Efficient Recovery of Phosphorus from Wastewater Using Calcium-Based Modified Biochar: Removal Performance, Adsorption Mechanism, and Resource Utilization
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Materials
2.2. Test Methods
2.2.1. Preparation of Adsorbents
2.2.2. Adsorption Test
- (1)
- Adsorption kinetics: C0 = 200 mg/L; V = 0.05 L; m = 0.05 g; pH = 2.00; t = 0–720 min; temperature = 298 K.
- (2)
- pH: initial concentration C0 = 200 mg/L; a total of 11 pH treatments (pH: 2.00–13.00); V = 0.05 L; m = 0.05 g; t = 720 min; temperature = 298 K.
- (3)
- Temperature: initial concentration C0 = 200 mg/L; temperature = 288, 298, 308, and 318 K; V = 0.05 L; m = 0.05 g; t = 720 min; temperature = 298 K; pH = 2.00.
- (4)
- Adsorption isotherm: C0 = 50–350 mg/L; V = 0.05 L; m = 0.05 g; t = 720 min; temperature = 298 K; pH = 2.00.
- (5)
- Anion types: NO3−, Cl−, HCO3−, SO42− (20–100 mmol/L); C0 = 200 mg/L; V = 0.05 L; m = 0.05 g; t = 720 min; temperature = 298 K; pH = 2.00.
- (6)
- Biochar produced under different pyrolysis temperatures and proportions of eggshell addition: C0 = 200 mg/L; V = 0.05 L; m = 0.05 g; t = 720 min; temperature = 298 K, pH = 2.00.
2.2.3. Potted Plant Experiment
2.2.4. Soil Physical and Chemical Properties and Growth and Development of Tobacco Plants
2.2.5. Characterization
2.2.6. Density Functional Theory Calculation Method
2.2.7. S rDNA Sequencing
3. Results and Discussion
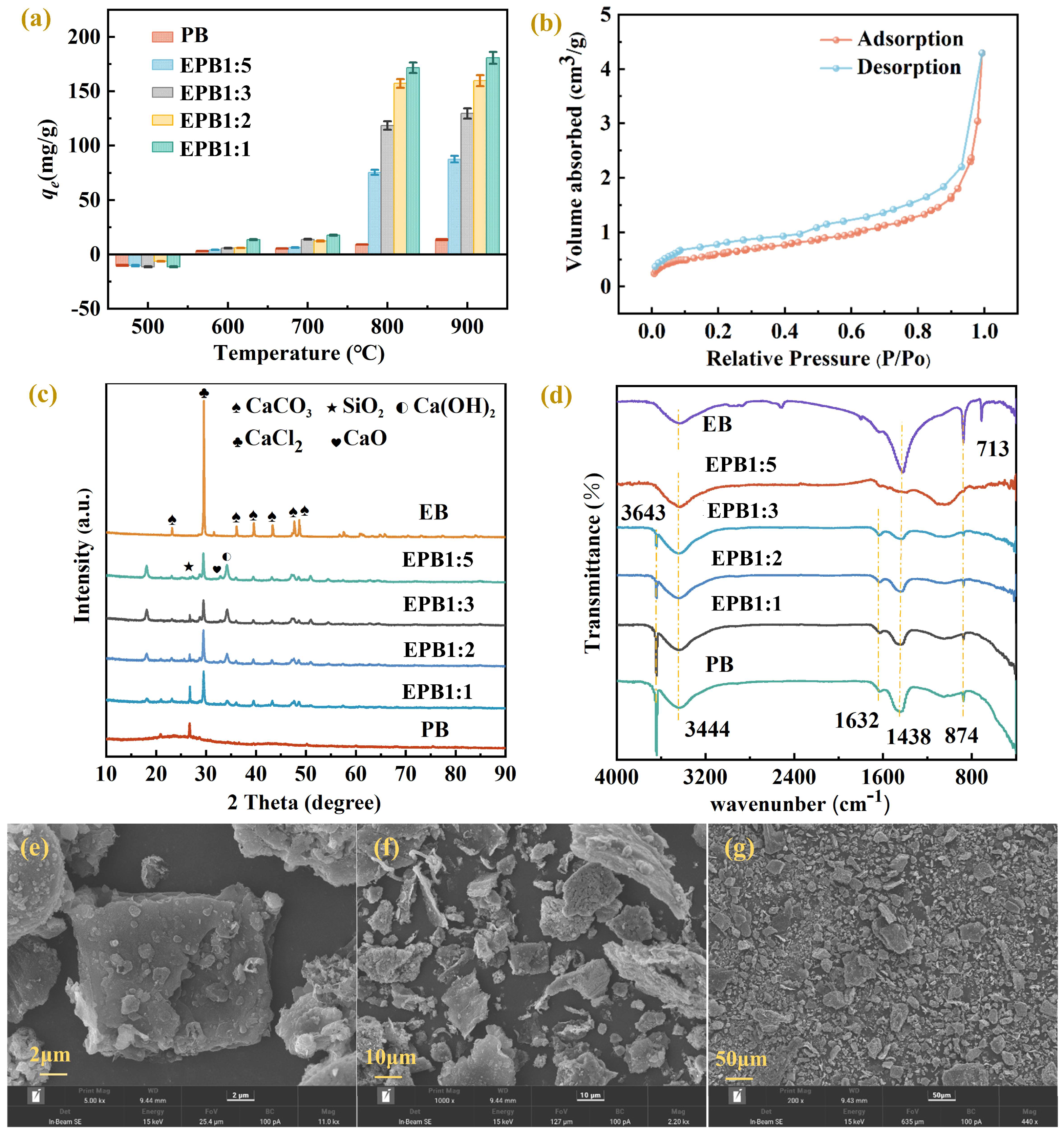
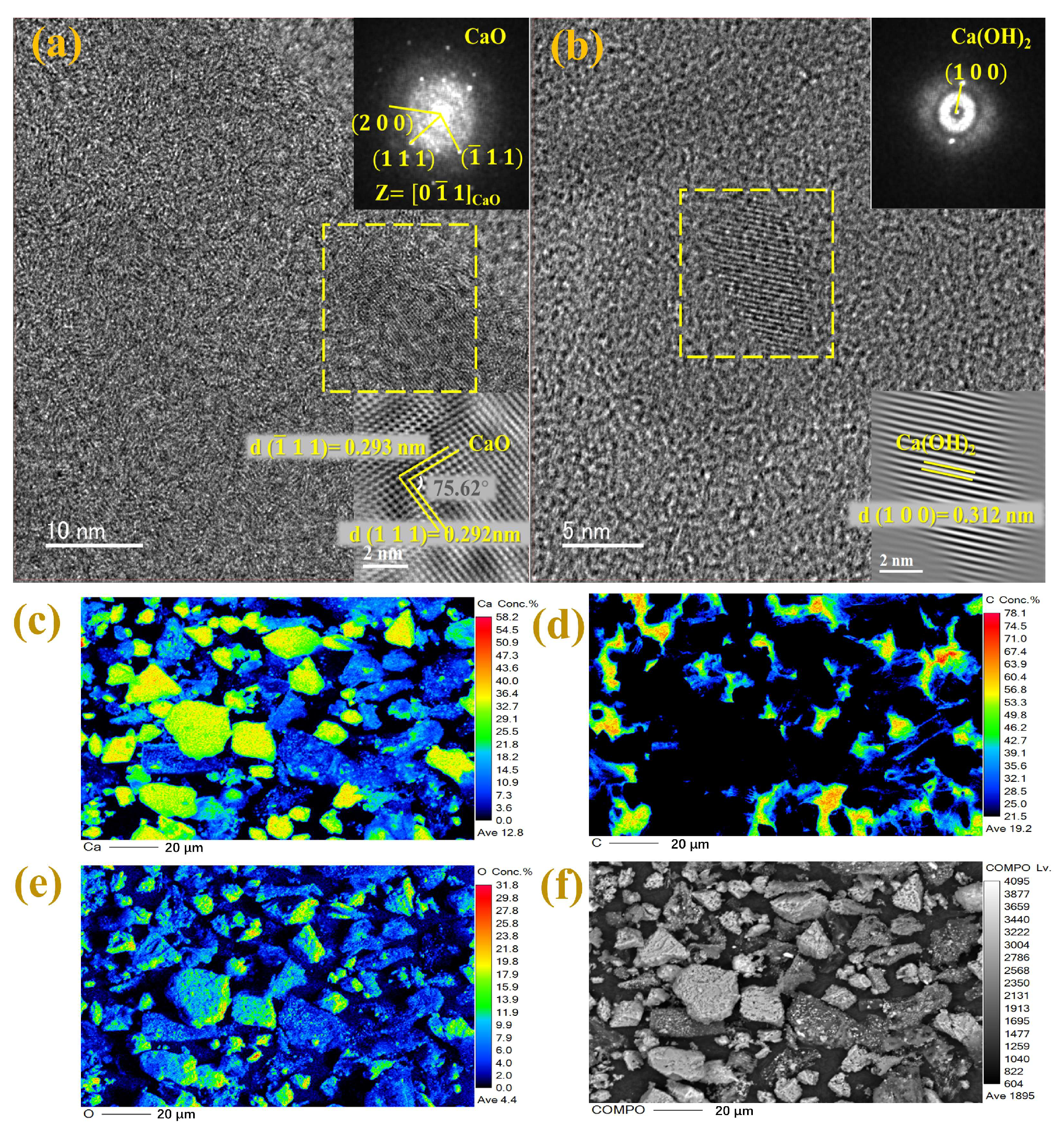
3.1. Preparation Mechanism of EPB
3.2. Phosphate Adsorption Performance of EPB
3.2.1. Adsorption Kinetics
3.2.2. Adsorption Isotherm
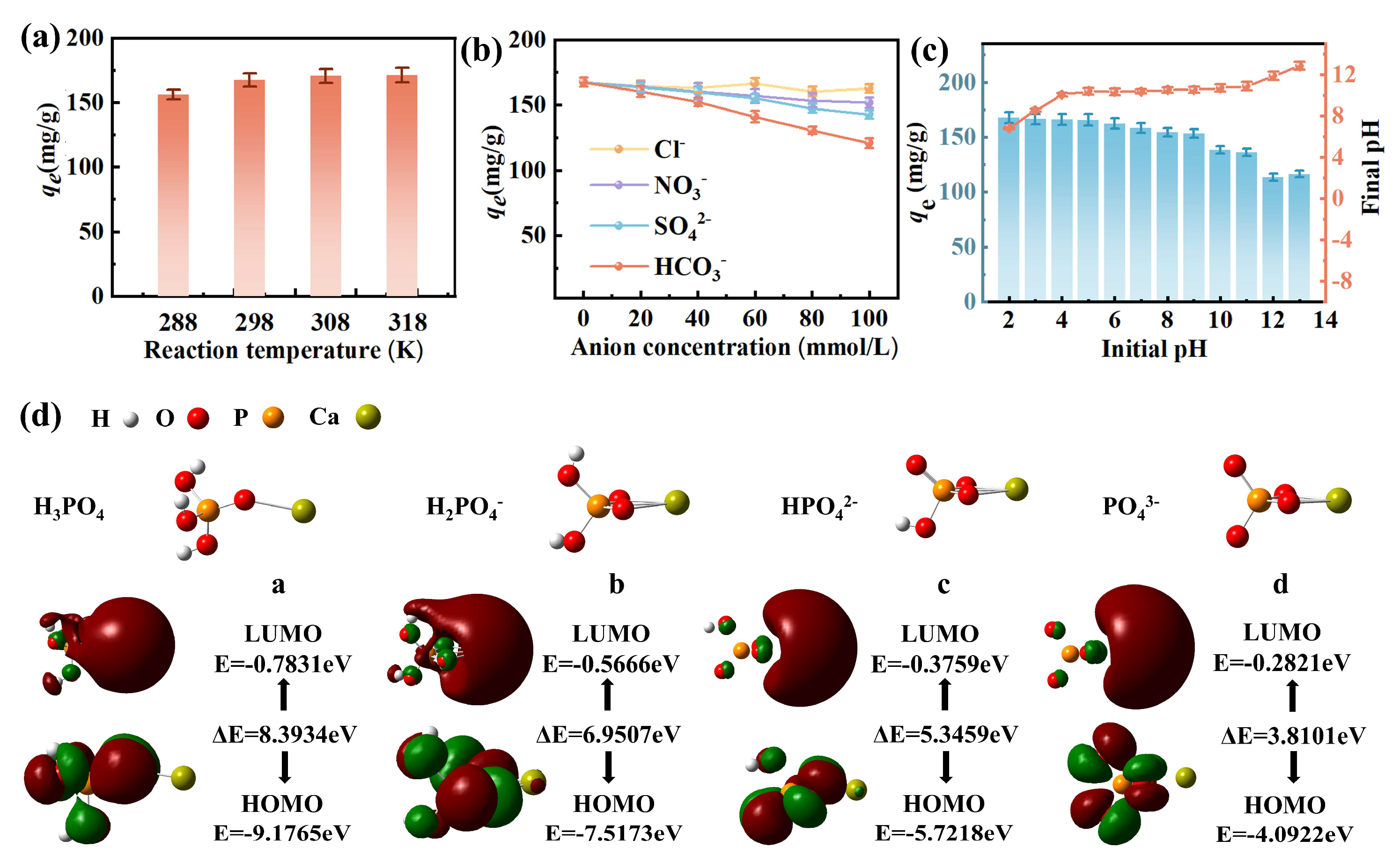
3.2.3. Factors Affecting Adsorption
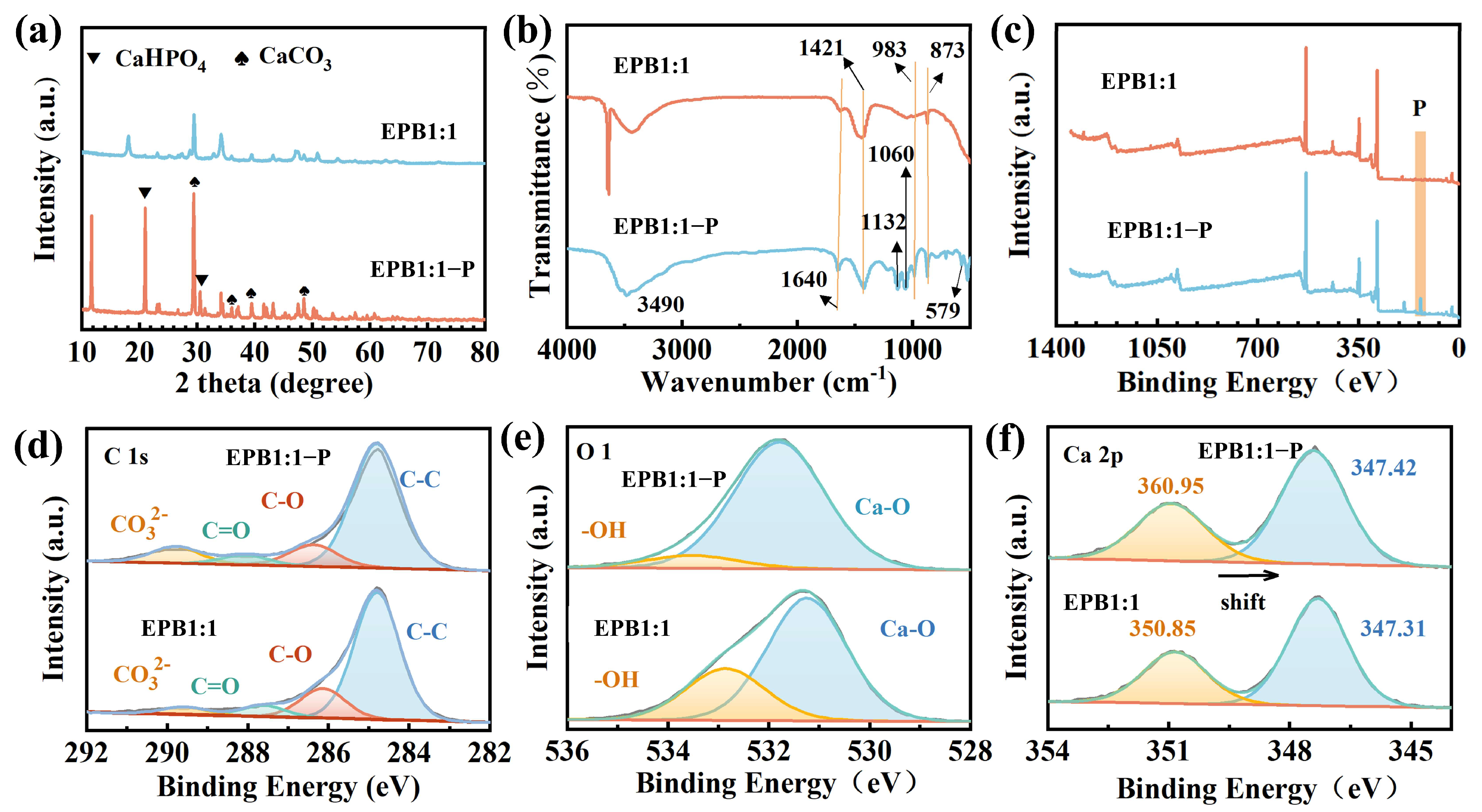
3.3. Adsorption Mechanism
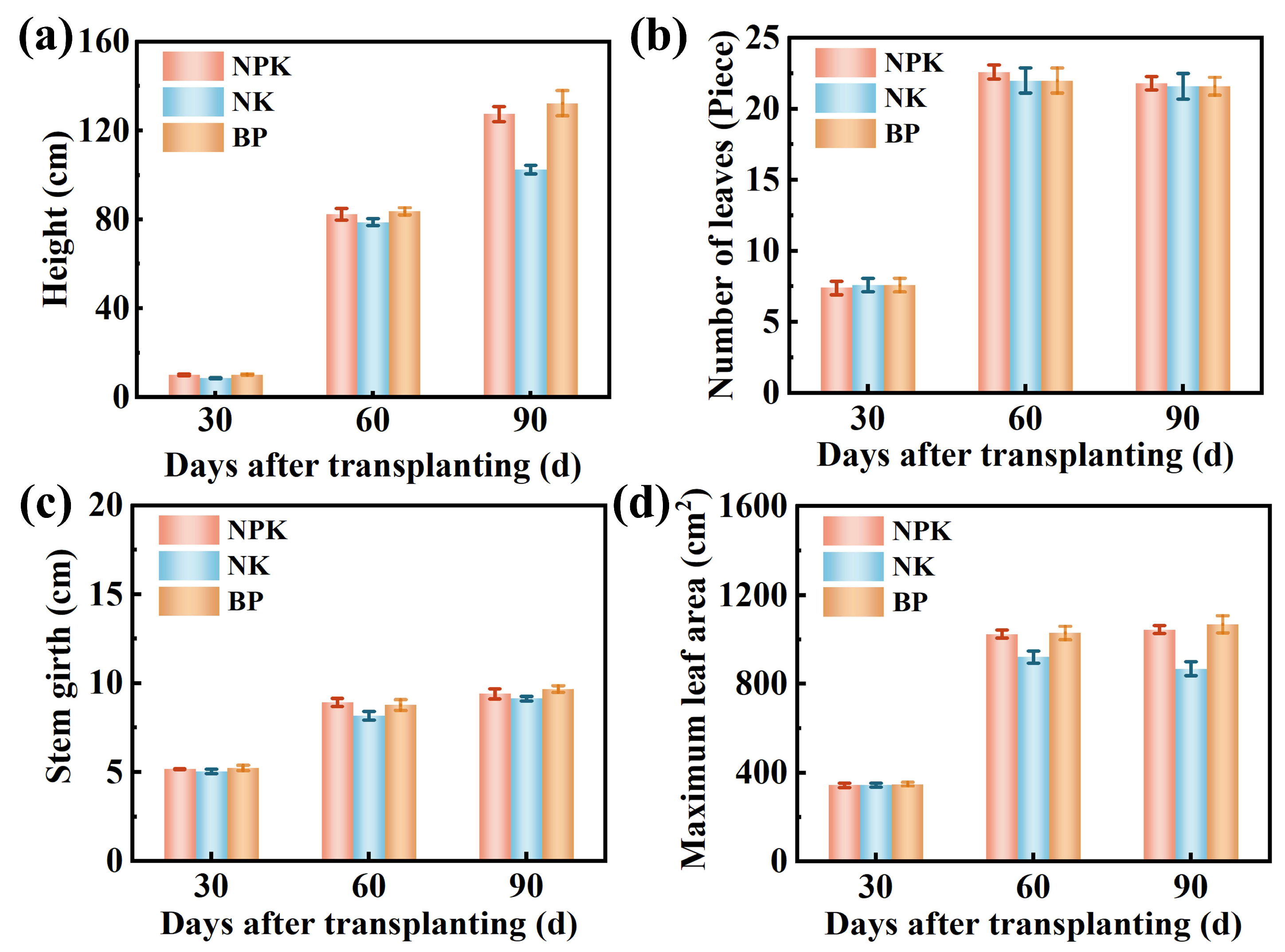
3.4. Effects of Calcium-Based Biochar on Basic Physical and Chemical Properties of Tobacco Soil and Tobacco Growth and Development
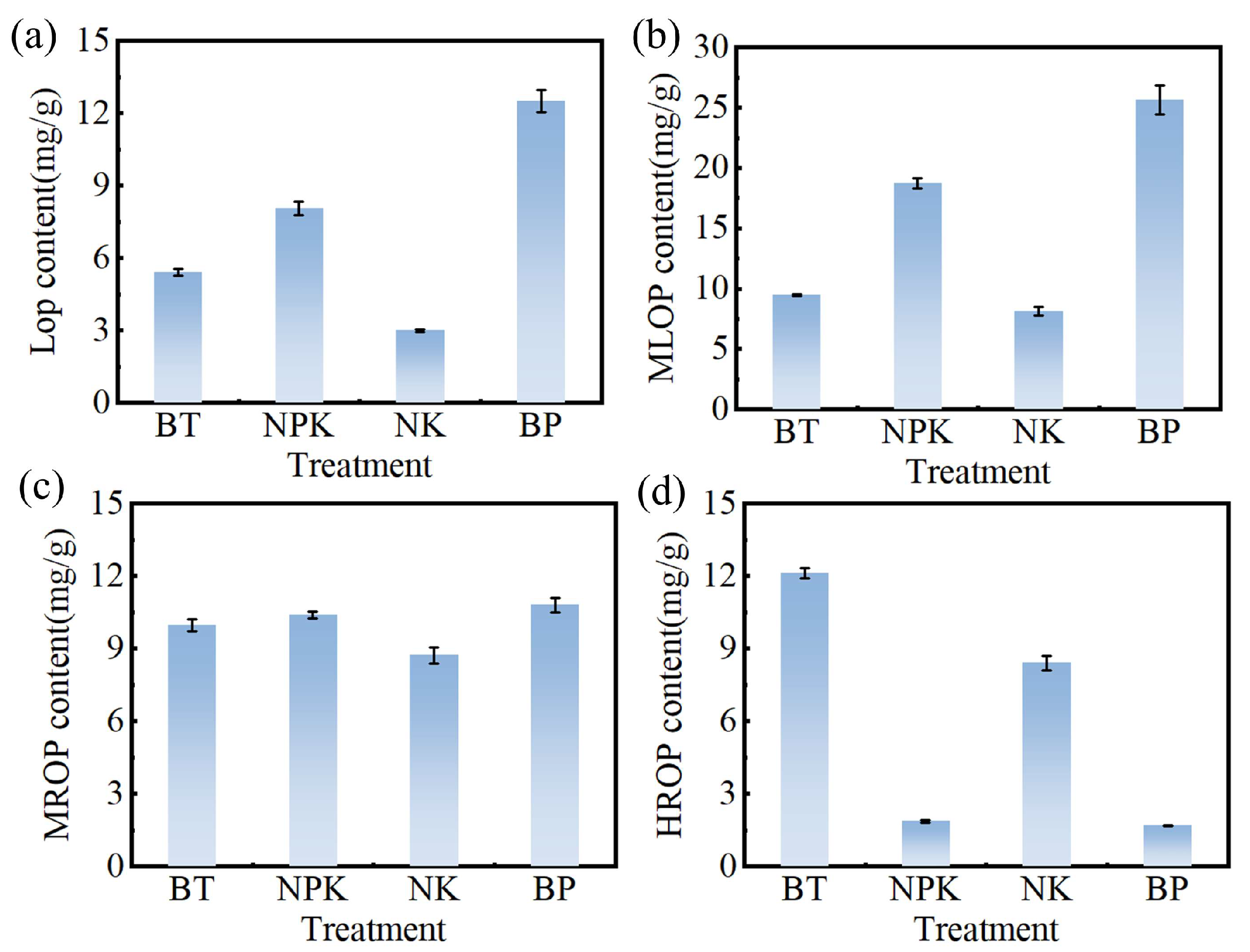
3.5. Effect of EPB1:1-P on Inorganic and Organic Phosphorus in Tobacco Soil
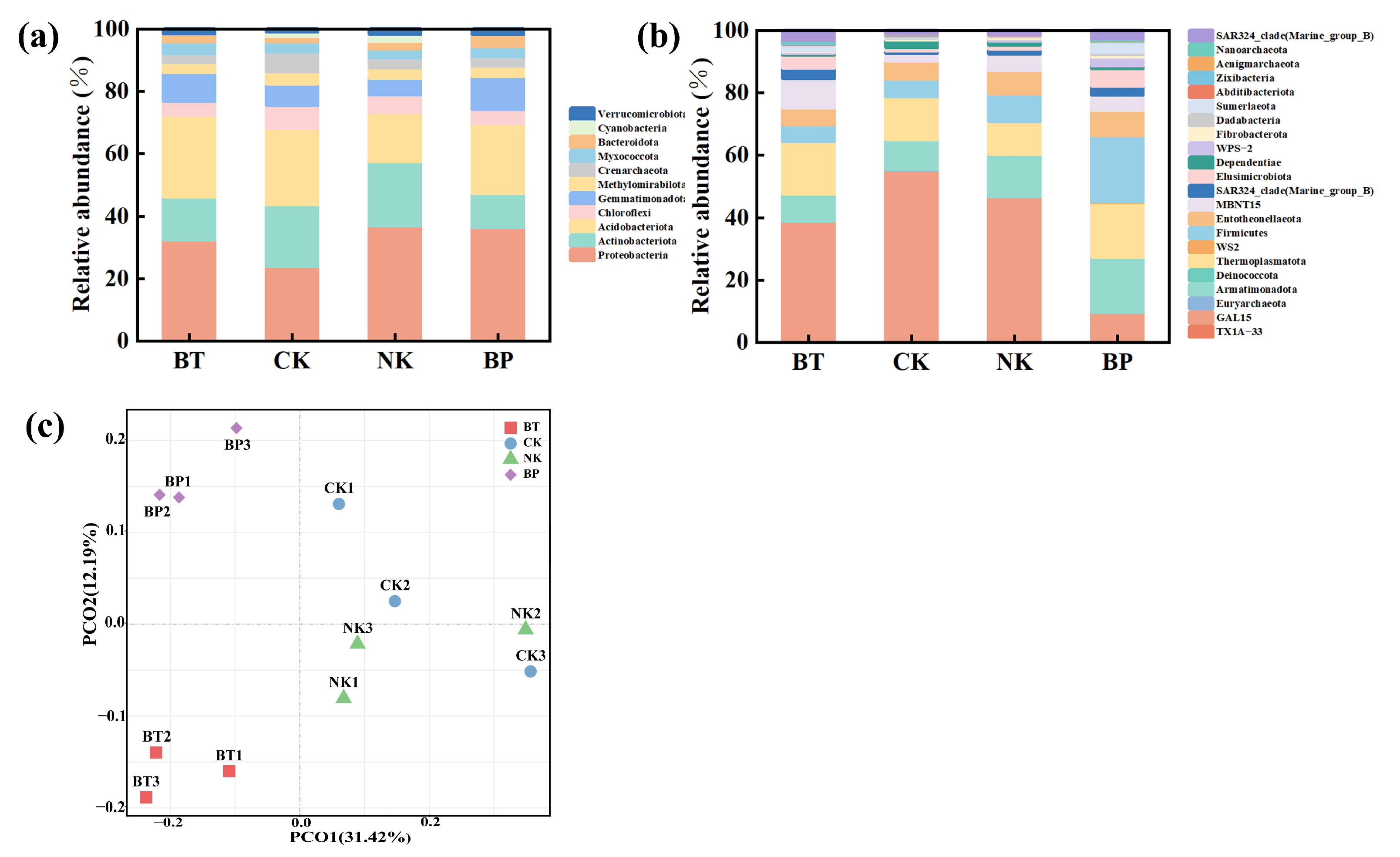
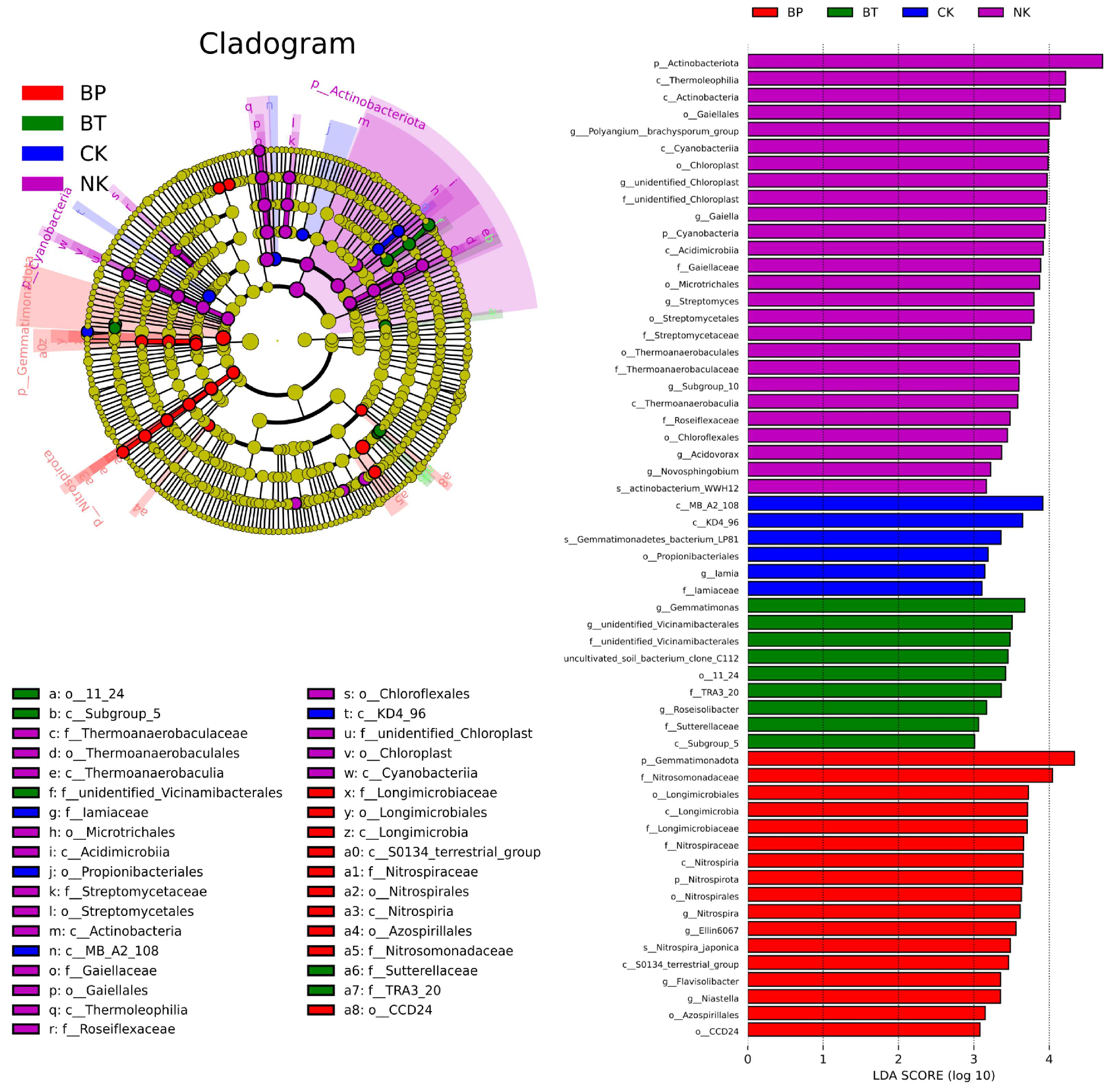
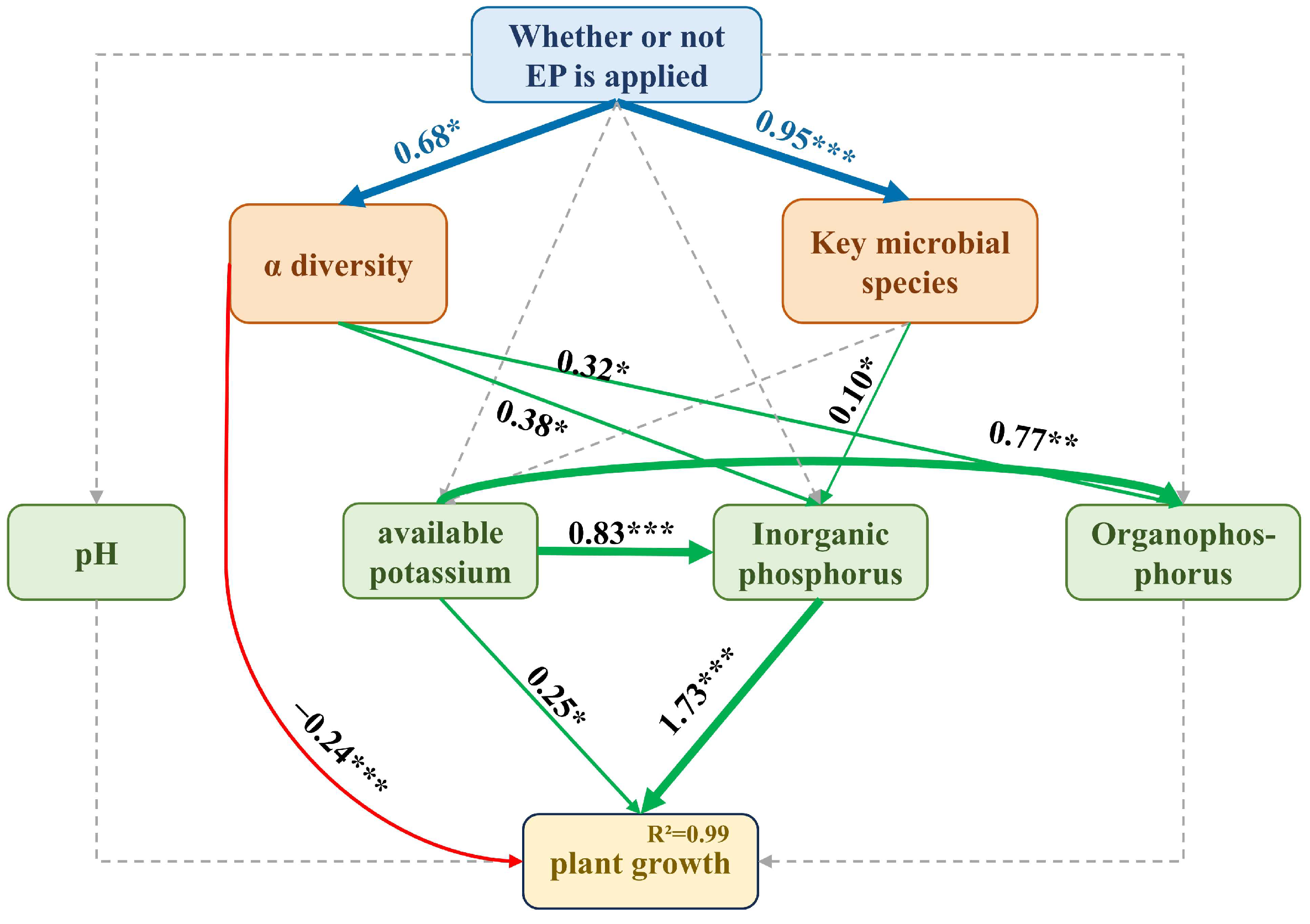
3.6. Effect of EPB1:1-P on Tobacco Rhizosphere Microorganisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elser, J.J.; Call, D.F.; Deaver, J.A.; Duckworth, O.W.; Mayer, B.K.; Mclamore, E.; Rittmann, B.; Mahmood, M.; Westerhoff, P. The phosphorus challenge: Biotechnology approaches for a sustainable phosphorus system. Curr. Opin. Biotechnol. 2024, 90, 103197. [Google Scholar] [CrossRef]
- Zhu, F.; Cakmak, E.K.; Cetecioglu, Z. Phosphorus recovery for circular economy: Application potential of feasible resources and engineering processes in europe. Chem. Eng. J. 2023, 454, 140153. [Google Scholar] [CrossRef]
