Multi- and Transgenerational Effects of Silver Ions (Ag+) in the ng/L Range on Life Cycle Parameters and Population Growth of the Midge Chironomus riparius (Diptera, Chironomidae)
Highlights
- Environmentally relevant Ag+ (0.01–µg/L) induced multigenerational and transgenerational toxicity in Chironomus riparius, including survival, reproduction, development and the population growth rate.
- Even the EU’s water quality standard for silver (0.01 µg/L) led to significant impairments in survival and reproduction
- Adverse effects persisted after exposure ceased, especially at ≥0.1 µg/L, indicating limited reversibility.
- Multigenerational assays are essential because acute and single-generation tests underestimate the long-term ecological risks of Ag+.
- Findings support stricter Ag thresholds and ecological risk assessments that account for recovery dynamics to better protect aquatic ecosystems.
Abstract
1. Introduction
2. Materials and Methods
2.1. The Model Organism and Culture Conditions
2.2. Test Chemical
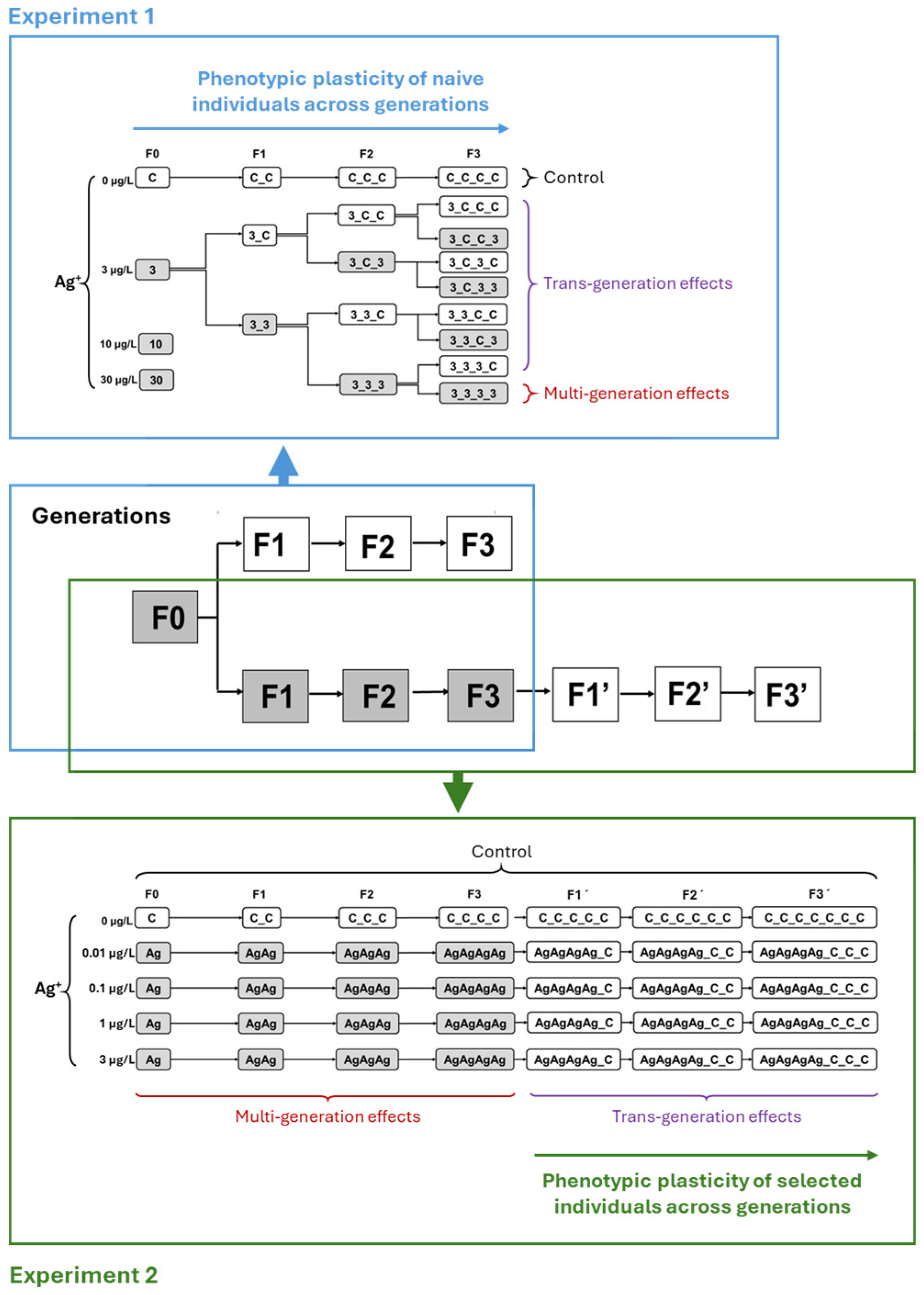
2.3. Experiment 1: Simulation of Contamination Scenarios with Multiple Generations Exposed to Pulses of Contamination and to Recovery Phases
2.4. Experiment 2: Simulation of Contamination Scenarios with Multiple Generations of Continuous Exposure to Low Concentrations of Ag+ and Subsequent Recovery
2.5. Endpoints in Chronic Toxicity Tests of Ag+
2.6. Data Analysis
3. Results
3.1. Experiment 1: Multiple Generations Exposed to Pulses of Contamination and to Recovery Phases
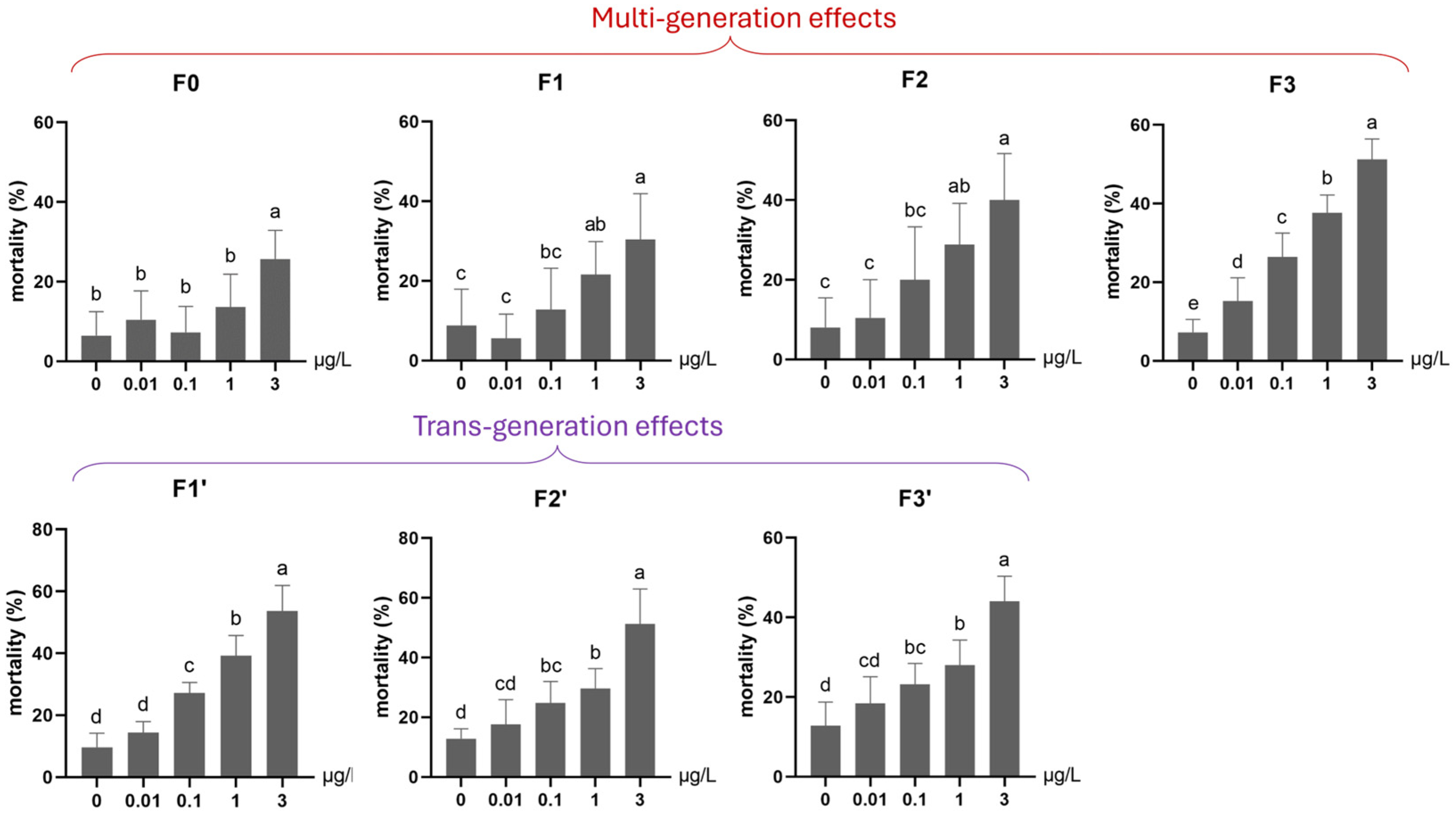
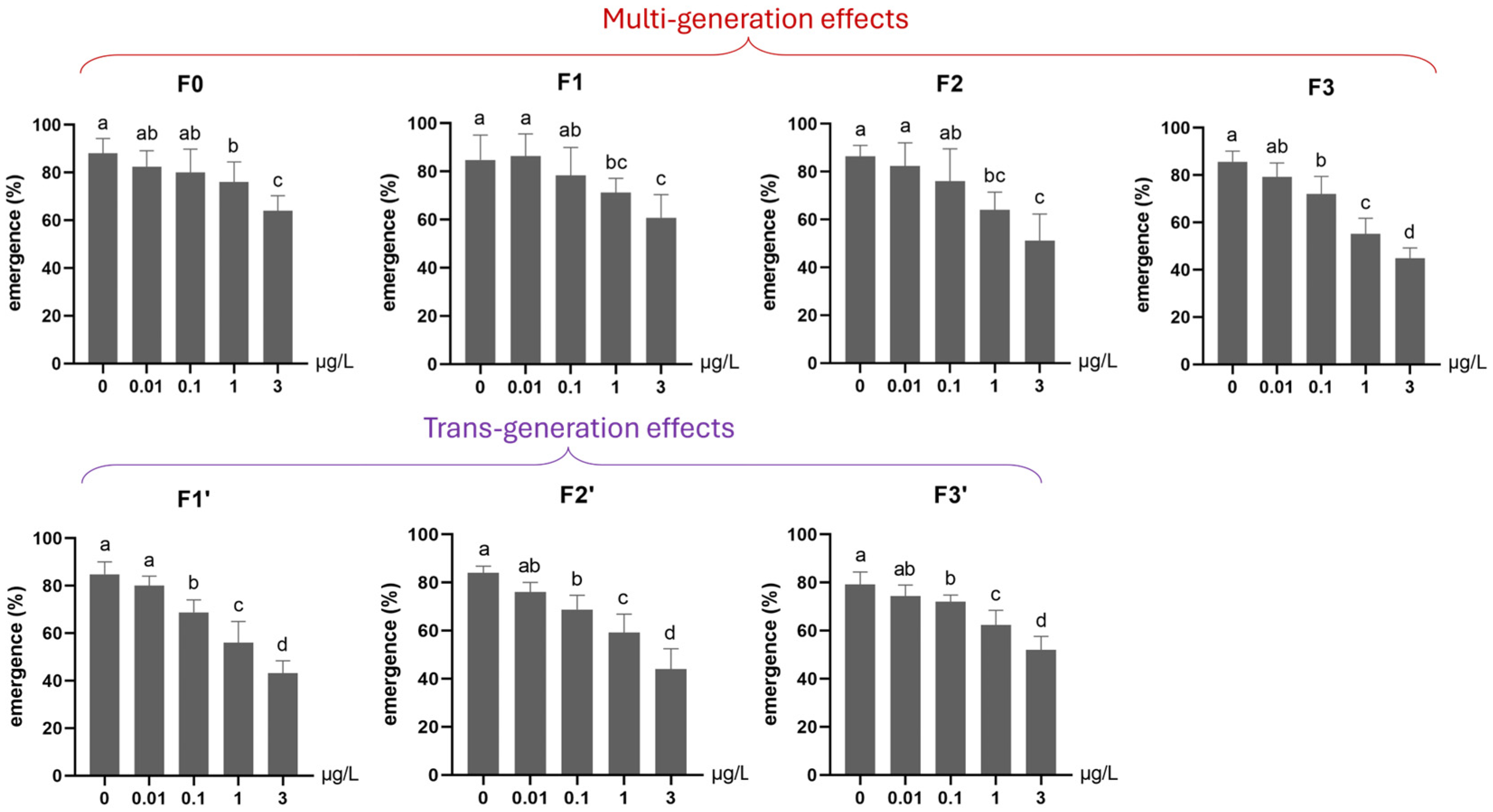
3.1.1. Survival (Mortality and Emergence)
3.1.2. Development (Emt50 and Development Rate)
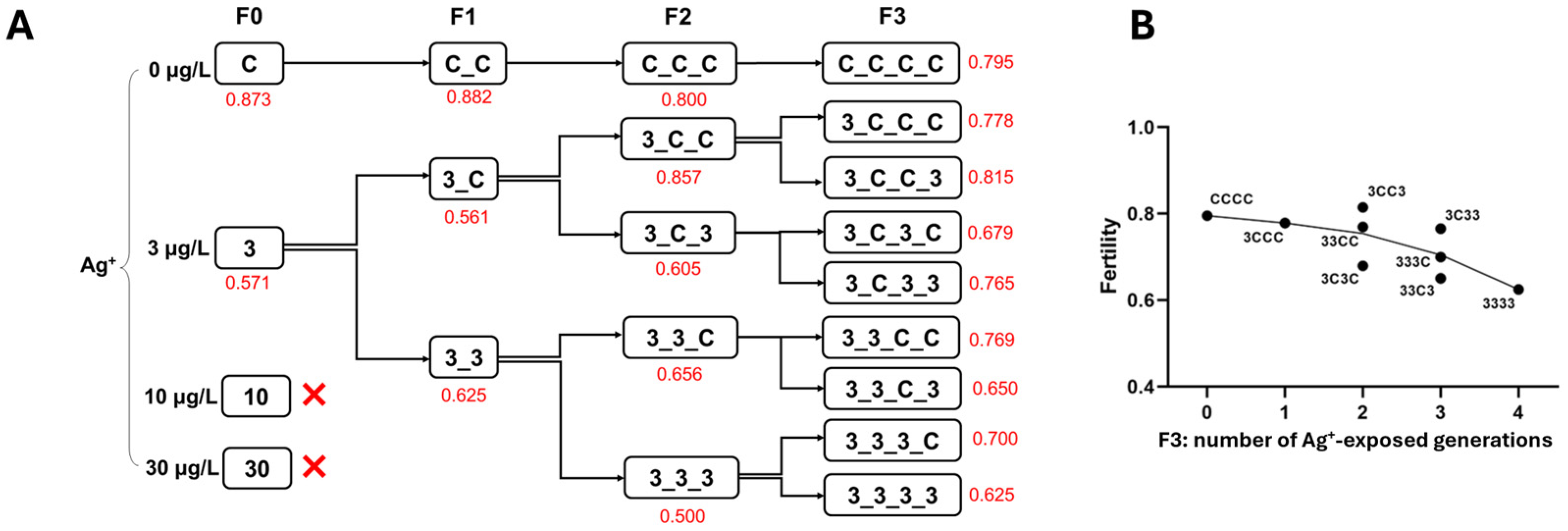
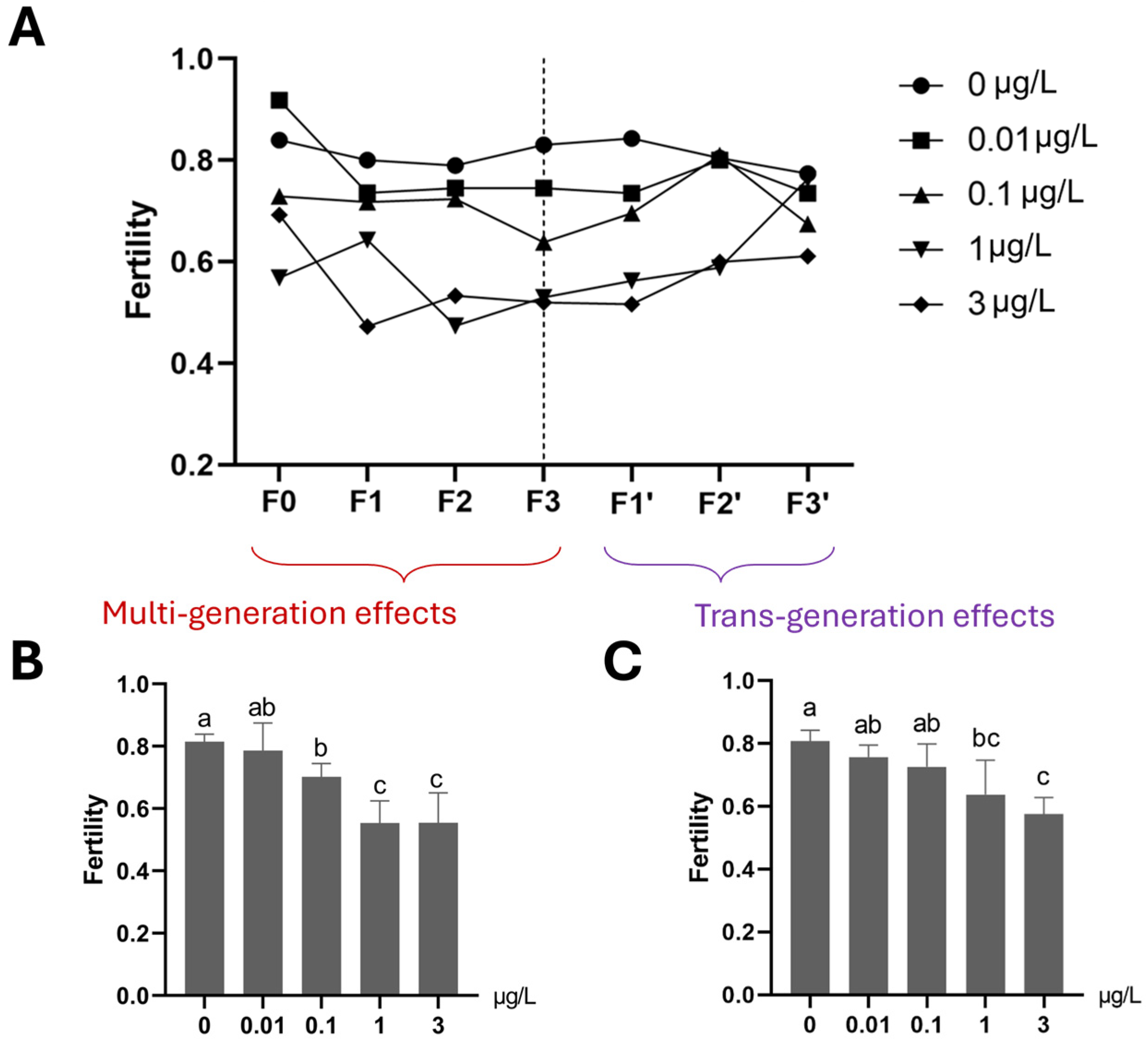
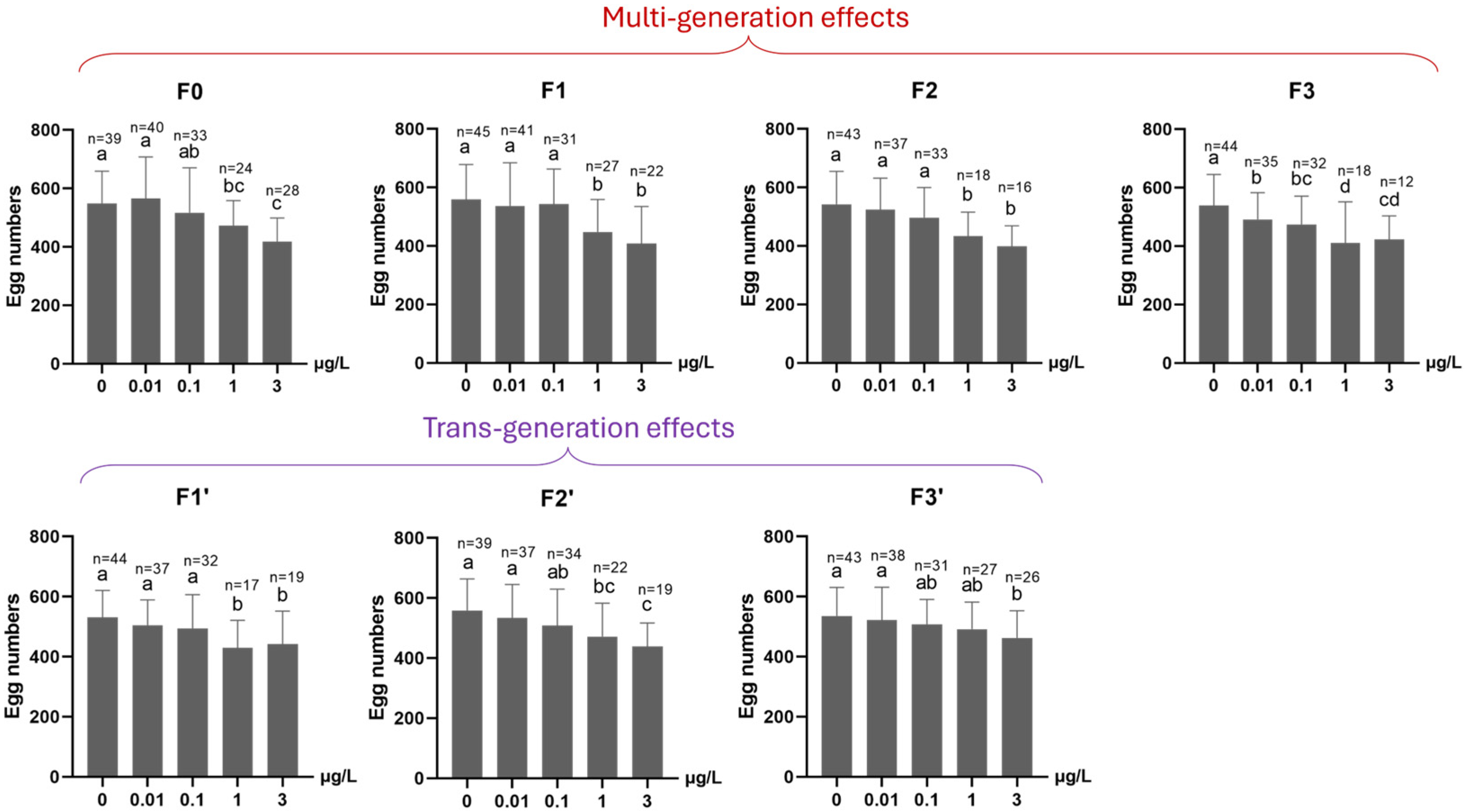
3.1.3. Reproduction (Fertility, Average Egg Numbers per Egg Rope and Female Percentage) and Growth (Based on Dry Body Weight)
3.1.4. Population Growth Rate (PGR)
3.2. Experiment 2: Simulation of Contamination Scenarios with Multiple Generations of Continuous Exposure and Subsequent Recovery
3.2.1. Survival (Mortality and Emergence)
3.2.2. Development (Emt50 and Development Rate)
3.2.3. Reproduction (Fertility, Average Egg Numbers per Egg Rope and Female Percentage) and Growth (Based on Dry Body Weight)
3.2.4. Population Growth Rate (PGR)
4. Discussion
4.1. Experiment 1
4.2. Experiment 2
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mertens, J.; Oorts, K.; Leverett, D.; Arijs, K. Effects of silver nitrate are a conservative estimate for the effects of silver nanoparticles on algae growth and Daphnia magna reproduction. Environ. Toxicol. Chem. 2019, 38, 1701–1713. [Google Scholar] [CrossRef]
- Sanudo-Wilhelmy, S.A.; Flegal, A.R. Anthropogenic silver in the Southern California Bight: A new tracer of sewage in coastal waters. Environ. Sci. Technol. 1992, 26, 2147–2151. [Google Scholar] [CrossRef]
