Non-Targeted PFAS Suspect Screening and Quantification of Drinking Water Samples Collected through Community Engaged Research in North Carolina’s Cape Fear River Basin
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Sample Collection
2.2. Materials
2.3. Sample Preparation
2.4. Quantitative Method
2.5. Quantitative Data Analysis and Quality Controls
2.6. Non-Targeted Data Dependent Suspect Screening Method
2.7. Non-Targeted Data Analysis
3. Results
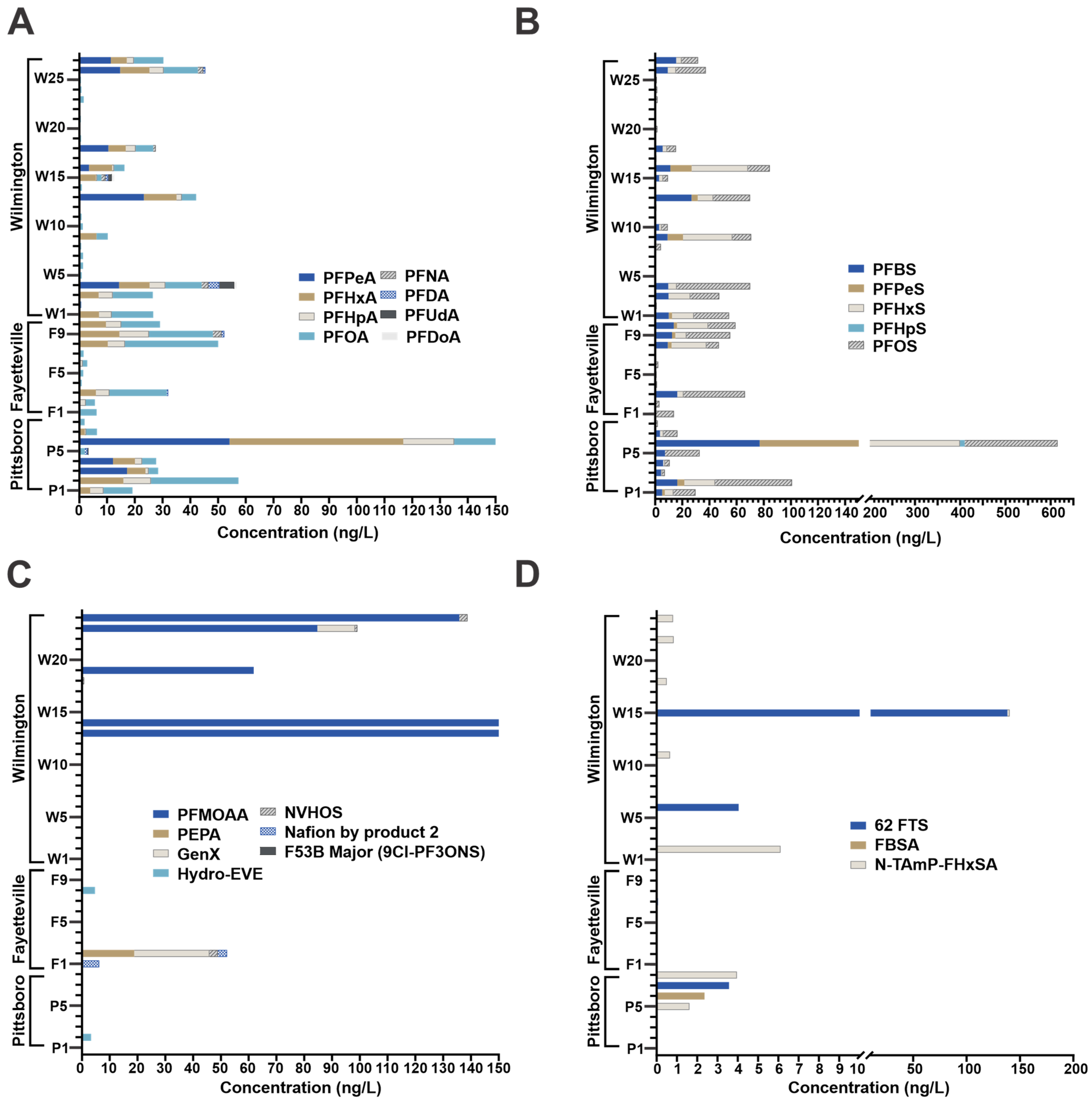
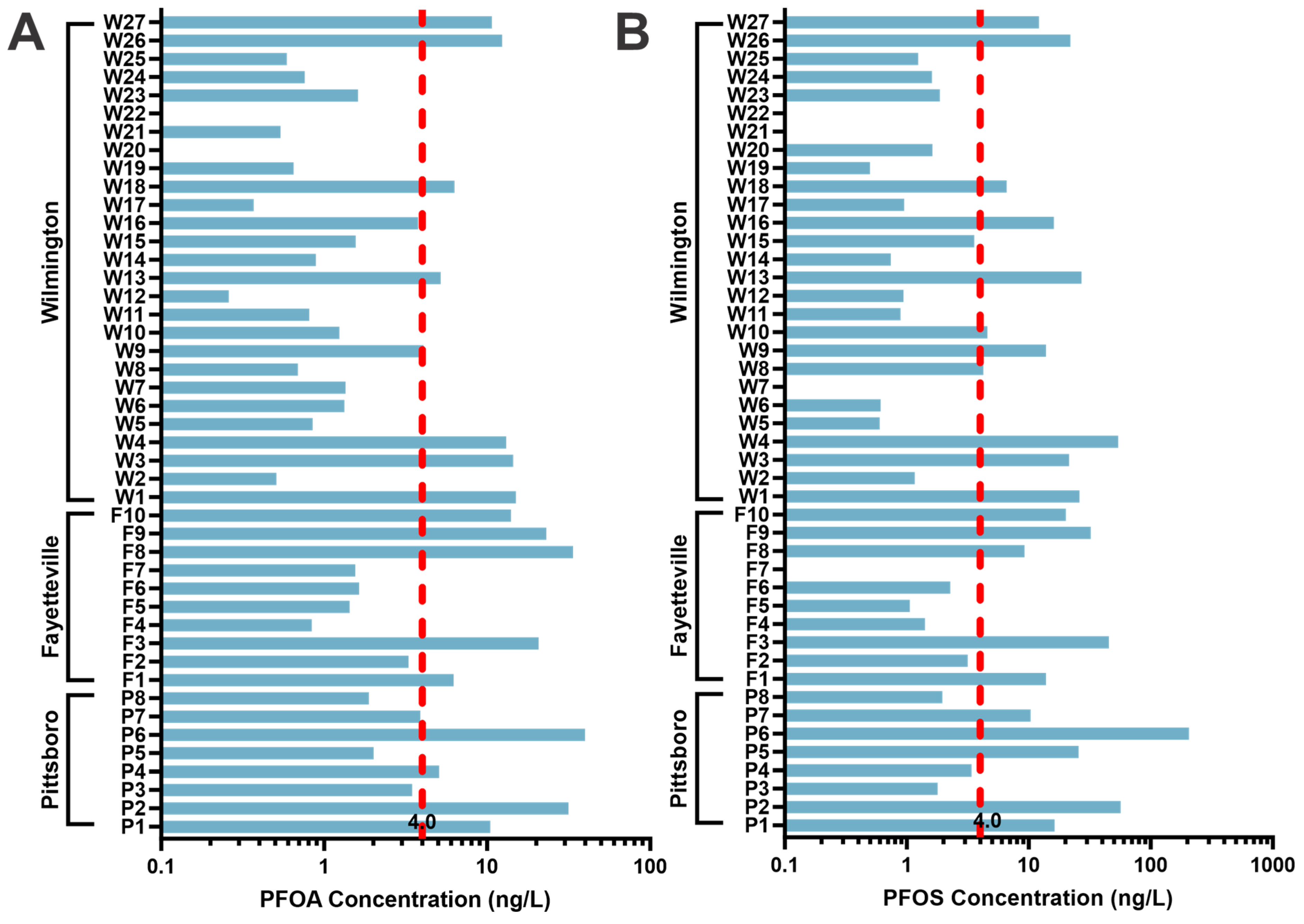
3.1. Quantitative Study
3.2. Data Dependent Suspect Screening Study
4. Discussion
4.1. High Resolution Quantitative Results
4.2. Data-Dependent Suspect Screening Study
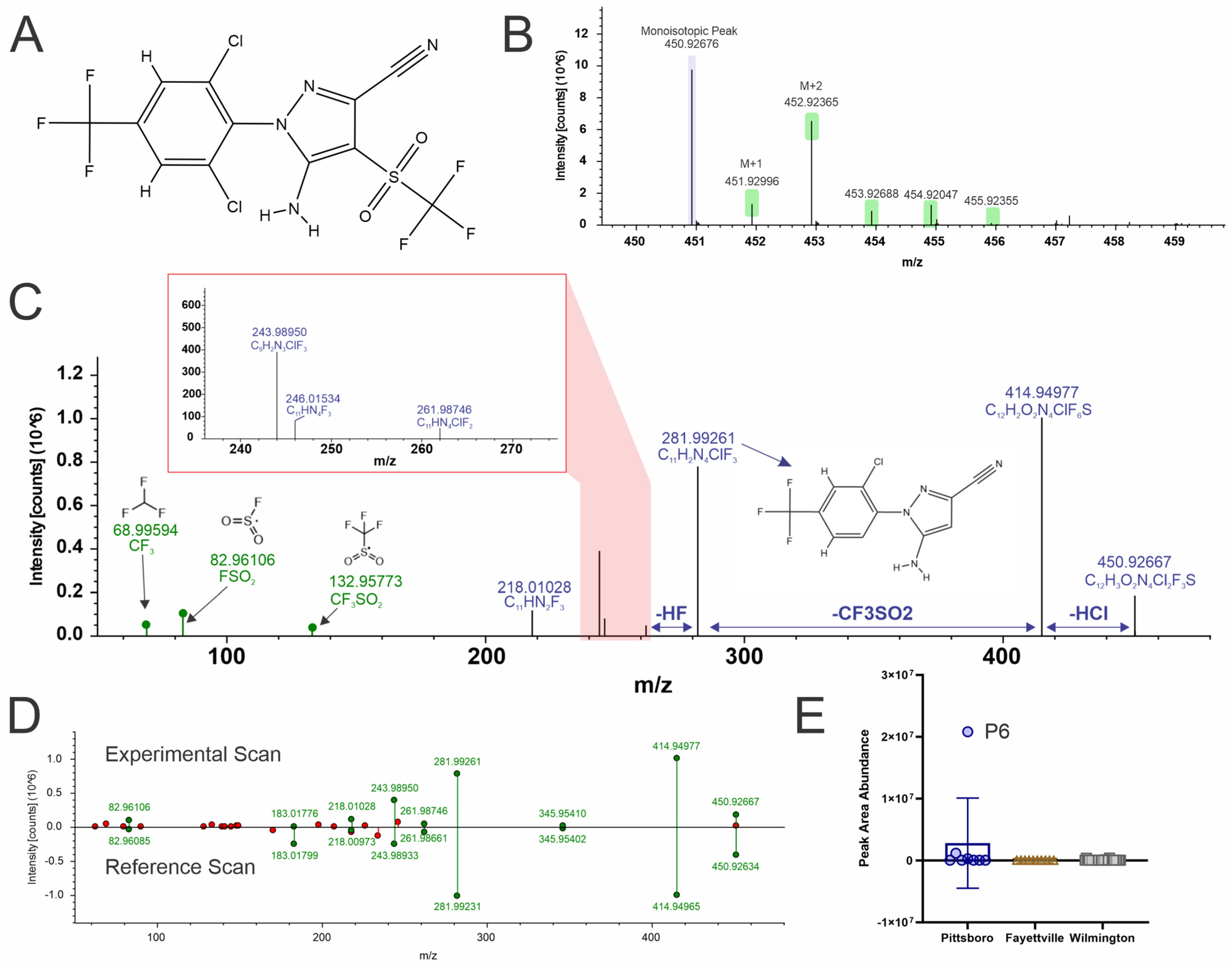
4.3. Fipronil Sulfone (Level 2a)
4.4. Bistriflimide (Level 2a)
4.5. 3-(Tridecafluoroundecyl)catechol (Level 2b)
