A Fenton Oxidation-Based Integrated Strategy for the Treatment of Raw Gasoline Alkali Residue in Kashi
Abstract
1. Introduction
1.1. Sources and Properties of the Gasoline Alkali Residue Stock Solution
1.2. Treatment Mode of Gasoline Alkali Residue Crude Fluid
1.3. Fenton Reagent Treatment of Gasoline Alkali Residue Stock Solution from Kashi Refinery
2. Experimental Section
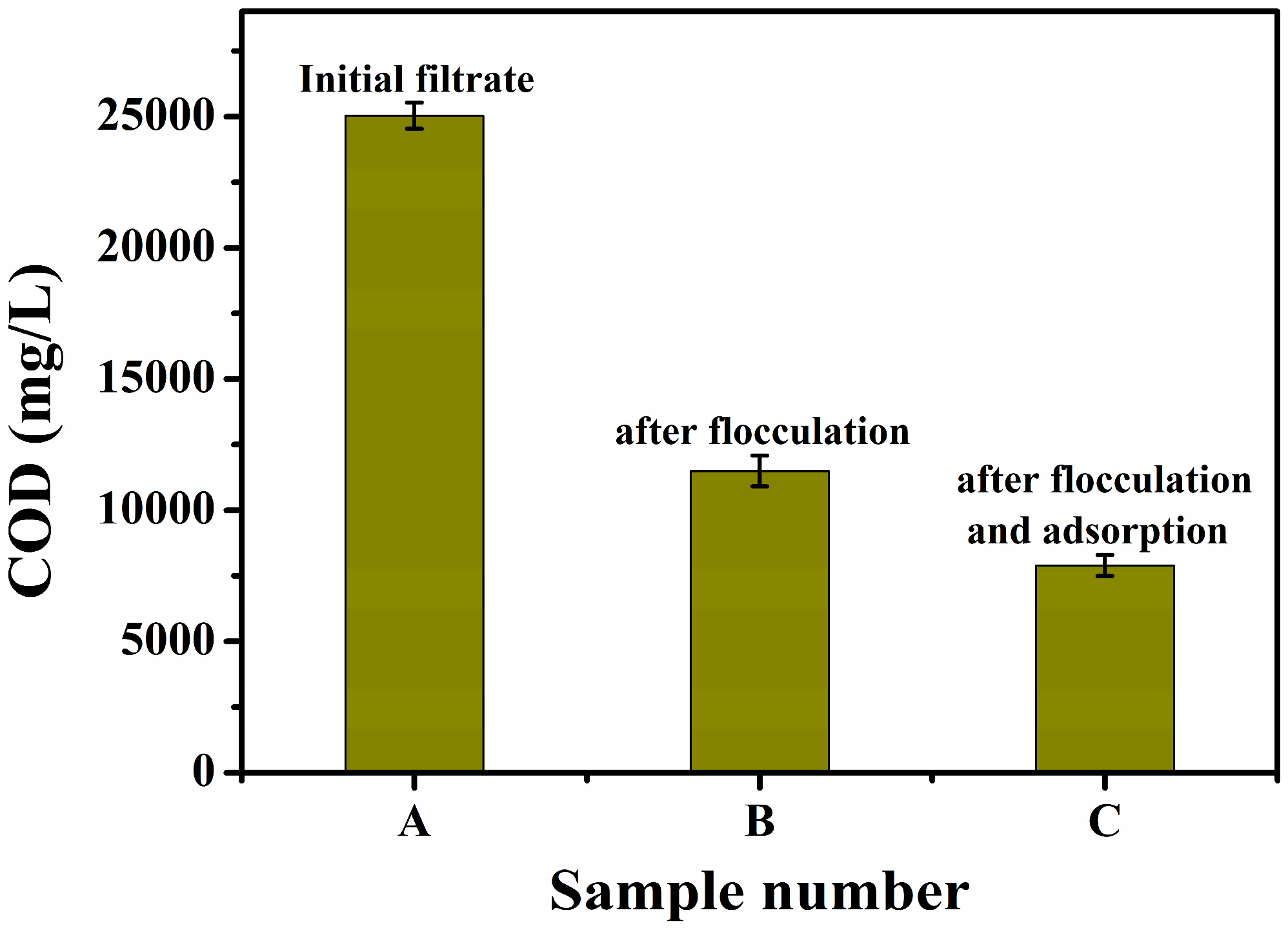
2.1. Pretreatment of the Alkali Residue Liquid
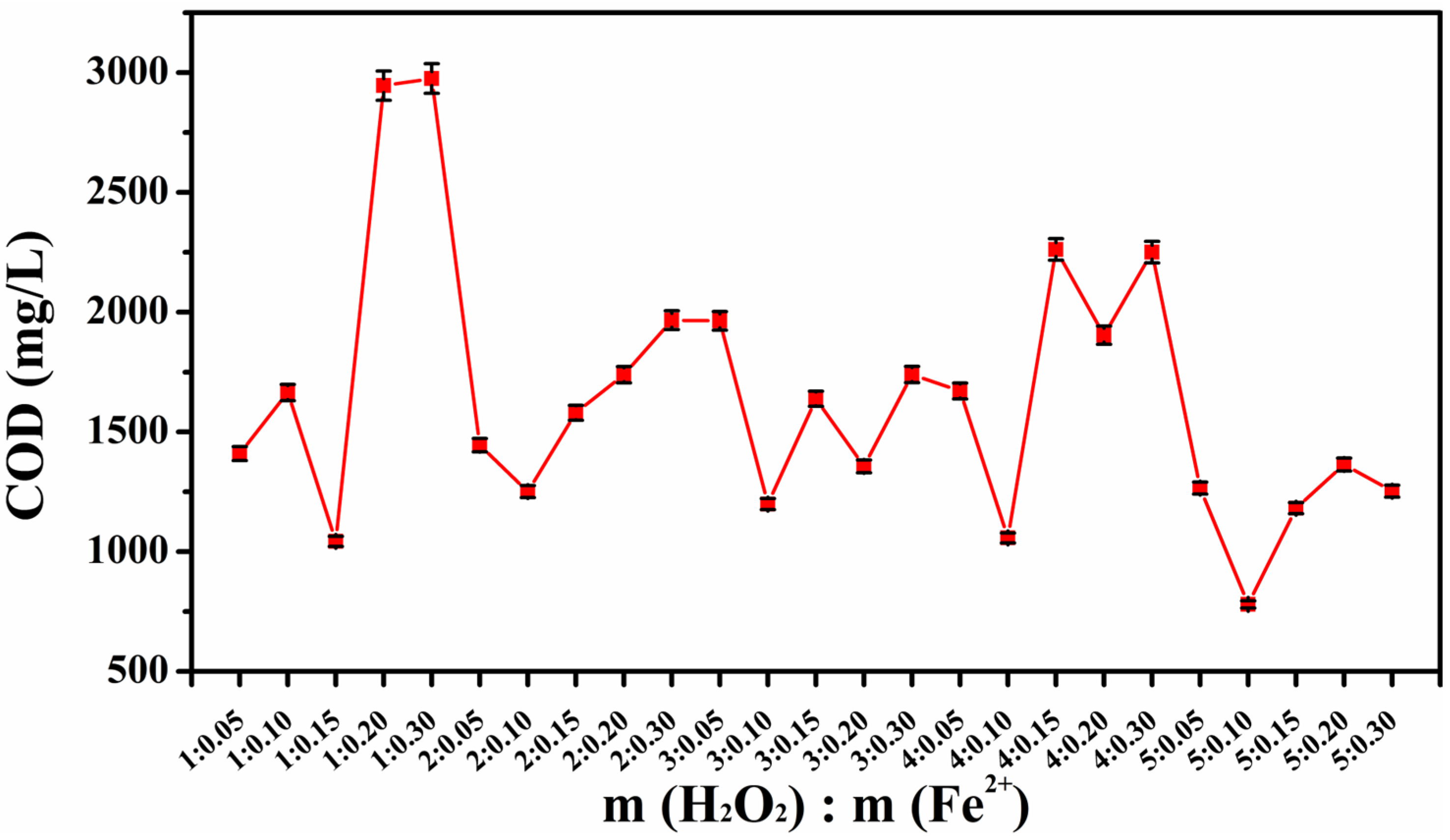
2.2. Orthogonal Test to Optimize the Fenton Oxidation Conditions
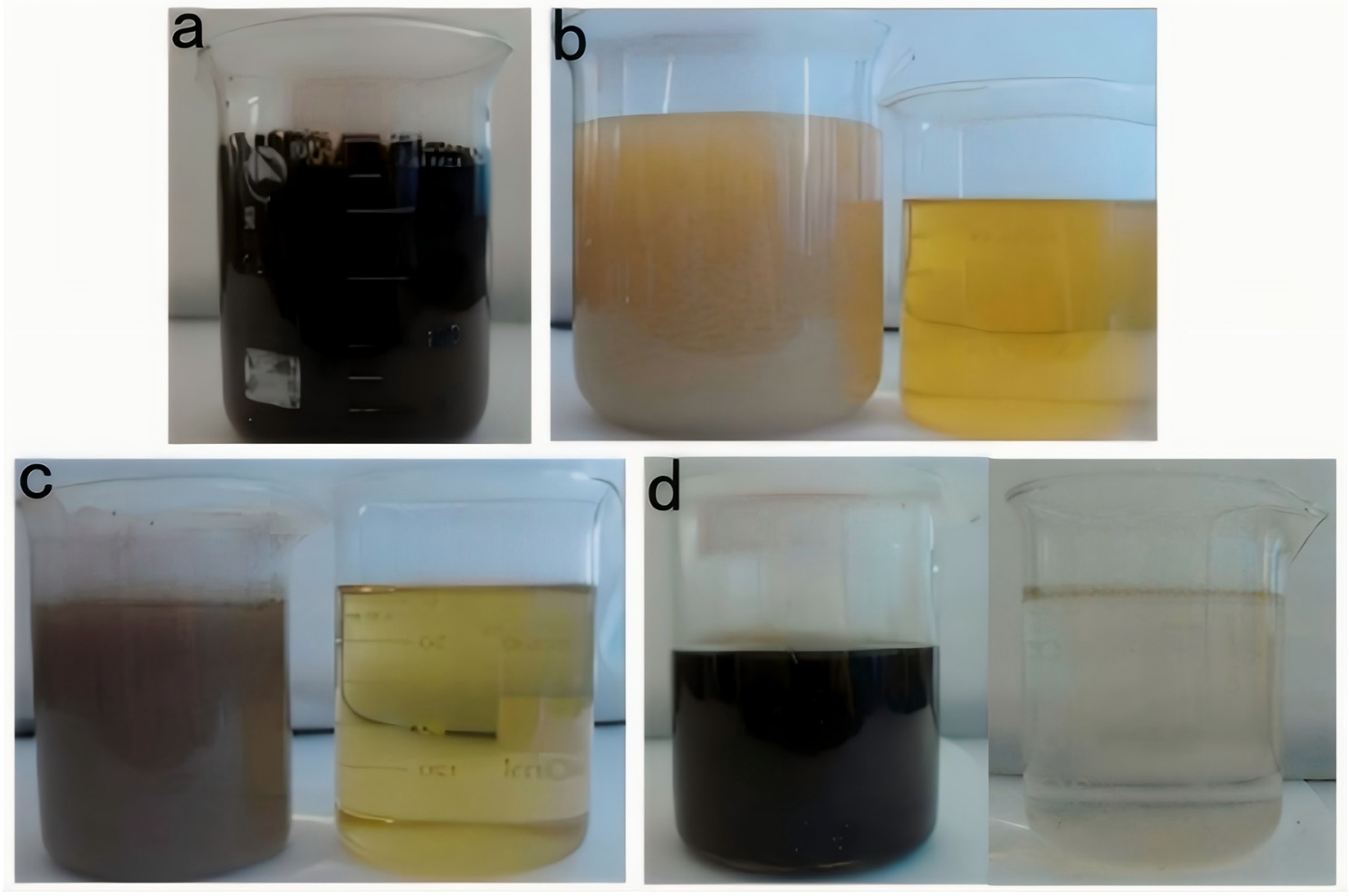
2.3. Comparison of the Composite Fenton Oxidizing Treatment Methods
- (1)
- Visible light–Fenton method
- (2)
- UV–Fenton method
- (3)
- Ozone–Fenton method
3. Results and Discussion
3.1. Optimization of the Fenton Oxidation Conditions
3.2. Oxidation Mechanism of the Fenton Reaction
3.2.1. Analysis of the Fenton Oxidation Mechanism
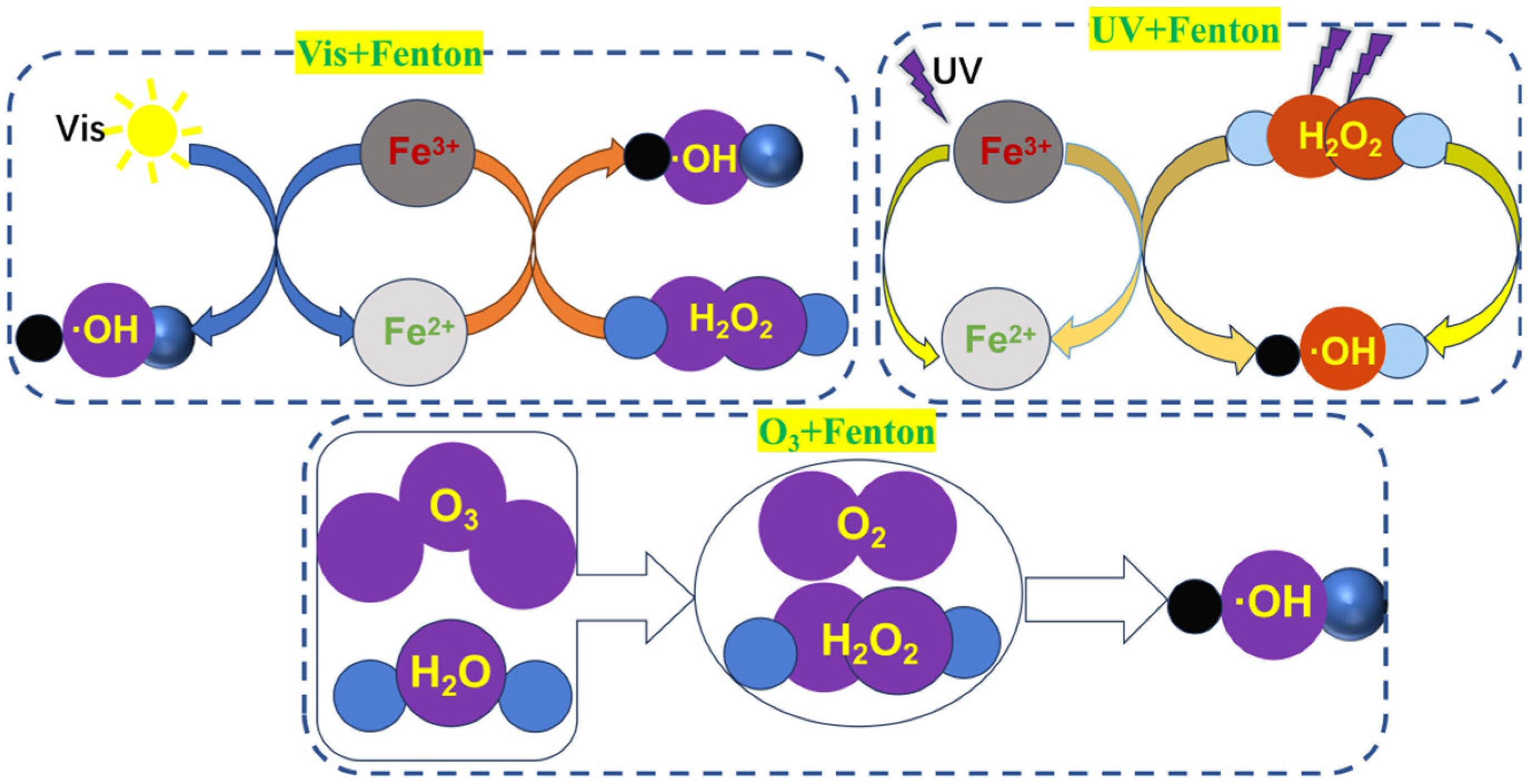
3.2.2. The Composite Fenton Oxidation System Mechanism
- (1)
- Visible light–Fenton method
- (2)
- Ultraviolet–Fenton (UV–Fenton)
- (3)
- Ozone-Fenton (O3-Fenton)
3.3. Treatment Results of the Composite Fenton Oxidation System
4. Conclusions
- (1)
- In the experiment for optimizing the H2O2/COD ratio, the H2O2 concentration was optimized. The ·OH generation rate was found to be positively correlated with the H2O2 concentration. The COD and TOC removal first increased and then decreased with the increased H2O2 concentration. The ·OH generation rate was higher for a higher H2O2 concentration. Excessive H2O2 could quickly oxidize Fe2+ to Fe3+ at the beginning of the reaction, and result in Fe3+-catalyzed oxidation, which consumed H2O2 and inhibited ·OH generation to gradually increase the COD value. The COD value was reduced to the lowest when the H2O2 concentration was 1.4 mol/L.
