Effects of Malic Acid on Cadmium Uptake and Translocation and Essential Element Accumulation in Rice
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Hydroponic Culture Test
2.3. Determination of Root Morphology
2.4. Determination of Cd and Essential Elements
2.5. Data Processing and Calculation Method
3. Results
3.1. Genotypic Differences in Cadmium Accumulation in Rice
3.2. Effects of Cd on Essential Element Accumulation in Rice Genotypes
3.3. Effects of Malic Acid on Cd Accumulation in Rice Genotypes
3.4. Effects of Malic Acid on Essential Elements in Rice Genotypes
3.5. Effect of Malic Acid on Root Morphology of Rice Genotypes Under Cd Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Cd | Cadmium |
| TCA | Tricarboxylic acid |
| CK | Control group |
| MA | Malic acid |
Appendix A
| Genotype | Mg | K | Ca | Mn | Fe | Zn | |
|---|---|---|---|---|---|---|---|
| Root | X24 | 1122 ± 280 bc | 12,358 ± 2461 a | 3480 ± 220 a | 128 ± 48 a | 2316 ± 146 b | 205 ± 47 b |
| 20 | 987 ± 76 c | 9681 ± 1324 d | 2555 ± 198 b | 45 ± 11 b | 4998 ± 126 a | 291 ± 32 b | |
| 58 | 1014 ± 79 bc | 16,099 ± 778 b | 2707 ± 107 b | 43 ± 3 b | 5757 ± 315 a | 281 ± 13 b | |
| 65 | 1586 ± 46 a | 20,130 ± 592 a | 2579 ± 148 b | 50 ± 8 b | 4951 ± 289 a | 514 ± 47 a | |
| Shoot | X24 | 6061 ± 414 a | 52,747 ± 1729 a | 6463 ± 479 a | 676 ± 33 a | 369 ± 138 b | 131 ± 16 b |
| 20 | 4842 ± 60 b | 45,501.7 ± 984 b | 5382 ± 166 bc | 469 ± 23 b | 454 ± 65 a | 119 ± 8 b | |
| 58 | 4893 ± 439 b | 54,039 ± 6137 a | 5616 ± 340 b | 384 ± 25 c | 424 ± 101 a | 104 ± 14 b | |
| 65 | 4775 ± 180 b | 48,533 ± 2423 b | 4717 ± 141 c | 411 ± 16 c | 338 ± 54 b | 263 ± 6 a |
| Genotype | Mg | K | Ca | Mn | Fe | Zn | |
|---|---|---|---|---|---|---|---|
| Root | X24 | 728 ± 22 d | 12,568 ± 1698 c | 3214 ± 157 a | 907 ± 106 a | 5562 ± 2089 a | 129 ± 13 d |
| 20 | 922 ± 20 b | 12,543 ± 257 c | 2779 ± 43 b | 70 ± 2 b | 3700 ± 73 ab | 220 ± 11 b | |
| 58 | 846 ± 26 c | 15,955 ± 776 b | 2709 ± 61 b | 71 ± 6 b | 4073 ± 257 ab | 201 ± 10 c | |
| 65 | 975 ± 41 a | 21,667 ± 1095 a | 1766 ± 190 c | 92 ± 41 b | 3422 ± 155 b | 310 ± 7 a | |
| Shoot | X24 | 5987 ± 112 a | 54,199 ± 885 a | 6255 ± 52 a | 353 ± 8 a | 201 ± 44 b | 93 ± 15 b |
| 20 | 4107 ± 330 b | 48,909 ± 3883 a | 5375 ± 450 b | 252 ± 22 b | 227 ± 24 b | 92 ± 9 b | |
| 58 | 4114 ± 301 b | 53,145 ± 3525 a | 5574 ± 272 b | 178 ± 11 c | 634 ± 335 a | 76 ± 4 b | |
| 65 | 3905 ± 140 b | 51,482 ± 490 a | 4155 ± 111 c | 182 ± 15 c | 225 ± 121 b | 239 ± 6 a |
| Genotype | Mg | K | Ca | Mn | Fe | Zn | |
|---|---|---|---|---|---|---|---|
| X24 | M0 | 728 ± 22 c | 12,568 ± 1698 a | 3214 ± 157 b | 907 ± 106 a | 5562 ± 2089 a | 129 ± 13 a |
| M0.5 | 1027 ± 52 b | 6317 ± 2087 b | 4634 ± 83 a | 863 ± 312 a | 4721 ± 369 a | 97 ± 12 b | |
| M1.5 | 1121 ± 59 a | 4264 ± 555 b | 4487 ± 71 a | 351 ± 12 b | 3403 ± 1138 a | 77 ± 11 b | |
