Association of Exposure to Phthalate Metabolites with Antenatal Depression in US Pregnant Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Exposure Assessment
2.3. Depression Score Assessment
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Unadjusted and Adjusted Associations of Phthalate Metabolites with Depression Scores
3.2. Unadjusted and Adjusted Association of Mixtures of Phthalate Metabolites with Depression Scores
3.3. Principal Findings
3.4. Results in the Context of What Is Known
3.5. Pathophysiology
3.6. Implications for Practice and/or Policy
4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCarron, R.M.; Shapiro, B.; Rawles, J.; Luo, J. Depression. Ann. Intern. Med. 2021, 174, ITC65–ITC80. [Google Scholar] [CrossRef]
- Jacobson, M.H.; Ghassabian, A.; Gore, A.C.; Trasande, L. Exposure to environmental chemicals and perinatal psychopathology. Biochem. Pharmacol. 2022, 195, 114835. [Google Scholar] [CrossRef]
- Gavin, N.I.; Gaynes, B.N.; Lohr, K.N.; Meltzer-Brody, S.; Gartlehner, G.; Swinson, T. Perinatal depression: A systematic review of prevalence and incidence. Obstet. Gynecol. 2005, 106 Pt 1, 1071–1083. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.; Weinberger, T.; Chandy, A.; Schmukler, S. Depression During Pregnancy and Postpartum. Curr. Psychiatry Rep. 2016, 18, 32. [Google Scholar] [CrossRef] [PubMed]
- Luca, D.L.; Margiotta, C.; Staatz, C.; Garlow, E.; Christensen, A.; Zivin, K. Financial Toll of Untreated Perinatal Mood and Anxiety Disorders Among 2017 Births in the United States. Am. J. Public Health 2020, 110, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Glover, V. Maternal depression, anxiety and stress during pregnancy and child outcome; what needs to be done. Best Pract. Res. Clin. Obstet. Gynaecol. 2014, 28, 25–35. [Google Scholar] [CrossRef]
- Kajta, M.; Wójtowicz, A.K. Impact of endocrine-disrupting chemicals on neural development and the onset of neurological disorders. Pharmacol. Rep. 2013, 65, 1632–1639. [Google Scholar] [CrossRef]
- Papalou, O.; Kandaraki, E.A.; Papadakis, G.; Diamanti-Kandarakis, E. Endocrine Disrupting Chemicals: An Occult Mediator of Metabolic Disease. Front. Endocrinol. 2019, 10, 112. [Google Scholar] [CrossRef]