- (2)
- The orthogonal test to optimize the mass ratios of H2O2/Fe2+ found that the COD removal first increased and then decreased as the Fe2+ concentration increased. COD had the most obvious reduced value when m(H2O2):m(Fe2+) was 5:0.10.
- (3)
- Effective pretreatment made the degradation effect of the Fenton oxidation treatment of the gasoline alkali residue more obvious. The UV–Fenton method had a more obvious degradation effect compared to the Vis–Fenton and O3–Fenton methods. Considering the production cost of this process, the highest H2O2 concentration was 1.0 mol/L and m(H2O2):m(Fe2+) was 5:0.10. A 302 nm UV–Fenton oxidation wavelength could be used to degrade the raw liquid to remove hazardous substances, and to treat gasoline alkali drugs in a low-investment, environment-friendly, and efficient way.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yadav, B.; Chavan, S.; Atmakuri, A.; Tyagi, R.D.; Drogui, P. A review on recovery of proteins from industrial wastewaters with special emphasis on PHA production process: Sustainable circular bioeconomy process development. Bioresour. Technol. 2020, 317, 124006. [Google Scholar] [CrossRef] [PubMed]
- Shokri, A.; Nasernejad, B. Treatment of spent caustic wastewater by electro-Fenton process: Kinetics and cost analysis. Process Saf. Environ. Prot. 2023, 172, 836–845. [Google Scholar] [CrossRef]
