; Elrod-Erickson, M.; Otter, R.R. Consistency of Morphological Endpoints Used to Assess Developmental Timing in Zebrafish (Danio Rerio) across a Temperature Gradient. Reprod. Toxicol. 2012, 34, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Selderslaghs, I.W.T.; Van Rompay, A.R.; De Coen, W.; Witters, H.E. Development of a Screening Assay to Identify Teratogenic and Embryotoxic Chemicals Using the Zebrafish Embryo. Reprod. Toxicol. 2009, 28, 308–320. [Google Scholar] [CrossRef]
- Sibly, R.M.; Calow, P. A Life-cycle Theory of Responses to Stress. Biol. J. Linn. Soc. 1989, 37, 101–116. [Google Scholar] [CrossRef]
- Wilson, L.B.; Truong, L.; Simonich, M.T.; Tanguay, R.L. Systematic Assessment of Exposure Variations on Observed Bioactivity in Zebrafish Chemical Screening. Toxics 2020, 8, 87. [Google Scholar] [CrossRef]
- OECD. Guidance Document on Aquatic Toxicity Testing of Difficult Substances and Mixtures. Series on Testing and Assessment No. 23 (2nd Edition). Organ. Econ. Co-Oper. Dev. 2019, 23, 1–81. [Google Scholar] [CrossRef]
- Hutchinson, T.H.; Shillabeer, N.; Winter, M.J.; Pickford, D.B. Acute and Chronic Effects of Carrier Solvents in Aquatic Organisms: A Critical Review. Aquat. Toxicol. 2006, 76, 69–92. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 231: Amphibian Metamorphosis Assay; OECD: Paris, France, 2009. [Google Scholar] [CrossRef]
- Henn, K.; Braunbeck, T. Dechorionation as a Tool to Improve the Fish Embryo Toxicity Test (FET) with the Zebrafish (Danio Rerio). Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 2011, 153, 91–98. [Google Scholar] [CrossRef]
- Mandrell, D.; Truong, L.; Jephson, C.; Sarker, M.R.; Moore, A.; Lang, C.; Simonich, M.T.; Tanguay, R.L. Automated Zebrafish Chorion Removal and Single Embryo Placement: Optimizing Throughput of Zebrafish Developmental Toxicity Screens. J. Lab. Autom. 2012, 17, 66–74. [Google Scholar] [CrossRef]
