Iron–Carbonate (Bi, Cu, Li) Composites with Antimicrobial Activity After Silver(I) Ion Adsorption
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Silver Institute and Metals Focus. 2025. Available online: https://silverinstitute.org/ (accessed on 12 July 2025).
- Jarchum, I. Turning silver dust into electronics. Nat. Biotechnol. 2017, 35, 723. [Google Scholar] [CrossRef]
- Ibrahim, N.; Akindoyo, J.O.; Mariatti, M. Recent development in silver-based ink for flexible electronics. J. Sci. Adv. Mater. Devices 2022, 7, 100395. [Google Scholar] [CrossRef]
- Skarżyński, K.; Krzemiński, J.; Jakubowska, M.; Słoma, M. Highly conductive electronics circuits from aerosol jet printed silver inks. Sci. Rep. 2021, 11, 18141. [Google Scholar] [CrossRef] [PubMed]
- Rout, S.; Jana, P.; Borra, C.R.; Önal, M.A.R. Unlocking silver from end-of-life photovoltaic panels: A concise review. Renew. Sustain. Energy Rev. 2025, 210, 115205. [Google Scholar] [CrossRef]
- Laugharne, A. Market Trend Report Silver’s Important Role in Solar Power; T.S. Institute: New York, NY, USA, 2020. [Google Scholar]
- Lo Piano, S.; Saltelli, A.; van der Sluijs, J.P. Silver as a constraint for a large-scale development of solar photovoltaics? Scenario-making to the year 2050 supported by expert engagement and global sensitivity analysis. Front. Energy Res. 2019, 7, 1–13. [Google Scholar] [CrossRef]
- Yazdani-Ahmadabadi, H.; Felix, D.F.; Yu, K.; Yeh, H.H.; Luo, H.D.; Khoddami, S.; Takeuchi, L.E.; Alzahrani, A.; Abbina, S.; Mei, Y.; et al. Durable surfaces from film-forming silver assemblies for long-term zero bacterial adhesion without toxicity. ACS Cent. Sci. 2022, 8, 546–561. [Google Scholar] [CrossRef]
- Rakhila, Y.; Elmchaouri, A.; Mestari, A.; Korili, S.; Abouri, M.; Gil, A. Adsorption recovery of Ag(I) and Au(III) from an electronics industry wastewater on a clay mineral composite. Int. J. Miner. Metall. Mater. 2019, 26, 673–680. [Google Scholar] [CrossRef]
- Sim, W.; Barnard, R.T.; Blaskovich, M.A.T.; Ziora, Z.M. Antimicrobial silver in medicinal and consumer applications: A patent review of the past decade (2007–2017). Antibiotics 2018, 7, 93. [Google Scholar] [CrossRef]
- Żyro, D.; Sikora, J.; Szynkowska-Jóźwik, M.I.; Ochocki, J. Silver, its salts and application in medicine and pharmacy. Int. J. Mol. Sci. 2023, 24, 15723. [Google Scholar] [CrossRef]
- Meher, A.; Tandi, A.; Moharana, S.; Chakroborty, S.; Mohapatra, S.S.; Mondal, A.; Dey, S.; Chandra, P. Silver nanoparticle for biomedical applications: A review. Hybrid. Adv. 2024, 6, 100184. [Google Scholar] [CrossRef]
- Medici, S.; Peana, M.; Nurchi, V.M.; Zoroddu, M.A. Medical uses of silver: History, myths, and scientific evidence. J. Med. Chem. 2019, 62, 5923–5943. [Google Scholar] [CrossRef] [PubMed]
- Adeeyo, A.O.; Bello, O.S.; Agboola, O.S.; Adeeyo, R.O.; Oyetade, J.A.; Alabi, M.A.; Edokpayi, J.N.; Makungo, R. Recovery of precious metals from processed wastewater: Conventional techniques nexus advanced and pragmatic alternatives. Water Reuse 2023, 13, 134–161. [Google Scholar] [CrossRef]
- Figueroa, G.; Valenzuela, J.L.; Parga, J.R.; Vazquez, V.; Valenzuela, A. Recovery of gold and silver and removal of copper, zinc and lead ions in pregnant and barren cyanide solutions. Mater. Sci. Appl. 2015, 6, 12. [Google Scholar] [CrossRef]
- Pikaar, I.; Guest, J.; Ganigue, R.; Jensen, P.; Rabaey, K.; Seviour, T.; Trimmer, J.; Kolk, O.; Vaneeckhaute, C.; Verstraete, W. Resource Recovery from Water: Principles and Application; IWA Publishing: London, UK, 2022. [Google Scholar]
