Unexpected High Blood Lead Levels in a Remote Indigenous Community in the Northeastern Peruvian Amazon
Abstract
1. Introduction
2. Materials and Methods
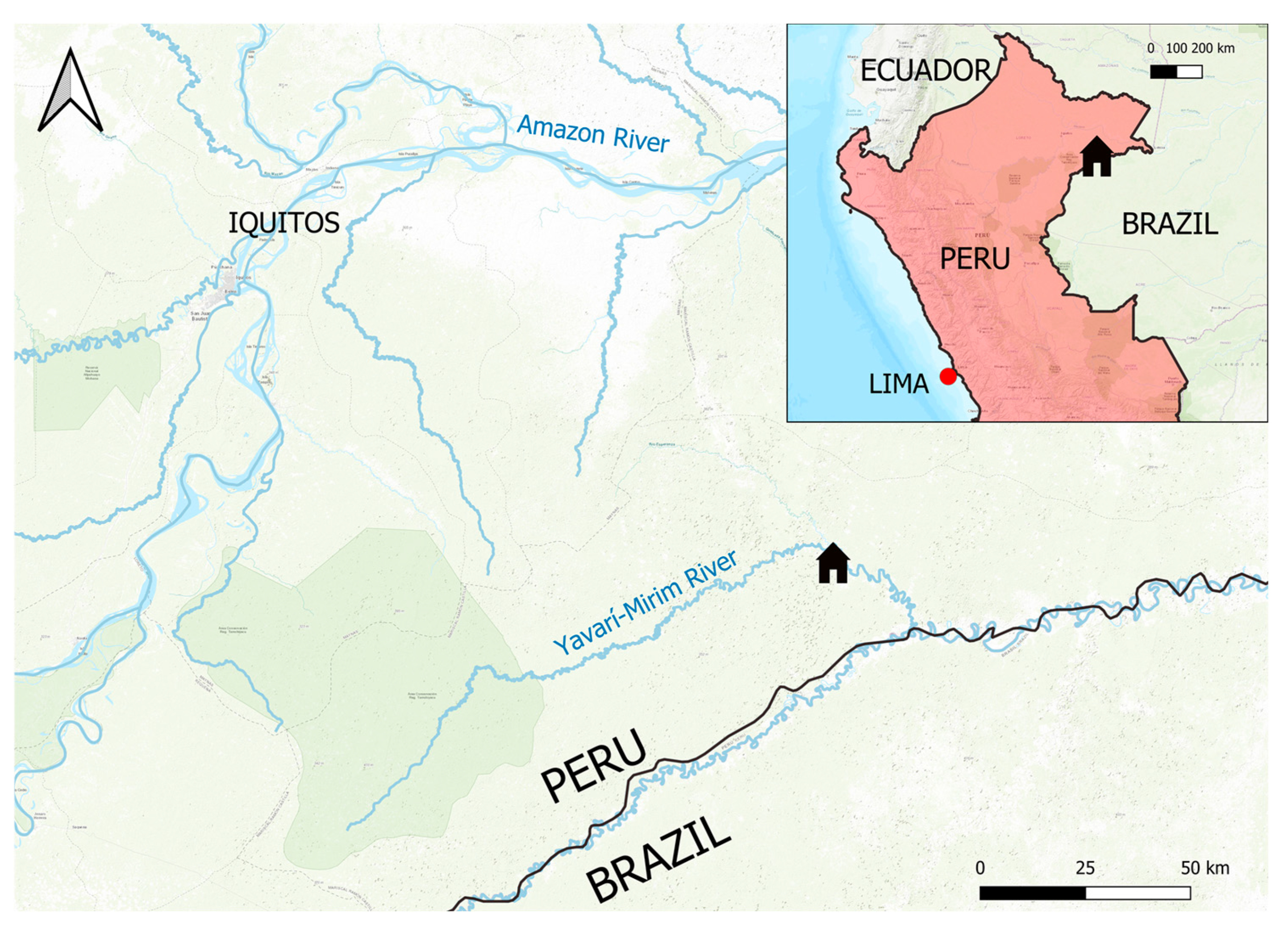
2.1. Study Population
2.2. Human Sampling
2.3. Environmental Sampling
2.4. Interviews for Risk Exposure
2.5. Laboratory Analysis
2.6. Data Analysis
2.7. Ethical Authorizations
3. Results
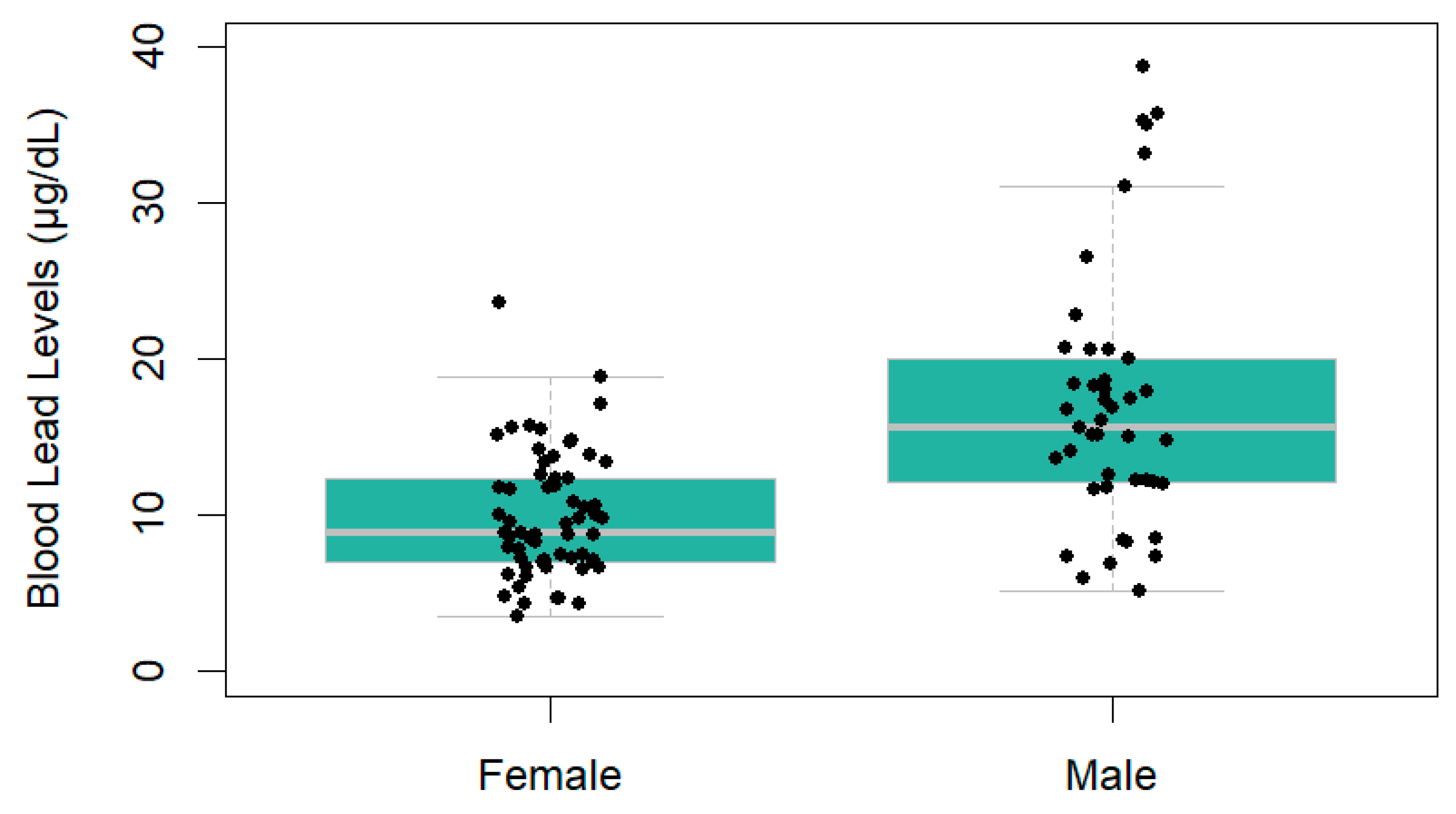
3.1. Human Lead Levels
3.2. Environmental Lead Levels
3.3. Isotopic Fingerprint
3.4. Dietary Patterns
3.5. Sociocultural Risk Factors
4. Discussion
4.1. High BLLs in Indigenous People
4.2. Sources and Exposure Routes for Pb
4.2.1. Lead Contribution Through Water
4.2.2. Lead Contribution Through Hunting Ammunition
4.2.3. Lead Contribution Through Alternative Uses of Shot Pellets
4.2.4. Lead Contribution Through Fish
4.2.5. Lead Contribution Through Soils
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, H.; Hu, Y. Lead (Pb) isotopic fingerprinting and its applications in lead pollution studies in China: A review. Environ. Pollut. 2010, 158, 1134–1146. [Google Scholar] [CrossRef]
- Canfield, R.L.; Henderson, C.R.; Cory-Slechta, D.A.; Cox, C.; Jusko, T.A.; Lanphear, B.P. Intellectual Impairment in Children with Blood Lead Concentrations below 10 μg per Deciliter. N. Engl. J. Med. 2003, 348, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Skerfving, S.; Bergdahl, I.A. Lead. In Handbook on the Toxicology of Metals; Nordberg, G.F., Fowler, B.A., Nordberg, M., Eds.; Academic Press: Amsterdam, The Netherlands; Elsevier: Amsterdam, The Netherlands, 2015; pp. 911–967. [Google Scholar] [CrossRef]
- Hu, H.; Shih, R.; Rothenberg, S.; Schwartz, B.S. The epidemiology of lead toxicity in adults: Measuring dose and consideration of other methodologic issues. Environ. Health Perspect. 2007, 115, 455. [Google Scholar] [CrossRef] [PubMed]
- UNEP. Final Reviews of Scientific Information on Lead—December 2010; United Nations Environment Programme: Nairobi, Kenya, 2010. Available online: https://wedocs.unep.org/handle/20.500.11822/27635 (accessed on 19 December 2024).