- Brownlie, W.J.; Alexander, P.; Cordell, D.; Maslin, M.; Metson, G.S.; Sutton, M.A.; Spears, B.M. National phosphorus planning for food and environmental security. Curr. Opin. Biotechnol. 2024, 90, 103226. [Google Scholar] [CrossRef]
- Brownlie, W.J. Phosphorus price spikes: A wake-up call for phosphorus resilience. Front. Sustain. Food Syst. 2023, 7, 8. [Google Scholar] [CrossRef]
- Scholz, R.W.; Wellmer, F.; Mew, M.; Steiner, G. The dynamics of increasing mineral resources and improving resource efficiency: Prospects for mid- and long-term security of phosphorus supply. Resour. Conserv. Recycl. 2025, 213, 107993. [Google Scholar] [CrossRef]
- Diamond, M.L.; de Wit, C.A.; Molander, S.; Scheringer, M.; Backhaus, T.; Lohmann, R.; Arvidsson, R.; Bergman, A.; Hauschild, M.; Holoubek, I.; et al. Exploring the planetary boundary for chemical pollution. Environ. Int. 2015, 78, 8. [Google Scholar] [CrossRef]
- Zheng, Y.; Wan, Y.; Zhang, Y.; Huang, J.; Yang, Y.; Tsang, D.C.W.; Wang, H.; Chen, H.; Gao, B. Recovery of phosphorus from wastewater: A review based on current phosphorous removal technologies. Crit. Rev. Environ. Sci. Technol. 2023, 53, 1148–1172. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, M.; Lv, Q.; Xue, J.; Yang, J.; Han, X. Effective co-treatment of synthetic acid mine drainage and domestic sewage using multi-unit passive treatment system supplemented with silage fermentation broth as carbon source. J. Environ. Manag. 2022, 310, 114803. [Google Scholar] [CrossRef]
- Xie, F.; Zhou, J.; Sun, S.; Li, H.; Xu, C.; Liu, H.; Yang, Z.; Liu, B.; Zhang, H.; Li, S. Differential adsorption and degradation of organic phosphorus by different interlayer cationic clay minerals in water-sediment system. J. Environ. Chem. Eng. 2025, 13, 118096. [Google Scholar] [CrossRef]
- Moulessehoul, A.; Harrache, D.; Gallart-Mateu, D.; de la Guardia, M.; Kameche, M. Phosphorus removal and recovery from water and wastewater by the struvite crystallization. Desalination Water Treat. 2024, 320, 100902. [Google Scholar] [CrossRef]
- Stocker, K.; Ellersdorfer, M. Simultaneous nutrient removal and selective recovery of nitrogen and phosphorus from municipal wastewater: A novel combined ion exchange–surface precipitation process from proof of concept to pilot scale. J. Environ. Chem. Eng. 2025, 13, 117408. [Google Scholar] [CrossRef]
- Zhou, M.; Han, Y.; Zhuo, Y.; Pu, B.; Li, L.; Liu, Y.; Peng, D. Bioinduced phosphorus precipitation in granular sludge undergoing denitrifying biological phosphorus removal: Phosphorus recovery from sewage as hydroxyapatite. Water Res. 2025, 281, 123590. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Zhao, S.; Xiu, X.; Yang, Q. A bioelectrochemical strategy for improving nitrogen and phosphorus removal and reversing blockage of constructed wetland. J. Environ. Chem. Eng. 2025, 13, 117517. [Google Scholar] [CrossRef]