- Hamm, J.T.; Ceger, P.; Allen, D.; Stout, M.; Maull, E.A.; Baker, G.; Zmarowski, A.; Padilla, S.; Perkins, E.; Planchart, A.; et al. Characterizing sources of variability in zebrafish embryo screening protocols. ALTEX 2019, 36, 103–120. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
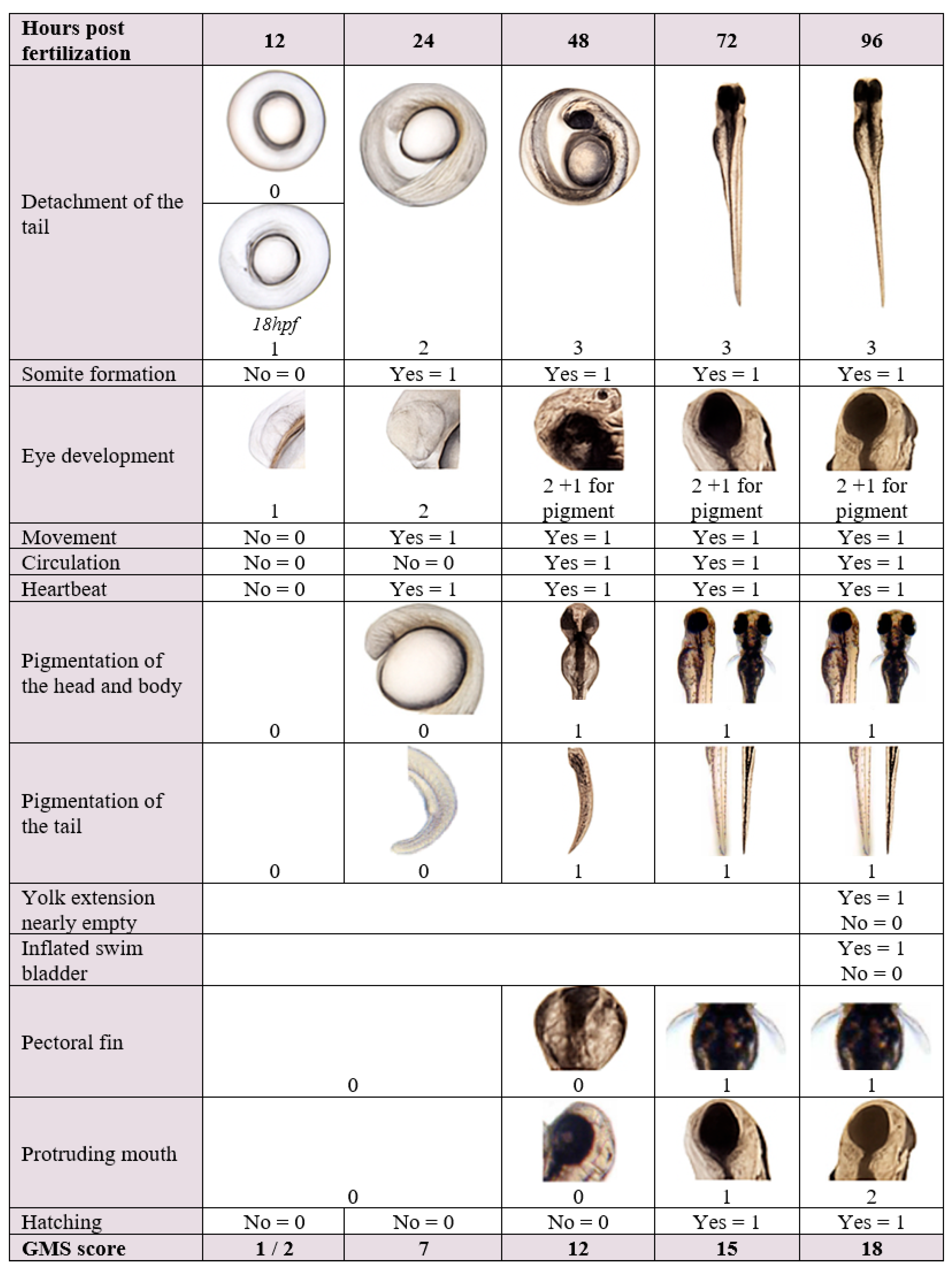
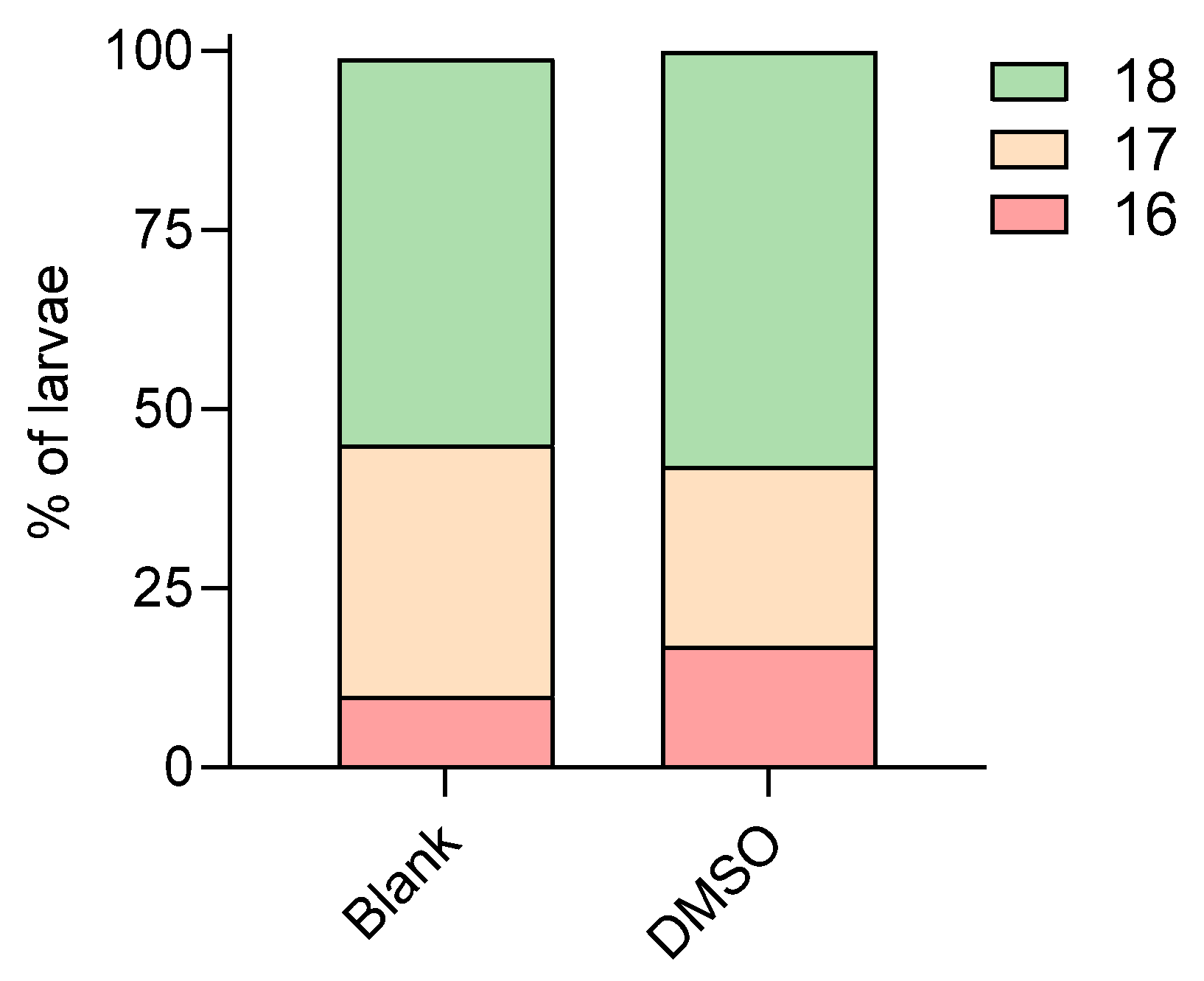



| Endpoints Assessed in the Embryo/Larvae | Description |
|---|---|
| Malformation of the head | Missing structures such as the jaw and the eyes. Uneven eye shape, head shape, edema, etc. |
| Malformation of the sacculi/otoliths | Missing structures, duplicated structures, or uneven shape. |
| Malformation of the tail | Kinked tails. |
| Malformation of the heart | Irregular shape due to edema or aplasia. Abnormal heartbeat. |
| Malformed body shape | Scoliosis and rachischisis. Also, the notochord presence and its morphology are assessed. |
| Yolk malformation | Edema. |
| Pectoral fins | Irregular shape or absence. |
| Control Treatment | Temp. (°C) | Well Plate | Refreshment Interval (h) | Eggs per Plate | Total Number of Plates | Total Number of Surviving Larvae | Survival (%) | |
|---|---|---|---|---|---|---|---|---|
| Mean | St Dev | |||||||
| Blank | 26 | 24 | 24 | 24 | 17 | 386 | 95 | 6.7 |
| 48 | 24 | 6 | 138 | 96 | 6.5 | |||
| 96 | 24 | 7 | 159 | 95 | 7.9 | |||
| 26 | 96 | 24 | 20 | 2 | 37 | 93 | 3.5 | |
| 28 | 24 | 24 | 24 | 10 | 231 | 96 | 5.0 | |
| 48 | 24 | 1 | 23 | 96 | n.a. | |||
| 96 | 24 | 3 | 72 | 100 | 0.0 | |||
| 96 | 24 | 20 | 2 | 35 | 88 | 3.5 | ||
| 0.5% v/v DMSO | 26 | 24 | 24 | 24 | 3 | 67 | 93 | 8.7 |
| 96 | 24 | 20 | 3 | 55 | 92 | 7.6 | ||