- Gilbert, S.G.; Weiss, B. A rationale for lowering the blood lead action level from 10 to 2 microg/dL. Neurotoxicology 2006, 27, 693–701. [Google Scholar] [CrossRef]
- Lanphear, B.P.; Rauch, S.; Auinger, P.; Allen, R.W.; Hornung, R.W. Low-level lead exposure and mortality in US adults: A population-based cohort study. Lancet Public Health 2018, 3, e177–e184. [Google Scholar] [CrossRef] [PubMed]
- Menke, A.; Muntner, P.; Batuman, V.; Sildergeld, E.K.; Guallar, E. Blood lead below 0.48 micromol/L (10 microg/dL) and mortality among US adults. Circulation 2006, 114, 1388–1394. [Google Scholar] [CrossRef]
- Shefa, S.T.; Héroux, P. Both physiology and epidemiology support zero tolerable blood lead levels. Toxicol. Lett. 2017, 280, 232–237. [Google Scholar] [CrossRef]
- Larsen, B.; Sánchez-Triana, E. Global health burden and cost of lead exposure in children and adults: A health impact and economic modelling analysis. Lancet Planet. Health 2023, 7, e831–e840. [Google Scholar] [CrossRef]
- Stanaway, J.D.; GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1923–1994. [Google Scholar] [CrossRef]
- Obeng-Gyasi, E. Sources of lead exposure in various countries. Rev. Environ. Health 2019, 34, 25–34. [Google Scholar] [CrossRef]
- Maurice, L.; Barraza, F.; Blondet, I.; Ho-A-Chuck, M.; Tablon, J.; Brousse, P.; Demar, M.; Schreck, E. Childhood lead exposure of Amerindian communities in French Guiana: An isotopic approach to tracing sources. Environ. Geochem. Health 2021, 43, 4741–4757. [Google Scholar] [CrossRef]
- Cartro-Sabate, M.; Mayor, P.; Orta-Martínez, M.; Rosell-Melé, A. Anthropogenic lead in Amazonian wildlife. Nat. Sustain. 2019, 2, 702–709. [Google Scholar] [CrossRef]
- Berky, A.J.; Robie, E.; Navio-Chipa, S.; Ortiz, E.J.; Palmer, E.J.; Rivera, N.A.; Morales-Avalos, A.M.; Meyer, J.N.; Hsu-Kim, H.; Pan, W.K. Risk of lead exposure from wild game consumption from cross-sectional studies in Madre de Dios, Peru. Lancet Reg. Health Am. 2022, 12, 100266. [Google Scholar] [CrossRef]