- Li, D.; Zhang, X.; Liang, X.; Liu, W.; Guo, K.; Zhang, Z.; Wang, S.; Xing, Y.; Li, Z.; Li, J.; et al. Simultaneous removal and conversion of silver ions from wastewater into antibacterial material through selective chemical precipitation. Arab. J. Chem. 2023, 16, 104836. [Google Scholar] [CrossRef]
- Chandrasekara Pillai, K.; Chung, S.J.; Moon, I.-S. Studies on electrochemical recovery of silver from simulated waste water from Ag(II)/Ag(I) based mediated electrochemical oxidation process. Chemosphere 2008, 73, 1505–1511. [Google Scholar] [CrossRef]
- Nawaz, T.; Sengupta, S.; Yang, C.-L. Silver recovery as Ag0 nanoparticles from ion-exchange regenerant solution using electrolysis. J. Environ. Sci. 2019, 78, 161–173. [Google Scholar] [CrossRef]
- Iglesias, M.; Torrent, L. Silver nanoparticles and ionic silver separation using a cation-exchange resin. Variables affecting their separation and improvements of AgNP characterization by SP-ICPMS. Nanomaterials 2021, 11, 2626. [Google Scholar] [CrossRef]
- Schwertfeger, D.; Velicogna, J.; Jesmer, A.; McShane, H.; Scroggins, R.; Princz, J. Ion exchange technique (IET) to characterise Ag+ exposure in soil extracts contaminated with engineered silver nanoparticles. Environ. Chem. 2017, 14, 123–133. [Google Scholar] [CrossRef]
- Wang, Q.; Li, M.; Xi, M.; Zhao, M.; Wang, X.; Chen, X.; Ding, L. Recovery of Ag(I) from wastewater by adsorption: Status and challenges. Toxics 2024, 12, 351. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Zhu, Z.; Qiu, Y.; Yu, F.; Zhou, T.; Ma, J.; Zhao, J. Dual-selective silver recovery strategy by simultaneous adsorption-reduction boosted by in-situ magnetic field. Green Energy Environ. 2025, 10, 433–440. [Google Scholar] [CrossRef]
- Cantuaria, M.; Nascimento, E.; Neto, A.A.; Santos, O.d.; Vieira, M. Removal and recovery of silver by dynamic adsorption on bentonite clay using a fixed-bed column system. Adsorpt. Sci. Technol. 2015, 33, 91–103. [Google Scholar] [CrossRef]
- Macena, M.; Pereira, H.; Cruz-Lopes, L.; Grosche, L.; Esteves, B. Competitive adsorption of metal ions by lignocellulosic materials: A review of applications, mechanisms and influencing factors. Separations 2025, 12, 70. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. npj Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Mokgehle, T.M.; Tavengwa, N.T. Recent developments in materials used for the removal of metal ions from acid mine drainage. Appl. Water Sci. 2021, 11, 42. [Google Scholar] [CrossRef]
- Antunes, E.; Jacob, M.V.; Brodie, G.; Schneider, P.A. Silver removal from aqueous solution by biochar produced from biosolids via microwave pyrolysis. J. Environ. Manag. 2017, 203, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Lech Cantuaria, M.; Neto, A.; Nascimento, E.; Vieira, M. Adsorption of silver from aqueous solution onto pre-treated bentonite clay: Complete batch system evaluation. J. Clean. Prod. 2015, 112, 1112–1121. [Google Scholar] [CrossRef]
- Jeon, C. Adsorption behavior of silver ions from industrial wastewater onto immobilized crab shell beads. J. Ind. Eng. Chem. 2015, 32, 195–200. [Google Scholar] [CrossRef]
- Jeon, C. Adsorption and recovery of immobilized coffee ground beads for silver ions from industrial wastewater. J. Ind. Eng. Chem. 2017, 53, 261–267. [Google Scholar] [CrossRef]
- Sarı, A.; Tüzen, M. Adsorption of silver from aqueous solution onto raw vermiculite and manganese oxide-modified vermiculite. Microporous Mesoporous Mater. 2013, 170, 155–163. [Google Scholar] [CrossRef]
