Phytoremediation of Meta-Cresol by Sunflower: Tolerance of Plant and Removal of M-Cresol
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Measuring Items and Methods
2.2.1. Photosynthetic Parameters and Chlorophyll Fluorescence Measurements
2.2.2. Determination of Chlorophyll Content
2.2.3. Determination of M-Cresol
2.2.4. Relative Growth Rate (RGR)
2.3. Statistical Analysis
3. Results
3.1. Effects of M-Cresol Stress on Photosynthetic Parameters of Sunflower
3.2. Effects of M-Cresol Stress on Chlorophyll Fluorescence Parameters of Sunflower
3.3. Effects of M-Cresol Stress on Relative Growth Rate and M-Cresol Removal of Sunflower.
4. Discussion
4.1. Photosynthetic Characteristics of Sunflower
4.2. Chlorophyll Fluorescence Parameters of Sunflower
4.3. Effect of M-Cresol on Chlorophyll Content of Sunflower
4.4. Decontamination Effect of Sunflower on M-Cresol
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gai, H.J.; Lin, Q.; Zhong, C.Y.; Zhang, X.W.; Xiao, M.; Song, H.B. A solvent based separation method for phenolic compounds from low-temperature coal tar. J. Clean. Prod. 2019, 223, 1–11. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Velis, C.A.; Roland, W.; Iacovidou, E.; Purnell, P. An overview of chemical additives presents in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef]
- Yao, C.X.; Jin, C.Y.; Wang, S.Z.; Wang, Y.H.; Zhang, Y.N.; Hou, Z.J.; Yu, Y.H.; Sun, C.L.; Wei, H.Z.; Wang, G.W. Analysis of the degradation of m-cresol with Fe/AC in catalytic wet peroxide oxidation enhanced by swirl flow. Chemosphere 2022, 298, 134356. [Google Scholar] [CrossRef]
- Keithl, H.; Telliard, W. Priority Pollutants: I. A Perspective View. Environ. Sci. Technol. 1979, 13, 416–423. [Google Scholar] [CrossRef]
- Tang, W.X.; Jianjian Wei, J.J.; Wang, S.T.; Cheng, Q.; Abid, A.; Gu, J.; Zheng, M.; Ma, D.H. Formation of highly toxic p-benzoquinones byproducts during ozonation of cresols. J. Environ. Sci. 2025, 154, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Ahamad, P.; Kunhi, A. Degradation of high concentrations of cresols by Pseudomonas sp. CP4. World. J. Microb. Biot. 1999, 15, 321–323. [Google Scholar] [CrossRef]
- Pedrazzani, R.; Cavallotti, I.; Bollati, E.; Ferreri, M.; Bertanza, G. The role of bioassays in the evaluation of ecotoxicological aspects within the PEF/OEF protocols: The case of WWTPs. Ecotox. Environ. Saf. 2018, 147, 742–748. [Google Scholar] [CrossRef]