| 58 | M0 | 846 ± 26 a | 15,955 ± 776 a | 2709 ± 61 b | 71 ± 6 c | 4073 ± 257 a | 201 ± 10 a |
| M0.5 | 805 ± 59 a | 9915 ± 699 b | 3602 ± 672 a | 230 ± 13 a | 2727 ± 226 b | 153 ± 11 b | |
| M1.5 | 856 ± 26 a | 4974 ± 250 c | 3235 ± 144 ab | 139 ± 7 b | 1628 ± 58 c | 171 ± 25 ab | |
| 65 | M0 | 975 ± 41 a | 21,667 ± 1095 a | 1766 ± 190 c | 92 ± 4 c | 3422 ± 155 a | 310 ± 7 a |
| M0.5 | 765 ± 61 b | 14,047 ± 715 b | 2596 ± 19 b | 205 ± 7 a | 2182 ± 98 b | 192 ± 5 c | |
| M1.5 | 831 ± 52 b | 9259 ± 919 c | 3292 ± 167 a | 176 ± 10 b | 1806 ± 34 c | 215 ± 4 b |
| Genotype | Mg | K | Ca | Mn | Fe | Zn | |
|---|---|---|---|---|---|---|---|
| X24 | M0 | 5987 ± 112 a | 54,199 ± 885 a | 6255 ± 52 a | 353 ± 8a | 201 ± 44a | 93 ± 15 a |
| M0.5 | 5816 ± 409 a | 51,404 ± 3270 ab | 7363 ± 841 a | 279 ± 27 b | 255 ± 106 a | 73 ± 8 a | |
| M1.5 | 5602 ± 393 a | 48,853 ± 2872 b | 7276 ± 609 a | 253 ± 7 b | 231 ± 126 a | 88 ± 6 a | |
| 58 | M0 | 4113 ± 301 a | 53,145 ± 3525 a | 5574 ± 272 b | 178 ± 11 b | 634 ± 335 a | 76 ± 4 a |
| M0.5 | 4267 ± 32 a | 48,831 ± 572 b | 5820 ± 54 b | 181 ± 3 b | 343 ± 6 ab | 66 ± 1 b | |
| M1.5 | 3923 ± 21 a | 43,179 ± 968 c | 6427 ± 171 a | 269 ± 3 a | 211 ± 20 b | 83 ± 5 a | |
| 65 | M0 | 3905 ± 140 a | 51,482 ± 490 a | 4155 ± 111 b | 182 ± 15 b | 225 ± 121 a | 239 ± 6 a |
| M0.5 | 3980 ± 318 a | 49,770 ± 4399 a | 4453 ± 381 b | 209 ± 16 b | 174 ± 56 a | 230 ± 16 a | |
| M1.5 | 3903 ± 121 a | 42,647 ± 1177 b | 4977 ± 169 a | 262 ± 18 a | 138 ± 33 a | 240 ± 19 a |
References
- Ahmad, S.; Sehrish, A.K.; Alomrani, S.O.; Zhang, L.; Waseem, M.; Noureen, S.; Ullah, I.; Tabassam, R.; Abbas, G.; Ali, S. Combined application of biochar and metal-tolerant bacteria alleviates cadmium toxicity by modulating the antioxidant defense mechanism and physicochemical attributes in rice (Oryza sativa L.) grown in cadmium-contaminated soil. Plant Stress 2024, 11, 100348. [Google Scholar] [CrossRef]
- Qin, Y.; Li, Z.; Sun, J.; Xu, M.; Gu, M.; Wei, Y.; Lei, J. Manganese (II) sulfate affects the formation of iron-manganese oxides in soil and the uptake of cadmium and arsenic by rice. Ecotoxicol. Environ. Saf. 2023, 263, 115360. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Chen, P.; Zhou, W.; Liu, H.; Cheng, K.; Xiao, X.; Tang, H.; Yi, Z. Response characteristics of soil Cd availability to microbes in paddy soil with long-term fertilization and its impact on Cd uptake in rice. Sci. Total Environ. 2024, 957, 177680. [Google Scholar] [CrossRef] [PubMed]
- Lwin, C.S.; Jung, H.; Kim, M.S.; Lee, E.J.; Lee, T.G. Geochemical speciation, uptake, and transportation mechanisms of arsenic, cadmium, and lead in soil–rice systems: Additional aspects and challenges. Antioxidants 2025, 14, 607. [Google Scholar] [CrossRef]
- Teng, Y.; Hu, J.; Sun, H.; Xiao, Y.; Guo, J.; Yu, H. Research on the impact of elevated atmospheric CO2 concentration on Cd absorption in the cell walls of rice roots. Process Saf. Environ. Prot. 2025, 200, 107379. [Google Scholar] [CrossRef]