- Hu, C.; Kang, S.; Xiong, B.; Zhou, S.; Tang, K. Selective recovery of Ag(I) from industrial wastewater using zeolite imidazolate framework-8: Performance and mechanisms. Environ. Sci. Pollut. Res. 2019, 26, 14214–14225. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Shen, T.; Zhao, Q.; Han, T.; Ding, F.; Jin, X.; Gao, M. Selective capture of silver ions from aqueous solution by series of azole derivatives-functionalized silica nanosheets. Chin. J. Chem. Eng. 2023, 57, 319–328. [Google Scholar] [CrossRef]
- Abbas, N.; Husnain, S.M.; Asim, U.; Shahzad, F.; Abbas, Y. A novel green synthesis of MnO2-Coal composite for rapid removal of silver and lead from wastewater. Water Res. 2024, 256, 121526. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Li, M.; Chen, H.; Liu, G.; Liu, D.; Chen, X.; Song, W.; Su, Y. Preparation of porous CuS/modified-diatomite composite via a facile in situ loading process for efficient recovery of silver ion from aqueous solution. Appl. Surf. Sci. 2024, 644, 158753. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, L.; Liu, J.; Yang, Z.; Su, G.; Song, H.; Xue, J.; Tang, A. Preparation of magnetic Fe3O4@SiO2@CaSiO3 composite for removal of Ag+ from aqueous solution. J. Mol. Liq. 2020, 299, 112222. [Google Scholar] [CrossRef]
- Beyler Çiğil, A.; Aydın Urucu, O.; Birtane, H.; Kahraman, M.V. Cellulose/cysteine based thiol-ene UV cured adsorbent: Removal of silver (I) ions from aqueous solution. Cellulose 2021, 28, 6439–6448. [Google Scholar] [CrossRef]
- Yin, X.; Long, J.; Xi, Y.; Luo, X. Recovery of silver from wastewater using a new magnetic photocatalytic ion-imprinted polymer. ACS Sustain. Chem. Eng. 2017, 5, 2090–2097. [Google Scholar] [CrossRef]
- Aljohani, M.S.; Alnoman, R.B.; Alharbi, H.Y.; Monier, M.; Youssef, I. Design and application of ion-imprinted chelating polymer for selective adsorption of silver ion. React. Funct. Polym. 2025, 208, 106162. [Google Scholar] [CrossRef]
- Li, X.G.; Huang, M.R. Milestones in Powerful Adsorbents of Heavy-Metal Ions; Cambridge Scholars Publishing: Newcastle upon Tyne, UK, 2024. [Google Scholar]
- Keshta, B.E.; Gemeay, A.H.; Kumar Sinha, D.; Elsharkawy, S.; Hassan, F.; Rai, N.; Arora, C. State of the art on the magnetic iron oxide nanoparticles: Synthesis, functionalization, and applications in wastewater treatment. Results Chem. 2024, 7, 101388. [Google Scholar] [CrossRef]
- Aydin, F.A.; Soylak, M. A novel multi-element coprecipitation technique for separation and enrichment of metal ions in environmental samples. Talanta 2007, 73, 134–141. [Google Scholar] [CrossRef]
- Patil, S.; Jagadale, S. Chapter 3—Co-precipitation methods for the synthesis of metal oxide nanostructures. In Solution Methods for Metal Oxide Nanostructures; Mane, R., Jadhav, V., Al-Enizi, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 39–60. [Google Scholar]
- Mohamed, D.S.; Abd El-Baky, R.M.; Sandle, T.; Mandour, S.A.; Ahmed, E.F. Antimicrobial activity of silver-treated cacteria against other multi-drug resistant pathogens in their environment. Antibiotics 2020, 9, 181. [Google Scholar] [CrossRef]
- Raza, S.; Wdowiak, M.; Grotek, M.; Adamkiewicz, W.; Nikiforow, K.; Mente, P.; Paczesny, J. Enhancing the antimicrobial activity of silver nanoparticles against ESKAPE bacteria and emerging fungal pathogens by using tea extracts. Nanoscale Adv. 2023, 5, 5786–5798. [Google Scholar] [CrossRef] [PubMed]
- Dube, E.; Okuthe, G.E. Silver nanoparticle-based antimicrobial coatings: Sustainable strategies for microbial contamination control. Microbiol. Res. 2025, 16, 110. [Google Scholar] [CrossRef]
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef]
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan, B.M.R.M.; Mitra, S.; Emran, T.B.; Dhama, K.; Ripon, M.K.H.; Gajdács, M.; Sahibzada, M.U.K.; et al. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. J. Infect. Public Health 2021, 14, 1750–1766. [Google Scholar]