- Zou, S.; He, Z. Electrodialysis recovery of reverse-fluxed fertilizer draw solute during forward osmosis water treatment. Chem. Eng. J. 2017, 330, 550–558. [Google Scholar] [CrossRef]
- Li, X.; Shen, S.; Xu, Y.; Guo, T.; Dai, H.; Lu, X. Application of membrane separation processes in phosphorus recovery: A review. Sci. Total Environ. 2021, 767, 144346. [Google Scholar] [CrossRef]
- Lin, H.; Wang, Y.; Dong, Y. A review of methods, influencing factors and mechanisms for phosphorus recovery from sewage and sludge from municipal wastewater treatment plants. J. Environ. Chem. Eng. 2024, 12, 111657. [Google Scholar] [CrossRef]
- Li, H.; Ding, K.; Zhang, T.; Yang, J.; Liu, C. Molding of the atp/5a/zif composite for simultaneous removal of nitrogen and phosphorus from wastewater: Investigation of adsorption mechanisms and performance evaluation. J. Water Process Eng. 2025, 72, 107470. [Google Scholar] [CrossRef]
- Huang, R.; Lu, X.; Li, W.; Xiong, J.; Yang, J. Progress on the adsorption characteristics of nzvi and other iron-modified biochar for phosphate adsorption in water bodies. Circ. Econ. 2024, 3, 100112. [Google Scholar] [CrossRef]
- Gotore, O.; Masere, T.P.; Muronda, M.T. The immobilization and adsorption mechanisms of agro-waste based biochar: A review on the effectiveness of pyrolytic temperatures on heavy metal removal. Environ. Chem. Ecotoxicol. 2024, 6, 92–103. [Google Scholar] [CrossRef]
- Nan, H.; Luo, D.; Zhang, X.; Wang, J.; Wang, C. Ca/la layered double hydroxide functionalized biochar: Preparation, characterization, phosphate adsorption mechanism, and post-evaluation of phosphorus availability. Mater. Today Commun. 2025, 48, 113296. [Google Scholar] [CrossRef]
- Zhang, Z.; Feng, C.; Wang, M.; Tang, K.H.D.; Li, R.; Qu, G.; Pan, J. Integrated phosphorus removal and recovery using mgo2-magnetic biochar: A circular approach combining fenton chemistry and soil fertility enhancement. J. Environ. Chem. Eng. 2025, 13, 118528. [Google Scholar] [CrossRef]
- Hui, K.; He, R.; Tian, Q.; Zhou, X.; Hou, L.; Zhang, X.; Jiang, Y.; Yao, H. Iron-based biochar materials for phosphorus recovery from agricultural runoff: Mechanism and potential application as a slow-release fertilizer. Sep. Purif. Technol. 2024, 347, 127597. [Google Scholar] [CrossRef]
- Luo, D.; Nan, H.; Zhang, Y.; Sher, F.; Wang, C. Phosphorus recovery from wastewater by ca-al layered double hydroxide/biochar as potential agricultural phosphorus for closed-loop phosphorus recycling. Process Saf. Environ. Prot. 2025, 194, 1538–1548. [Google Scholar]
- Dorozhkin, S.V. Calcium orthophosphates (capo4): Occurrence and properties. Prog. Biomater. 2016, 5, 9–70. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yue, Y. Phosphate adsorption from phosphorus-polluted wastewater by peanut hull-derived biochar functionalized with eggshell-based calcium chloride: Preparation, adsorption performance and mechanism. Desalination Water Treat. 2024, 320, 100880. [Google Scholar] [CrossRef]