- Ncanana, Z.S.; Pullabhotla, V.S.R. Oxidative degradation of m-cresol using ozone in the presence of pure γ-Al2O3, SiO2 and V2O5 catalysts. Environ. Chem. Eng. 2019, 7, 103072. [Google Scholar] [CrossRef]
- Yang, W.W.; Wu, T.T. Investigation of Matrix Effects in Laboratory Studies of Catalytic Ozonation Processes. Ind. Eng. Chemi. Res. 2019, 58, 3468–3477. [Google Scholar] [CrossRef]
- Rita, D.S.; Jacqueline, V.K.; Bruno, V.V.; Antoine, D.C.; Philippe, B.; Norbert, L.; Raymond, V. Toxicity of free p-cresol: A prospective and cross-sectional analysis. Clin. Chem. 2002, 49, 470–478. [Google Scholar] [CrossRef]
- Fu, X.Y.; Zhao, W.; Xiong, A.S.; Tian, Y.S.; Yao, Q.H. Phytoremediation of triphenylmethane dyes by overexpressing a Citrobacter sp. triphenylmethane reductase in transgenic Arabidopsis. Appl. Microbiol. Biot. 2013, 97, 1799–1806. [Google Scholar] [CrossRef] [PubMed]
- Töre, G.Y.; Özkoç, Ö.B. Recent developments in aquatic macrophytes for environmental pollution control: A case study on heavy metal removal from lake water and agricultural return wastewater with the use of duckweed (Lemnacea). In Phytoremediation Technology for the Removal of Heavy Metals and Other Contaminants from Soil and Water; Elsevier: Amsterdam, The Netherlands, 2022; pp. 75–127. [Google Scholar] [CrossRef]
- Xie, H.C.; Zhao, T.R.; Han, K.J.; Wang, Z. Phytoremediation of wastewater containing phenol by Salix matsudana seedlings and their physiological response. Glob. J. Bot. Sci. 2020, 8, 53–58. [Google Scholar] [CrossRef]
- Fu, G.L.; Wang, Z.; Xie, H.C.; Wang, L. Bacillus thuringiensis A1 improve phenol tolerance and phytoextraction by Acorus calamus L. Int. J. Phytoremediat. 2022, 24, 1251–1258. [Google Scholar] [CrossRef]
- Liu, Z.L. Research on Phytoremediation of Nitrobenzene and Aniline Contaminated Soil. Master’s Thesis, Lanzhou University, Lanzhou, China, 2012. [Google Scholar]
- Pooja, S.; Sonam, T.; Diane, P.; Ram, C. Integrating phytoremediation into treatment of pulp and paper industry wastewater: Field observations of native plants for the detoxification of metals and their potential as part of a multidisciplinary strategy. J. Environ. Chem. Eng. 2021, 9, 105547. [Google Scholar] [CrossRef]
- Lu, Y.P. Research on heavy metal stress and stress resistance in sunflower. Hebei Agr. Mach. 2016, 5, 63–65. [Google Scholar] [CrossRef]