- Dubey, P.; Reddy, S.Y.; Singh, V.; Shi, T.; Coltharp, M.; Clegg, D.; Dwivedi, A.K. Association of Exposure to Phthalate Metabolites With Sex Hormones, Obesity, and Metabolic Syndrome in US Women. JAMA Netw. Open 2022, 5, e2233088. [Google Scholar] [CrossRef]
- Pacyga, D.C.; Sathyanarayana, S.; Strakovsky, R.S. Dietary Predictors of Phthalate and Bisphenol Exposures in Pregnant Women. Adv. Nutr. 2019, 10, 803–815. [Google Scholar] [CrossRef]
- Calafat, A.M.; Longnecker, M.P.; Koch, H.M.; Swan, S.H.; Hauser, R.; Goldman, L.R.; Lanphear, B.P.; Rudel, R.A.; Engel, S.M.; Teitelbaum, S.L.; et al. Optimal Exposure Biomarkers for Nonpersistent Chemicals in Environmental Epidemiology. Environ. Health Perspect. 2015, 123, A166–A168. [Google Scholar] [CrossRef]
- Kim, K.N.; Choi, Y.H.; Lim, Y.H.; Hong, Y.C. Urinary phthalate metabolites and depression in an elderly population: National Health and Nutrition Examination Survey 2005–2012. Environ. Res. 2016, 145, 61–67. [Google Scholar] [CrossRef]
- Eatman, J.A.; Dunlop, A.L.; Barr, D.B.; Corwin, E.J.; Hill, C.C.; Brennan, P.A.; Ryan, P.B.; Panuwet, P.; Taibl, K.R.; Tan, Y.; et al. Exposure to phthalate metabolites, bisphenol A, and psychosocial stress mixtures and pregnancy outcomes in the Atlanta African American maternal-child cohort. Environ. Res. 2023, 233, 116464. [Google Scholar] [CrossRef] [PubMed]
- Vilmand, M.; Beck, I.H.; Bilenberg, N.; Andersson, A.M.; Juul, A.; Schoeters, G.; Boye, H.; Frederiksen, H.; Jensen, T.K. Prenatal and current phthalate exposure and cognitive development in 7-year-old children from the Odense child cohort. Neurotoxicol. Teratol. 2023, 96, 107161. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ren, T.; Zhang, N.; Xia, W.; Xiang, M.; Ran, J.; Zhang, J. Poly-and perfluoroalkyl substances exposure during pregnancy and postpartum depression: Evidence from the Shanghai birth cohort. Chemosphere 2023, 318, 137941. [Google Scholar] [CrossRef] [PubMed]
- Welch, B.M.; Keil, A.P.; Buckley, J.P.; Calafat, A.M.; Christenbury, K.E.; Engel, S.M.; O’Brien, K.M.; Rosen, E.M.; James-Todd, T.; Zota, A.R.; et al. Associations Between Prenatal Urinary Biomarkers of Phthalate Exposure and Preterm Birth: A Pooled Study of 16 US Cohorts. JAMA Pediatr. 2022, 176, 895–905. [Google Scholar] [CrossRef]