- Monsalve, J.L.P. Depredador depredado: Cacerías y comercio de jaguar en dos cuencas andino amazônicas. Novos Cad. 2009, 12, 109–134. [Google Scholar] [CrossRef]
- Bernárdez-Rodríguez, G.B.; Bowler, M.; Braga-Pereira, F.; McNaughton, M.; Mayor, P. Conservation education promotes positive short- and medium-term changes in perceptions and attitudes towards a threatened primate species. Ethnobiol. Conserv. 2021, 10, 31. [Google Scholar] [CrossRef]
- Pitman, N.; Vriesendorp, C.; Moskovits, D. Rapid Biological Inventory 11: Peru: Yavari; The Field Museum: Chicago, IL, USA, 2003. [Google Scholar]
- Bergen, N.; Labonte, R. “Everything Is Perfect, and We Have No Problems”: Detecting and Limiting Social Desirability Bias in Qualitative Research. Qual. Health Res. 2020, 30, 783–792. [Google Scholar] [CrossRef]
- Gilbert-Barness, E.; Spicer, D.E.; Steffensen, T.S. Handbook of Pediatric Autopsy Pathology, 2nd ed.; Springer: New York, NY, USA, 2014. [Google Scholar]
- Orta-Martínez, M.; Mayor, P.; Cartro-Sabate, M.; Rosell-Melé, A. Reply to: Improper estimation of lead contamination. Nat. Sustain. 2021, 4, 19–20. [Google Scholar] [CrossRef]
- Eslava, P. Estimation of yield and nutritional value of bobo mullet (Joturus pichardi Poey, 1860) (Pisces: Mugilidae). Rev. MVZ Córdoba 2009, 14, 1576–1586. [Google Scholar] [CrossRef]
- Bardales-García, J.; Bendayan, N.; Verdi, L. Técnicas de preservación y factor de conversión de fauna silvestre en la región Loreto, Perú. In Proceedings of the VI Congreso Internacional Sobre Manejo de Fauna Silvestre en la Amazonía y Latinoamérica, Iquitos, Perú, 5–10 September 2004. [Google Scholar]
- Alarcon, W.A.; Davidson, S.; Dufour, B.; Roach, M.; Tsang, K.; Payne, S.F.; de Loreto, A.M.; St. Louis, T.; Rajagopalan, S.; Watkins, S.H.; et al. Elevated Blood Lead Levels Among Employed Adults—United States, 1994–2013. Morb. Mortal. Wkly. Rep. 2016, 63, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Health Canada. Final Human Health State of the Science Report on Lead. Report No. 120152; Health Canada: Ottawa, ON, Canada, 2013; Available online: https://www.canada.ca/content/dam/hc-sc/migration/hc-sc/ewh-semt/alt_formats/pdf/pubs/contaminants/dhhssrl-rpecscepsh/dhhssrl-rpecscepsh-eng.pdf (accessed on 19 December 2024).