- Purcell, T.W.; Peters, J.J. Sources of silver in the environment. Environ. Toxicol. Chem. 1998, 17, 539–546. [Google Scholar] [CrossRef]
- Wood, C.M.; Playle, R.C.; Hogstrand, C. Physiology and modeling of mechanisms of silver uptake and toxicity in fish. Environ. Toxicol. Chem. 1999, 18, 71–83. [Google Scholar] [CrossRef]
- Howe, P.D.; Dobson, S. Silver and Silver Compounds: Environmental Aspects; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Funck, J.A.; Danger, M.; Gismondi, E.; Cossu-Leguille, C.; Guérold, F.; Felten, V. Behavioural and physiological responses of Gammarus fossarum (Crustacea Amphipoda) exposed to silver. Aquat. Toxicol. 2013, 142, 73–84. [Google Scholar] [CrossRef]
- Ndungu, K. Dissolved silver in the Baltic Sea. Environ. Res. 2011, 111, 45–49. [Google Scholar] [CrossRef]
- Deshmukh, S.P.; Patil, S.; Mullani, S.; Delekar, S. Silver nanoparticles as an effective disinfectant: A review. Mater. Sci. Eng. C 2019, 97, 954–965. [Google Scholar] [CrossRef]
- Hamad, A.; Khashan, K.S.; Hadi, A. Silver nanoparticles and silver ions as potential antibacterial agents. J. Inorg. Organomet. Polym. Mater. 2020, 30, 4811–4828. [Google Scholar] [CrossRef]
- Künniger, T.; Gerecke, A.C.; Ulrich, A.; Huch, A.; Vonbank, R.; Heeb, M.; Wichser, A.; Haag, R.; Kunz, P.; Faller, M. Release and environmental impact of silver nanoparticles and conventional organic biocides from coated wooden façades. Environ. Pollut. 2014, 184, 464–471. [Google Scholar] [CrossRef]
- Tremiliosi, G.C.; Simoes, L.G.P.; Minozzi, D.T.; Santos, R.I.; Vilela, D.C.; Durigon, E.L.; Machado, R.R.G.; Medina, D.S.; Ribeiro, L.K.; Rosa, I.L.V. Ag nanoparticles-based antimicrobial polycotton fabrics to prevent the transmission and spread of SARS-CoV-2. bioRxiv 2020. [Google Scholar] [CrossRef]
- Balagna, C.; Perero, S.; Percivalle, E.; Nepita, E.V.; Ferraris, M. Virucidal effect against coronavirus SARS-CoV-2 of a silver nanocluster/silica composite sputtered coating. Open Ceram. 2020, 1, 100006. [Google Scholar] [CrossRef]
- Vazquez-Munoz, R.; Lopez-Ribot, J.L. Nanotechnology as an alternative to reduce the spread of COVID-19. Challenges 2020, 11, 15. [Google Scholar] [CrossRef]
- Geranio, L.; Heuberger, M.; Nowack, B. The behavior of silver nanotextiles during washing. Environ. Sci. Technol. 2009, 43, 8113–8118. [Google Scholar] [CrossRef]
- Liu, J.; Hurt, R.H. Ion release kinetics and particle persistence in aqueous nano-silver colloids. Environ. Sci. Technol. 2010, 44, 2169–2175. [Google Scholar] [CrossRef]
- Völker, C.; Kämpken, I.; Boedicker, C.; Oehlmann, J.; Oetken, M. Toxicity of silver nanoparticles and ionic silver: Comparison of adverse effects and potential toxicity mechanisms in the freshwater clam Sphaerium corneum. Nanotoxicology 2015, 9, 677–685. [Google Scholar] [CrossRef]