- Chavez-Esquivel, G.; Cervantes-Cuevas, H.; Ybieta-Olvera, L.F.; Castañeda Briones, M.T.; Acosta, D.; Cabello, J. Antimicrobial activity of graphite oxide doped with silver against Bacillus subtilis, Candida albicans, Escherichia coli, and Staphylococcus aureus by agar well diffusion test: Synthesis and characterization. Mater. Sci. Eng. C 2021, 123, 111934. [Google Scholar] [CrossRef]
- Madhumitha, G.; Elango, G.; Roopan, S.M. Bio-functionalized doped silver nanoparticles and its antimicrobial studies. J. Sol-Gel Sci. Technol. 2015, 73, 476–483. [Google Scholar] [CrossRef]
- Anees Ahmad, S.; Sachi Das, S.; Khatoon, A.; Tahir Ansari, M.; Afzal, M.; Saquib Hasnain, M.; Kumar Nayak, A. Bactericidal activity of silver nanoparticles: A mechanistic review. Mater. Sci. Energy Technol. 2020, 3, 756–769. [Google Scholar] [CrossRef]
- Monte-Serrano, M.; Fernandez-Saiz, P.; Ortí-Lucas, R.; Hernando, B. Effective antimicrobial coatings containing silver-based nanoclays and zinc pyrithione. J. Microb. Biochem. Technol. 2015, 7, 398–403. [Google Scholar] [CrossRef]
- Kadirvelu, L.; Sivaramalingam, S.S.; Jothivel, D.; Chithiraiselvan, D.D.; Karaiyagowder Govindarajan, D.; Kandaswamy, K. A review on antimicrobial strategies in mitigating biofilm-associated infections on medical implants. Curr. Res. Microb. Sci. 2024, 6, 100231. [Google Scholar] [CrossRef] [PubMed]
- Pascu, B.; Negrea, A.; Ciopec, M.; Duteanu, N.; Negrea, P.; Nemeş, N.S.; Seiman, C.; Marian, E.; Micle, O. A green, simple and facile way to synthesize silver nanoparticles using soluble starch. pH studies and antimicrobial applications. Materials 2021, 14, 4765. [Google Scholar] [CrossRef]
- Su, T.-L.; Chen, T.-P.; Liang, J. Green in-situ synthesis of silver coated textiles for wide hygiene and healthcare applications. Colloids Surf. A Physicochem. Eng. Asp. 2023, 657, 130506. [Google Scholar] [CrossRef]
- Chandra Joshi, H.; Dutta, D.; Gaur, N.; Singh, G.S.; Dubey, R.; Dwivedi, S.K. Silver-doped active carbon spheres and their application for microbial decontamination of water. Heliyon 2022, 8, e09209. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Donia, D.T.; Sabbatella, G.; Antiochia, R. Silver nanoparticles in polymeric matrices for fresh food packaging. J. King Saud Univ.—Sci. 2016, 28, 273–279. [Google Scholar] [CrossRef]
- Kraśniewska, K.; Galus, S.; Gniewosz, M. Biopolymers-based materials containing silver nanoparticles as active packaging for food applications–A Review. Int. J. Mol. Sci. 2020, 21, 698. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Lin, H.; Zhou, J.; Lin, Z.; Huang, Y.; Chen, G.; Zhang, Y.; Lv, J.; Chen, J.; Liu, G.; et al. Silver nanocomposites with enhanced shelf-life for fruit and vegetable preservation: Mechanisms, advances, and prospects. Nanomaterials 2024, 14, 1244. [Google Scholar] [CrossRef]
- Shahzadi, P.; Majeed, M.A.; Ibrahim, S.; Asif, S.; Kalsoom, R.; Hussain, I. Polymeric coating doped with nanomaterials for functional impact on different substrates. Sci. Rep. 2024, 14, 578. [Google Scholar] [CrossRef] [PubMed]
- Ganguli, P.; Chaudhuri, S. Nanomaterials in antimicrobial paints and coatings to prevent biodegradation of man-made surfaces: A review. Mater. Today Proc. 2021, 45, 3769–3777. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Über die adsorption in lösungen. Z. Phys. Chem. 1907, 57, 385–470. [Google Scholar] [CrossRef]
- Sips, R. On the structure of a catalyst surface. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Lagergren, S. About the theory of so-called adsorption of soluble substances. K. Sven. Vetenskapsakademiens Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.-S. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef]