- Schober, S.E.; Mirel, L.B.; Graubard, B.I.; Brody, D.J.; Flegal, K.M. Blood lead levels and death from all causes, cardiovascular disease, and cancer: Results from the NHANES III mortality study. Environ. Health Perspect. 2006, 114, 1538–1541. [Google Scholar] [CrossRef]
- WHO. Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks; World Health Organization: Geneva, Switzerland, 2009. Available online: https://iris.who.int/bitstream/handle/10665/44203/9789241563871_eng.pdf?sequence=1 (accessed on 19 December 2024).
- WHO. Preventing Disease Through Healthy Environments: Exposure to Lead: A Major Public Health Concern; World Health Organization: Geneva, Switzerland, 2010. Available online: https://www.who.int/publications/i/item/9789240078130 (accessed on 19 December 2024).
- WHO. Lead in Drinking-Water. Background Document for Development of WHO Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2011. Available online: https://cdn.who.int/media/docs/default-source/wash-documents/wash-chemicals/lead-background-feb17.pdf?sfvrsn=fc50727b_4 (accessed on 19 December 2024).
- Council on Environmental Health. Prevention of childhood lead toxicity. Pediatrics 2016, 138, e20161493. [Google Scholar] [CrossRef]
- Lanphear, B.P.; Hornung, R.; Khoury, J.; Yolton, K.; Baghurst, P.; Bellinger, D.C.; Canfield, R.L.; Dietrich, K.N.; Bornschein, R.; Greene, T.; et al. Low-level environmental lead exposure and children’s intellectual function: An international pooled analysis. Environ. Health Perspect. 2005, 113, 894–899. [Google Scholar] [CrossRef] [PubMed]
- ATSDR. Toxicological Profile: Lead; Agency for Toxic Substances & Disease Registry: Atlanta, Georgia, 2007. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp13.pdf (accessed on 10 August 2025).