- Levard, C.; Hotze, E.M.; Colman, B.P.; Dale, A.L.; Truong, L.; Yang, X.; Bone, A.J.; Brown, G.E., Jr.; Tanguay, R.L.; Di Giulio, R.T. Sulfidation of silver nanoparticles: Natural antidote to their toxicity. Environ. Sci. Technol. 2013, 47, 13440–13448. [Google Scholar] [CrossRef]
- Ratte, H.T. Bioaccumulation and toxicity of silver compounds: A review. Environ. Toxicol. Chem. 1999, 18, 89–108. [Google Scholar] [CrossRef]
- Wijnhoven, S.W.; Peijnenburg, W.J.; Herberts, C.A.; Hagens, W.I.; Oomen, A.G.; Heugens, E.H.; Roszek, B.; Bisschops, J.; Gosens, I.; Van De Meent, D. Nano-silver—A review of available data and knowledge gaps in human and environmental risk assessment. Nanotoxicology 2009, 3, 109–138. [Google Scholar] [CrossRef]
- Hogstrand, C.; Wood, C.M. Toward a better understanding of the bioavailability, physiology, and toxicity of silver in fish: Implications for water quality criteria. Environ. Toxicol. Chem. 1998, 17, 547–561. [Google Scholar] [CrossRef]
- Eisler, R. Silver Hazards to Fish, Wildlife, and Invertebrates: A Synoptic Review; US Department of the Interior, National Biological Service: Washington, DC, USA, 1996. [Google Scholar]
- European Commission. Regulation (EC) 1272/2008 of the European Parliament and the Council of 16 December 2008 on classification, labelling, and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. Off. J. Eur. Union 2008, L353, 1–1355. [Google Scholar]
- Arijs, K.; Nys, C.; Van Sprang, P.; De Schamphelaere, K.; Mertens, J. Setting a protective threshold value for silver toward freshwater organisms. Environ. Toxicol. Chem. 2021, 40, 1678–1693. [Google Scholar] [CrossRef]
- Anzecc, A. Australian and New Zealand Guidelines for Fresh and Marine Water Quality; Australian and New Zealand Environment and Conservation Council and Agriculture and Resource Management Council of Australia and New Zealand: Canberra, Australia, 2000; Volume 1, pp. 1–314. [Google Scholar]
- U.S. Environmental Protection Agency. National Primary Drinking Water Regulations; U.S. Environmental Protection Agency: Washington, DC, USA, 1991; Volume 56, pp. 3573–3574.
- U.S. Environmental Protection Agency. Ambient Water Quality Criteria for Silver; EPA 440/5-80-071; U.S. Environmental Protection Agency: Washington, DC, USA, 1980.
- Scott, M.; Vighi, M.; Borges, T.; Duarte-Davidson, R.; Hoet, P.; de Voogt, P.; Backhaus, T.; Johnson, A.; Linders, J.; SCHEER (Scientific Committee on Health, Environmental and Emerging Risks). Final Opinion on “Draft Environmental Quality Standards for Priority Substances Under the Water Framework Directive", Silver and Its Compounds; European Commission: Brussels, Belgium, 2021. [Google Scholar]
- Wang, N.; Ingersoll, C.G.; Kunz, J.L.; Brumbaugh, W.G.; Kane, C.M.; Evans, R.B.; Alexander, S.; Walker, C.; Bakaletz, S. Toxicity of sediments potentially contaminated by coal mining and natural gas extraction to unionid mussels and commonly tested benthic invertebrates. Environ. Toxicol. Chem. 2013, 32, 207–221. [Google Scholar] [CrossRef]