- Haider, F.U.; Liqun, C.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.; Wenjun, M.; Farooq, M. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887. [Google Scholar] [CrossRef]
- Zhang, Z.; Qin, Y.; Ya, F.; Ma, Y.; Pan, G.; Peng, S.; Liang, S.; Feng, Y.; Jiang, Z.; Gu, M.; et al. Foliar spraying of a novel biosynthesized nano-selenium: Enhancing cadmium reduction in rice grains and regulating in vitro bioavailability with mechanistic insights. J. Food Compos. Anal. 2025, 148, 108280. [Google Scholar] [CrossRef]
- Guo, R.; Ren, R.; Wang, L.; Zhi, Q.; Yu, T.; Hou, Q.; Yang, Z. Using machine learning to predict selenium and cadmium contents in rice grains from black shale-distributed farmland area. Sci. Total Environ. 2024, 912, 168802. [Google Scholar] [CrossRef]
- Hafez, E.M.; Gao, Y.; La, H.G.; Alharbi, K.; Hamada, M.M.; Omara, A.E.-D.; Alshaal, T. Enhancing rice productivity in wastewater-irrigated saline Cd-contaminated soils using microbial-nanoparticle synergy. Environ. Technol. Innov. 2025, 39, 104253. [Google Scholar] [CrossRef]
- Iqbal, A.; Ligeng, J.; Mo, Z.; Adnan, M.; Lal, R.; Zaman, M.; Usman, S.; Hua, T.; Imran, M.; Pan, S.; et al. Substation of vermicompost mitigates Cd toxicity, improves rice yields and restores bacterial community in a Cd-contaminated soil in Southern China. J. Hazard. Mater. 2024, 465, 133118. [Google Scholar] [CrossRef]
- Roy, R.; Hossain, A.; Sarker, T.; Asaduzzaman, M. Alleviation of heavy metal accumulation in rice: A synergistic planetary health approach of organic amendment and phytoremediation in cadmium contaminated soil. J. Hazard. Mater. Adv. 2025, 19, 100864. [Google Scholar] [CrossRef]
- Dou, X.; Zhang, C.; Zhang, J.; Ma, D.; Chen, L.; Zhou, G.; Duan, Y.; Tao, L.; Chen, J. Relationship between calcium forms and organic carbon content in aggregates of calcareous soils in northern China. Soil Tillage Res. 2024, 244, 106210. [Google Scholar] [CrossRef]
- Wu, X.; Lin, Q.; Li, G.; Guo, C.; Li, L.; Wang, J. Evaluating water management efficiency in regulating cadmium and arsenic accumulation in rice in typical japonica paddy soils at varied pH levels. Agriculture 2024, 14, 407. [Google Scholar] [CrossRef]
- Veeken, A.; Hamelers, B. Sources of Cd, Cu, Pb and Zn in biowaste. Sci. Total Environ. 2002, 300, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, M.; Wang, H.; Li, C.; Zhang, Y.; Dong, Z.; Fu, C.; Ye, Y.; Wang, F.; Chen, X.; et al. Effects of different phosphorus fertilizers on cadmium absorption and accumulation in rice under low-phosphorus and rich-cadmium soil. Environ. Sci. Pollut. Res. 2024, 31, 11898–11911. [Google Scholar] [CrossRef]
- Mukta, T.A.; Hoque, M.A.; Shimo, F.J.; Islam, S. Cadmium contamination in rice and associated human health risk. Agric. (Pol’nohospodárstvo) 2024, 70, 38–52. [Google Scholar] [CrossRef]