| 28 | 24 | 24 | 24 | 3 | 67 | 93 | 6.4 | |
| 96 | 24 | 20 | 3 | 52 | 87 | 2.9 | ||
| Control Treatment | Temp. (°C) | Well Plate | Refreshment Interval (h) | Eggs per Plate | GMS at Time of Exposure 1 | |||
|---|---|---|---|---|---|---|---|---|
| 24 h (7) | 48 h (12) | 72 h (15) | 96 h 2 (18) | |||||
| Blank | 26 | 24 | 24 | 24 | 7 | 12 | 15 | 18 |
| 48 | 24 | 7 | 12 | 14 | 18 | |||
| 96 | 24 | 7 | 12 | 15 | 16 | |||
| 26 | 96 | 24 | 20 | 7 | 12 | 15 | 17 | |
| 28 | 24 | 24 | 24 | 7 | 12 | 15 | 18 | |
| 48 3 | 24 3 | 7 3 | 12 3 | 15 3 | 18 3 | |||
| 96 | 24 | 7 | 12 | 15 | 17 | |||
| 96 | 24 | 20 | 7 | 12 | 15 | 17 | ||
| 0.5% v/v DMSO | 26 | 24 | 24 | 24 | 7 | 12 | 15 | 18 |
| 96 | 24 | 20 | 7 | 11 | 15 | 18 | ||
| 28 | 24 | 24 | 24 | 7 | 12 | 15 | 18 | |
| 96 | 24 | 20 | 7 | 12 | 15 | 17 | ||
| Control Treatment | Temp. (°C) | Well Plate | Refreshment Interval (h) | Total Number of Plates | % of Larvae Showing a Given Score | ||
|---|---|---|---|---|---|---|---|
| 16 | 17 | 18 | |||||
| Blank | 26 | 24 | 24 | 17 | 0 | 41 | 59 |
| 48 | 6 | 0 | 0 | 100 | |||
| 96 | 7 | 71 | 29 | 0 | |||
| 26 | 96 | 24 | 2 | 0 | 100 | 0 | |
| 28 | 24 | 24 | 10 | 0 | 20 | 80 | |
| 48 | 1 | 0 | 0 | 100 | |||
| 96 | 3 | 0 | 67 | 33 | |||
| 96 | 24 | 2 | 0 | 100 | 0 | ||
| 0.5% v/v DMSO | 26 | 24 | 24 | 3 | 0 | 0 | 100 |
| 96 | 24 | 3 | 33 | 33 | 33 | ||
| 28 | 24 | 24 | 3 | 0 | 0 | 100 | |
| 96 | 24 | 3 | 33 | 67 | 0 | ||
| Control Treatment | Temp. (°C) | Well Plate | Refreshment Interval (h) | Average Length (mm) 1 | Std Dev | Number of Examined Larvae |
|---|---|---|---|---|---|---|
| Blank | 26 | 24 | 24 | 3.04 | 1.08 | 210 |
| 48 | 3.66 | 0.22 | 30 | |||
| 96 | 1.66 | 0.18 | 70 | |||
| 96 | 24 | 3.58 | 0.24 | 60 | ||
| 28 | 24 | 24 | 3.49 | 0.85 | 160 | |
| 48 | 3.63 | 0.30 | 30 | |||