- Sher, A.; Suleman, M.; Qayyum , A.; Sattar, A.; Wasaya, A.; Ijaz, M.; Nawaz, A. Ridge sowing of sunflower (Helianthus annuus L.) in a minimum till system improves the productivity, oil quality, and profitability on a sandy loam soil under an arid climate. Environ. Sci. Pollut. R. 2018, 25, 11905–11912. [Google Scholar] [CrossRef]
- Zeng, X.B.; Tang, J.M.; Zhu, C.H.; Zou, R.; Shi, Y.C.; Wei, X.; Cai, L.H. Effects of heavy metal nickel stress on physiological and biochemical characteristics of sunflower seedlings. Guangxi Plants 2019, 39, 1702–1709. [Google Scholar] [CrossRef]
- Ma, H. Experimental Study on the In-Situ Remediation of Cd and Zn Contaminated Sites by Castor and Sunflower. Master’s Thesis, Shanghai University, Shanghai, China, 2015. [Google Scholar]
- Yan, F.; Wang, Q.L.; Guo, Y.Y.; Zhang, Y.J.; Hou, L.Y. Effect of NaCl stress on photosynthetic chlorophyll fluorescence characteristics of wild yellow rhizome leaves in Qilian Mountains. Northwest J. Bot. 2016, 36, 1182–1189. [Google Scholar] [CrossRef]
- Sun, Y.M.; Ning, G.H.; Liu, S.Q.; Wang, Q.Q.; Yang, S.S.; Yang, Z.X. Enrichment characteristics of cadmium in tolerantplants oilseed sunflower and cotton. J. Soil Water Conserv. 2015, 29, 281–286. [Google Scholar] [CrossRef]
- Jing, T.; Xie, H.C.; Sun, J.W.; Liu, H.D.; Li, H. Photosynthetic physiological response of sunflower to aniline wastewater and its purification effect. J. Ecol. 2017, 37, 6091–6098. [Google Scholar] [CrossRef]
- Jha, P.; Jobby, R.; Kudale, S.; Modi, N.; Dhaneshwar, A.; Desai, N. Biodegradation of phenol using hairy roots of Helianthus annuus L. Int. Biodeter. Biodegr. 2013, 77, 106–113. [Google Scholar] [CrossRef]
- In, Y.J.; Mi, C.K.; Jin-Kyoo, K.; Lee, S.O.; Cho, K.; Lee, K. Mutation of rpoS enhances Pseudomonas sp. KL28 growth at higher concentrations of m-cresol and changes its surface-related phenotypes. Fems Microbiol. Lett. 2010, 269, 97–103. [Google Scholar] [CrossRef]
- Bai, J.; Wen, J.P.; Li, H.M.; Jiang, Y. Kinetic modeling of growth and biodegradation of phenol and m-cresol using Alcaligenes faecal is. Process Biochem. 2007, 42, 510–517. [Google Scholar] [CrossRef]
- Basheer, F.; Farooqi, I.H. Biodegradation of m-cresol by aerobic granules in a sequencing batch reactor. Environ. Technol. 2012, 33, 1847–1856. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, L.; Fu, Y.; Fu, G.; Fu, D.; Li, H.; Su, S.; Xie, H.; Tian, H.; Wang, R.; et al. Exogenous Salicylic Acid Alleviates Physiological Stress in Salix matsudana Seedlings and Increases 2,4-Dinitrophenol Removal. Forests 2024, 15, 1392. [Google Scholar] [CrossRef]