- Li, J.; Cao, L.; Li, B.; Huang, H.; Yu, W.; Sun, C.; Long, K.; Young, B. Utilization of activated sludge and shell wastes for the preparation of ca-loaded biochar for phosphate removal and recovery. J. Clean. Prod. 2023, 382, 135395. [Google Scholar] [CrossRef]
- Quisperima, A.; Pérez, S.; Flórez, E.; Acelas, N. Valorization of potato peels and eggshells wastes: Ca-biocomposite to remove and recover phosphorus from domestic wastewater. Bioresour. Technol. 2022, 343, 126106. [Google Scholar] [CrossRef]
- Zhou, Q.; Luo, J.; Wu, H.; Zhu, G.; Feng, J.; Dong, Z.; Sun, S. Fe-modified porous biochar for efficient adsorption of low-concentration phosphate from municipal wastewater: Performance, mechanism, and fixed-bed behavior prediction. Sep. Purif. Technol. 2025, 379, 134903. [Google Scholar] [CrossRef]
- Bao, S.D. Soil and Agricultural Chemistry Analysis; China Agricultural Press: Beijing, China, 2000. [Google Scholar]
- Bowman, R.A.; Cole, C.V. An exploratory method for fractionation of organic phosphorus from grassland soils. Soil. Sci. 1978, 125, 95–101. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, K.; He, X. Characteristics and mechanism of adsorption of tartaric acid by carbide slag ascertained and applied to prepare a binder. J. Clean. Prod. 2022, 337, 130477. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Mills, D.A. Next-generation approaches to the microbial ecology of food fermentations. BMB Rep. 2012, 45, 377. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Muscle: Multiple sequence alignment with improved accuracy and speed. In Proceedings of the 2004 IEEE Computational Systems Bioinformatics Conference 2004, CSB 2004, Stanford, CA, USA, 19 August 2004. [Google Scholar]
- Sun, C.; Cao, H.; Huang, C.; Wang, P.; Yin, J.; Liu, H.; Tian, H.; Xu, H.; Zhu, J.; Liu, Z. Eggshell based biochar for highly efficient adsorption and recovery of phosphorus from aqueous solution: Kinetics, mechanism and potential as phosphorus fertilizer. Bioresour. Technol. 2022, 362, 127851. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Wu, X.; Syed-Hassan, S.S.A.; Zhang, S.; Mood, S.H.; Milan, Y.J.; Garcia-Perez, M. Characteristics and mechanisms of phosphorous adsorption by rape straw-derived biochar functionalized with calcium from eggshell. Bioresour. Technol. 2020, 318, 124063. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Wei, H.; Huang, Y.; Song, J.; Gao, M.; Zhang, Z.; Teng, R.; Jing, S. Phosphorus adsorption by calcium chloride-modified buckwheat hulls biochar and the potential application as a fertilizer. J. Clean. Prod. 2024, 444, 141233. [Google Scholar] [CrossRef]