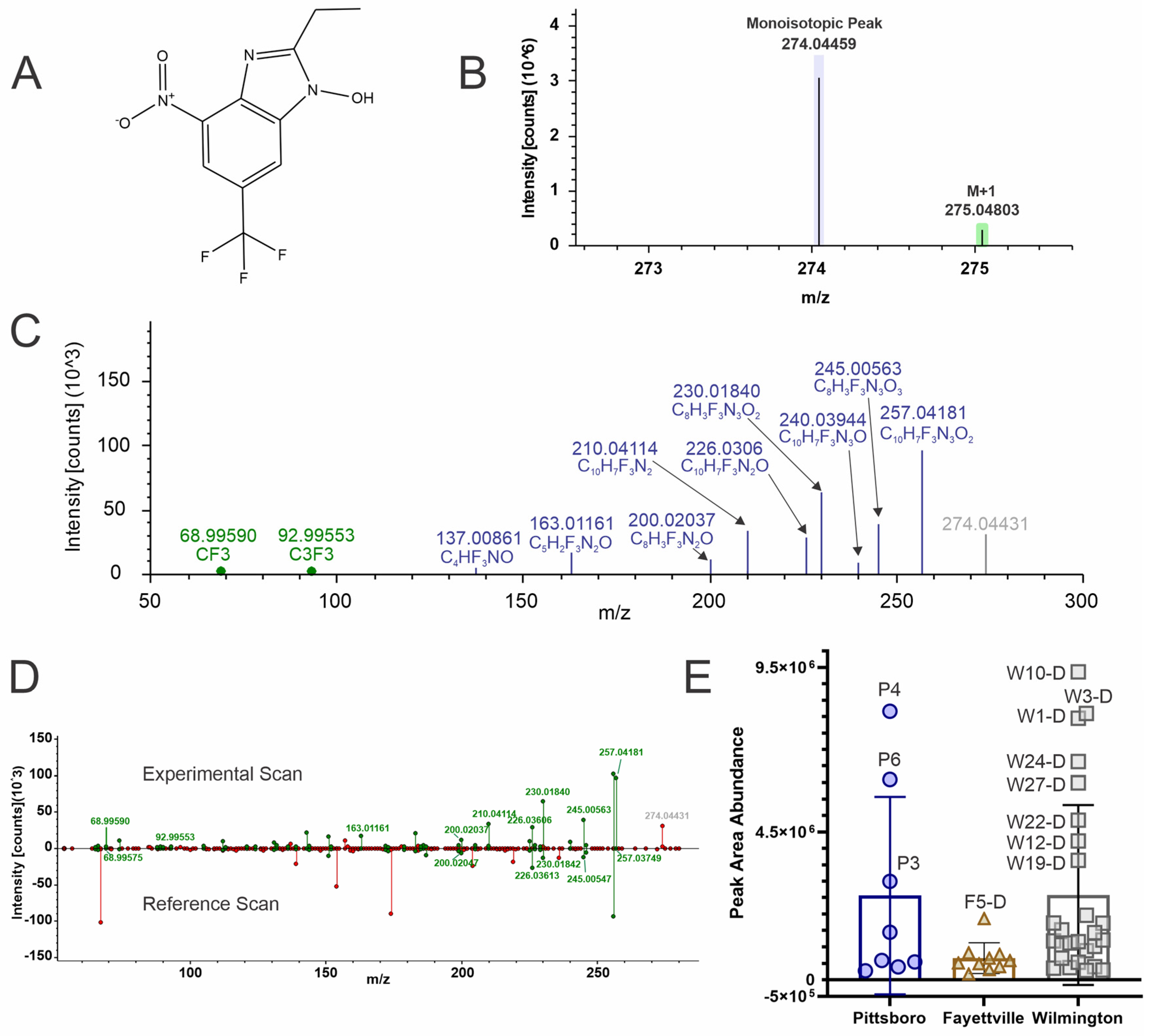
4.6. 2-Ethyl-4-Nitro-6-(Trifluoromethyl)-1H-Benzimidazol-1-ol (Level 2b)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abunada, Z.; Alazaiza, M.Y.D.; Bashir, M.J.K. An Overview of Per- and Polyfluoroalkyl Substances (PFAS) in the Environment: Source, Fate, Risk and Regulations. Water 2020, 12, 3590. [Google Scholar] [CrossRef]
- Giesy, J.P.; Kannan, K. Global distribution of perfluorooctane sulfonate in wildlife. Environ. Sci. Technol. 2001, 35, 1339–1342. [Google Scholar] [CrossRef]
- Kurwadkar, S.; Dane, J.; Kanel, S.R.; Nadagouda, M.N.; Cawdrey, R.W.; Ambade, B.; Struckhoff, G.C.; Wilkin, R. Per- and polyfluoroalkyl substances in water and wastewater: A critical review of their global occurrence and distribution. Sci. Total Environ. 2022, 809, 151003. [Google Scholar] [CrossRef]