- Weber, W.J., Jr.; Morris, J.C. Equilibria and capacities for adsorption on carbon. J. Sanit. Eng. Div. 1964, 90, 79–108. [Google Scholar] [CrossRef]
- Atkins, P.; de Paula, J.; Keeler, J. Atkins’ Physical Chemistry, 12th ed.; Oxford University Press: Oxford, UK, 2023. [Google Scholar]
- Parimalam, B.S.; MacIntosh, A.D.; Kadam, R.; Lucht, B.L. Decomposition reactions of anode solid electrolyte interphase (SEI) components with LiPF6. J. Phys. Chem. C 2017, 121, 22733–22738. [Google Scholar] [CrossRef]
- Pasierb, P.; Komornicki, S.; Rokita, M.; Rȩkas, M. Structural properties of Li2CO3–BaCO3 system derived from IR and Raman spectroscopy. J. Mol. Struct. 2001, 596, 151–156. [Google Scholar] [CrossRef]
- Hadj Mokhtar, H.; Boukoussa, B.; Hamacha, R.; Bengueddach, A.; El Abed, D. CuCO3–CuO nanocomposite as a novel and environmentally friendly catalyst for triazole synthesis. RSC Adv. 2015, 5, 93438–93446. [Google Scholar] [CrossRef]
- Zhao, M.; Fu, Y.; Ma, H.; Ma, C.; Dong, X.; Zhang, X. Synthesis, characterization and photoreactivity of hierarchically N-doped (BiO)2CO3/Bi2S3 with highly exposed {001} facets. Mater. Des. 2016, 93, 1–8. [Google Scholar] [CrossRef]
- Astuti, Y.; Fauziyah, A.; Nurhayati, S.; Wulansari, A.D.; Andianingrum, R.; Hakim, A.R.; Bhaduri, G. Synthesis of α-Bismuth oxide using solution combustion method and its photocatalytic properties. IOP Conf. Ser. Mater. Sci. Eng. 2016, 107, 012006. [Google Scholar] [CrossRef]
- Huang, H.; Ji, Y.; Qiao, Z.; Zhao, C.; He, J.; Zhang, H. Preparation, characterization, and application of magnetic Fe-SBA-15 mesoporous silica molecular sieves. J. Anal. Methods Chem. 2010, 2010, 323509. [Google Scholar] [CrossRef]
- Tadakamalla, V.K.; Chary, A.; Bhardwaj, S.; Reddy, S. Dielectric relaxation, ionic conduction and complex impedance studies on NaNo3 fast ion conductor. Int. J. Mater. Sci. Appl. 2013, 2, 173. [Google Scholar] [CrossRef]
- Elmelouky, A.; Mortadi, A.; Chahid, E.; Elmoznine, R. Impedance spectroscopy as a tool to monitor the adsorption and removal of nitrate ions from aqueous solution using zinc aluminum chloride anionic clay. Heliyon 2018, 4, e00536. [Google Scholar] [CrossRef] [PubMed]
- Padmaningrum, R.; Louise, I.; Yunita, I.; Sugiyarto, K. Infrared spectral and magnetic properties of basic copper(II) nitrate produced by slow titration method. Malays. J. Sci. 2022, 41, 106–116. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2001. [Google Scholar]
- Farahmandjou, M.; Soflaee, F. Low temperature synthesis of α-Fe2O3 nano-rods using simple chemical route. J. Nanostructures 2015, 2, 413. [Google Scholar]
- Sedlaček, M.; Gregorčič, P.; Podgornik, B. Use of the roughness parameters Ssk and Sku to control friction—A method for designing surface texturing. Tribol. Trans. 2016, 60, 260–266. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Anitas, E. Small-Angle Scattering from mass and surface fractals. In Complexity in Biological and Physical Systems—Bifurcations, Solitons and Fractals; López-Ruiz, R., Ed.; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar]
- Svergun, D.I.; Feigin, L.A.; Taylor, G.W. Structure Analysis by Small-Angle X-Ray and Neutron Scattering; Plenum Press: New York, NY, USA, 1987. [Google Scholar]
- Huber, K. Basic X-ray scattering applied to soft matter. By Wim H. de Jeu. Angew. Chem. Int. Ed. 2016, 55, 13645. [Google Scholar] [CrossRef]
- Henry, C.K.; Sandoz-Rosado, E.; Roenbeck, M.R.; Magagnosc, D.J.; Palmese, G.R.; Strawhecker, K.E.; Alvarez, N.J. Direct measure of crystalline domain size, distribution, and orientation in polyethylene fibers. Polymer 2020, 202, 122589. [Google Scholar] [CrossRef]