- Almomani, F.; Bhosale, R.; Kumar, A.; Khraisheh, M. Potential use of solar photocatalytic oxidation in removing emerging pharmaceuticals from wastewater: A pilot plant study. Sol. Energy 2018, 172, 128–140. [Google Scholar] [CrossRef]
- Duan, Y.W.; Wang, S.Z.; Liu, K.; Huang, X.Y.; Mi, Z.X.; Lui, H.; Li, Y.H.; Wang, Y.L. Experimental study on the treatment of printing and dyeing wastewater by supercritical water oxidation based on response surface methodology. Environ. Prog. Sustain. Energy 2025, 44, 14617. [Google Scholar] [CrossRef]
- Apul, O.G.; Arrowsmith, S.; Hall, C.A.; Miranda, E.M.; Alam, F.; Dahlen, P.; Sra, K.; Kamath, R.; Mcmillen, S.J.; Sihota, N. Biodegradation of petroleum hydrocarbons in a weathered, unsaturated soil is inhibited by peroxide Oxidants. J. Hazard. Mater. 2022, 433, 128770. [Google Scholar] [CrossRef]
- Li, C.; Di, H.; Yang, T.; Huang, T.; Deng, W.; Du, F.; Luo, H. Fe/N/S Co-doped porous carbon from the Co-processing residue of coal and heavy oil for an efficient oxygen reduction reaction. Ind. Eng. Chem. Res. 2023, 62, 2536–2547. [Google Scholar] [CrossRef]
- Wang, Y.X.; Zhang, T.A.; Lyu, G.Z.; Guo, F.F.; Zhang, W.G.; Zhang, Y.H. Recovery of alkali and alumina from bauxite residue (red mud) and complete reuse of the treated residue. J. Clean. Prod. 2018, 188, 456–465. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Jia, X.; Wang, M.R.; Zhang, H.; Song, X.F.; Wang, C.; Han, Z.N.; Xu, G.W. Fundamentals of NO reduction by liquid wastes: The first application in hazardous waste incineration. J. Clean. Prod. 2024, 449, 141714. [Google Scholar] [CrossRef]