- El-Mamoon, M.F.; Abdel-Salam, A.A.; Abdel-Hmied, A.H.; Moursy, A.A.; Hekal, M.A. Concentrations of Cd, Co, and Pb in soil and taro plant (Colocasia esculenta) at various distances from a phosphate fertilizer factory. Benha J. Appl. Sci. 2023, 8, 75–78. [Google Scholar] [CrossRef]
- Shao, X.; Yao, H.; Cui, S.; Peng, Y.; Gao, X.; Yuan, C.; Chen, X.; Hu, Y.; Mao, X. Activated low-grade phosphate rocks for simultaneously reducing the phosphorus loss and cadmium uptake by rice in paddy soil. Sci. Total Environ. 2021, 780, 146550. [Google Scholar] [CrossRef]
- Xia, F.; Zhao, Z.; Niu, X.; Wang, Z. Integrated pollution analysis, pollution area identification and source apportionment of heavy metal contamination in agricultural soil. J. Hazard. Mater. 2024, 465, 133215. [Google Scholar] [CrossRef]
- Wang, S.; Huang, D.; Zhu, Q.; Zhu, H.; Liu, S.; Luo, Z.; Cao, X.; Wang, J.; Rao, Z.; Shen, X. Speciation and phytoavailability of cadmium in soil treated with cadmium-contaminated rice straw. Environ. Sci. Pollut. Res. 2015, 22, 2679–2686. [Google Scholar] [CrossRef]
- Yan, T.; Gao, Z.; Wang, T.; Lu, X.; Yang, L.; Shen, L.; Zhang, Q.; Hu, J.; Ren, D.; Zhang, G.; et al. Appropriate supply of ammonium nitrogen and ammonium nitrate reduces cadmium content in rice seedlings by inhibiting cadmium uptake and transport. Rice Sci. 2024, 31, 587–602. [Google Scholar] [CrossRef]
- Song, J.; Song, Q.; Wang, D.; Liu, Y. Mitigation strategies for excessive cadmium in rice. Compr. Rev. Food Sci. Food Saf. 2023, 22, 3847–3869. [Google Scholar] [CrossRef]
- Madomguia, D.; Basokdou, G.B.; Appoline Isabelle, K.W.; Dongo, P.K.; Nya, E.; Togouet, S.H.Z. Accumulation of heavy metals in maga-pouss rice fields (far-north region, Cameroon) and transfer to rice grains. Agric. Sci. 2024, 15, 311–326. [Google Scholar] [CrossRef]
- Jiang, K.; Chen, S.; Xiang, N.; Hu, J.; Cheng, Y. Deciphering the translocation of cadmium in indica rice under high-cadmium soil conditions. Environ. Geochem. Health 2025, 47, 303. [Google Scholar] [CrossRef] [PubMed]
- Dabgar, S.; Bhavsar, S.; Modi, N. Effects of lead and cadmium on shoot length and root length of Cascabela thevetia (L.) lippold. Int. Assoc. Biol. Comput. Digest 2023, 3, 301–306. [Google Scholar] [CrossRef]
- Barman, F.; Majumdar, S.; Arzoo, S.H.; Kundu, R. Genotypic variation among 20 rice cultivars/landraces in response to cadmium stress grown locally in West Bengal, India. Plant Physiol. Biochem. 2020, 148, 193–206. [Google Scholar] [CrossRef]
- Liu, T.; Hu, W.; Weng, L.; Deng, L.; Li, J.; Yu, J.; Zhou, Z.; Liu, Y.; Chen, C.; Sheng, T.; et al. Phenotypic and genetic dissection of the contents of important metallic elements in hybrid rice grown in cadmium-contaminated paddy fields. Heliyon 2023, 9, e19919. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Wang, C.; Li, S.; Li, B.; Li, Q.; Chen, G.; Chen, W.; Wang, F. Cadmium adsorption, chelation and compartmentalization limit root-to-shoot translocation of cadmium in rice (Oryza sativa L.). Environ. Sci. Pollut. Res. 2017, 24, 11319–11330. [Google Scholar] [CrossRef]