- Jacobson, M.H.; Hamra, G.B.; Monk, C.; Crum, R.M.; Upadhyaya, S.; Avalos, L.A.; Bastain, T.M.; Barrett, E.S.; Bush, N.R.; Dunlop, A.L.; et al. Prenatal Exposure to Nonpersistent Environmental Chemicals and Postpartum Depression. JAMA Psychiatry 2023, 81, 67–76. [Google Scholar] [CrossRef]
- Svechnikova, I.; Svechnikov, K.; Soder, O. The influence of di-(2-ethylhexyl) phthalate on steroidogenesis by the ovarian granulosa cells of immature female rats. J. Endocrinol. 2007, 194, 603–609. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Bourguignon, J.P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef]
- Geraghty, S.R.; Khoury, J.C.; Morrow, A.L.; Lanphear, B.P. Reporting individual test results of environmental chemicals in breastmilk: Potential for premature weaning. Breastfeed. Med. 2008, 3, 207–213. [Google Scholar] [CrossRef]
- Warner, G.R.; Dettogni, R.S.; Bagchi, I.C.; Flaws, J.A.; Graceli, J.B. Placental outcomes of phthalate exposure. Reprod. Toxicol. 2021, 103, 1–17. [Google Scholar] [CrossRef]
- Xu, X.; Yang, Y.; Wang, R.; Wang, Y.; Ruan, Q.; Lu, Y. Perinatal exposure to di-(2-ethylhexyl) phthalate affects anxiety- and depression-like behaviors in mice. Chemosphere 2015, 124, 22–31. [Google Scholar] [CrossRef]
- Bustamante-Montes, L.P.; Hernández-Valero, M.A.; Flores-Pimentel, D.; García-Fábila, M.; Amaya-Chávez, A.; Barr, D.B.; Borja-Aburto, V.H. Prenatal exposure to phthalates is associated with decreased anogenital distance and penile size in male newborns. J. Dev. Orig. Health Dis. 2013, 4, 300–306. [Google Scholar] [CrossRef]
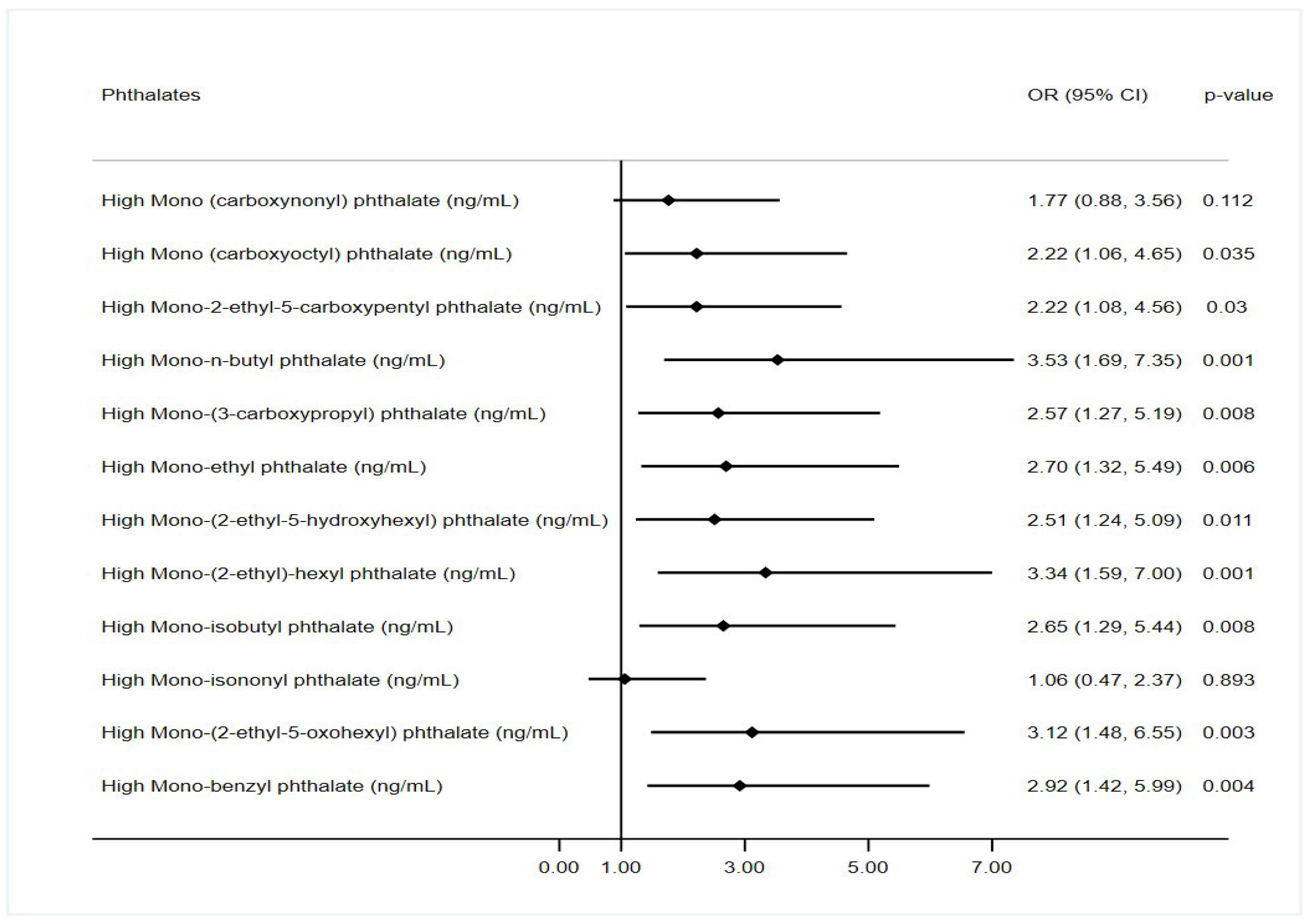
| Factor | Value | Depression Score | p-Value |
|---|---|---|---|
| N | 208 | Median (IQR) | |
| Age | |||
| 18–30 years | 145 (69.71%) | 3.00 (1.00, 5.00) | 0.37 |
| ≥31 years | 63 (30.29%) | 3.00 (1.00, 6.00) | |
| Marital Status | |||
| Married | 130 (62.50%) | 3.00 (1.00, 5.00) | 0.26 |
| Others | 78 (37.50%) | 3.00 (1.00, 7.00) | |
| Ethnicity | |||
| Hispanic | 13 (6.25%) | 2.00 (2.00, 4.00) | 0.70 |
| Non-Hispanic | 31 (14.90%) | 3.00 (1.00, 6.00) | |
| Others/Unknown/missing | 164 (78.85%) | 3.00 (1.00, 5.00) | |
| Income | |||
| 0 to 44,999 | 48 (23.08%) | 4.00 (1.00, 6.50) | 0.18 |
| 45,000 to 99,999 | 26 (12.50%) | 2.00 (1.00, 4.00) | |
| 100,000 and above | 13 (6.25%) | 2.00 (1.00, 3.00) | |
| Unknown | 121 (58.17%) | 3.00 (1.00, 5.00) | |
| Education | |||