- Buck, R.C.; Franklin, J.; Berger, U.; Conder, J.M.; Cousins, I.T.; de Voogt, P.; Jensen, A.A.; Kannan, K.; Mabury, S.A.; van Leeuwen, S.P. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr. Environ. Assess. Manag. 2011, 7, 513–541. [Google Scholar] [CrossRef]
- Crone, B.C.; Speth, T.F.; Wahman, D.G.; Smith, S.J.; Abulikemu, G.; Kleiner, E.J.; Pressman, J.G. Occurrence of per- and polyfluoroalkyl substances (PFAS) in source water and their treatment in drinking water. Crit. Rev. Environ. Sci. Technol. 2019, 49, 2359–2396. [Google Scholar] [CrossRef] [PubMed]
- Guillette, T.C.; Jackson, T.W.; Guillette, M.; McCord, J.; Belcher, S.M. Blood concentrations of per- and polyfluoroalkyl substances are associated with autoimmune-like effects in American alligators from Wilmington, North Carolina. Front. Toxicol. 2022, 4, 1010185. [Google Scholar] [CrossRef] [PubMed]
- Kirkwood, K.I.; Fleming, J.; Nguyen, H.; Reif, D.M.; Baker, E.S.; Belcher, S.M. Utilizing Pine Needles to Temporally and Spatially Profile Per- and Polyfluoroalkyl Substances (PFAS). Environ. Sci. Technol. 2022, 56, 3441–3451. [Google Scholar] [CrossRef] [PubMed]
- Mahinroosta, R.; Senevirathna, L. A review of the emerging treatment technologies for PFAS contaminated soils. J. Environ. Manag. 2020, 255, 109896. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.K.; Brecher, R.W.; Cousins, I.T.; DeWitt, J.; Fiedler, H.; Kannan, K.; Kirman, C.R.; Lipscomb, J.; Priestly, B.; Schoeny, R.; et al. Grouping of PFAS for human health risk assessment: Findings from an independent panel of experts. Regul. Toxicol. Pharm. 2022, 134, 105226. [Google Scholar] [CrossRef]
- Panieri, E.; Baralic, K.; Djukic-Cosic, D.; Djordjevic, A.B.; Saso, L. PFAS Molecules: A Major Concern for the Human Health and the Environment. Toxics 2022, 10, 44. [Google Scholar] [CrossRef]
- Sunderland, E.M.; Hu, X.D.C.; Dassuncao, C.; Tokranov, A.K.; Wagner, C.C.; Allen, J.G. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Vandermeyden, C.; Hagerty, V. Managing PFAS: A North Carolina Utility Story. J. Am. Water Work. 2020, 112, 10–18. [Google Scholar] [CrossRef]
- Orellana, M.A.; Olawuyi, D.S.; Boyd, D.R.; Fakhri, M.; Arrojo-Agudo, P. Mandates of the Special Rapporteur on the Implications for Human Rights of the Environmentally Sound Management and Disposal of Hazardous Substances and Wastes. AL USA 26/2023, United Nations to United States of America. Available online: https://spcommreports.ohchr.org/TMResultsBase/DownLoadPublicCommunicationFile?gId=28341 (accessed on 15 April 2024).