- Siddique, M.N.I.; Munaim, M.S.A.; Wahid, Z.B.A. The combined effect of ultrasonic and microwave pre-treatment on bio-methane generation from co-digestion of petrochemical wastewater. J. Clean. Prod. 2017, 145, 303–309. [Google Scholar] [CrossRef]
- Tian, X.M.; Song, Y.D.; Shen, Z.Q.; Zhou, Y.X.; Wang, K.J.; Jin, X.G.; Han, Z.F.; Liu, T. A comprehensive review on toxic petrochemical wastewater retreatment and advanced treatment. J. Clean. Prod. 2020, 245, 118692. [Google Scholar] [CrossRef]
- Maroudas, A.; Pandis, P.K.; Chatzopoulou, A.; Davellas, L.R.; Sourkouni, G.; Argirusis, C. Synergetic decolorization of azo dyes using ultrasounds, photocatalysis and photo-fenton reaction. Ultrason. Sonochem. 2021, 71, 105367. [Google Scholar] [CrossRef]
- Tiburtius, E.R.L.; Zamora, P.P.; Emmel, A. Treatment of gasoline-contaminated waters by advanced oxidation processes. J. Hazard. Mater. 2005, 126, 86–90. [Google Scholar] [CrossRef]
- Jiménez, S.; Andreozzi, M.; Micó, M.M.; Álvarez, M.G.; Contreras, S. Produced water treatment by advanced oxidation processes. Sci. Total Environ. 2019, 666, 12–21. [Google Scholar] [CrossRef]
- Grbić, J.; Mladenović, D.; Pavlović, S.; Lazović, S.; Mojović, L.; Vuković, A.D. Advanced oxidation processes in the treatment of corn stalks. Sustain. Chem. Pharm. 2023, 32, 100962. [Google Scholar] [CrossRef]
- Dang, T.T.; Do, V.M.; Trinh, V.T. Nano-catalysts in ozone-based advanced oxidation processes for wastewater treatment. Curr. Pollut. Rep. 2020, 6, 217–229. [Google Scholar] [CrossRef]
- Fenton, H.J.H. Oxidation of tartaric acid in presence of iron. J. Chem. Soc. Trans. 1894, 65, 899–910. [Google Scholar] [CrossRef]