- Lanphear, B.P.; Hornung, R.; Ho, M.; Howard, C.R.; Eberly, S.; Knauf, K. Environmental lead exposure during early childhood. J. Pediatr. 2002, 140, 40–47. [Google Scholar] [CrossRef] [PubMed]
- WHO. Childhood Lead Poisoning; World Health Organization: Geneva, Switzerland, 2010. Available online: https://www.who.int/publications/i/item/childhood-lead-poisoning (accessed on 19 December 2024).
- Espinoza, R.; Hernández-Avila, M.; Narciso, J.; Castañaga, C.; Moscoso, S.; Ortiz, G.; Carbajal, L.; Wegner, S.; Noonan, G. Determinants of blood-lead levels in children in Callao and Lima metropolitan area. Salud Publ. Mex. 2003, 45, 209–219. [Google Scholar] [CrossRef]
- Olivero-Verbel, J.; Duarte, D.; Echenique, M.; Guette, J.; Johnson-Restrepo, B.; Parsons, P. Blood lead levels in children aged 5-9 years living in Cartagena, Colombia. Sci. Total Environ. 2007, 372, 707–716. [Google Scholar] [CrossRef]
- Bierkens, J.; Smolders, R.; Van Holderbeke, M.; Cornelis, C. Predicting blood lead levels from current and past environmental data in Europe. Sci. Total Environ. 2011, 409, 5101–5110. [Google Scholar] [CrossRef]
- Dignam, T.; Kaufmann, R.B.; LeStourgeon, L.; Brown, M.J. Control of Lead Sources in the United States, 1970-2017: Public Health Progress and Current Challenges to Eliminating Lead Exposure. J. Public Health Manag. Pract. 2019, 25, 13–22. [Google Scholar] [CrossRef]
- Tsoi, M.-F.; Cheung, C.-L.; Tsang Cheung, T.; Yung Cheung, B.M. Continual Decrease in Blood Lead Level in Americans: United States National Health Nutrition and Examination Survey 1999–2014. Am. J. Med. 2016, 129, 1213–1218. [Google Scholar] [CrossRef]
- O’Callaghan-Gordo, C.; Rosales, J.; Lizárraga, P.; Barclay, F.; Okamoto, T.; Papoulias, D.M.; Espinosa, A.; Orta-Martinez, M.; Kogevinas, M.; Astete, J. Blood lead levels in indigenous peoples living close to oil extraction areas in the Peruvian Amazon. Environ. Int. 2021, 154, 106639. [Google Scholar] [CrossRef]
- Barbosa, F.; Fillion, M.; Lemire, M.; Passos, C.J.; Rodrigues, J.L.; Philibert, A.; Guimaraes, J.R.; Mergler, D. Elevated blood lead levels in a riverside population in the Brazilian Amazon. Environ. Res. 2009, 109, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Bandeira, I.C.N.; Adamy, A.; Andretta, E.R.; Costa de Caonceiçao, R.A.; Nogueira de Andrade, M.M. Terras caídas: Fluvial erosion or distinct phenomenon in the Amazon? Environ. Earth Sci. 2018, 77, 222. [Google Scholar] [CrossRef]
- EPA. Edition of the Drinking Water Standards and Health Advisories; U.S. Environmental Protection Agency: Washington, DC, USA, 2012. Available online: https://rais.ornl.gov/documents/2012_drinking_water.pdf (accessed on 10 August 2025).
- Federal-Provincial-Territorial Committee on Drinking Water. Lead in Drinking Water. Document for Public Consultation; Federal-Provincial-Territorial Committee on Drinking Water: Ottawa, ON, Canada, 2017; Available online: https://www.canada.ca/content/dam/hc-sc/healthy-canadians/migration/health-system-systeme-sante/consultations/lead-drinking-water-plomb-eau-potable/alt/lead-drinking-water-plomb-eau-potable-03-01-2017-eng.pdf (accessed on 19 December 2024).