- Orellana, M.A.; Olawuyi, D.S.; Boyd, D.R.; Fakhri, M.; Arrojo-Agudo, P. Mandates of the Special Rapporteur on the Implications for Human Rights of the Environmentally Sound Management and Disposal of Hazardous Substances and Wastes. AL OTH 113/2023, United Nations to Chemours. 2023. Available online: https://spcommreports.ohchr.org/TMResultsBase/DownLoadPublicCommunicationFile?gId=28409 (accessed on 15 April 2024).
- Nakayama, S.; Strynar, M.J.; Helfant, L.; Egeghy, P.; Ye, X.B.; Lindstrom, A.B. Perfluorinated compounds in the Cape Fear Drainage Basin in North Carolina. Environ. Sci. Technol. 2007, 41, 5271–5276. [Google Scholar] [CrossRef] [PubMed]
- Herkert, N.J.; Merrill, J.; Peters, C.; Bollinger, D.; Zhang, S.; Hoffman, K.; Ferguson, P.L.; Knappe, D.R.U.; Stapleton, H.M. Assessing the Effectiveness of Point-of-Use Residential Drinking Water Filters for Perfluoroalkyl Substances (PFASs). Environ. Sci. Technol. Lett. 2020, 7, 178–184. [Google Scholar] [CrossRef]
- Hopkins, Z.R.; Sun, M.; DeWitt, J.C.; Knappe, D.R.U. Recently Detected Drinking Water Contaminants: GenX and Other Per- and Polyfluoroalkyl Ether Acids. J. Am. Water Work Assoc. 2018, 110, 13–28. [Google Scholar] [CrossRef]
- Pétré, M.A.; Genereux, D.P.; Koropeckyj-Cox, L.; Knappe, D.R.U.; Duboscq, S.; Gilmore, T.E.; Hopkins, Z.R. Per- and Polyfluoroalkyl Substance (PFAS) Transport from Groundwater to Streams near a PFAS Manufacturing Facility in North Carolina, USA. Environ. Sci. Technol. 2021, 55, 5848–5856. [Google Scholar] [CrossRef]
- Pétré, M.A.; Salk, K.R.; Stapleton, H.M.; Ferguson, P.L.; Tait, G.; Obenour, D.R.; Knappe, D.R.U.; Genereux, D.P. Per- and polyfluoroalkyl substances (PFAS) in river discharge: Modeling loads upstream and downstream of a PFAS manufacturing plant in the Cape Fear watershed, North Carolina. Sci. Total Environ. 2022, 831, 154763. [Google Scholar] [CrossRef]
- Sun, M.; Arevalo, E.; Strynar, M.; Lindstrom, A.; Richardson, M.; Kearns, B.; Pickett, A.; Smith, C.; Knappe, D.R.U. Legacy and Emerging Perfluoroalkyl Substances Are Important Drinking Water Contaminants in the Cape Fear River Watershed of North Carolina. Environ. Sci. Technol. Lett. 2016, 3, 415–419. [Google Scholar] [CrossRef]