- Polli, F.; Zingaretti, D.; Crognale, S.; Pesciaroli, L.; Annibale, A.D.; Petruccioli, M.; Baciocchi, R. Impact of the Fenton-like treatment on the microbial community of a diesel-contaminated soil. Chemosphere 2018, 191, 580–588. [Google Scholar] [CrossRef]
- Xu, J.L.; Du, J.; Li, L.; Zhang, Q.J.; Chen, Z.W. Fast-stimulating bioremediation of macro crude oil in soils using matching Fenton pre-oxidation. Chemosphere 2020, 252, 126622. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, Z.N.; Wang, Y.H.; Xu, J.L. Efficient removal of heavily oil-contaminated soil using a combination of fenton pre-oxidation with biostimulated iron and bioremediation. J. Environ. Manag. 2022, 308, 114590. [Google Scholar] [CrossRef]
- Rivas, F.J.; Gimeno, O.; Portela, J.R.; Ossa, E.M.D.; Beltrán, F.J. Supercritical water oxidation of olive oil mill wastewater. Ind. Eng. Chem. Res. 2001, 40, 3670–3674. [Google Scholar] [CrossRef]
- Wongfaed, N.; Kongjan, P.; Prasertsan, P.; Thong, S.O. Effect of oil and derivative in palm oil mill effluent on the process imbalance of biogas production. J. Clean. Prod. 2020, 247, 119110. [Google Scholar] [CrossRef]
- Nasrin, A.B.; Raman, A.A.A.; Bukhari, N.A.; Sukiran, M.A.; Buthiyappan, A.; Subramaniam, V.; Aziz, A.A.; Loh, S.K. A critical analysis on biogas production and utilisation potential from palm oil mill effluent. J. Clean. Prod. 2022, 361, 132040. [Google Scholar] [CrossRef]
- Ayare, S.D.; Gogate, P.R. Sonophotocatalytic oxidation based treatment of phthalocyanine pigment containing industrial wastewater intensified using oxidising agents. Sep. Purif. Technol. 2020, 233, 115979. [Google Scholar] [CrossRef]