| 96 | 1.65 | 0.17 | 40 | |||
| 96 | 24 | 3.37 | 0.27 | 60 |
| Control Treatment | Temp. (°C) | Well Plate | Refreshment Interval (h) | Number of Examined Larvae | Percentage of Larvae Showing Given Malformations 1 | Average Teratogenic Score 2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Head | Sacculi/Otholiths | Tail | Heart | Body Shape | Yolk | ||||||
| Blank | 26 | 24 | 24 | 386 | 0.52 (2) | 0.26 (1) | 4.7 (18) | 11 (41) | 3.9 (15) | 4.7 (18) | 0.25 |
| 48 | 138 | 0.72 (1) | 0 | 1.4 (2) | 3.6 (5) | 1.4 (2) | 20 (27) | 0.27 | |||
| 96 | 159 | 0 | 0 | 1.3 (2) | 20 (32) | 3.1 (5) | 14 (22) | 0.39 | |||
| 26 | 96 | 24 | 37 | 0 | 0 | 2.7 (1) | 41 (15) | 5.4 (2) | 27 (10) | 0.74 | |
| 28 | 24 | 24 | 231 | 1.3 (3) | 0.87 (2) | 2.6 (6) | 9.1 (21) | 2.6 (6) | 2.6 (6) | 0.19 | |
| 48 | 23 | 0 | 0 | 0 | 8.7 (2) | 0 | 4.3 (1) | 0.13 | |||
| 96 | 72 | 0 | 0 | 0 | 8.3 (6) | 0 | 13 (9) | 0.21 | |||
| 96 | 24 | 35 | 2.9 (1) | 0 | 0 | 14 (5) | 5.7 (2) | 14 (5) | 0.37 | ||
| 0.5% v/v DMSO | 26 | 24 | 24 | 67 | 1.5 (1) | 0 | 4.5 (3) | 4.5 (3) | 3.0 (2) | 3.0 (2) | 0.16 |
| 96 | 24 | 55 | 0 | 0 | 5.5 (3) | 9.1 (5) | 0 | 5.5 (3) | 0.19 | ||
| 28 | 24 | 24 | 67 | 0 | 0 | 3.0 (2) | 3.0 (2) | 0 | 0 | 0.056 | |
| 96 | 24 | 52 | 9.6 (5) | 0 | 3.8 (2) | 31 (16) | 15 (8) | 7.7 (4) | 0.68 | ||
| Hours of Exposure (Maximal Possible Score) | % Zebrafish with a Given Score | Total Examined | Mortality (%) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 4 | 5 | 6 | 7 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |||
| 24 (7) | 0.21 | 0.41 | 0.41 | 13 | 86 | 486 1 | 2.9 | ||||||||||
| 48 (12) | 0.20 | 0.20 | 0.60 | 0.20 | 4.4 | 94 | 503 | 3.3 | |||||||||
| 72 (15) | 0.21 | 0.21 | 0.21 | 2.1 | 40 | 57 | 485 2 | 3.3 | |||||||||
| 96 (18) | 0.20 | 0.20 | 0.80 | 1.2 | 6.6 | 29 | 62 | 502 | 3.5 | ||||||||