- Hall, S.M.; Zhang, S.R.; Tait, G.H.; Hoffman, K.; Collier, D.N.; Hoppin, J.A.; Stapleton, H.M. PFAS levels in paired drinking water and serum samples collected from an exposed community in Central North Carolina. Sci. Total Environ. 2023, 895, 165091. [Google Scholar] [CrossRef]
- Kotlarz, N.; McCord, J.; Collier, D.; Lea, C.S.; Strynar, M.; Lindstrom, A.B.; Wilkie, A.A.; Islam, J.Y.; Matney, K.; Tarte, P.; et al. Measurement of Novel, Drinking Water-Associated PFAS in Blood from Adults and Children in Wilmington, North Carolina. Environ. Health Perspect. 2020, 128, 077005. [Google Scholar] [CrossRef]
- Poothong, S.; Papadopoulou, E.; Padilla-Sánchez, J.A.; Thomsen, C.; Haug, L.S. Multiple pathways of human exposure to poly- and perfluoroalkyl substances (PFASs): From external exposure to human blood. Environ. Int. 2020, 134, 105244. [Google Scholar] [CrossRef] [PubMed]
- De Silva, A.O.; Armitage, J.M.; Bruton, T.A.; Dassuncao, C.; Heiger-Bernays, W.; Hu, X.C.; Kärrman, A.; Kelly, B.; Ng, C.; Robuck, A.; et al. PFAS Exposure Pathways for Humans and Wildlife: A Synthesis of Current Knowledge and Key Gaps in Understanding. Environ. Toxicol. Chem. 2021, 40, 631–657. [Google Scholar] [CrossRef] [PubMed]
- Vestergren, R.; Cousins, I.T. Tracking the Pathways of Human Exposure to Perfluorocarboxylates. Environ. Sci. Technol. 2009, 43, 5565–5575. [Google Scholar] [CrossRef]
- North Carolina Department of Environmental Quality. “DEQ PFAS Sampling of Public Water Systems”. Available online: https://www.deq.nc.gov/news/key-issues/emerging-compounds/understanding-pfas/deq-pfas-sampling-public-water-systems (accessed on 26 April 2024).
- Cape Fear Public Utility Authority. Sweeney Treatment Enhancements Project. Available online: https://www.cfpua.org/775/Sweeney-Treatment-Enhancements-Project (accessed on 26 April 2024).
- Town of Pittsboro. Water Quality & GAC. Available online: https://pittsboronc.gov/514/Water-Quality-GAC (accessed on 10 April 2024).