- Xie, H.; Fu, Y.; Fu, D.; Lin, D.; Zhou, H.; Fu, G.; Li, H.; Liu, J.; Zheng, X.; Li, K. The Physiological Responseof Salix matsudana for Water Pollution by 2,4-Dinitrophenol. Toxics 2024, 12, 763. [Google Scholar] [CrossRef]
- Schreiber, U.; Schliwa, U.; Bilger, W. Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynthe. Res. 1986, 10, 51–62. [Google Scholar] [CrossRef]
- Braun, G.; Malkin, S. Regulation of the imbalance in light excitation between Photosystem II and Photosystem I by cations and by the energized state of the thylakoid membrane. Biochim. Biophys. Acta Bioenerg. 1990, 1017, 79–90. [Google Scholar] [CrossRef]
- Barbara, D.D.; Lii, W.W.A.; Barker, D.H.; Logan, B.A.; Verhoeven, A.S. Using chlorophyll fluorescence to assess the fraction of absorbed light allocated to thermal dissipation of excess excitation. Physiol. Plant. 1996, 98, 253–264. [Google Scholar] [CrossRef]
- Gao, J.S.; Cai, Y.P. Experimental Guide to Plant Physiology; China Agricultural University Press: Beijing, China, 2018; pp. 33–34. [Google Scholar]
- Klibanova, M.; Albertib, N.; Morrise, D.; Felshinl, M. Enzymatic removalof toxic phenols and anilines from wastewaters. J. Appl. Biochem. 1980, 2, 414–421. Available online: https://www.researchgate.net/publication/236357015 (accessed on 1 October 2025).
- Ucisika, S.; Trapp, S. Uptake, removal, accumulation, and phytotoxicity of 4-chlorophenol in willow trees. Arch Environ. Contam. Toxicol. 2008, 54, 619–627. [Google Scholar] [CrossRef]
- Li, K.; Li, H.; Li, C.R.; Xie, H.C. Phytoremediation of aniline by Salix babylonica cuttings: Removal, accumulation, andphotosynthetic response. Ecotox. Environ. Safe 2021, 214, 112124. [Google Scholar] [CrossRef]
- Farquharg, D.; Sharkeyt, D. Stomatal conductance and photosynthesis. Annu. Rev. Plant Physiol. 1982, 33, 317–345. [Google Scholar] [CrossRef]
- Gao, G.L.; Zhang, X.Y.; Yu, T.F.; Chang, J.F.; Zhao, H. Simulation of stomatal conductance of poplar leaves under extreme drought conditions. Geogr. Arid Reg. 2016, 39, 607–612. [Google Scholar] [CrossRef]
- Perez-Martin, A.; Michelazzo, C.; Torres-Ruiz, J.M.; Flexas, J.; Fernandez, J.E.; Sebastiani, L.; Diaz-Espejo, A. Regulation of photosynthesis and stomatal and mesophyll conductance under water stress and recovery in olive trees: Correlation with gene expression of carbonic anhydrase and aquaporins. J. Exp. Bot. 2014, 65, 3143–3156. [Google Scholar] [CrossRef]