- Pan, B.; Cai, Y.; Liu, B.; Cai, K.; Lv, W.; Tian, J.; Wang, W. Abatement of Cd in rice grain and toxic risks to human health by the split application of silicon at transplanting and jointing period. J. Environ. Manag. 2022, 302, 114039. [Google Scholar] [CrossRef]
- Hakeem, K.R.; Alharby, H.F.; Pirzadah, T.B. Exogenously applied calcium regulates antioxidative system and reduces cadmium-uptake in Fagopyrum esculentum. Plant Physiol. Biochem. 2022, 180, 17–26. [Google Scholar] [CrossRef]
- Uraguchi, S.; Mori, S.; Kuramata, M.; Kawasaki, A.; Arao, T.; Ishikawa, S. Root-to-shoot Cd translocation via the xylem is the major process determining shoot and grain cadmium accumulation in rice. J. Exp. Bot. 2009, 60, 2677–2688. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Huang, D.; Zhang, Q.; Zhu, H.; Xu, C.; Li, B.; Zhu, Q. Meta-analysis of the effects of liming on soil pH and cadmium accumulation in crops. Ecotoxicol. Environ. Saf. 2021, 223, 112621. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, C.; Zhao, Y.; Sun, S.; Liu, Z. Effects of growing seasons and genotypes on the accumulation of cadmium and mineral nutrients in rice grown in cadmium contaminated soil. Sci. Total Environ. 2017, 579, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, W.; Xiao, H.; Xin, J.; Zhao, C.; Tian, R. Photosynthetic responses of Pontederia cordata to cadmium stress: Anatomical structure, ultrastructure, physiology, and gene expression. Plants 2025, 14, 1344. [Google Scholar] [CrossRef]
- Che, S.; Wang, J.; Zhou, Y.; Yue, C.; Zhou, X.; Xu, Y.; Tian, S.; Cao, Z.; Wei, X.; Li, S.; et al. The adsorption and fixation of Cd and Pb by the microbial consortium weakened the toxic effect of heavy metal-contaminated soil on rice. Chem. Eng. J. 2024, 497, 154684. [Google Scholar] [CrossRef]
- Huang, G.; Huang, Y.; Ding, X.; Ding, M.; Wang, P.; Wang, Z.; Jiang, Y.; Zou, L.; Zhang, W.; Li, Z. Effects of high manganese-cultivated seedlings on cadmium uptake by various rice (Oryza sativa L.) genotypes. Ecotoxicol. Environ. Saf. 2023, 264, 115440. [Google Scholar] [CrossRef]
- Gulzar, U.; Hussain, A.; Hamayun, M.; Iqbal, A.; Seleiman, M.F.; Alotaibi, M.; Lee, B. Gibberellins producing endophytic Aspergillus nidulans DSE-2 biosorbs Cd and down-regulates OsNRAMP5 and OsCd1 genes to improve rice growth in contaminated soil. Plant Physiol. Biochem. 2025, 221, 109650. [Google Scholar] [CrossRef]
- Ghouri, F.; Shahid, M.J.; Liu, J.; Lai, M.; Sun, L.; Wu, J.; Liu, X.; Ali, S.; Shahid, M.Q. Polyploidy and zinc oxide nanoparticles alleviated Cd toxicity in rice by modulating oxidative stress and expression levels of sucrose and metal-transporter genes. J. Hazard. Mater. 2023, 448, 130991. [Google Scholar] [CrossRef]