- Kausar, A.; Naeem, K.; Hussain, T.; Nazli, Z.-i.-H.; Bhatti, H.N.; Jubeen, F.; Nazir, A.; Iqbal, M. Preparation and characterization of chitosan/clay composite for direct Rose FRN dye removal from aqueous media: Comparison of linear and non-linear regression methods. J. Mater. Res. Technol. 2019, 8, 1161–1174. [Google Scholar] [CrossRef]
- Faure, G. Principles and Applications of Inorganic Geochemistry: A Comprehensive Textbook for Geology Students; Macmillan Pub. Co.: New York, NY, USA, 1991. [Google Scholar]
- Ochs, M.; Vielle-Petit, L.; Wang, L.; Mallants, D.; Leterme, B. Additional Sorption Parameters for the Cementitious Barriers of a Near-surface Repository; ONDRAF/NIRAS: Brussels, Belgium, 2011. [Google Scholar]
- Phothitontimongkol, T.; Sanuwong, K.; Siebers, N.; Sukpirom, N.; Unob, F. Functionalized hectorite clay mineral for Ag(I) ions extraction from wastewater and preparation of silver nanoparticles supported clay. Appl. Clay Sci. 2013, 80–81, 346–350. [Google Scholar] [CrossRef]
- Zhang, S.; Ning, S.; Liu, H.; Zhou, J.; Wang, S.; Zhang, W.; Wang, X.; Wei, Y. Highly-efficient separation and recovery of ruthenium from electroplating wastewater by a mesoporous silica-polymer based adsorbent. Microporous Mesoporous Mater. 2020, 303, 110293. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Iftekhar, S.; Srivastava, V.; Sillanpää, M. Enrichment of lanthanides in aqueous system by cellulose based silica nanocomposite. Chem. Eng. J. 2017, 320, 151–159. [Google Scholar] [CrossRef]
- Sobhani-Nasab, A.; Behpour, M. Synthesis and characterization of AgO nanostructures by precipitation method and its photocatalyst application. J. Mater. Sci. Mater. Electron. 2016, 27, 1191–1196. [Google Scholar] [CrossRef]
- Waterhouse, G.I.N.; Bowmaker, G.A.; Metson, J.B. The thermal decomposition of silver (I, III) oxide: A combined XRD, FT-IR and Raman spectroscopic study. Phys. Chem. Chem. Phys. 2001, 3, 3838–3845. [Google Scholar] [CrossRef]
- Kumar, A.; Boyer, C.; Nebhani, L.; Wong, E.H.H. Highly bactericidal macroporous antimicrobial polymeric gel for point-of-use water disinfection. Sci. Rep. 2018, 8, 7965. [Google Scholar] [CrossRef]

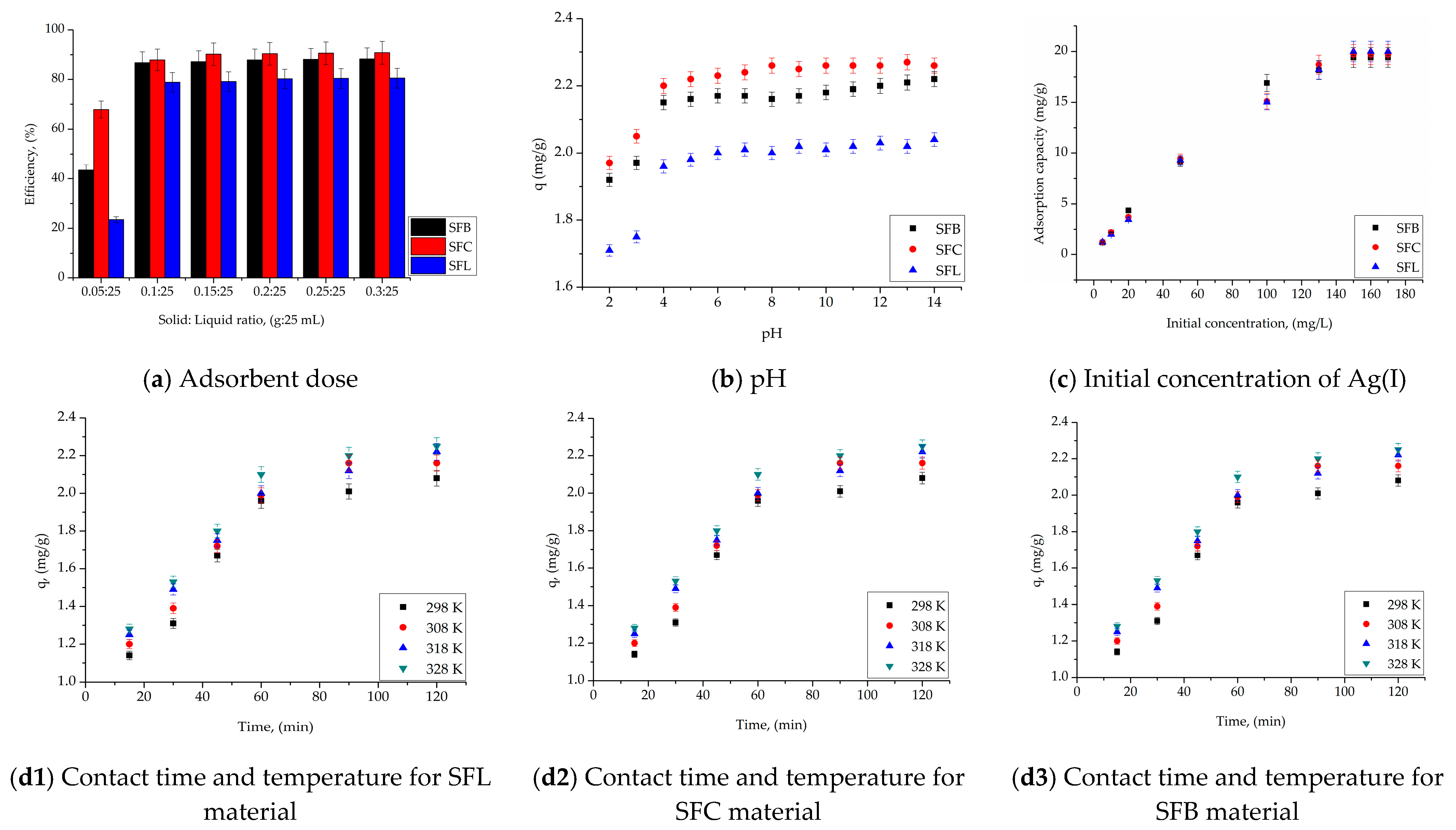
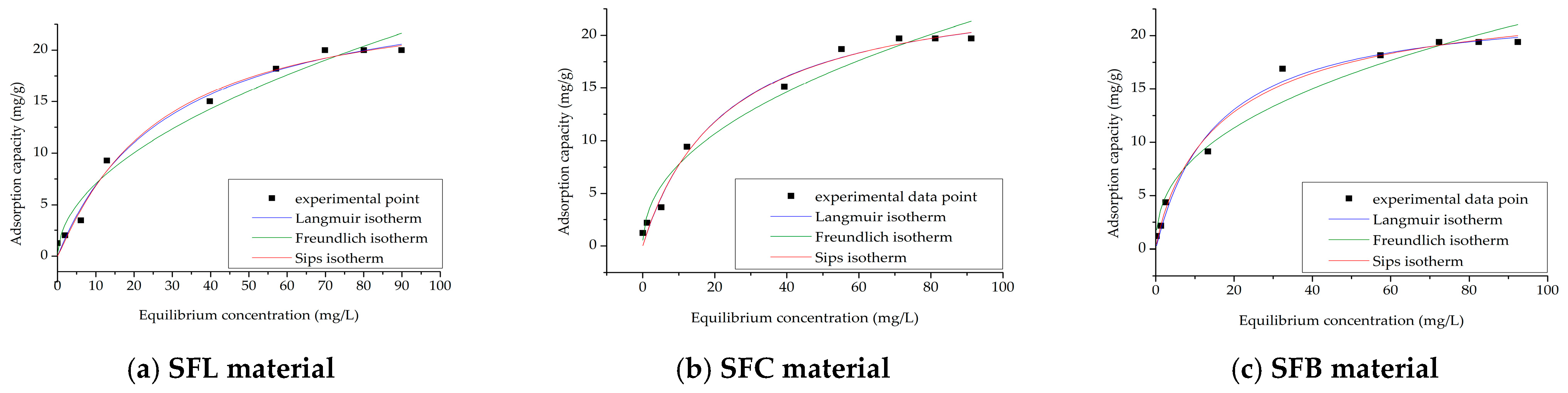


| Adsorbents | Adsorption Capacity, mg/g | References |
|---|---|---|
| Biochar | 43.9 | [28] |
| Bentonite | 55.6 | [29] |
| Immobilized crab shell beads | 2.9 | [30] |
| Immobilized coffee ground beads A | 36.3 | [31] |
| Vermiculite | 69.2 | [32] |
| Zeolite | 446.7 | [33] |
| Silica nanosheets functionalized by azole derivatives | 139.5 | [34] |
| MgO2-coal | 93.57 | [35] |
| MOFs (Fe3O4@UiO−66) | 226.88 | [36] |
| Fe3O4@SiO2@CaSiO3 | 127.84 | [37] |
| Biomass Adsorbent | 23.92 | [38] |
| Fe3O4@SiO2@TiO2−IIP | 35.475 | [39] |
| Ag-imprinted polymer, Ag-IIP | 296 | [40] |
| Poly(o-phenylenediamine) microparticles | 533 | [41] |
| SFC | 19.7 | This paper |
| SFB | 19.3 | This paper |
| SFL | 19.9 | This paper |
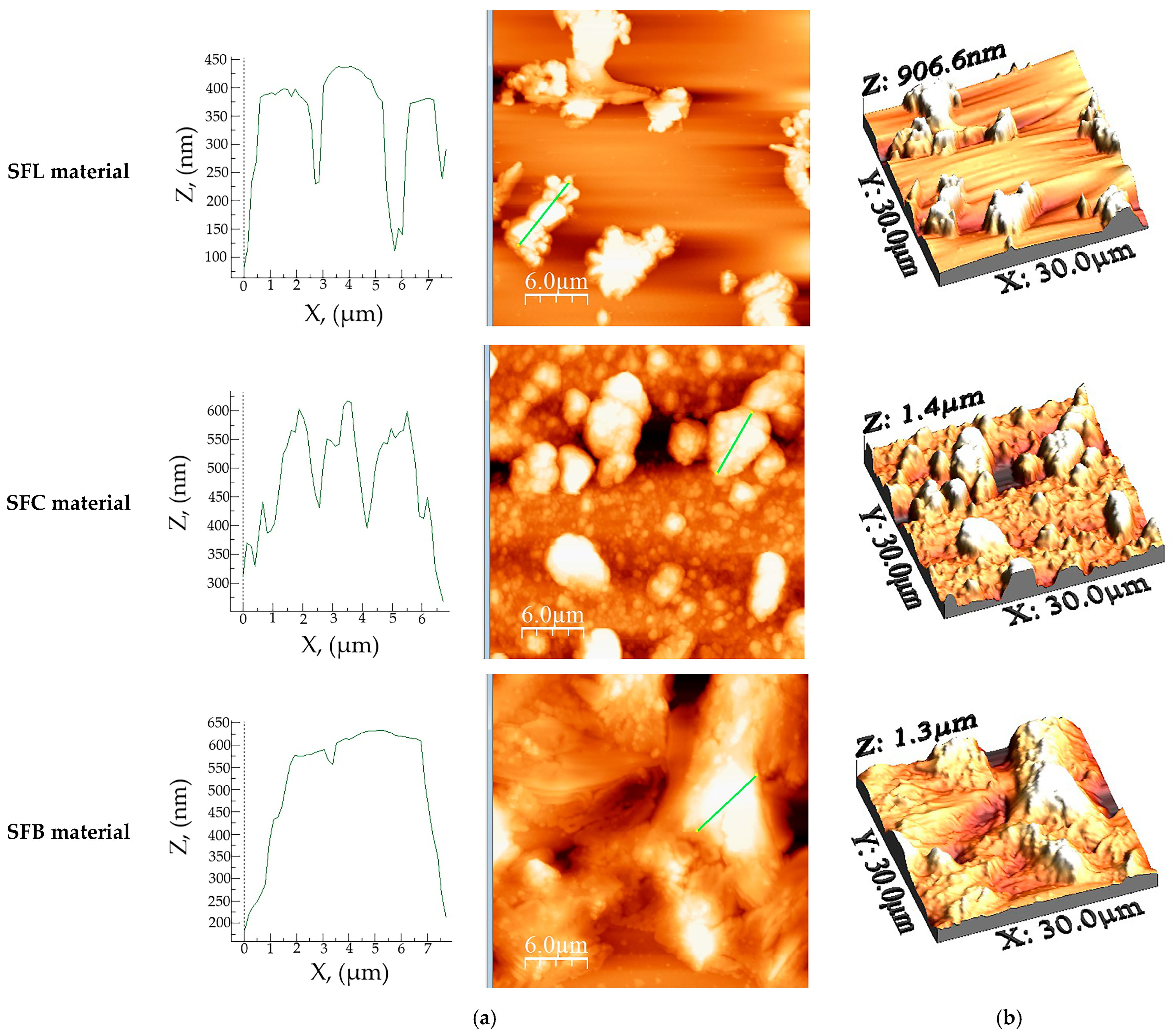
| Sample Name | Ironed Area, (µm2) | Sa, (nm) | Sq, (nm) | Sp, (nm) | Sv, (nm) | Sy, (nm) | Sku | Ssk |
|---|---|---|---|---|---|---|---|---|
| SFL | 945 | 119.8 | 166 | 442.6 | −464 | 906.6 | 3.592 | 0.762 |
| SFC | 1024 | 221 | 287 | 703 | −674 | 1377 | 3.062 | 0.438 |
| SFB | 939 | 193.7 | 251 | 634 | −619 | 1253 | 3.074 | 0.176 |
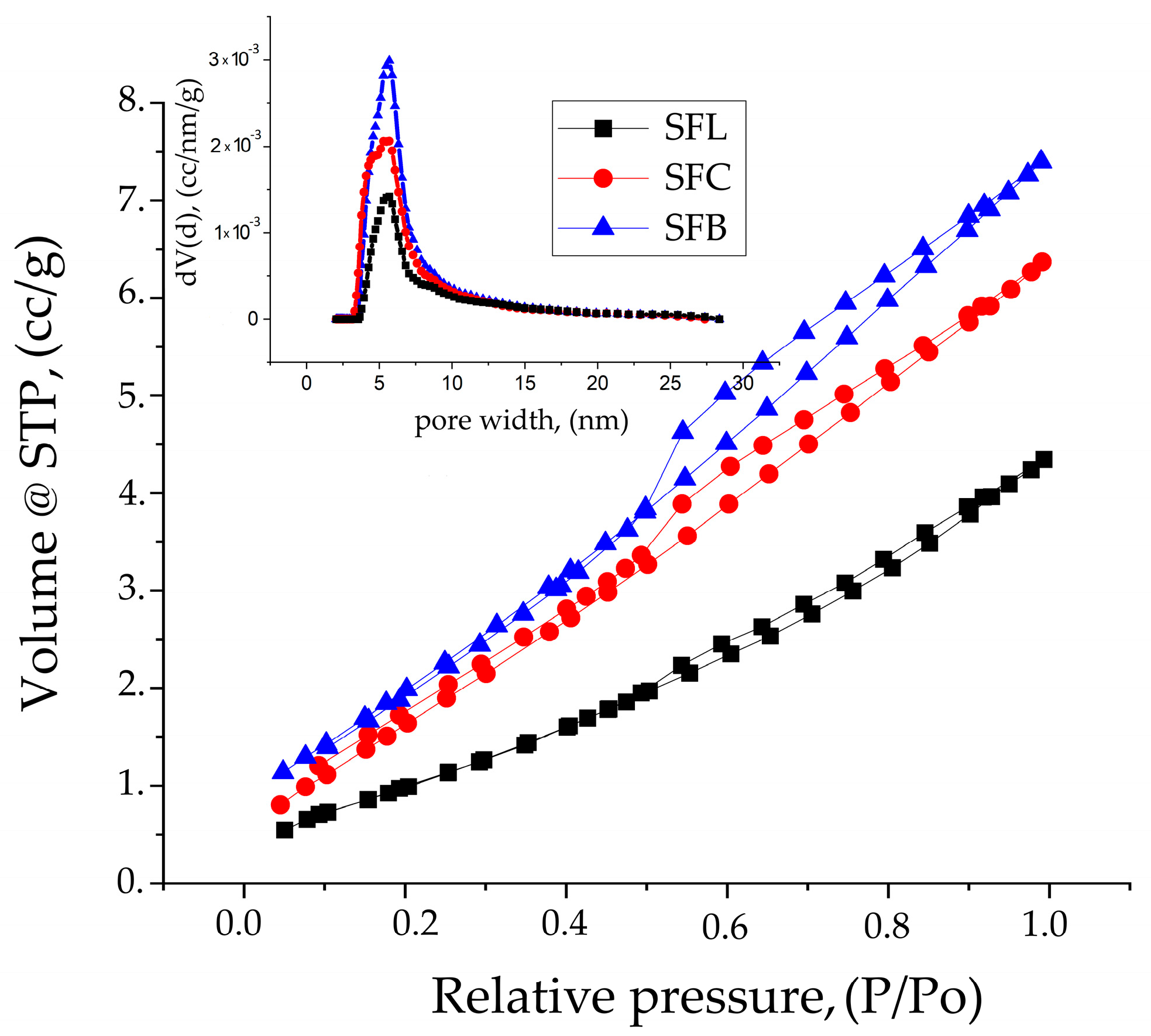
| Sample Name | Surface Area, (m2/g) | Pore Width DFT, (nm) | Total Pore Volume, (cc/g) | FHH, D |
|---|---|---|---|---|
| SFL | 4.4 | 5.682 | 6.454 × 10−3 for pores smaller than 87.8 nm | 1.9603 |