- Ding, R.; Zhang, D.D.; Gao, Y.X.; Chen, X.; Yang, M. Characteristics of refractory organics in industrial wastewater treated using a Fenton-coagulation process. Environ. Technol. 2021, 42, 3432–3440. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Ganiyu, S.O.; Huitle, C.A.M.; Mousset, E.; Vargas, H.O.; Trellu, C.; Zhou, M.H.; Oturan, M.A. Recent advances in electro-Fenton process and its emerging applications. Crit. Rev. Environ. Sci. Technol. 2023, 53, 887–913. [Google Scholar] [CrossRef]
- Marta, V.M.; Fresnedo, S.R.; Inmaculada, O.; Angel, I. Overview of the PCDD/Fs degradation potential and formation risk in the application of advanced oxidation processes (AOPs) to wastewater treatment. Chemosphere 2015, 118, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, A.C.; Santos, A.; Romero, A.; Dominguez, C.M. Application of chelating agents to enhance Fenton process in soil remediation: A review. Catalysts 2021, 11, 722. [Google Scholar] [CrossRef]
- Hara, J. Oxidative degradation of benzene rings using iron sulfide activated by hydrogen peroxide/ozone. Chemosphere 2017, 189, 382–389. [Google Scholar] [CrossRef]
- Tang, T.T.; Jin, B.; Zhao, P. Preparation of the photo-Fenton agent MIL-100 (Fe) with high performance in the degradation of nitro explosives. New J. Chem. 2023, 47, 8566–8577. [Google Scholar] [CrossRef]
- Hou, C.C.; Lu, G.; Zhao, L.; Yin, P.H.; Zhu, L.F. Estrogenicity assessment of membrane concentrates from landfill leachate treated by the UV-Fenton process using a human breast carcinoma cell line. Chemosphere 2017, 180, 192–200. [Google Scholar] [CrossRef]
- Ikhlaq, A.; Munir, H.M.S.; Khan, A.; Javed, F.; Joya, K.S. Comparative study of catalytic ozonation and Fenton-like processes using iron-loaded rice husk ash as catalyst for the removal of methylene blue in wastewater. Ozone Sci. Eng. 2019, 3, 41. [Google Scholar] [CrossRef]