- Shahzad, M.; Peng, D.; Khan, A.; Ayyaz, A.; Askri, S.M.H.; Naz, S.; Huang, B.; Zhang, G. Sufficient manganese supply is necessary for OsNramp5 knockout rice plants to ensure normal growth and less Cd uptake. Ecotoxicol. Environ. Saf. 2024, 288, 117386. [Google Scholar] [CrossRef]
- Feng, X.; Pang, Z.; Liu, J.; Peng, H.; Liang, Y.; Guo, B. Chemical and microbial mechanisms underpinning calcium-regulated suppression of cadmium bioavailability in alkaline paddy soil and cadmium accumulation in rice grain. Ecotoxicol. Environ. Saf. 2025, 302, 118620. [Google Scholar] [CrossRef]
- Wang, S.; Dong, Q.; Wang, Z. Differential effects of citric acid on cadmium uptake and accumulation between tall fescue and Kentucky bluegrass. Ecotoxicol. Environ. Saf. 2017, 145, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Hawrylak-Nowak, B.; Dresler, S.; Matraszek, R. Exogenous malic and acetic acids reduce cadmium phytotoxicity and enhance cadmium accumulation in roots of sunflower plants. Plant Physiol. Biochem. 2015, 94, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Mnasri, M.; Ghabriche, R.; Fourati, E.; Zaier, H.; Sabally, K.; Barrington, S.; Lutts, S.; Abdelly, C.; Ghnaya, T. Cd and Ni transport and accumulation in the halophyte Sesuvium portulacastrum: Implication of organic acids in these processes. Front. Plant Sci. 2015, 6, 156. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Shi, Q.; Li, Z.; Deng, H.; Zhou, Q.; Li, L.; Zhao, L.; Yuan, S.; Liu, S.; Chen, Y. Rhodopseudomonas palustris shapes bacterial community, reduces Cd bioavailability in Cd contaminated flooding paddy soil, and improves rice performance. Sci. Total Environ. 2024, 926, 171824. [Google Scholar] [CrossRef]
- Kováčik, J.; Klejdus, B.; Babula, P.; Hedbavny, J. Ascorbic acid affects short-term response of Scenedesmus quadricauda to cadmium excess. Algal Res. 2017, 24, 354–359. [Google Scholar] [CrossRef]
- Guo, H.; Chen, H.; Hong, C.; Jiang, D.; Zheng, B. Exogenous malic acid alleviates cadmium toxicity in Miscanthus sacchariflorus through enhancing photosynthetic capacity and restraining ROS accumulation. Ecotoxicol. Environ. Saf. 2017, 141, 119–128. [Google Scholar] [CrossRef]
- Liu, D.; Gao, Z.; Li, J.; Yao, Q.; Tan, W.; Xing, W.; Lu, Z. Effects of cadmium stress on the morphology, physiology, cellular ultrastructure, and BvHIPP24 gene expression of sugar beet (Beta vulgaris L.). Int. J. Phytoremediat. 2023, 25, 455–465. [Google Scholar] [CrossRef]
- Das, S.; Sengupta, S.; Patra, P.K.; Dey, P. Limestone and yellow gypsum can reduce cadmium accumulation in groundnut (Arachis hypogaea): A study from a three-decade old landfill site. Chemosphere 2024, 353, 141645. [Google Scholar] [CrossRef]