- Poscheneieder, C.; Gunse, B.; Barcelo, J. Influence of cadmium on water relations, stomatal resistance and abscisic acid content in expanding bean leaves. Plant Physiol. 1989, 90, 1365–1371. [Google Scholar] [CrossRef]
- Lavergne, A.; Voelker, S.; Csank, A.; Graven, H.; Boer, H.; Daux, V.; Robertson, L.; Dorado, I.; Sancho, E.; Battipaglia, G.; et al. Historical changes in the stomatal limitation of photosynthesis: Empirical support for an optimality. New Phytol. 2020, 225, 2484–2497. [Google Scholar] [CrossRef]
- Li, H.; Zhang, G.C.; Xie, H.C.; Xu, J.W.; Li, C.R.; Sun, J.W. Effect of phenol wastewater on photosynthetic physiological parameters of weeping willow seedling leaves. J. Bot. 2016, 51, 31–39. [Google Scholar] [CrossRef]
- Wang, L.; Shi, Y.X. Effects of cadmium, lead and their combined pollution on the physiological and biochemical characteristics of radish. Chin. J. Eco-Agric. 2008, 16, 411–414. [Google Scholar] [CrossRef]
- Banksj, M. Chlorophyll fluorescence as a tool to identify drought stress in Acer genotypes. Envir. Exp. Bot. 2018, 155, 118–127. [Google Scholar] [CrossRef]
- Bussotti, F.; Gerosa, G.; Digrado, A.; Gerosa, G.; Digrado, A.; Digrado, A.; Pollastrini, M.; Pollastrini, M. Selection of chlorophyll fluorescence parameters as indicators of photosynthetic efficiency in large scale plant ecological studies. Ecol. Indic. 2019, 108, 105686. [Google Scholar] [CrossRef]
- Kalajih, M.; Baba, W.; Gediga, K.; Goltsev, V.; Samborska, I.; Cetner, M.; Dimitrova, S.; Piszcz, U.; Bielecki, K.; Karmowska, K. Chlorophyll fluorescence as a tool for nutrient status identification in rapeseed plants. Photosynth. Res. 2018, 136, 329–343. [Google Scholar] [CrossRef]
- Ruhle, T.; Reiter, B.; Leister, D. Chlorophyll fluorescence video imaging: A versatile tool for identifying factors related to photosynthesis. Front. Plant Sci. 2018, 9, 55. [Google Scholar] [CrossRef]
- Gotoh, E.; Suetsugu, N.; Yamori, W.; Ishishita, K.; Kiyabu, R.; Fukuda, M.; Higa, T.; Sshirouchi, B.; Wada, M. Chloroplast accumulation response enhances leaf photosynthesis and plant biomass production. Plant Physiol. 2018, 178, 1358–1369. [Google Scholar] [CrossRef]
- Umetani, I.; Janka, E.; Sposob, M.; Hulattc, J.; Kleiven, S.; Bakke, R. Bicarbonate for microalgae cultivation: A case study in a chlorophyte, Tetradesmus wisconsinensis isolated from a Norwegian lake. J. Appl. Phycol. 2021, 33, 1341–1352. [Google Scholar] [CrossRef]