- Huguenot, D.; Mousset, E.; Hullebusch, E.D.; Oturan, M.A. Combination of surfactant enhanced soil washing and electro-Fenton process for the treatment of soils contaminated by petroleum hydrocarbons. J. Environ. Manag. 2015, 153, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.H.; Yu, L.; Chen, Z.F.; He, H.P.; Ye, F.; Cheng, G.; Zhang, Q.X. Microwave-assisted synthesis of Fe3O4 nano-crystals with predominantly exposed facets and their heterogeneous UVA/Fenton catalytic activity. ACS Appl. Mater. Interfaces 2017, 9, 29203–29212. [Google Scholar]





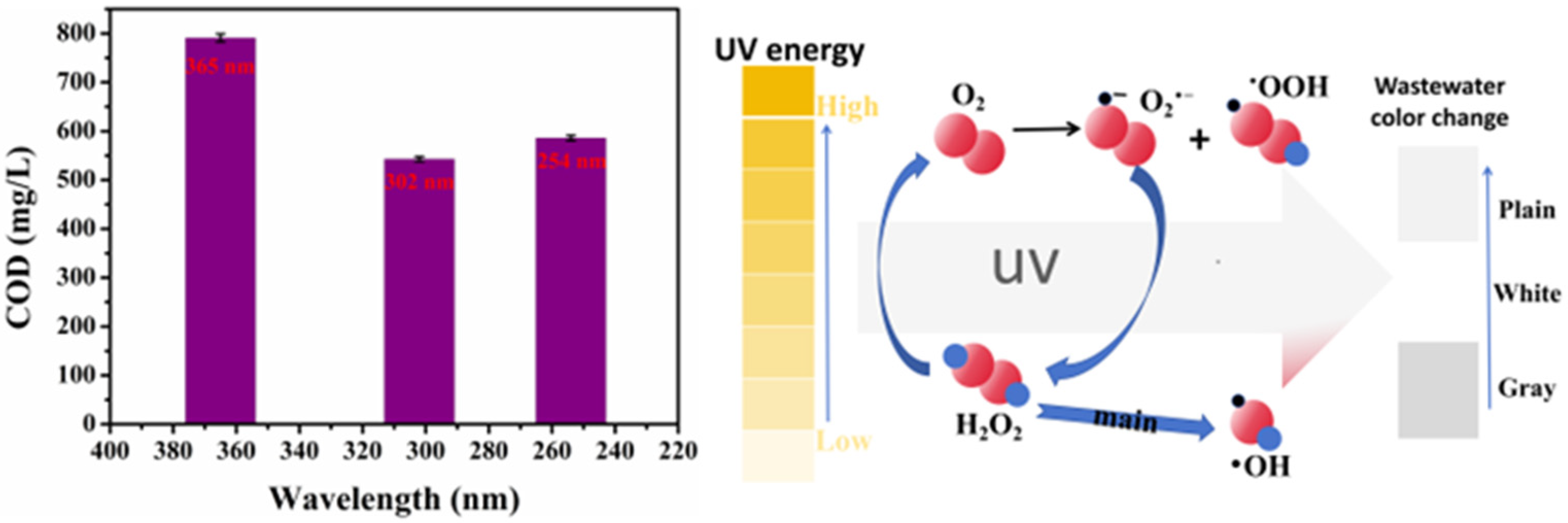
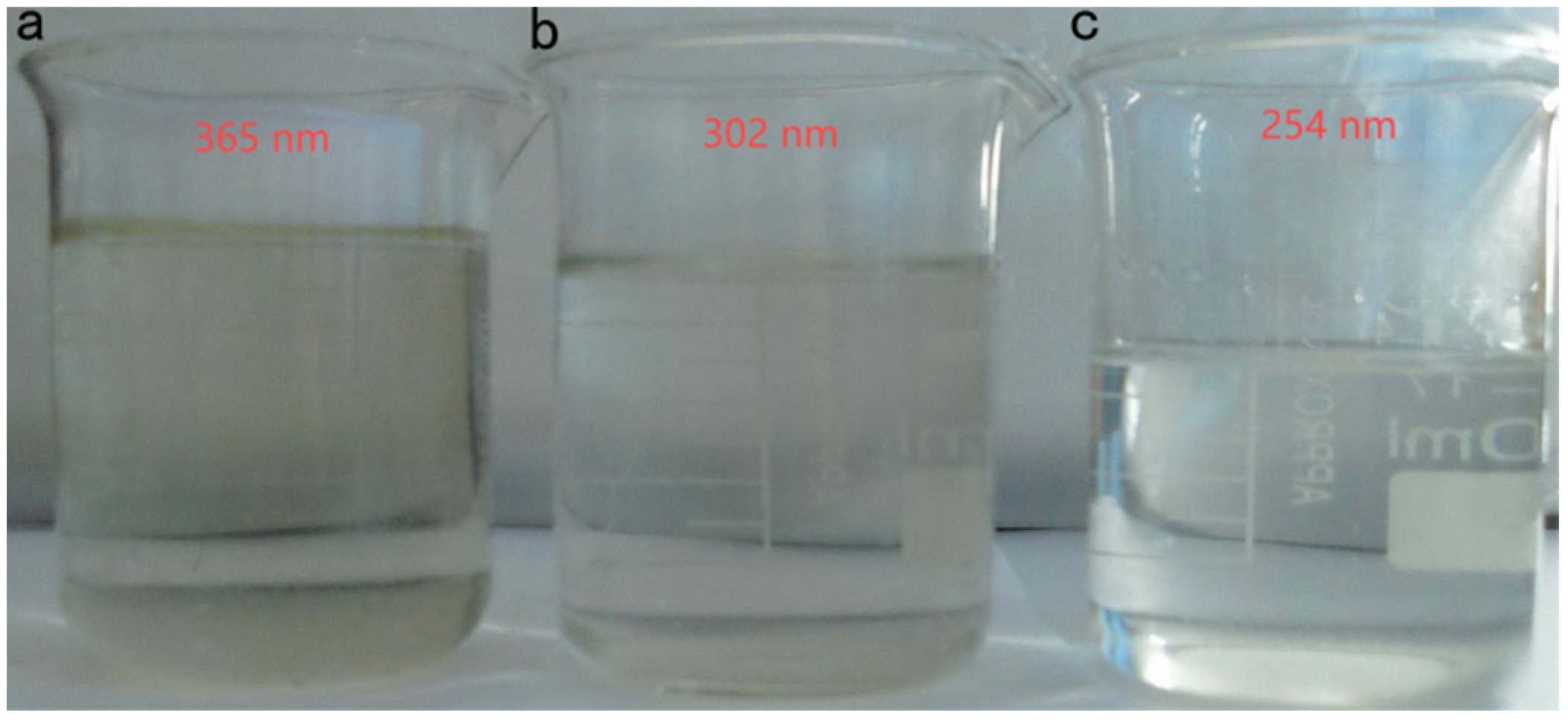
| Sample Number | H2O2 Concentration (mol/L) | COD Values (mg/L) |
|---|---|---|
| 1 | 0.2 mol/L | 3816.5 |
| 2 | 0.4 mol/L | 3252.1 |
| 3 | 0.6 mol/L | 2702.4 |
| 4 | 0.8 mol/L | 1975.3 |
| 5 | 1.0 mol/L | 1639.7 |
| 6 | 1.2 mol/L | 1489.2 |
| 7 | 1.4 mol/L | 1321.6 |
| 8 | 1.6 mol/L | 2943.2 |
| 9 | 1.8 mol/L | 3424.9 |
| Sample Number | m(H2O2):m(Fe2+) | COD Values (mg/L) | Sample Number | m(H2O2):m(Fe2+) | COD Values (mg/L) |
|---|---|---|---|---|---|
| 1 | 1:0.05 | 1454.7 | 16 | 3:0.20 | 1511.3 |
| 2 | 1:0.10 | 1360.2 | 17 | 3:0.25 | 1601.2 |
| 3 | 1:0.15 | 1815.4 | 18 | 3:0.30 | 1703.2 |
| 4 | 1:0.20 | 2042.3 | 19 | 4:0.05 | 1733.7 |
| 5 | 1:0.25 | 2404.2 | 20 | 4:0.10 | 1081.3 |
| 6 | 1:0.30 | 2529.8 | 21 | 4:0.15 | 1748.1 |
| 7 | 2:0.05 | 1505.4 | 22 | 4:0.20 | 1966.2 |
| 8 | 2:0.10 | 1334.9 | 23 | 4:0.25 | 2184.5 |
| 9 | 2:0.15 | 1646.3 | 24 | 4:0.30 | 2394.5 |