| Total Number of Malformations per Larvae | Number of Larvae (% of Total) | % of Surviving Larvae |
|---|---|---|
| 1 | 26 (68) | 5.2 |
| 2 | 5 (13) | 1.0 |
| 3 | 3 (7.9) | 0.60 |
| 4 | 2 (5.3) | 0.40 |
| 5 | 0 (0) | 0.0 |
| 6 | 1 (2.6) | 0.20 |
| 7 | 1 (2.6) | 0.20 |
| Total larvae | 38 | 7.6 |
| Malformation | Number of Malformations (% of Total) | % of Surviving Larvae |
|---|---|---|
| Sacculi/otholiths | 1 (1.5) | 0.20 |
| Body shape | 4 (6.1) | 0.80 |
| Pectoral fins | 6 (9.1) | 1.20 |
| Head | 8 (12) | 1.59 |
| Heart | 13 (20) | 2.59 |
| Tail | 14 (21) | 2.79 |
| Yolk | 20 (30) | 3.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van den Oetelaar, D.; Tobor-Kapłon, M.A.; Reijnaers, M.; Beekhuijzen, M. Zebrafish Embryo Developmental Toxicity Assay (ZEDTA) for Regulatory Testing—Protocol Optimization and Historical Control Data. Toxics 2025, 13, 874. https://doi.org/10.3390/toxics13100874
van den Oetelaar D, Tobor-Kapłon MA, Reijnaers M, Beekhuijzen M. Zebrafish Embryo Developmental Toxicity Assay (ZEDTA) for Regulatory Testing—Protocol Optimization and Historical Control Data. Toxics. 2025; 13(10):874. https://doi.org/10.3390/toxics13100874
Chicago/Turabian Stylevan den Oetelaar, Daphne, Marysia Agnieszka Tobor-Kapłon, Mèlanie Reijnaers, and Manon Beekhuijzen. 2025. "Zebrafish Embryo Developmental Toxicity Assay (ZEDTA) for Regulatory Testing—Protocol Optimization and Historical Control Data" Toxics 13, no. 10: 874. https://doi.org/10.3390/toxics13100874
APA Stylevan den Oetelaar, D., Tobor-Kapłon, M. A., Reijnaers, M., & Beekhuijzen, M. (2025). Zebrafish Embryo Developmental Toxicity Assay (ZEDTA) for Regulatory Testing—Protocol Optimization and Historical Control Data. Toxics, 13(10), 874. https://doi.org/10.3390/toxics13100874