- Bernalte, E.; Arévalo, S.; Pérez-Taborda, J.; Wenk, J.; Estrela, P.; Avila, A.; Di Lorenzo, M. Rapid and on-site simultaneous electrochemical detection of copper, lead and mercury in the Amazon river. Sens. Actuators B-Chem. 2020, 307, 127620. [Google Scholar] [CrossRef]
- Agudelo Calderón, C.A.; García-Ubaqie, J.C.; Robledo Martínez, R.; García-Ubaque, C.A.; Quiroz Arcentales, L. Evaluación de condiciones ambientales: Aire, agua y suelos en áreas de actividad minera en Boyacá, Colombia. Rev. Salud Púb. 2016, 18, 50–60. [Google Scholar] [CrossRef]
- Macías Socha, C.; García Colmenares, M.; Chaparro, S.P. Determinación electroquímica de plomo y cadmio en aguas superficiales. Luna Azul 2017, 44, 27–38. [Google Scholar] [CrossRef]
- Anticona, C.; Bergdahl, I.A.; Lundh, T.; Alegre, Y.; Sebastian, M.S. Lead exposure in indigenous communities of the Amazon basin, Peru. Int. J. Hyg. Environ. Health 2011, 215, 59–63. [Google Scholar] [CrossRef]
- Fustinoni, S.; Sucato, S.; Consonni, D.; Mannucci, P.M.; Moretto, A. Blood lead levels following consumption of game meat in Italy. Environ. Res. 2017, 155, 36–41. [Google Scholar] [CrossRef]
- Iqbal, S.; Blumenthal, W.; Kennedy, C.; Yip, F.Y.; Pickard, S.; Flanders, W.D.; Loringer, K.; Kruger, K.; Caldwell, K.L.; Brown, M.J. Hunting with lead: Association between blood lead levels and wild game consumption. Environ. Res. 2009, 109, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Mayor, P.; Pérez-Peña, P.; Bowler, M.; Puertas, M.; Kirkland, M.; Bodmer, R. Effects of selecting logging on large mammal populations in a remote indigenous territory in the northern Peruvian Amazon. Ecol. Soc. 2015, 20, 36. [Google Scholar] [CrossRef]
- National Research Council. Potential Health Risks to DOD Firing-Range Personnel from Recurrent Lead Exposure; The National Academies Press: Washington, DC, USA, 2013. [Google Scholar] [CrossRef]
- Anticona, C.; Bergdahl, I.A.; San Sebastian, M. Sources and risk factors for lead exposure in indigenous children of the Peruvian Amazon, disentangling connections with oil activity. Int. J. Occup. Environ. Health 2012, 18, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Amici, A.; Danieli, P.P.; Russo, C.; Primi, R.; Ronchi, B. Concentrations of some toxic and trace elements in wild boar (Sus scrofa) organs and tissues in different areas of the province of Viterbo, central Italy. Ital. J. Anim. Sci. 2012, 11, e65. [Google Scholar] [CrossRef]
- Bilandžić, N.; Sedak, M.; Vratarić, D.; Perić, T.; Šimić, B. Lead and cadmium in red deer and wild boar from different hunting grounds in Croatia. Sci. Total Environ. 2009, 407, 4243–4247. [Google Scholar] [CrossRef]
- Reglero, M.M.; Monsalve-González, L.; Tagart, M.; Mateo, R. Transfer of metals to plants and red deer in an old lead mining area in Spain. Sci. Total Environ. 2008, 406, 287–297. [Google Scholar] [CrossRef]
- Bustamante, J.; Chaparro, A.; Peláez, M. Impacto de las actividades antrópicas derivadas de la industria petrolera en relación con la presencia de metales pesados en la ganadería bovina colombiana. Toxicología 2015, 32, 124–130. [Google Scholar]
- Fa, J.E.; Funk, S.M.; Nasi, R. Hunting Wildlife in the Tropics and Subtropics; Cambridge University Press: Cambridge, UK, 2022. [Google Scholar]
- Nielsen, M.R.; Meilby, H.; Smith-Hall, C.; Pouliot, M.; Treue, T. The Importance of wild meat in the Global South. Ecol. Econ. 2018, 146, 696–705. [Google Scholar] [CrossRef]