- Pallottini, M.; Cappelletti, D.; Fabrizi, A.; Gaino, E.; Goretti, E.; Selvaggi, R.; Céréghino, R. Macroinvertebrate functional trait responses to chemical pollution in agricultural–industrial landscapes. River Res. Appl. 2017, 33, 505–513. [Google Scholar] [CrossRef]
- Besser, J.M.; Steevens, J.; Kunz, J.L.; Brumbaugh, W.G.; Ingersoll, C.G.; Cox, S.; Mebane, C.; Balistrieri, L.; Sinclair, J.; MacDonald, D. Characterizing toxicity of metal-contaminated sediments from the Upper Columbia River, Washington, USA, to benthic invertebrates. Environ. Toxicol. Chem. 2018, 37, 3102–3114. [Google Scholar] [CrossRef]
- Hiemstra, A.-F.; Rambonnet, L.; Gravendeel, B.; Schilthuizen, M. The effects of COVID-19 litter on animal life. Anim. Biol. 2021, 71, 215–231. [Google Scholar] [CrossRef]
- Balian, E.V.; Segers, H.; Martens, K.; Lévéque, C. The Freshwater Animal Diversity Assessment: An Overview of the Results; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar] [CrossRef]
- Charles, S.; Ferreol, M.; Chaumot, A.; Péry, A. Food availability effect on population dynamics of the midge Chironomus riparius: A Leslie modeling approach. Ecol. Model. 2004, 175, 217–229. [Google Scholar] [CrossRef]
- Cranston, P.S.; Armitage, P.; Pinder, L. The Chironomidae: Biology and Ecology of Non-Biting Midges; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1995. [Google Scholar] [CrossRef]
- Park, K.; Kwak, I.-S. Characterization of heat shock protein 40 and 90 in Chironomus riparius larvae: Effects of di (2-ethylhexyl) phthalate exposure on gene expressions and mouthpart deformities. Chemosphere 2008, 74, 89–95. [Google Scholar] [CrossRef]
- Lilley, T.M.; Ruokolainen, L.; Pikkarainen, A.; Laine, V.N.; Kilpimaa, J.; Rantala, M.J.; Nikinmaa, M. Impact of tributyltin on immune response and life history traits of Chironomus riparius: Single and multigeneration effects and recovery from pollution. Environ. Sci. Technol. 2012, 46, 7382–7389. [Google Scholar] [CrossRef]
- Bulut, B.; Rigano, L.; Doria, H.B.; Gemüth, G.; Pfenninger, M. A multigenerational study can detect the evolutionary response to BaP exposure in the non-biting freshwater midge Chironomus riparius. Chemosphere 2024, 358, 142242. [Google Scholar] [CrossRef]
- EPA (Environmental Protection Agency). Ecological Effects Test Guidelines; Gammarid Amphipod Acute Toxicity Test 850; EPA: Washington, DC, USA, 1996. [Google Scholar]
- OECD. Test No. 233: Sediment-Water Chironomid Life-Cycle Toxicity Test Using Spiked Water or Spiked Sediment; OECD: Paris, France, 2010. [Google Scholar] [CrossRef]
- Dabrin, A.; Durand, C.L.; Garric, J.; Geffard, O.; Ferrari, B.J.; Coquery, M. Coupling geochemical and biological approaches to assess the availability of cadmium in freshwater sediment. Sci. Total Environ. 2012, 424, 308–315. [Google Scholar] [CrossRef]