| less than 9th grade | 18 (8.65%) | 2.00 (0.00, 5.00) | 0.077 |
| 9th to 12th grade | 90 (43.27%) | 4.00 (1.00, 7.00) | |
| college or above | 100 (48.08%) | 2.00 (1.00, 4.00) | |
| US born | |||
| US born | 37 (17.79%) | 3.00 (1.00, 5.00) | 0.17 |
| Non US born | 19 (9.13%) | 2.00 (1.00, 3.00) | |
| Unknown | 152 (73.08%) | 3.00 (1.00, 5.50) | |
| Ever alcohol | |||
| No | 49 (23.56%) | 2.00 (1.00, 5.00) | 0.17 |
| Yes | 137 (65.87%) | 3.00 (1.00, 6.00) | |
| Unknown | 22 (10.58%) | 3.50 (2.00, 6.00) | |
| Smoking Status | |||
| No | 145 (69.71%) | 3.00 (1.00, 5.00) | 0.071 |
| Yes | 53 (25.48%) | 4.00 (1.00, 7.00) | |
| Unknown | 10 (4.81%) | 3.00 (2.00, 8.00) | |
| Physical activity | |||
| No | 64 (30.77%) | 3.00 (1.00, 5.00) | 0.41 |
| Yes | 32 (15.38%) | 3.50 (1.50, 6.00) | |
| Unknown | 112 (53.85%) | 3.00 (1.00, 5.00) | |
| Obesity | |||
| No | 134 (64.73%) | 3.00 (1.00, 5.00) | 0.44 |
| Yes | 73 (35.27%) | 3.00 (1.00, 6.00) |
| Phthalates | Median (IQR) | RC * (95% CI) | p-Value |
|---|---|---|---|
| Mono (carboxynonyl) phthalate (ng/mL) | 1.88 (1.04, 3.76) | 0.13 (0.03, 0.24) | 0.014 |
| Mono (carboxyoctyl) phthalate (ng/mL) | 4.75 (2.60, 11.70) | 0.06 (−0.02, 0.14) | 0.158 |
| Mono-2-ethyl-5-carboxypentyl phthalate (ng/mL) | 15.45 (6.60, 34.60) | 0.08 (0.00, 0.17) | 0.042 |
| Mono-n-butyl phthalate (ng/mL) | 14.70 (7.05, 31.90) | 0.17 (0.09, 0.26) | <0.001 |
| Mono-(3-carboxypropyl) phthalate (ng/mL) | 1.60 (0.70, 2.87) | 0.16 (0.07, 0.26) | 0.001 |
| Mono-ethyl phthalate (ng/mL) | 59.95 (21.41, 228.69) | 0.07 (0.00, 0.13) | 0.045 |
| Mono-(2-ethyl-5-hydroxyhexyl) phthalate (ng/mL) | 9.95 (3.85, 21.35) | 0.08 (0.01, 0.16) | 0.029 |
| Mono-(2-ethyl)-hexyl phthalate (ng/mL) | 1.92 (0.85, 5.20) | 0.08 (0.00, 0.16) | 0.049 |
| Mono-isobutyl phthalate (ng/mL) | 6.40 (2.50, 11.95) | 0.12 (0.03, 0.21) | 0.006 |
| Mono-isononyl phthalate (ng/mL) | 0.87 (0.67, 0.87) | 0.03 (−0.10, 0.16) | 0.644 |
| Mono-(2-ethyl-5-oxohexyl) phthalate (ng/mL) | 8.20 (3.55, 15.90) | 0.09 (0.02, 0.17) | 0.018 |
| Mono-benzyl phthalate (ng/mL) | 6.48 (2.68, 16.37) | 0.14 (0.06, 0.22) | <0.001 |
| Phthalates | aRC * (95% CI) | p-Value |
|---|---|---|
| Mono (carboxynonyl) phthalate (ng/mL) | 0.11 (0.00, 0.22) | 0.055 |
| Mono (carboxyoctyl) phthalate (ng/mL) | 0.05 (−0.03, 0.14) | 0.226 |