- Wu, W.; Yang, W.; Zheng, F.; Zhang, Q.; Ma, Q.; Zhao, Y.; Luo, S.; Yang, Y.; Zeng, Q.; Deng, X. Strategic attenuation of Cd accumulation in rice through stage-specific flooding: Synergistic coordination of rhizospheric Cd bioavailability, microbial communities, and iron plaque speciation. Environ. Pollut. 2025, 377, 126455. [Google Scholar] [CrossRef]
- Zhang, S.; Han, W.; Liu, T.; Feng, C.; Jiang, Q.; Zhang, B.; Chen, Y.; Zhang, Y. Tetracycline inhibits the nitrogen fixation ability of soybean (Glycine max (L.) Merr.) nodules in black soil by altering the root and rhizosphere bacterial communities. Sci. Total Environ. 2024, 908, 168047. [Google Scholar] [CrossRef]
- Li, Q.; Guo, J.; Zhang, X.; Yu, H.; Huang, F.; Zhang, L.; Zhang, M.; Li, T. Changes of non-protein thiols in root and organic acids in xylem sap involved in cadmium translocation of cadmium-safe rice line (Oryza sative L.). Plant Soil 2019, 439, 475–486. [Google Scholar] [CrossRef]
- Pan, J.; Guan, M.; Xu, P.; Chen, M.; Cao, Z. Salicylic acid reduces cadmium (Cd) accumulation in rice (Oryza sativa L.) by regulating root cell wall composition via nitric oxide signaling. Sci. Total Environ. 2021, 797, 149202. [Google Scholar] [CrossRef] [PubMed]
- Akter, S.; Samad, R. Effects of kinetin and gibberellic acid on growth and ion transport of rice under cadmium stress. Bangladesh J. Bot. 2024, 53, 209–216. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, J.; Li, S.; Shao, J.; Shen, R.; Zhu, X. Salicylic acid minimize cadmium accumulation in rice through regulating the fixation capacity of the cell wall to cadmium. Plant Sci. 2023, 336, 111839. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, H.; Ding, S.; Hu, Z.; Tie, B.; Luo, S. A new insight into the straw decomposition associated with minerals: Promoting straw humification and Cd immobilization. J. Environ. Sci. 2025, 148, 553–566. [Google Scholar] [CrossRef]
- Ubeynarayana, N.; Jeyakumar, P.; Bishop, P.; Pereira, R.C.; Anderson, C.W.N. Effect of soil cadmium on root organic acid secretion by forage crops. Environ. Pollut. 2021, 268, 115839. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, S.; Du, X.; Bao, Z.; Xu, F.; Awadelseid, S.F.; Yaisamut, O. Effects and mechanisms of different exogenous organic matters on selenium and cadmium uptake by rice in natural selenium-cadmium-rich soil. Heliyon 2024, 10, e37740. [Google Scholar] [CrossRef]
- Lu, H.; Chen, L.; Liu, J.; Tang, X.; Chen, C.; Sheteiwy, M.S.; Yu, J.; Wang, X.; Han, J.; Wu, Y.; et al. Periphytic biofilm (PB) in paddy field: A natural cadmium barrier for rice (Oryza sativa L.) safe production through physiological detoxification and gene regulation. J. Hazard. Mater. 2025, 495, 139136. [Google Scholar] [CrossRef]
- Wu, J.; Guo, J.; Hu, Y.; Gong, H. Distinct physiological responses of tomato and cucumber plants in silicon-mediated alleviation of cadmium stress. Front. Plant Sci. 2015, 6, 453. [Google Scholar] [CrossRef]