| SFC | 7.1 | 5.682 | 9.874 × 10−3 for pores smaller than 220.7 nm | 1.9118 |
| SFB | 8.2 | 5.682 | 1.147 × 10−2 for pores smaller than 191.6 nm | 1.9382 |
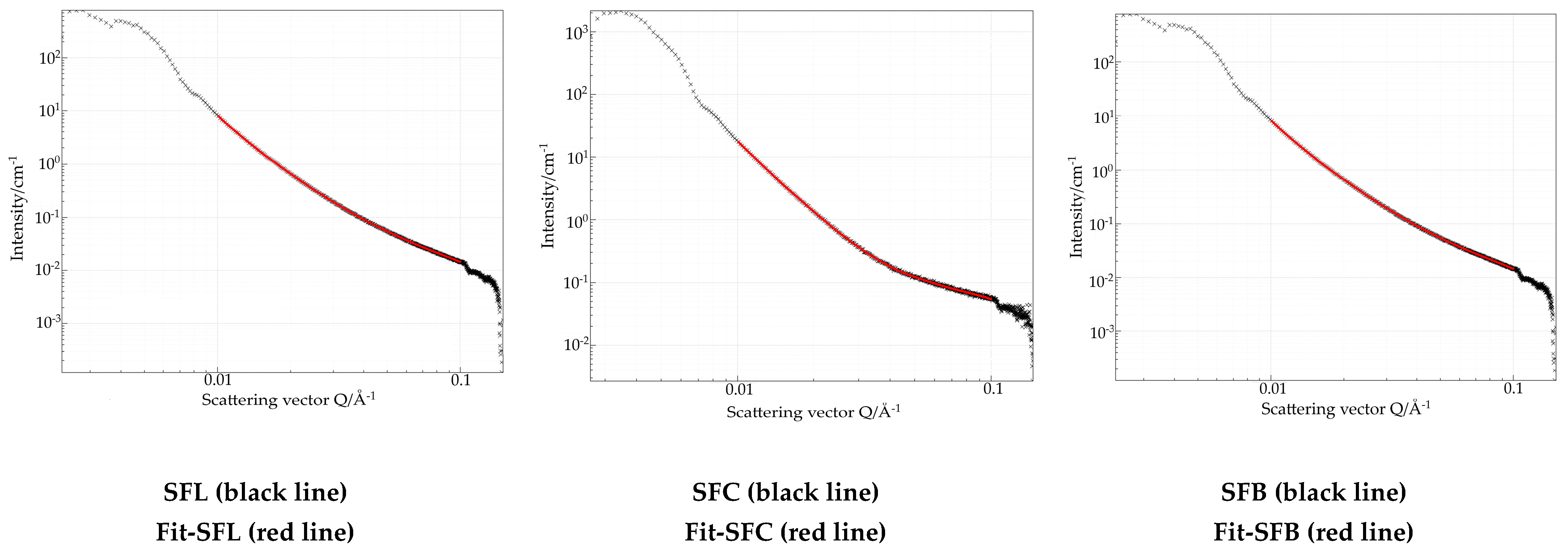
| Sample Name | Median of the Distribution, (nm) | Surface Equivalent Mean Size, (nm) | Fractal Dimension |
|---|---|---|---|
| SFL | 460 | 15 | 2.6 |
| SFC | 289 | 9 | 2.3 |
| SFB | 552 | 28 | 2.6 |
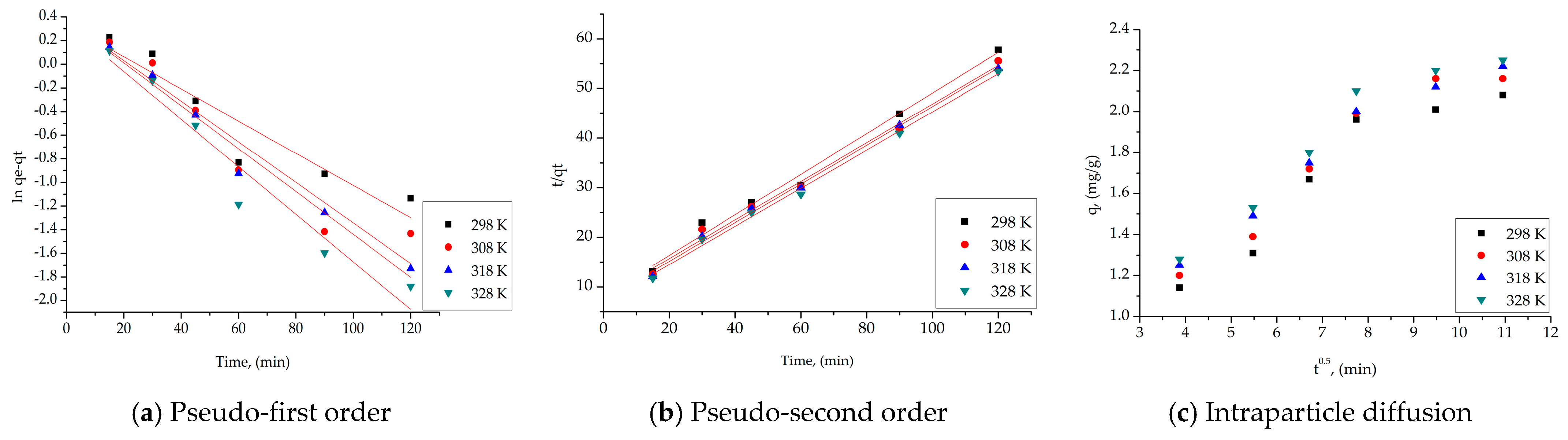
| SFL material | |||||
|---|---|---|---|---|---|
| Pseudo-first order | |||||
| Temperature (K) | qe,exp (mg g−1) | k1 (min−1) | qe,calc (mg g−1) | R2 | |
| 298 | 2.01 | 0.0136 | 1.39 | 0.8838 | |
| 308 | 2.16 | 0.0161 | 1.42 | 0.9141 | |
| 318 | 2.17 | 0.0181 | 1.45 | 0.9683 | |
| 328 | 2.20 | 0.0201 | 1.46 | 0.9451 | |
| Pseudo-second order | |||||
| Temperature (K) | qe,exp (mg g−1) | k2 (g mg−1∙min−1) | qe,calc (mg g−1) | R2 | |
| 298 | 2.01 | 0.73 | 2.44 | 0.9902 | |
| 308 | 2.16 | 0.81 | 2.53 | 0.9918 | |
| 318 | 2.17 | 0.89 | 2.56 | 0.9960 | |
| 328 | 2.20 | 0.99 | 2.60 | 0.9956 | |
| Intraparticle diffusion model (IPD) | |||||
| Temperature (K) | Kdiff (mg·g−1 min−1/2) | C | R2 | ||
| 298 | 0.053 | 1.21 | 0.8531 | ||
| 308 | 0.076 | 1.28 | 0.8754 | ||
| 318 | 0.089 | 1.31 | 0.8890 | ||
| 328 | 0.096 | 1.46 | 0.8632 | ||
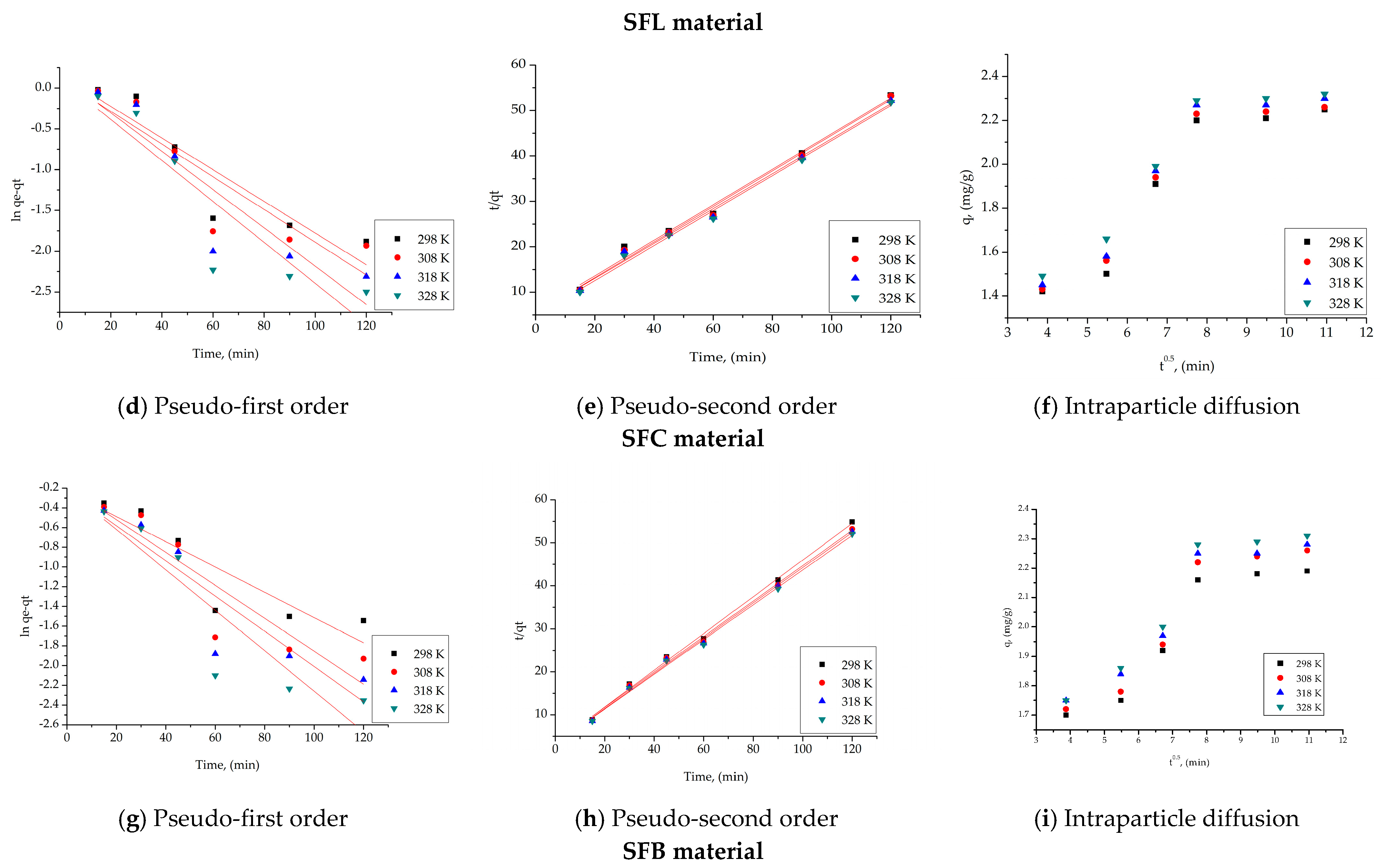
| SFC material | |||||
| Pseudo-first order | |||||
| Temperature (K) | qe,exp (mg g−1) | k1 (min−1) | qe,calc (mg g−1) | R2 | |
| 298 | 2.21 | 0.0194 | 1.17 | 0.8376 | |
| 308 | 2.24 | 0.0201 | 1.10 | 0.8086 | |
| 318 | 2.27 | 0.0235 | 1.18 | 0.8338 | |
| 328 | 2.30 | 0.0251 | 1.124 | 0.8287 | |
| Pseudo-second order | |||||
| Temperature (K) | qe,exp (mg g−1) | k2 (g mg−1∙min−1) | qe,calc (mg g−1) | R2 | |
| 298 | 2.21 | 1.13 | 2.55 | 0.9899 | |
| 308 | 2.24 | 1.22 | 2.56 | 0.9915 | |
| 318 | 2.27 | 1.26 | 2.60 | 0.9919 | |
| 328 | 2.30 | 1.39 | 2.59 | 0.9939 | |
| Intraparticle diffusion model (IPD) | |||||
| Temperature (K) | Kdiff (mg·g−1 min−1/2) | C | R2 | ||
| 298 | 0.083 | 1.44 | 0.8726 | ||
| 308 | 0.086 | 1.48 | 0.8430 | ||
| 318 | 0.089 | 1.54 | 0.8915 | ||
| 328 | 0.092 | 1.56 | 0.8751 | ||
| SFB material | |||||
| Pseudo-first order | |||||
| Temperature (K) | qe,exp (mg g−1) | k1 (min−1) | qe,calc (mg g−1) | R2 | |
| 298 | 2.18 | 0.0128 | 1.23 | 0.8049 | |
| 308 | 2.24 | 0.0167 | 1.24 | 0.8270 | |
| 318 | 2.25 | 0.0178 | 1.25 | 0.8284 | |
| 328 | 2.29 | 0.0205 | 1.23 | 0.8381 | |
| Pseudo-second order | |||||
| Temperature (K) | qe,exp (mg g−1) | k2 (g mg−1∙min−1) | qe,calc (mg g−1) | R2 | |
| 298 | 2.18 | 1.68 | 2.34 | 0.9969 | |
| 308 | 2.24 | 1.69 | 2.42 | 0.9961 | |
| 318 | 2.25 | 1.83 | 2.44 | 0.9969 | |
| 328 | 2.29 | 1.89 | 2.47 | 0.9968 | |
| Intraparticle diffusion model (IPD) | |||||
| Temperature (K) | Kdiff (mg·g−1 min−1/2) | C | R2 | ||
| 298 | 0.080 | 1.37 | 0.8229 | ||
| 308 | 0.083 | 1.39 | 0.8310 | ||
| 318 | 0.087 | 1.43 | 0.8305 | ||
| 328 | 0.088 | 1.47 | 0.8351 | ||
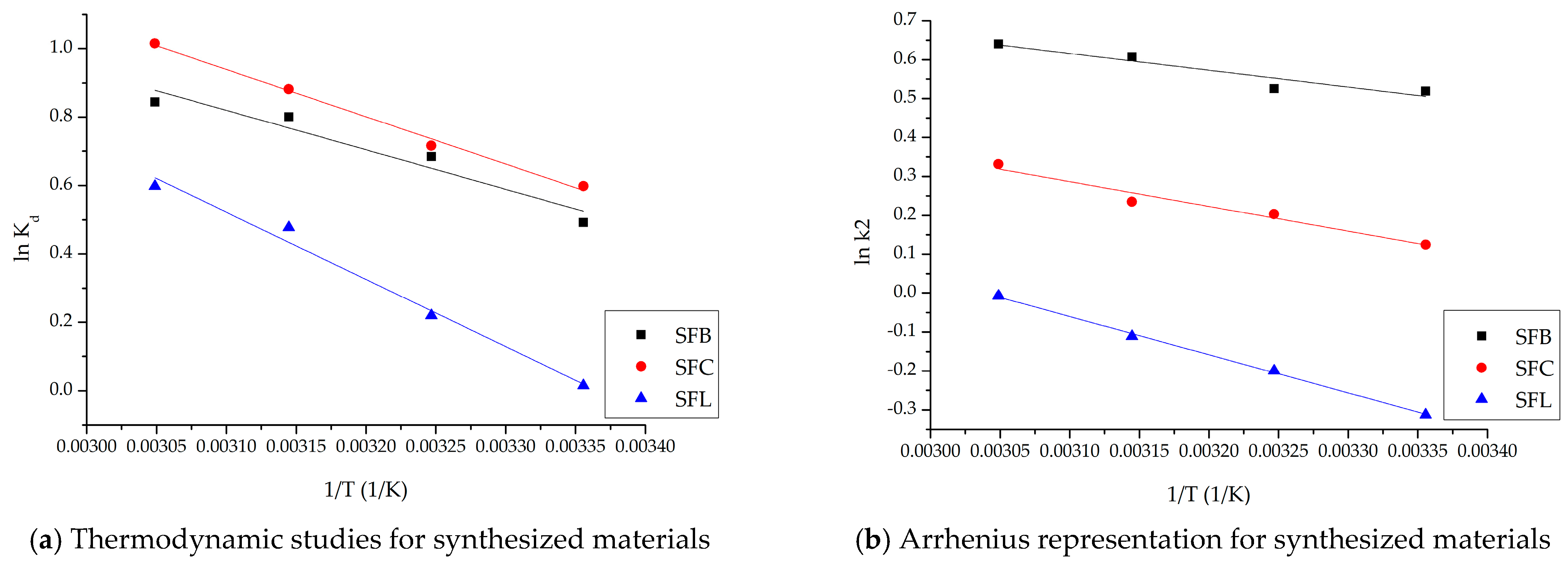
| ΔH° (kJ/mol) | ΔS° (J/mol∙K) | ΔG° (kJ/mol) | R2 | ||||
|---|---|---|---|---|---|---|---|
| 298 K | 308 K | 318 K | 328 K | ||||
| SFL material | |||||||
| 16.33 | 54.98 | −16.3 | −16.9 | −17.4 | −18.0 | 0.9868 | |
| SFC material | |||||||
| 11.49 | 45.46 | −12.9 | −13.3 | −13.8 | −14.2 | 0.9940 | |
| SFB material | |||||||
| 9.59 | 36.57 | −10.8 | −11.2 | −11.6 | −11.9 | 0.9802 | |
| Materials | Ea (kJ/mol) | R2 |
|---|---|---|
| SFL | 1.32 | 0.9984 |
| SFC | 1.60 | 0.9642 |
| SFB | 4.15 | 0.9904 |
| Materials | Langmuir Isotherm | |||
|---|---|---|---|---|
| qm,exp (mg/g) | KL (L/mg) | qL (mg/g) | R2 | |
| SFL | 19.9 | 0.0336 | 24.4 | 0.9882 |