| 10 | 2:0.20 | 1797.1 | 25 | 5:0.05 | 1302.5 |
| 11 | 2:0.25 | 1914.1 | 26 | 5:0.10 | 810.7 |
| 12 | 2:0.30 | 2056.4 | 27 | 5:0.15 | 1215.1 |
| 13 | 3:0.05 | 2038.1 | 28 | 5:0.20 | 1425.1 |
| 14 | 3:0.10 | 1233.4 | 29 | 5:0.25 | 1499.4 |
| 15 | 3:0.15 | 1423.5 | 30 | 5:0.30 | 1582.8 |
| Oxidizing Agent | Chemical Reaction Equation | Redox Potential/V |
|---|---|---|
| ·OH | ·OH + H+ + e− = H2O | 28.10 |
| O3 | O3 + 2H+ + 2e− = H2O + O2 | 22.07 |
| H2O2 | H2O2 + 2H+ + 2e− = 2H2O | 1.77 |
| MnO4− | MnO4− + 8H+ + 5e− = Mn2+ + 4H2O | 1.52 |
| ClO2 | ClO2 + 4H+ + 5e− = Cl− + 2H2O | 1.51 |
| Cl2 | Cl2 + 2e− = 2Cl− | 1.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zha, X.; Zhang, Z.; Guo, Y.; Yang, S.; Qiu, H.; Li, Z. A Fenton Oxidation-Based Integrated Strategy for the Treatment of Raw Gasoline Alkali Residue in Kashi. Toxics 2025, 13, 871. https://doi.org/10.3390/toxics13100871
Zhang Y, Zha X, Zhang Z, Guo Y, Yang S, Qiu H, Li Z. A Fenton Oxidation-Based Integrated Strategy for the Treatment of Raw Gasoline Alkali Residue in Kashi. Toxics. 2025; 13(10):871. https://doi.org/10.3390/toxics13100871
Chicago/Turabian StyleZhang, Yucai, Xianghao Zha, Zhuo Zhang, Yangyang Guo, Shuying Yang, Haonan Qiu, and Zhiwei Li. 2025. "A Fenton Oxidation-Based Integrated Strategy for the Treatment of Raw Gasoline Alkali Residue in Kashi" Toxics 13, no. 10: 871. https://doi.org/10.3390/toxics13100871
APA StyleZhang, Y., Zha, X., Zhang, Z., Guo, Y., Yang, S., Qiu, H., & Li, Z. (2025). A Fenton Oxidation-Based Integrated Strategy for the Treatment of Raw Gasoline Alkali Residue in Kashi. Toxics, 13(10), 871. https://doi.org/10.3390/toxics13100871