- Niemuth, N.J.; Curtis, B.J.; Hang, M.N.; Gallagher, M.J.; Fairbrother, D.H.; Hamers, R.J.; Klaper, R.D. Next-generation complex metal oxide nanomaterials negatively impact growth and development in the benthic invertebrate Chironomus riparius upon settling. Environ. Sci. Technol. 2019, 53, 3860–3870. [Google Scholar] [CrossRef]
- Pastorino, P.; Prearo, M.; Bertoli, M.; Abete, M.C.; Dondo, A.; Salvi, G.; Zaccaroni, A.; Elia, A.C.; Pizzul, E. Accumulation of As, Cd, Pb, and Zn in sediment, chironomids and fish from a high-mountain lake: First insights from the Carnic Alps. Sci. Total Environ. 2020, 729, 139007. [Google Scholar] [CrossRef]
- Lee, S.-W.; Park, S.-Y.; Kim, Y.; Im, H.; Choi, J. Effect of sulfidation and dissolved organic matters on toxicity of silver nanoparticles in sediment dwelling organism, Chironomus riparius. Sci. Total Environ. 2016, 553, 565–573. [Google Scholar] [CrossRef]
- Marczylo, E.L.; Jacobs, M.N.; Gant, T.W. Environmentally induced epigenetic toxicity: Potential public health concerns. Crit. Rev. Toxicol. 2016, 46, 676–700. [Google Scholar] [CrossRef]
- Li, Y.; Guo, L.; Huang, D.; Jawad, A.; Chen, Z.; Yang, J.; Liu, W.; Shen, Y.; Wang, M.; Yin, G. Support-dependent active species formation for CuO catalysts: Leading to efficient pollutant degradation in alkaline conditions. J. Hazard. Mater. 2017, 328, 56–62. [Google Scholar] [CrossRef]
- Barata, C.; Campos, B.; Rivetti, C.; LeBlanc, G.A.; Eytcheson, S.; McKnight, S.; Tobor-Kaplon, M.; de Vries Buitenweg, S.; Choi, S.; Choi, J. Validation of a two-generational reproduction test in Daphnia magna: An interlaboratory exercise. Sci. Total Environ. 2017, 579, 1073–1083. [Google Scholar] [CrossRef]
- Im, J.; Chatterjee, N.; Choi, J. Genetic, epigenetic, and developmental toxicity of Chironomus riparius raised in metal-contaminated field sediments: A multi-generational study with arsenic as a second challenge. Sci. Total Environ. 2019, 672, 789–797. [Google Scholar] [CrossRef]
- Mendes, L.A.; Maria, V.L.; Scott-Fordsmand, J.J.; Amorim, M.J. Multigenerational exposure of Folsomia candida to silver: Effect of different contamination scenarios (continuous versus pulsed and recovery). Sci. Total Environ. 2018, 631, 326–333. [Google Scholar] [CrossRef]
- Guimarães, B.; Römbke, J.; Amorim, M. On the importance of longer-term exposure to stressors-A critical review and proposal for multigenerational testing in standard soil invertebrates. Sci. Total Environ. 2023, 854, 158680. [Google Scholar] [CrossRef]
- Doria, H.B.; Pfenninger, M. A multigenerational approach can detect early Cd pollution in Chironomus riparius. Chemosphere 2021, 262, 127815. [Google Scholar] [CrossRef]
- Amorim, M.; Pereira, C.; Soares, A.; Scott-Fordsmand, J. Does long term low impact stress cause population extinction? Environ. Pollut. 2017, 220, 1014–1023. [Google Scholar] [CrossRef]