- Kang, H.M.; Liu, C.B.; Bo, W.; Wang, J. Effects of drought stress on water physiology and chlorophyll fluorescence parameters of four ground cover plants. Shanxi Agric. Sci. 2020, 48, 1767–1771. [Google Scholar] [CrossRef]
- Song, Q.; Liu, Y.; Pang, J.; Yong, J.; Lambere, H. Supplementary calcium restores peanut (Arachis hypogaea) growth and photosynthetic capacity under low nocturnal temperature. Front. Plant Sci. 2019, 10, 1637. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.C.; Chu, Z.; Zhu, L.F.; Zhang, J.H.; Hussain, S.; Wu, L.H.; Jin, Q.Y. Glycine increases cold tolerance in rice via the regulation of N uptake, physiological characteristics, and photosynthesis. Plant Physiol. Biochem. 2017, 112, 251–260. [Google Scholar] [CrossRef]
- Shi, Y.J.; Luo, Q.H.; Song, F.H.; Yu, T.; Kou, Y.L. Effects of high temperature stress on photosynthetic parameters andchlorophyll fluorescence of Xinjiang hazelnut. J. Appl. Ecol. 2012, 23, 2477–2482. [Google Scholar] [CrossRef]
- Khan, S.; Sun, J.S.; Brudvigg, W. Cation effects on the electron-acceptor side of photosystem II. J. Phys. Chem. B. 2015, 119, 7722–7728. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.L.; Zuo, G.Q.; Li, Y.; Zheng, D.F.; Feng, N.J. Regulation of photosynthetic characteristics and protective enzymeactivities of soybean seedlings under salinity stress by calcium regioleate. Chinese J. Oil Crop. Sci. 2019, 41, 741–749. [Google Scholar] [CrossRef]
- Pezeshki, S.R.; Hester, M.W.; Lin, Q. The effects of oil spill and clean-up on dominant US Gulf coast marsh macrophytes: A review. Environ. Pollut. 2000, 108, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Jesse, K.; Helen, E.C.; Jonathan, H.P. Breeding on the leading edge of a northward range expansion: Differences in morphology and the stress response in the arctic Gambel’s white-crowned sparrow. Oecologia 2016, 180, 33–44. [Google Scholar] [CrossRef]
- Seyma, H.Y.; Mahmut, K.; Ridvan, T.; Semih, Y. Antioxidant enzyme response of sorghum plant upon exposure to aluminum, chromium and lead heavy metals. Turk. J. Biochem. 2017, 42, 503–512. [Google Scholar] [CrossRef]

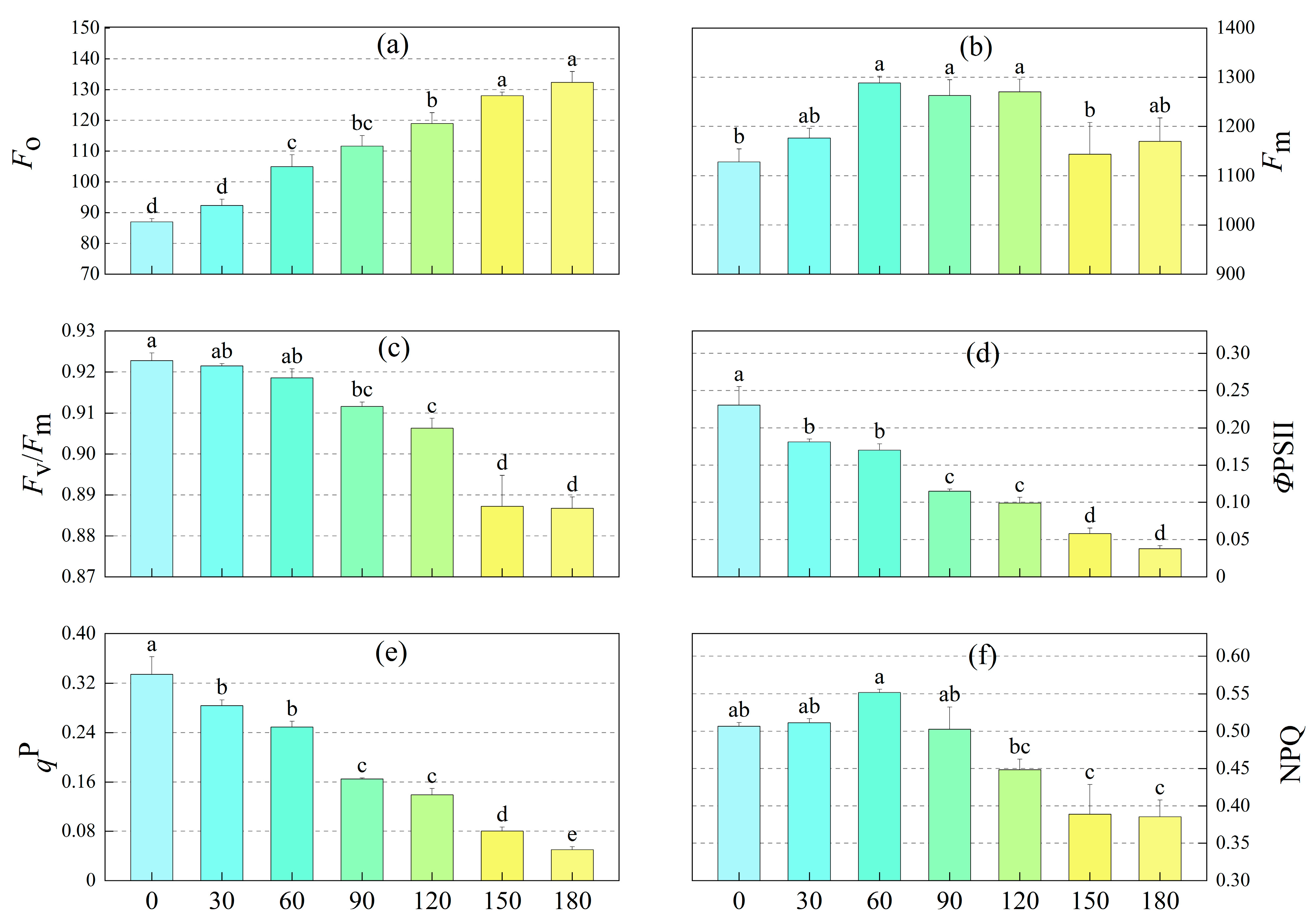
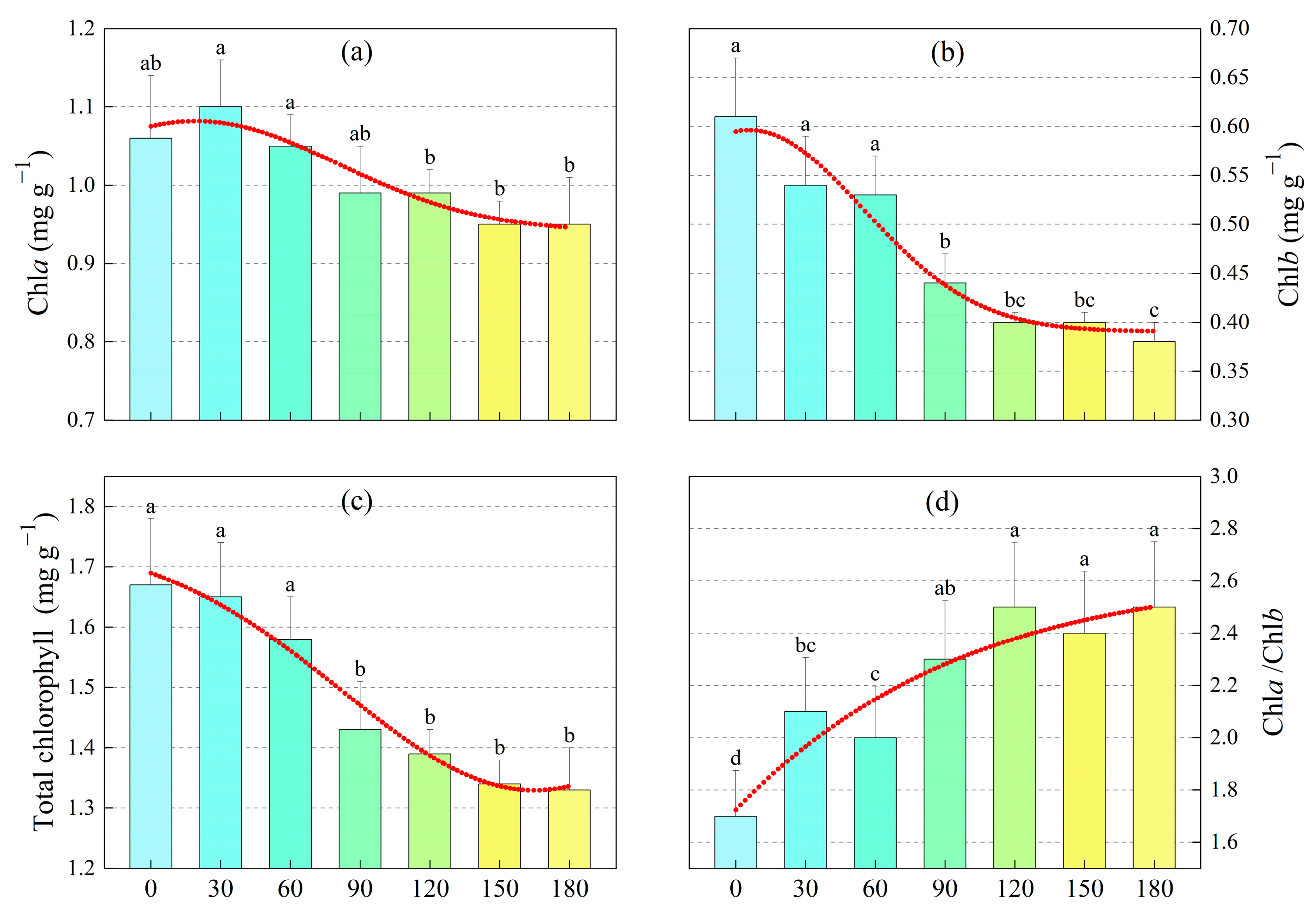
| Treatment | Y (NPQ) | Y (NO) | f | α | β | P | D | Ex |
|---|---|---|---|---|---|---|---|---|