- Wang, K.; Wang, F.; Yu, Y.; Yang, S.; Han, Y.; Yao, H. Microplastics and soil microbiomes. BMC Biol. 2025, 23, 273. [Google Scholar] [CrossRef]
- Huang, L.; Li, W.; Tam, N.F.Y.; Ye, Z. Effects of root morphology and anatomy on cadmium uptake and translocation in rice (Oryza sativa L.). J. Environ. Sci. 2019, 75, 296–306. [Google Scholar] [CrossRef]
- Yoshihara, T.; Goto, F.; Shoji, K.; Kohno, Y. Cross relationships of Cu, Fe, Zn, Mn, and Cd accumulations in common japonica and indica rice cultivars in Japan. Environ. Exp. Bot. 2010, 68, 180–187. [Google Scholar] [CrossRef]
- Fontanili, L.; Lancilli, C.; Suzui, N.; Dendena, B.; Yin, Y.; Ferri, A.; Ishii, S.; Kawachi, N.; Lucchini, G.; Fujimaki, S.; et al. Kinetic analysis of zinc/cadmium reciprocal competitions suggests a possible Zn-insensitive pathway for root-to-shoot cadmium translocation in rice. Rice 2016, 9, 16. [Google Scholar] [CrossRef]
- Zhang, X.; Xue, W.; Qi, L.; Zhang, C.; Wang, C.; Huang, Y.; Wang, Y.; Peng, L.; Liu, Z. Malic acid inhibits accumulation of cadmium, lead, nickel and chromium by down-regulation of OsCESA and up-regulation of OsGLR3 in rice plant. Environ. Pollut. 2024, 341, 122934. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xu, J.; Peng, L.; Cheng, Z.; Kuang, X.; Li, D.; Peng, C.; Song, H. Cadmium accumulation in rice grains is mitigated by duckweed-like hydrophyte through adsorption and increased ammonia nitrogen. Sci. Total Environ. 2023, 890, 164510. [Google Scholar] [CrossRef] [PubMed]


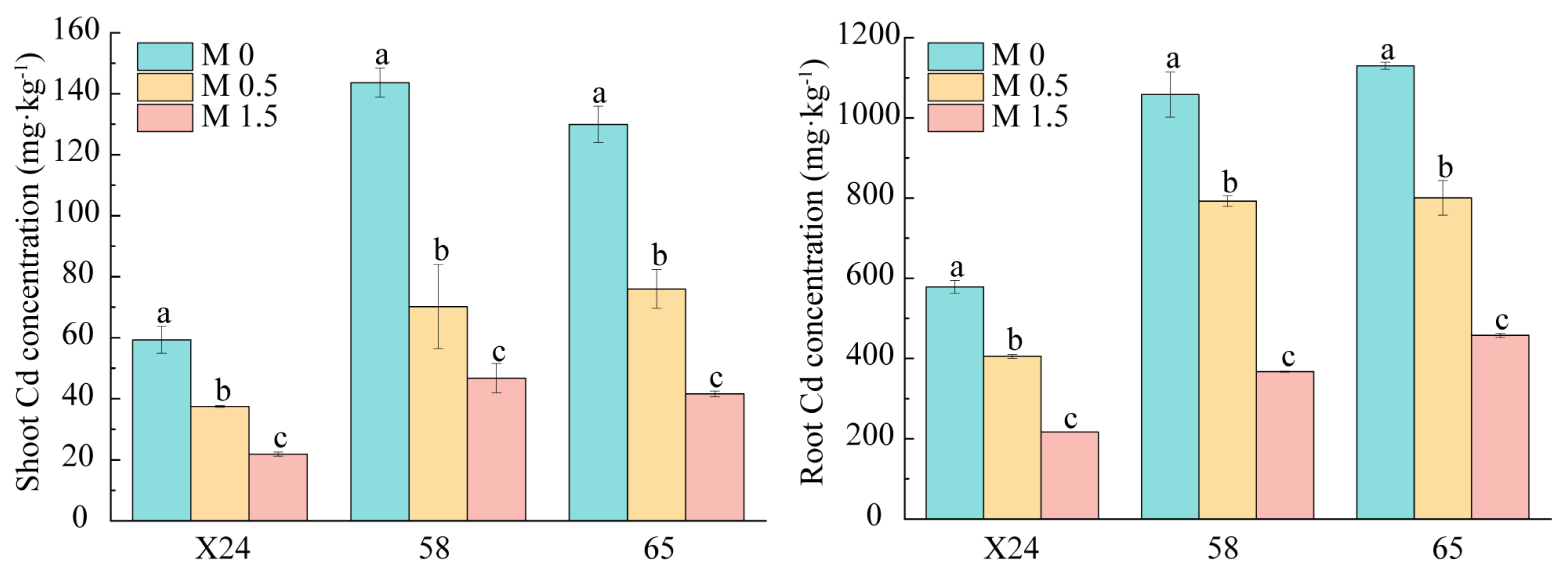
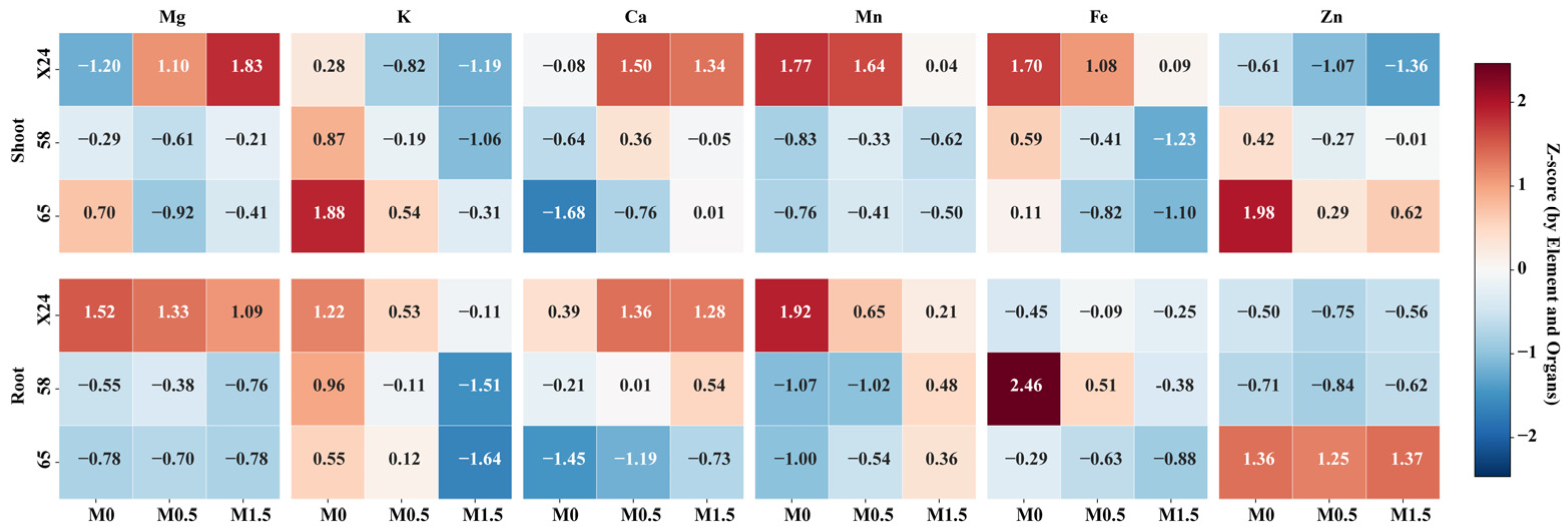


| List | Genotype |
|---|---|
| X24 | Xiang zao shan 24 |
| 20 | Nan xiong zao you zhan |
| 58 | Zhu zhen B |
| 65 | Zhen shan 97B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Zhang, Y.; Lv, G.; Liu, T.; Liu, Z.; Jiang, Y.; Hao, Y.; Yu, Y.; Dong, W.; Qian, C. Effects of Malic Acid on Cadmium Uptake and Translocation and Essential Element Accumulation in Rice. Toxics 2025, 13, 811. https://doi.org/10.3390/toxics13100811
Zhang S, Zhang Y, Lv G, Liu T, Liu Z, Jiang Y, Hao Y, Yu Y, Dong W, Qian C. Effects of Malic Acid on Cadmium Uptake and Translocation and Essential Element Accumulation in Rice. Toxics. 2025; 13(10):811. https://doi.org/10.3390/toxics13100811
Chicago/Turabian StyleZhang, Shuo, Yiteng Zhang, Guoyi Lv, Tianqi Liu, Zhongqi Liu, Yubo Jiang, Yubo Hao, Yang Yu, Wenjun Dong, and Chunrong Qian. 2025. "Effects of Malic Acid on Cadmium Uptake and Translocation and Essential Element Accumulation in Rice" Toxics 13, no. 10: 811. https://doi.org/10.3390/toxics13100811
APA StyleZhang, S., Zhang, Y., Lv, G., Liu, T., Liu, Z., Jiang, Y., Hao, Y., Yu, Y., Dong, W., & Qian, C. (2025). Effects of Malic Acid on Cadmium Uptake and Translocation and Essential Element Accumulation in Rice. Toxics, 13(10), 811. https://doi.org/10.3390/toxics13100811