- Leon Paumen, M.; Steenbergen, E.; Kraak, M.H.; Van Straalen, N.M.; Van Gestel, C.A. Multigeneration exposure of the springtail Folsomia candida to phenanthrene: From dose− response relationships to threshold concentrations. Environ. Sci. Technol. 2008, 42, 6985–6990. [Google Scholar] [CrossRef]
- van Gestel, C.A.; De Lima E Silva, C.; Lam, T.; Koekkoek, J.C.; Lamoree, M.H.; Verweij, R.A. Multigeneration toxicity of imidacloprid and thiacloprid to Folsomia candida. Ecotoxicology 2017, 26, 320–328. [Google Scholar] [CrossRef]
- OECD. Test No. 202: Testing of Chemicals: Daphnia sp., Acute Immobilisation Test, Method; OECD: Paris, France, 2004. [Google Scholar] [CrossRef]
- Krais, S.; Anthes, N.; Huppertsberg, S.; Knepper, T.P.; Peschke, K.; Ruhl, A.S.; Schmieg, H.; Schwarz, T.; Köhler, H.-R.; Triebskorn, R. Polystyrene microplastics modulate the toxicity of the hydrophilic insecticide Thiacloprid for Chironomid larvae and also influence their burrowing behavior. Microplastics 2022, 1, 505–519. [Google Scholar] [CrossRef]
- OECD. Test No. 219: Testing of Chemicals, Sediment-Water Chironomid Toxicity Test Using Spiked Water; OECD: Paris, France, 2004. [Google Scholar] [CrossRef]
- OECD. Test No. 218: Testing of Chemicals, Sediment-Water Chironomid Toxicity Test Using Spiked Sediment; OECD: Paris, France, 2004. [Google Scholar] [CrossRef]
- Day, K.; Kirby, R.S.; Reynoldson, T. Sexual dimorphism in Chironomus riparius (Meigen): Impact on interpretation of growth in whole-sediment toxicity tests. Environ. Toxicol. Chem. 1994, 13, 35–39. [Google Scholar] [CrossRef]
- Marinković, M.; de Bruijn, K.; Asselman, M.; Bogaert, M.; Jonker, M.J.; Kraak, M.H.; Admiraal, W. Response of the nonbiting midge Chironomus riparius to multigeneration toxicant exposure. Environ. Sci. Technol. 2012, 46, 12105–12111. [Google Scholar] [CrossRef]
- da Silva Pinto, T.J.; Rocha, G.S.; Moreira, R.A.; da Silva, L.C.M.; Yoshii, M.P.C.; Goulart, B.V.; Montagner, C.C.; Daam, M.A.; Espindola, E.L.G. Multi-generational exposure to fipronil, 2, 4-D, and their mixtures in Chironomus sancticaroli: Biochemical, individual, and population endpoints. Environ. Pollut. 2021, 283, 117384. [Google Scholar] [CrossRef]
- Watts, M.; Pascoe, D. A comparative study of Chironomus riparius Meigen and Chironomus tentans Fabricius (Diptera: Chironomidae) in aquatic toxicity tests. Arch. Environ. Contam. Toxicol. 2000, 39, 299–306. [Google Scholar] [CrossRef]
- Vogt, C.; Pupp, A.; Nowak, C.; Jagodzinski, L.S.; Baumann, J.; Jost, D.; Oetken, M.; Oehlmann, J. Interaction between genetic diversity and temperature stress on life-cycle parameters and genetic variability in midge Chironomus riparius populations. Clim. Res. 2007, 33, 207–214. [Google Scholar] [CrossRef]
- Fisher, J.; Benner, S.; Golden, P.; Edwards, R. Silver Toxicity: A Brief Overview—Literature Review. 2015. Available online: https://archive.legmt.gov/content/Committees/Interim/2019-2020/Water-Policy/Meetings/Jan-2020/Ag%20Toxicity%20literature%20review%20(12-16-15).pdf (accessed on 7 October 2025).