- Arnemo, J.M.; Andersen, O.; Stokke, S.; Thomas, V.G.; Krone, O.; Pain, D.J.; Mateo, R. Health and Environmental Risks from Lead-based Ammunition: Science Versus Socio-Politics. Ecohealth 2016, 13, 618–622. [Google Scholar] [CrossRef]
- de Souza-Araujo, J.; Hussey, N.E.; Hauser-Davis, R.A.; Rosa, A.H.; de Oliveira Lima, M.; Giarrizzo, T. Human risk assessment of toxic elements (As, Cd, Hg, Pb) in marine fish from the Amazon. Chemosphere 2022, 301, 134575. [Google Scholar] [CrossRef]
- Rodriguez-Levy, I.E.; Van Damme, P.A.; Carvajal-Vallejos, F.M.; Bervoets, L. Trace element accumulation in different edible fish species from the Bolivian Amazon and the risk for human consumption. Heliyon 2022, 8, e11649. [Google Scholar] [CrossRef]
- Fuentes-Gandara, F.; Pinedo-Hernández, J.; Marrugo-Negrete, J.; Diéz, S. Human health impacts of exposure to metals through extreme consumption of fish from the Colombian Caribbean Sea. Environ. Geochem. Health 2016, 40, 229–242. [Google Scholar] [CrossRef]
- Rodríguez Africano, P.E.; Vergara Estupiñán, E.J. Presencia de mercurio, plomo y cobre en tejidos de Orechromis niloticus: Sector de la cuenca alta del Río Chicamocha, vereda Volcán, Paipa, Colombia. Prod. Más Limpia 2016, 10, 114–126. [Google Scholar] [CrossRef]
- Gallego-Ríos, S.E.; Ramírez-Botero, C.M.; López-Marín, B.E.; Velásquez-Rodríguez, C.M. Evaluation of mercury, lead, and cadmium in the waste material of crevalle jack fish from the Gulf of Urabá, Colombian Caribbean, as a possible raw material in the production of sub-products. Environ. Monit. Assess. 2018, 190, 115. [Google Scholar] [CrossRef]
- EPA. Updated Soil Lead Guidance for CERCLA Sites and RCRA Corrective Action Facilities; U.S. Environmental Protection Agency: Washington, DC, USA, 2024. Available online: https://semspub.epa.gov/work/HQ/100003435.pdf (accessed on 19 December 2024).
- de Andrade Lima, L.R.P.; Menezes Filho, J.A.; Mertens, F.; Passos, C.J.S. Investigation of lead sources in manioc flour from riparian communities in the Tapajós Region, Brazilian Amazon. Environ. Earth Sci. 2021, 80, 158. [Google Scholar] [CrossRef]
- Correia, A.; Freydier, R.; Delmas, R.J.; Simões, J.C.; Taupin, J.-D.; Dupré, B.; Artaxo, P. Trace elements in South America aerosol during 20th century inferred from a Nevado Illimani ice core, Eastern Bolivian Andes (6350 m asl). Atmos. Chem. Phys. 2003, 3, 1352. [Google Scholar] [CrossRef]
- Bollhöfer, A.; Rosman, K.J. Isotopic source signatures for atmospheric lead: The Southern Hemisphere. Geochim. Cosmochim. Acta 2000, 64, 3251–3262. [Google Scholar] [CrossRef]
- Eichler, A.; Gramlich, G.; Kellerhals, T.; Tobler, L.; Schwikowski, M. Pb pollution from leaded gasoline in South America in the context of a 2000-year metallurgical history. Sci. Adv. 2015, 1, 8. [Google Scholar] [CrossRef]
- Azeh-Engwa, G.; Udoka Ferdinand, P.; Nweke Nwalo, F.; Unachukwu, M.N. Mechanism and Health Effects of Heavy Metal Toxicity in Humans. In Poisoning in the Modern World—New Tricks for an Old Dog? Karcioglu, O., Arslan, B., Eds.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Gremse, C.; Rieger, S. Lead from hunting ammunition in wild game meat: Research initiatives and current legislation in Germany and the EU. In Proceedings of the Oxford Lead Symposium: Lead Ammunition: Understanding and Minimizing the Risks to Human and Environmental Health, Oxford, UK, 10 December 2014; E dward Grey Institute, The University of Oxford: Oxford, UK, 2014. [Google Scholar]
- Kanstrup, N.; Thomas, V.G.; Krone, O.; Gremse, C. The transition to non-lead rifle ammunition in Denmark: National obligations and policy considerations. Ambio 2016, 45, 621–628. [Google Scholar] [CrossRef]