| SFC | 19.7 | 0.0435 | 25.4 | 0.9878 |
| SFB | 19.3 | 0.0648 | 23.1 | 0.9854 |
| Freundlich isotherm | ||||
| KF (mg/g) | 1/nF | R2 | ||
| SFL | 2.13 | 0.51 | 0.9715 | |
| SFC | 2.67 | 0.46 | 0.9722 | |
| SFB | 3.38 | 0.40 | 0.9613 | |
| Sips isotherm | ||||
| KS | qS (mg/g) | 1/nS | R2 | |
| SFL | 0.03 | 25.7 | 0.07 | 0.9865 |
| SFC | 0.04 | 25.7 | 0.02 | 0.9858 |
| SFB | 0.07 | 25.0 | 0.03 | 0.9843 |
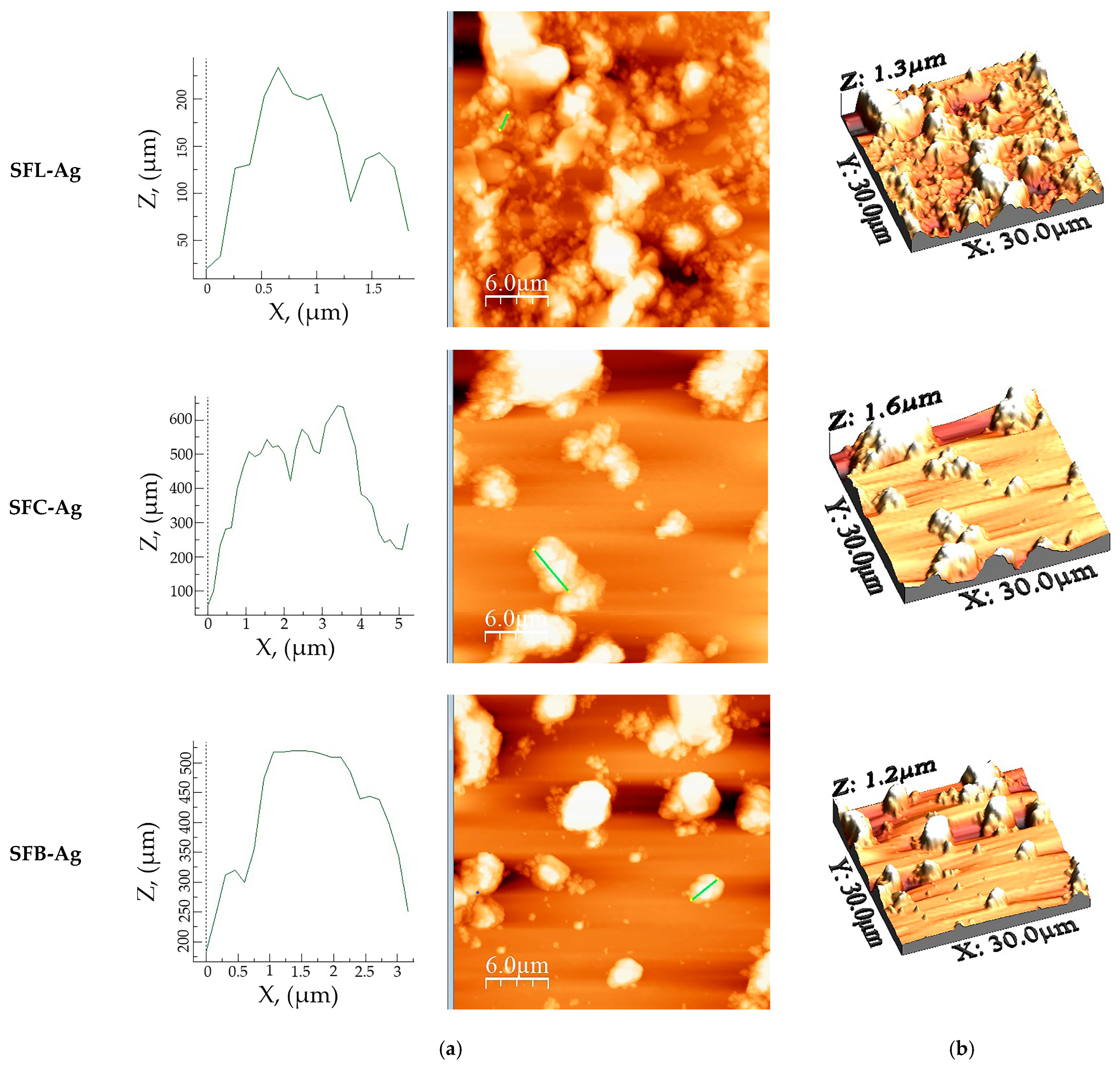
| Sample Name | Ironed Area (µm2) | Sa (nm) | Sq (nm) | Sp (nm) | Sv (nm) | Sy (nm) | Sku | Ssk |
|---|---|---|---|---|---|---|---|---|
| SFL-Ag | 1010 | 222 | 279 | 681 | −667 | 1348 | 2.821 | 0.390 |
| SFC-Ag | 973 | 188 | 275 | 710 | −846 | 1556 | 4.061 | 0.316 |
| SFB-Ag | 960 | 147 | 216 | 604 | −562 | 1166 | 3.936 | 0.724 |
| Material | Surface Area, (m2/g) | DFT Pore Width, (nm) | Total Pore Volume, (cc/g) | FHH, D |
|---|---|---|---|---|
| SFL-Ag | 30.9 | 5.483 | 3.726 × 10−2 cc/g for pores smaller than 249.6 nm | 2.1057 |
| SFC-Ag | 53.1 | 5.483 | 6.028 × 10−2 cc/g for pores smaller than 160.4 nm | 2.1137 |
| SFB-Ag | 4.9 | 5.682 | 1.037 × 10−2 cc/g for pores smaller than 161.6 nm | 1.7725 |
| Material | Median of the Distribution (nm) | Surface Equivalent Mean Size (nm) | Fractal Dimension |
|---|---|---|---|
| SFL-Ag | 436 | 7 | 3.8 |
| SFC-Ag | 258 | 11 | 3.3 |
| SFB-Ag | 593 | 66 | 2.2 |
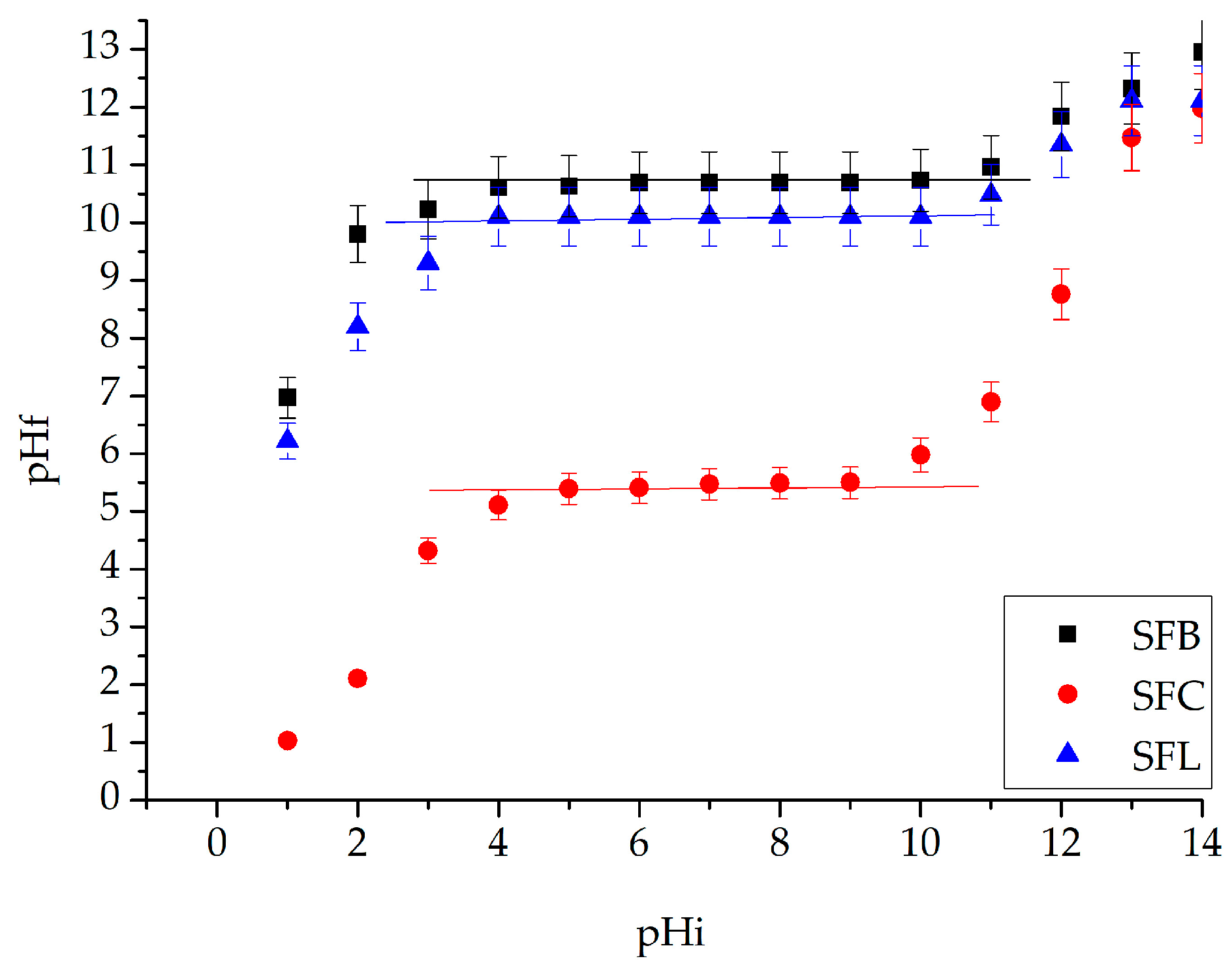
| Material | Ag mg/L | Inhibition Rate (%) | OBS. | |||
|---|---|---|---|---|---|---|
| S. aureus ATCC 25923 | P. aeruginosa ATCC 27853 | E. coli ATCC 25922 | C. albicans ATCC 10231 | |||
| SFL-Ag | 1 | 50 | 50 | 20 | 100 | Slightly better bactericidal effect on Gram-negative bacteria Very good antifungal activity |
| SFL-Ag | 10 | 20 | 50 | 100 | 100 | |
| SFL-Ag | 50 | 20 | 100 | 100 | 100 | |
| SFL-Ag | 150 | 20 | 100 | 100 | 100 | |
| SFC-Ag | 1 | 100 | 100 | 100 | 100 | Very good antibacterial and antifungal activity |
| SFC-Ag | 10 | 100 | 100 | 100 | 100 | |
| SFC-Ag | 50 | 100 | 100 | 100 | 100 | |
| SFC-Ag | 150 | 100 | 100 | 100 | 100 | |
| SFB-Ag | 1 | 20 | 20 | 20 | 75 | Good antifungal activity Lower antibacterial activity regardless of the type of bacteria |
| SFB-Ag | 10 | 20 | 20 | 20 | 75 | |
| SFB-Ag | 50 | 20 | 20 | 30 | 90 | |
| SFB-Ag | 150 | 20 | 60 | 30 | 100 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berbentea, A.; Ciopec, M.; Negrea, A.; Negrea, P.; Nemeş, N.S.; Pascu, B.; Svera, P.; Duţeanu, N.; Ianăşi, C.; Verdes, O.; et al. Iron–Carbonate (Bi, Cu, Li) Composites with Antimicrobial Activity After Silver(I) Ion Adsorption. Toxics 2025, 13, 825. https://doi.org/10.3390/toxics13100825
Berbentea A, Ciopec M, Negrea A, Negrea P, Nemeş NS, Pascu B, Svera P, Duţeanu N, Ianăşi C, Verdes O, et al. Iron–Carbonate (Bi, Cu, Li) Composites with Antimicrobial Activity After Silver(I) Ion Adsorption. Toxics. 2025; 13(10):825. https://doi.org/10.3390/toxics13100825
Chicago/Turabian StyleBerbentea, Alexandra, Mihaela Ciopec, Adina Negrea, Petru Negrea, Nicoleta Sorina Nemeş, Bogdan Pascu, Paula Svera, Narcis Duţeanu, Cătălin Ianăşi, Orsina Verdes, and et al. 2025. "Iron–Carbonate (Bi, Cu, Li) Composites with Antimicrobial Activity After Silver(I) Ion Adsorption" Toxics 13, no. 10: 825. https://doi.org/10.3390/toxics13100825
APA StyleBerbentea, A., Ciopec, M., Negrea, A., Negrea, P., Nemeş, N. S., Pascu, B., Svera, P., Duţeanu, N., Ianăşi, C., Verdes, O., Suba, M., Duda-Seiman, D. M., & Muntean, D. (2025). Iron–Carbonate (Bi, Cu, Li) Composites with Antimicrobial Activity After Silver(I) Ion Adsorption. Toxics, 13(10), 825. https://doi.org/10.3390/toxics13100825