- Baas, J.; Jager, T.; Kooijman, B. Understanding toxicity as processes in time. Sci. Total Environ. 2010, 408, 3735–3739. [Google Scholar] [CrossRef]
- Jager, T.; Albert, C.; Preuss, T.G.; Ashauer, R. General unified threshold model of survival-a toxicokinetic-toxicodynamic framework for ecotoxicology. Environ. Sci. Technol. 2011, 45, 2529–2540. [Google Scholar] [CrossRef]
- Swain, S.; Wren, J.; Stürzenbaum, S.; Kille, P.; Jager, T.; Jonker, M.; Hankard, P.; Svendsen, C.; Chaseley, J.; Hedley, B. Linking toxicants mechanism of action and physiological mode of action in Caenorhabditis elegans. BMC Syst. Biol. 2010, 4, 32. [Google Scholar] [CrossRef]
- Luoma, S.N. Silver Nanotechnologies and the Environment; The project on emerging nanotechnologies report; The Pew Charitable Trusts: Philadelphia, PA, USA, 2008; Volume 15, pp. 12–13. [Google Scholar]
- Bielmyer, G.K.; Grosell, M.; Brix, K.V. Toxicity of silver, zinc, copper, and nickel to the copepod Acartia tonsa exposed via a phytoplankton diet. Environ. Sci. Technol. 2006, 40, 2063–2068. [Google Scholar] [CrossRef]
- Silva, P.V.; Santos, C.S.; Papadiamantis, A.G.; Gonçalves, S.F.; Prodana, M.; Verweij, R.A.; Lynch, I.; van Gestel, C.A.; Loureiro, S. Toxicokinetics of silver and silver sulfide nanoparticles in Chironomus riparius under different exposure routes. Sci. Total Environ. 2023, 865, 161087. [Google Scholar] [CrossRef]
- Diez-Ortiz, M.; Lahive, E.; George, S.; Ter Schure, A.; Van Gestel, C.A.; Jurkschat, K.; Svendsen, C.; Spurgeon, D.J. Short-term soil bioassays may not reveal the full toxicity potential for nanomaterials; bioavailability and toxicity of silver ions (AgNO3) and silver nanoparticles to earthworm Eisenia fetida in long-term aged soils. Environ. Pollut. 2015, 203, 191–198. [Google Scholar] [CrossRef]
- Waalewijn-Kool, P.L.; Klein, K.; Forniés, R.M.; van Gestel, C.A. Bioaccumulation and toxicity of silver nanoparticles and silver nitrate to the soil arthropod Folsomia candida. Ecotoxicology 2014, 23, 1629–1637. [Google Scholar] [CrossRef]
- Völker, C.; Boedicker, C.; Daubenthaler, J.; Oetken, M.; Oehlmann, J. Comparative toxicity assessment of nanosilver on three Daphnia species in acute, chronic and multi-generation experiments. PLoS ONE 2013, 8, e75026. [Google Scholar] [CrossRef] [PubMed]
- Postma, J.F.; Davids, C. Tolerance induction and life cycle changes in cadmium-exposed Chironomus riparius (Diptera) during consecutive generations. Ecotoxicol. Environ. Saf. 1995, 30, 195–202. [Google Scholar] [CrossRef]
- Tassou, K.T.; Schulz, R. Effects of the insect growth regulator pyriproxyfen in a two-generation test with Chironomus riparius. Ecotoxicol. Environ. Saf. 2009, 72, 1058–1062. [Google Scholar] [CrossRef] [PubMed]
- Tassou, K.T.; Schulz, R. Low field-relevant tebufenozide concentrations affect reproduction in Chironomus riparius (Diptera: Chironomidae) in a long-term toxicity test. Environ. Sci. Pollut. Res. 2013, 20, 3735–3742. [Google Scholar] [CrossRef]
- Campos, D.; Silva, A.R.R.; Loureiro, S.; Grabicová, K.; Staňová, A.V.; Soares, A.M.; Pestana, J.L. Two-generational effects of Benzophenone-3 on the aquatic midge Chironomus riparius. Sci. Total Environ. 2019, 669, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Vandegehuchte, M.B.; Lemière, F.; Vanhaecke, L.; Vanden Berghe, W.; Janssen, C.R. Direct and transgenerational impact on Daphnia magna of chemicals with a known effect on DNA methylation. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2010, 151, 278–285. [Google Scholar] [CrossRef]
- Vandegehuchte, M.B.; De Coninck, D.; Vandenbrouck, T.; De Coen, W.M.; Janssen, C.R. Gene transcription profiles, global DNA methylation and potential transgenerational epigenetic effects related to Zn exposure history in Daphnia magna. Environ. Pollut. 2010, 158, 3323–3329. [Google Scholar] [CrossRef] [PubMed]
- Oppold, A.; Kreß, A.; Vanden Bussche, J.; Diogo, J.; Kuch, U.; Oehlmann, J.; Vandegehuchte, M.; Müller, R. Epigenetic alterations and decreasing insecticide sensitivity of the Asian tiger mosquito Aedes albopictus. Ecotoxicol. Environ. Saf. 2015, 122, 45–53. [Google Scholar] [CrossRef]
- Anway, M.D.; Memon, M.A.; Uzumcu, M.; Skinner, M.K. Transgenerational effect of the endocrine disruptor vinclozolin on male spermatogenesis. J. Androl. 2006, 27, 868–879. [Google Scholar] [CrossRef]
- Nair, P.M.G.; Choi, J. Modulation in the mRNA expression of ecdysone receptor gene in aquatic midge, Chironomus riparius upon exposure to nonylphenol and silver nanoparticles. Environ. Toxicol. Pharmacol. 2012, 33, 98–106. [Google Scholar] [CrossRef]
- Barata, C.; Baird, D.J.; Soares, A.M. Demographic responses of a tropical cladoceran to cadmium: Effects of food supply and density. Ecol. Appl. 2002, 12, 552–564. [Google Scholar]
- Jameel, M.; Alam, M.F.; Younus, H.; Jamal, K.; Siddique, H.R. Hazardous sub-cellular effects of Fipronil directly influence the organismal parameters of Spodoptera litura. Ecotoxicol. Environ. Saf. 2019, 172, 216–224. [Google Scholar] [CrossRef]
- Manlik, O. The importance of reproduction for the conservation of slow-growing animal populations. In Reproductive Sciences in Animal Conservation; Springer: Cham, Switzerland, 2019; pp. 13–39. [Google Scholar] [CrossRef]