| 0 | 0.45 ± 0.021 b | 0.32 ± 0.021 e | 0.33 ± 0.031 a | 0.25 ± 0.024 a | 0.76 ± 0.015 e | 0.23 ± 0.015 a | 0.31 ± 0.027 b | 0.46 ± 0.016 e |
| 30 | 0.48 ± 0.012 a | 0.34 ± 0.012 e | 0.28 ± 0.013 b | 0.22 ± 0.012 b | 0.78 ± 0.006 d | 0.18 ± 0.004 b | 0.36 ± 0.008 a | 0.46 ± 0.008 e |
| 60 | 0.45 ± 0.005 b | 0.38 ± 0.013 d | 0.25 ± 0.014 b | 0.20 ± 0.011 b | 0.80 ± 0.007 d | 0.17 ± 0.007 b | 0.31 ± 0.007 b | 0.51 ± 0.004 d |
| 90 | 0.41 ± 0.012 c | 0.48 ± 0.013 c | 0.17 ± 0.004 c | 0.14 ± 0.003 c | 0.86 ± 0.004 c | 0.12 ± 0.004 c | 0.30 ± 0.008 b | 0.58 ± 0.005 c |
| 120 | 0.37 ± 0.011 d | 0.53 ± 0.021 b | 0.14 ± 0.009 c | 0.12 ± 0.010 c | 0.88 ± 0.010 c | 0.10 ± 0.006 c | 0.29 ± 0.006 b | 0.61 ± 0.008 c |
| 150 | 0.31 ± 0.022 e | 0.64 ± 0.024 a | 0.08 ± 0.007 d | 0.07 ± 0.008 d | 0.93 ± 0.009 b | 0.06 ± 0.007 d | 0.28 ± 0.025 b | 0.66 ± 0.030 b |
| 180 | 0.29 ± 0.009 e | 0.67 ± 0.006 a | 0.05 ± 0.004 d | 0.04 ± 0.003 e | 0.95 ± 0.004 a | 0.04 ± 0.002 d | 0.24 ± 0.008 c | 0.72 ± 0.007 a |
| Concentration of M-Cresol (mg L−1) | Relative Growth Rate (RGR) | Percent Removal (%) |
|---|---|---|
| 0 | 0.025 ± 0.0024 | - |
| 30 | 0.018 ± 0.0012 | 84.91 |
| 60 | 0.009 ± 0.0011 | 57.28 |
| 90 | 0.001 ± 0.0009 | 40.11 |
| 120 | −0.023 ± 0.0021 | 21.45 |
| 150 | −0.029 ± 0.0029 | 15.59 |
| 180 | −0.029 ± 0.0034 | 11.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Su, S.; Jiang, Y.; Chen, H.; Zhang, L.; Li, Y.; Ma, S.; Liu, J.; Li, H.; Fu, D.; et al. Phytoremediation of Meta-Cresol by Sunflower: Tolerance of Plant and Removal of M-Cresol. Toxics 2025, 13, 845. https://doi.org/10.3390/toxics13100845
Li H, Su S, Jiang Y, Chen H, Zhang L, Li Y, Ma S, Liu J, Li H, Fu D, et al. Phytoremediation of Meta-Cresol by Sunflower: Tolerance of Plant and Removal of M-Cresol. Toxics. 2025; 13(10):845. https://doi.org/10.3390/toxics13100845
Chicago/Turabian StyleLi, Hui, Shuai Su, Yujia Jiang, Hong Chen, Liudong Zhang, Yi Li, Shengguo Ma, Jiaxin Liu, Haitao Li, Degang Fu, and et al. 2025. "Phytoremediation of Meta-Cresol by Sunflower: Tolerance of Plant and Removal of M-Cresol" Toxics 13, no. 10: 845. https://doi.org/10.3390/toxics13100845
APA StyleLi, H., Su, S., Jiang, Y., Chen, H., Zhang, L., Li, Y., Ma, S., Liu, J., Li, H., Fu, D., Li, K., & Xie, H. (2025). Phytoremediation of Meta-Cresol by Sunflower: Tolerance of Plant and Removal of M-Cresol. Toxics, 13(10), 845. https://doi.org/10.3390/toxics13100845