- Rahmani-Sani, A.; Singh, P.; Raizada, P.; Lima, E.C.; Anastopoulos, I.; Giannakoudakis, D.A.; Sivamani, S.; Dontsova, T.A.; Hosseini-Bandegharaei, A. Use of chicken feather and eggshell to synthesize a novel magnetized activated carbon for sorption of heavy metal ions. Bioresour. Technol. 2020, 297, 122452. [Google Scholar] [CrossRef]
- Liu, X.; Shen, F.; Qi, X. Adsorption recovery of phosphate from aqueous solution by cao-biochar composites prepared from eggshell and rice straw. Sci. Total Environ. 2019, 666, 694–702. [Google Scholar] [CrossRef]
- Florez, S.; Bernacki, M. Efficient and accurate simulation of the smith–zener pinning mechanism during grain growth using a front-tracking numerical framework. Comput. Mater. Sci. 2025, 256, 113958. [Google Scholar] [CrossRef]
- Zeng, J.; Chen, D.; Zhu, J.; Long, C.; Qing, T.; Feng, B.; Zhang, P. Phosphate recovery using activated sludge cyanophycin: Adsorption mechanism and utilization as nitrogen-phosphorus fertilizer. Chem. Eng. J. 2023, 476, 146607. [Google Scholar] [CrossRef]
- Cheng, N.; Wang, B.; Feng, Q.; Zhang, X.; Chen, M. Co-adsorption performance and mechanism of nitrogen and phosphorus onto eupatorium adenophorum biochar in water. Bioresour. Technol. 2021, 340, 125696. [Google Scholar] [CrossRef]
- Pan, F.; Wei, H.; Huang, Y.; Guo, W.; Song, J.; Zhang, Z.; Teng, R.; Gao, M.; Jing, S.; Shi, B. Biochar-enabled phosphorus recovery from aqueous: Advances, applications and challenges. Environ. Res. 2025, 285, 122329. [Google Scholar] [CrossRef]
- Rizwan, M.; Lin, Q.; Chen, X.; Li, Y.; Li, G.; Zhao, X.; Tian, Y. Synthesis, characterization and application of magnetic and acid modified biochars following alkaline pretreatment of rice and cotton straws. Sci. Total Environ. 2021, 714, 136532. [Google Scholar] [CrossRef]
- Korybska-Sadło, I.; Sitarz, M.; Król, M.; Gunia, P. Vibrational spectroscopic characterization of the magnesium borate-phosphate mineral lüneburgite. Spectrosc. Lett. 2016, 49, 606–612. [Google Scholar] [CrossRef]
- Zhuo, S.-N.; Dai, T.-C.; Ren, H.-Y.; Liu, B.-F. Simultaneous adsorption of phosphate and tetracycline by calcium modified corn stover biochar: Performance and mechanism. Bioresour. Technol. Biomass Bioenergy Biowastes Convers. Technol. Biotransformations Prod. Technol. 2022, 359, 127477. [Google Scholar] [CrossRef]
- López-Arredondo, D.L.; Leyva-González, M.A.; González-Morales, S.I.; López-Bucio, J.; Herrera-Estrella, L. Phosphate nutrition: Improving low-phosphate tolerance in crops. Annu. Rev. Plant Biol. 2014, 65, 95–123. [Google Scholar] [CrossRef]
- Liu, C.; Chen, H.; Li, B.; Wu, Q.; Zhou, W.; Chen, D.; Ao, J. The effects and underlying mechanisms of modified biochar combined with nitrification inhibitors on nitrous oxide mitigation in acidic soils. Ecotoxicol. Environ. Saf. 2025, 303, 118876. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, T.A.; Gomes Filho, R.R.; Wisniewski, A.; Mašek, O. Biochar physical degradation: Long-term effects as soil amendments. Biomass Bioenergy 2025, 203, 108284. [Google Scholar] [CrossRef]