| Mono-2-ethyl-5-carboxypentyl phthalate (ng/mL) | 0.10 (0.01, 0.18) | 0.030 |
| Mono-n-butyl phthalate (ng/mL) | 0.17 (0.07, 0.26) | 0.001 |
| Mono-(3-carboxypropyl) phthalate (ng/mL) | 0.15 (0.05, 0.25) | 0.003 |
| Mono-ethyl phthalate (ng/mL) | 0.07 (0.00, 0.14) | 0.046 |
| Mono-(2-ethyl-5-hydroxyhexyl) phthalate (ng/mL) | 0.09 (0.01, 0.16) | 0.033 |
| Mono-(2-ethyl)-hexyl phthalate (ng/mL) | 0.08 (0.00, 0.17) | 0.049 |
| Mono-isobutyl phthalate (ng/mL) | 0.10 (0.01, 0.19) | 0.037 |
| Mono-isononyl phthalate (ng/mL) | 0.05 (−0.09, 0.19) | 0.472 |
| Mono-(2-ethyl-5-oxohexyl) phthalate (ng/mL) | 0.10 (0.02, 0.18) | 0.018 |
| Mono-benzyl phthalate (ng/mL) | 0.13 (0.04, 0.21) | 0.004 |
| Factor | Exposure | RC (95% CI) | p-Value |
|---|---|---|---|
| Primary analysis | |||
| Unadjusted analysis | Composite scores of all phthalate metabolites | 0.30 (0.13, 0.47) | <0.001 |
| Adjusted analysis | Composite scores of all phthalate metabolites | 0.22 (0.03, 0.41) | 0.029 |
| Validation analysis | |||
| Unadjusted analysis | Composite scores of selected phthalates metabolites | 0.29 (0.13, 0.45) | <0.001 |
| Adjusted analysis | Composite scores of selected phthalate metabolites | 0.18 (0.00, 0.36) | 0.049 |
| Adjusted analysis | Composite scores of selected phthalate metabolites | 0.25 (0.12, 0.38) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dubey, P.; Thangavel, C.; Yousif, A.; Kim, S.; Reddy, S. Association of Exposure to Phthalate Metabolites with Antenatal Depression in US Pregnant Women. Toxics 2025, 13, 838. https://doi.org/10.3390/toxics13100838
Dubey P, Thangavel C, Yousif A, Kim S, Reddy S. Association of Exposure to Phthalate Metabolites with Antenatal Depression in US Pregnant Women. Toxics. 2025; 13(10):838. https://doi.org/10.3390/toxics13100838
Chicago/Turabian StyleDubey, Pallavi, Chinthana Thangavel, Abdelrahman Yousif, Sophie Kim, and Sireesha Reddy. 2025. "Association of Exposure to Phthalate Metabolites with Antenatal Depression in US Pregnant Women" Toxics 13, no. 10: 838. https://doi.org/10.3390/toxics13100838
APA StyleDubey, P., Thangavel, C., Yousif, A., Kim, S., & Reddy, S. (2025). Association of Exposure to Phthalate Metabolites with Antenatal Depression in US Pregnant Women. Toxics, 13(10), 838. https://doi.org/10.3390/toxics13100838