- Vogt, C.; Nowak, C.; Diogo, J.B.; Oetken, M.; Schwenk, K.; Oehlmann, J. Multi-generation studies with Chironomus riparius–effects of low tributyltin concentrations on life history parameters and genetic diversity. Chemosphere 2007, 67, 2192–2200. [Google Scholar] [CrossRef] [PubMed]
- Heye, K.; Becker, D.; Eversloh, C.L.; Durmaz, V.; Ternes, T.A.; Oetken, M.; Oehlmann, J. Effects of carbamazepine and two of its metabolites on the non-biting midge Chironomus riparius in a sediment full life cycle toxicity test. Water Res. 2016, 98, 19–27. [Google Scholar] [CrossRef]
- Sildanchandra, W.; Crane, M. Influence of sexual dimorphism in Chironomus riparius Meigen on toxic effects of cadmium. Environ. Toxicol. Chem. 2000, 19, 2309–2313. [Google Scholar] [CrossRef]
- Santos, F.C.; Verweij, R.A.; Soares, A.M.; Scott-Fordsmand, J.J.; van Gestel, C.A.; Amorim, M.J. Multigenerational exposure of Ag materials (nano and salt) in soil–environmental hazards in Enchytraeus crypticus (Oligochaeta). Nanoscale Adv. 2024, 6, 826–831. [Google Scholar] [CrossRef]
- Wamucho, A.; Unrine, J.; May, J.; Tsyusko, O. Global DNA adenine methylation in Caenorhabditis elegans after multigenerational exposure to silver nanoparticles and silver nitrate. Int. J. Mol. Sci. 2023, 24, 6168. [Google Scholar] [CrossRef]
- Townsend, K.R.; Pettigrove, V.J.; Hoffmann, A.A. Food limitation in Chironomus tepperi: Effects on survival, sex ratios and development across two generations. Ecotoxicol. Environ. Saf. 2012, 84, 1–8. [Google Scholar] [CrossRef]
- Nair, P.M.G.; Park, S.Y.; Choi, J. Evaluation of the effect of silver nanoparticles and silver ions using stress responsive gene expression in Chironomus riparius. Chemosphere 2013, 92, 592–599. [Google Scholar] [CrossRef]
- Li, H.; Shi, L.; Wang, D.; Wang, M. Impacts of mercury exposure on life history traits of Tigriopus japonicus: Multigeneration effects and recovery from pollution. Aquat. Toxicol. 2015, 166, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Schultz, C.L.; Wamucho, A.; Tsyusko, O.V.; Unrine, J.M.; Crossley, A.; Svendsen, C.; Spurgeon, D.J. Multigenerational exposure to silver ions and silver nanoparticles reveals heightened sensitivity and epigenetic memory in Caenorhabditis elegans. Proc. R. Soc. B Biol. Sci. 2016, 283, 20152911. [Google Scholar] [CrossRef]
- Suarez-Ulloa, V.; Gonzalez-Romero, R.; Eirin-Lopez, J.M. Environmental epigenetics: A promising venue for developing next-generation pollution biomonitoring tools in marine invertebrates. Mar. Pollut. Bull. 2015, 98, 5–13. [Google Scholar] [CrossRef]
- Shen, M.-H.; Zhou, X.-X.; Yang, X.-Y.; Chao, J.-B.; Liu, R.; Liu, J.-F. Exposure medium: Key in identifying free Ag+ as the exclusive species of silver nanoparticles with acute toxicity to Daphnia magna. Sci. Rep. 2015, 5, 9674. [Google Scholar] [CrossRef]
- Singh, A.; Hou, W.-C.; Lin, T.-F.; Zepp, R.G. Roles of silver–chloride complexations in sunlight-driven formation of silver nanoparticles. Environ. Sci. Technol. 2019, 53, 11162–11169. [Google Scholar] [CrossRef]
- Ben Yahia, M.; Ben Yahia, M. Physico-chemical study of complexation of silver ion (Ag(+)) by macrocyclic molecules (hexa-Helicenes) based on statistical physics theory: New description of a cancer drug. Sci. Rep. 2020, 10, 10328. [Google Scholar] [CrossRef] [PubMed]
- Merritt, R.W.; Cummins, K.W. An Introduction to the Aquatic Insects of North America; Kendall Hunt: Dubuque, IA, USA, 1996. [Google Scholar] [CrossRef]
- Ferrari, B.J.; Fabure, J. Field assessment of reproduction-related traits of chironomids using a newly developed emergence platform (E-Board). Ecotoxicol. Environ. Saf. 2017, 137, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Lilley, T.; Ruokolainen, L.; Vesterinen, E.; Paasivirta, L.; Norrdahl, K. Sediment organic tin contamination promotes impoverishment of non-biting midge species communities in the Archipelago Sea, SW Finland. Ecotoxicology 2012, 21, 1333–1344. [Google Scholar] [CrossRef] [PubMed]
- Sayer, C.D.; Hoare, D.J.; Simpson, G.L.; Henderson, A.C.; Liptrot, E.R.; Jackson, M.J.; Appleby, P.G.; Boyle, J.F.; Jones, J.I.; Waldock, M.J. TBT causes regime shift in shallow lakes. Environ. Sci. Technol. 2006, 40, 5269–5275. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, J.; Krais, S.; Li, Z.; Triebskorn, R.; Köhler, H.-R. Multi- and Transgenerational Effects of Silver Ions (Ag+) in the ng/L Range on Life Cycle Parameters and Population Growth of the Midge Chironomus riparius (Diptera, Chironomidae). Toxics 2025, 13, 855. https://doi.org/10.3390/toxics13100855
Ding J, Krais S, Li Z, Triebskorn R, Köhler H-R. Multi- and Transgenerational Effects of Silver Ions (Ag+) in the ng/L Range on Life Cycle Parameters and Population Growth of the Midge Chironomus riparius (Diptera, Chironomidae). Toxics. 2025; 13(10):855. https://doi.org/10.3390/toxics13100855
Chicago/Turabian StyleDing, Jingyun, Stefanie Krais, Zequn Li, Rita Triebskorn, and Heinz-R. Köhler. 2025. "Multi- and Transgenerational Effects of Silver Ions (Ag+) in the ng/L Range on Life Cycle Parameters and Population Growth of the Midge Chironomus riparius (Diptera, Chironomidae)" Toxics 13, no. 10: 855. https://doi.org/10.3390/toxics13100855
APA StyleDing, J., Krais, S., Li, Z., Triebskorn, R., & Köhler, H.-R. (2025). Multi- and Transgenerational Effects of Silver Ions (Ag+) in the ng/L Range on Life Cycle Parameters and Population Growth of the Midge Chironomus riparius (Diptera, Chironomidae). Toxics, 13(10), 855. https://doi.org/10.3390/toxics13100855