- Hou, J.; Yi, G.; Hao, Y.; Li, L.; Shen, L.; Zhang, Q. The effect of combined application of biochar and phosphate fertilizers on phosphorus transformation in saline-alkali soil and its microbiological mechanism. Sci. Total Environ. 2024, 951, 175610. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Deluca, T.H. Wood biochar impacts soil phosphorus dynamics and microbial communities in organically-managed croplands. Soil. Biol. Biochem. 2018, 126, 144–150. [Google Scholar] [CrossRef]
- Wang, C.; Pollet, S.; Howell, K.; Cornelis, J. Placing cropping systems under suboptimal phosphorus conditions promotes plant nutrient acquisition and microbial carbon supply without compromising biomass. Soil. Biol. Biochem. 2025, 204, 109753. [Google Scholar] [CrossRef]
- Zhu, J.; Qu, B.; Li, M. Phosphorus mobilization in the yeyahu wetland: Phosphatase enzyme activities and organic phosphorus fractions in the rhizosphere soils. Int. Biodeterior. Biodegrad. 2017, 124, 304–313. [Google Scholar] [CrossRef]
- Lu, H.; Yang, Y.; Huang, K.; Huang, G.; Hu, S.; Pan, D.; Liu, T.; Li, X. Transformation kinetics of exogenous lead in an acidic soil during anoxic-oxic alteration: Important roles of phosphorus and organic matter. Environ. Pollut. 2023, 335, 122271. [Google Scholar] [CrossRef]
- Lauricella, D.; Butterly, C.R.; Clark, G.J.; Sale, P.W.G.; Li, G.; Tang, C. Effectiveness of innovative organic amendments in acid soils depends on their ability to supply p and alleviate al and mn toxicity in plants. J. Soils Sediments 2020, 20, 3951–3962. [Google Scholar] [CrossRef]
- Chrysochoou, M.; Dermatas, D.; Grubb, D.G. Phosphate application to firing range soils for pb immobilization: The unclear role of phosphate. J. Hazard. Mater. 2007, 144, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Dermatas, D.; Chrysochoou, M.; Grubb, D.G.; Xu, X. Phosphate treatment of firing range soils: Lead fixation or phosphorus release? J. Environ. Qual. 2008, 37, 47. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Liang, X.; He, M.; Liu, Y.; Tian, G.; Shi, J. Manure biochar influence upon soil properties, phosphorus distribution and phosphatase activities: A microcosm incubation study. Chemosphere 2016, 142, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Lin, M.; Du, C.; Hao, Y.; Zhang, Y.; Wang, Z. The use of manure shifts the response of α-diversity and network while not β-diversity of soil microbes to altered irrigation regimes. Appl. Soil. Ecol. 2022, 174, 104423. [Google Scholar] [CrossRef]