- Kanstrup, N.; Swift, J.; Stroud, D.A.; Lewis, M. Hunting with lead ammunition is not sustainable: European perspectives. Ambio 2018, 47, 846–857. [Google Scholar] [CrossRef]
- Dobrowolska, A.; Melosik, M. Bullet-derived lead in tissues of the wild boar (Sus scrofa) and red deer (Cervus elaphus). Eur. J. Wildl. Res. 2008, 54, 231–235. [Google Scholar] [CrossRef]
- Pain, D.J.; Cromie, R.L.; Newth, J.; Brown, M.J.; Crutcher, E.; Hardman, P.; Hurst, L.; Mateo, R.; Meharg, A.A.; Moran, A.C.; et al. Potential Hazard to Human Health from Exposure to Fragments of Lead Bullets and Shot in the Tissues of Game Animals. PLoS ONE 2010, 5, e10315. [Google Scholar] [CrossRef] [PubMed]
- Levallois, P.; Barn, P.; Valcke, M.; Gauvin, D.; Kosatsky, T. Public Health Consequences of Lead in Drinking Water. Curr. Environ. Health Rep. 2018, 5, 255–262. [Google Scholar] [CrossRef]

| Sample | Description | Code | Pb |
|---|---|---|---|
| Community | Soil samples collected from various locations within the community | S1 | 36.11 |
| S2 | 24.43 | ||
| S3 | 25.76 | ||
| S4 | 34.16 | ||
| Agricultural Lands | Soil samples from agricultural plots | S5 | 14.35 |
| S6 | 10.14 | ||
| Hunting hotspots | Soil samples from sites frequently used for subsistence hunting (e.g., mineral salt licks) | S7 | 6.37 |
| S8 | 6.93 | ||
| S9 | 11.95 | ||
| S10 | 20.5 | ||
| S11 | 7.04 | ||
| S12 | 7.63 |
| Sample | Code | Treatment | Description | Pb |
|---|---|---|---|---|
| YMR | W1 | Untreated | River water used directly for drinking | 3.85 |
| W2 | Untreated | River water used directly for drinking | 3.4 | |
| W3 | Treated | River water left to settle before use | 0.53 | |
| W4 | Treated | River water left to settle before use | 0.43 | |
| W5 | Treated | River water centrifuged in the laboratory | <0.04 | |
| W6 | Treated | River water that is boiled before use | 0.93 | |
| W7 | Treated | River water that is boiled before use | 0.82 | |
| Small nearby river | W8 | Untreated | Stream water collected directly | 0.15 |
| W9 | Treated | Sedimented stream sample | 0.07 | |
| Rainwater | W10 | Untreated | Collected from rooftops using metal gutters from different houses | 1.66 |
| W11 | Untreated | Collected from rooftops using metal gutters from different houses | 0.88 | |
| W12 | Treated | Rainwater left to settle before use | 0.21 | |
| W13 | Treated | Rainwater left to settle before use | 0.17 | |
| W14 | Treated | Rainwater that is boiled before use | 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayor, P.; Rius-Taberner, G.; Ulloa, G.M.; Orta-Martínez, M. Unexpected High Blood Lead Levels in a Remote Indigenous Community in the Northeastern Peruvian Amazon. Toxics 2025, 13, 826. https://doi.org/10.3390/toxics13100826
Mayor P, Rius-Taberner G, Ulloa GM, Orta-Martínez M. Unexpected High Blood Lead Levels in a Remote Indigenous Community in the Northeastern Peruvian Amazon. Toxics. 2025; 13(10):826. https://doi.org/10.3390/toxics13100826
Chicago/Turabian StyleMayor, Pedro, Guillem Rius-Taberner, Gabriela M. Ulloa, and Martí Orta-Martínez. 2025. "Unexpected High Blood Lead Levels in a Remote Indigenous Community in the Northeastern Peruvian Amazon" Toxics 13, no. 10: 826. https://doi.org/10.3390/toxics13100826
APA StyleMayor, P., Rius-Taberner, G., Ulloa, G. M., & Orta-Martínez, M. (2025). Unexpected High Blood Lead Levels in a Remote Indigenous Community in the Northeastern Peruvian Amazon. Toxics, 13(10), 826. https://doi.org/10.3390/toxics13100826