- Nan, Q.; Chi, W.; Yang, X.; Li, S.; Qin, Y.; Wang, L.; Wu, W. Long-term impacts of straw and biochar applications on microbial diversity and soil functions in paddy soils. Environ. Pollut. 2025, 384, 126943. [Google Scholar] [CrossRef]
- Wang, W.; Liu, A.; Fu, W.; Peng, D.; Wang, G.; Ji, J.; Jin, C.; Guan, C. Tobacco-associated with methylophilus sp. Fp-6 enhances phytoremediation of benzophenone-3 through regulating soil microbial community, increasing photosynthetic capacity and maintaining redox homeostasis of plant. J. Hazard. Mater. 2022, 431, 128588. [Google Scholar] [CrossRef]
- Yang, Z.; Gu, K.; Wu, G.; Su, J.; Jiang, Y.; Hu, B.; Wang, W.; Zhao, M.; Ren, K.; Chen, Y. Mechanisms of tobacco yield enhancement in winter crop-tobacco rotations: Enhancing soil aggregate stability, organic carbon content, and microbial diversity. Appl. Soil. Ecol. 2025, 214, 106352. [Google Scholar] [CrossRef]
- Shin, J.; Rho, H.; Cho, Y.; Son, C.; Kwak, J.; Kim, S.; Ki, S.; Kim, H.J.; Lee, Y.G.; Park, Y.; et al. Functionalization of coffee waste biochars with Ca/Fe layered double hydroxides for enhanced removal and selectivity of phosphate ions: Mechanisms and reusability. J. Water Process Eng. 2025, 76, 108123. [Google Scholar] [CrossRef]
- Chen, M.; Liu, Y.; Pan, J.; Jiang, Y.; Zou, X.; Wang, Y. Low-cost Ca/Mg co-modified biochar for effective phosphorus recovery: Adsorption mechanisms, resourceful utilization, and life cycle assessment. Chem. Eng. J. 2024, 502, 157993. [Google Scholar] [CrossRef]
- Cheng, F.; Wang, Y.; Fan, Y.; Huang, D.; Pan, J.; Li, W. Optimized Ca-Al-La modified biochar with rapid and efficient phosphate removal performance and excellent pH stability. Arab. J. Chem. 2023, 16, 104880. [Google Scholar] [CrossRef]
- Yang, Z.; Zou, Z.; Akhtar, M.A.; Niu, W.; Ren, L.; Zhang, S.; Liu, N.; Cao, H. Synergistic effects of N-containing heterocyclic and Ca ligand structures on the phosphorus adsorption of N/Ca co-doped biochar. J. Clean. Prod. 2024, 485, 144392. [Google Scholar] [CrossRef]
- Wang, Q.; Xiao, Y.; Qi, J.; Wei, H.; Zhao, J.; Lei, X.; Yang, D.; Huang, Y.; Gao, P. A novel strategy for preparing porous Fe/Ca-loaded biochar transformed from municipal sludge towards phosphate removal. J. Water Process Eng. 2024, 66, 106109. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, Y.; Huang, Y.; Song, L.; Chen, H.; Zhu, S.; Tang, C. Enhanced adsorption of phosphate on orange peel-based biochar activated by Ca/Zn composite: Adsorption efficiency and mechanisms. Colloids Surf. A Physicochem. Eng. Asp. 2022, 651, 129728. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, R.; Gao, J.; Han, M.; Qin, S.; Liu, K.; Shu, Y.; Zhang, R.; Shi, C.; Zheng, Y. The role of silica in biomass for calcium-modified biochar: Phosphorus removal mechanism and potential as a phosphate fertilizer application. J. Environ. Sci. 2025, 158, 242–253. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, Q.; Zhang, R.; Zhang, Y.; Wang, F.; He, M.; Guo, X.; Yang, J.; Zhang, X.; Mu, J. Pyrolysis of Ca/Fe-rich antibiotic fermentation residues into biochars for efficient phosphate removal/recovery from wastewater: Turning hazardous waste to phosphorous fertilizer. Sci. Total Environ. 2023, 869, 161732. [Google Scholar] [CrossRef]


| Treatment | Organic Material | Chemical Fertilizer | ||
|---|---|---|---|---|
| NH4NO3 | Ca(H2PO4)2 | K2SO4 | ||
| (kg/hm2) | (kg/hm2) | (kg/hm2) | ||
| NPK | - | 188.57 | 217.52 | 366.51 |
| NK | - | 188.57 | - | 366.51 |
| BP | BP1:1-P 1295 kg/hm2 | 188.57 | - | 366.51 |
| Parameter | Surface Area (m2/g) | Pore Volume (×10−2 cm3/g) | Average Pore Size (nm) | Grain Size |
|---|---|---|---|---|
| EPB1:1 | 2.19 | 0.66 | 12.13 | 5.97 |
| EPB1:2 | 1.85 | 0.47 | 10.08 | 20.97 |
| EPB1:3 | 2.45 | 0.77 | 12.65 | 44.07 |
| EPB1:5 | 87.3 4 | 0.14 | 6.59 | 77.12 |
| BP | 94.30 | 12.07 | 5.12 | - |
| Model | Parameter | |
|---|---|---|
| Pseudo-first-order model | qe (mg·g−1) | 164.13 ± 3.89 |
| k1 (min−1) | 0.038 ± 0.003 | |
| R2 | 0.980 | |
| RMSE | 8.77 | |
| Pseudo-second-order model | qe (mg·g−1) | 179.08 ± 2.43 |
| k2 (g·(mg·min)−1) | 2.95 × 10−4 ± 2.09 × 10−5 | |
| R2 | 0.998 | |
| RMSE | 4.23 | |
| Elovich model | α | 27.12 ± 9.17 |
| β | 0.033 ± 0.003 | |
| R2 | 0.954 | |
| RMSE | 13.21 | |
| Isotherm Model | Parameter | |
|---|---|---|
| Freundlich | KF (mg(1−n)·Ln/g) | 81.45 ± 10.32 |
| 1/n | 6.193 | |
| R2 | 0.790 | |
| Langmuir | KL (L/mg) | 2.37 ± 0.31 |
| RMSE | 6.48 | |
| Qmax (mg/g) | 167.97 ± 6.91 | |
| R2 | 0.97 | |
| RMSE | 2.61 | |
| Temkin | A (L/mg) | 104.11 |
| B (J·g/mg) | 130.88 | |
| R2 | 0.89 | |
| RMSE | 5.06 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, Y.; Yuan, R.; Li, H.; Huang, H. Efficient Recovery of Phosphorus from Wastewater Using Calcium-Based Modified Biochar: Removal Performance, Adsorption Mechanism, and Resource Utilization. Toxics 2025, 13, 808. https://doi.org/10.3390/toxics13100808
Qin Y, Yuan R, Li H, Huang H. Efficient Recovery of Phosphorus from Wastewater Using Calcium-Based Modified Biochar: Removal Performance, Adsorption Mechanism, and Resource Utilization. Toxics. 2025; 13(10):808. https://doi.org/10.3390/toxics13100808
Chicago/Turabian StyleQin, Yihe, Run Yuan, Han Li, and Haiming Huang. 2025. "Efficient Recovery of Phosphorus from Wastewater Using Calcium-Based Modified Biochar: Removal Performance, Adsorption Mechanism, and Resource Utilization" Toxics 13, no. 10: 808. https://doi.org/10.3390/toxics13100808
APA StyleQin, Y., Yuan, R., Li, H., & Huang, H. (2025). Efficient Recovery of Phosphorus from Wastewater Using Calcium-Based Modified Biochar: Removal Performance, Adsorption Mechanism, and Resource Utilization. Toxics, 13(10), 808. https://doi.org/10.3390/toxics13100808