- Key, K.D.; Furr-Holden, D.; Lewis, E.Y.; Cunningham, R.; Zimmerman, M.A.; Johnson-Lawrence, V.; Selig, S. The Continuum of Community Engagement in Research: A Roadmap for Understanding and Assessing Progress. Prog. Community Health Partnersh. Res. Educ. Action 2019, 13, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Ross, L.F.; Loup, A.; Nelson, R.M.; Botkin, J.R.; Kost, R.; Smith, G.R.; Gehlert, S. The Challenges of Collaboration for Academic and Community Partners in a Research Partnership: Points to Consider. J. Empir. Res. Hum. Res. 2010, 5, 19–31. [Google Scholar] [CrossRef]
- Weed, R.A.; Boatman, A.K.; Enders, J.R. Recovery of per- and polyfluoroalkyl substances after solvent evaporation. Environ. Sci.-Proc. Imp. 2022, 24, 2263–2271. [Google Scholar] [CrossRef] [PubMed]
- Enders, J.R.; Weed, R.A.; Griffith, E.H.; Muddiman, D.C. Development and validation of a high resolving power absolute quantitative per- and polyfluoroalkyl substances method incorporating Skyline data processing. Rapid Commun. Mass Spectrom. 2022, 36, e9295. [Google Scholar] [CrossRef] [PubMed]
- Henry, H.; Sobhi, H.R.; Scheibner, O.; Bromirski, M.; Nimkar, S.B.; Rochat, B. Comparison between a high-resolution single-stage Orbitrap and a triple quadrupole mass spectrometer for quantitative analyses of drugs. Rapid Commun. Mass. Spectrom. 2012, 26, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Grund, B.; Marvin, L.; Rochat, B. Quantitative performance of a quadrupole-orbitrap-MS in targeted LC-MS determinations of small molecules. J. Pharm. Biomed. 2016, 124, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Herrero, P.; Cortes-Francisco, N.; Borrull, F.; Caixach, J.; Pocurull, E.; Marcé, R.M. Comparison of triple quadrupole mass spectrometry and Orbitrap high-resolution mass spectrometry in ultrahigh performance liquid chromatography for the determination of veterinary drugs in sewage: Benefits and drawbacks. J. Mass. Spectrom. 2014, 49, 585–596. [Google Scholar] [CrossRef]
- Munoz, G.; Duy, S.V.; Budzinski, H.; Labadie, P.; Liu, J.X.; Sauvé, S. Quantitative analysis of poly- and perfluoroalkyl compounds in water matrices using high resolution mass spectrometry: Optimization for a laser diode thermal desorption method. Anal. Chim. Acta 2015, 881, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Charbonnet, J.A.; McDonough, C.A.; Xiao, F.; Schwichtenberg, T.; Cao, D.P.; Kaserzon, S.; Thomas, K.V.; Dewapriya, P.; Place, B.J.; Schymanski, E.L.; et al. Communicating Confidence of Per- and Polyfluoroalkyl Substance Identification via High-Resolution Mass Spectrometry. Environ. Sci. Technol. Lett. 2022, 9, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying Small Molecules via High Resolution Mass Spectrometry: Communicating Confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.G.; Letcher, R.J. A targeted and non-targeted discovery screening approach for poly-and per-fluoroalkyl substances in model environmental biota samples. J. Chromatogr. A 2024, 1715, 464584. [Google Scholar] [CrossRef] [PubMed]
- Koelmel, J.P.; Stelben, P.; McDonough, C.A.; Dukes, D.A.; Aristizabal-Henao, J.J.; Nason, S.L.; Li, Y.; Sternberg, S.; Lin, E.; Beckmann, M.; et al. FluoroMatch 2.0-making automated and comprehensive non-targeted PFAS annotation a reality. Anal. Bioanal. Chem. 2022, 414, 1201–1215. [Google Scholar] [CrossRef] [PubMed]
- Koelmel, J.P.; Paige, M.K.; Aristizabal-Henao, J.J.; Robey, N.M.; Nason, S.L.; Stelben, P.J.; Li, Y.; Kroeger, N.M.; Napolitano, M.P.; Savvaides, T.; et al. Toward Comprehensive Per- and Polyfluoroalkyl Substances Annotation Using FluoroMatch Software and Intelligent High-Resolution Tandem Mass Spectrometry Acquisition. Anal. Chem. 2020, 92, 11186–11194. [Google Scholar] [CrossRef] [PubMed]
- Kind, T.; Liu, K.H.; Lee, D.Y.; DeFelice, B.; Meissen, J.K.; Fiehn, O. LipidBlast in silico tandem mass spectrometry database for lipid identification. Nat. Methods 2013, 10, 755–758. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Buser, A.M.; Cousins, I.T.; Demattio, S.; Drost, W.; Johansson, O.; Ohno, K.; Patlewicz, G.; Richard, A.M.; Walker, G.W.; et al. A New OECD Definition for Per- and Polyfluoroalkyl Substances. Environ. Sci. Technol. 2021, 55, 15575–15578. [Google Scholar] [CrossRef] [PubMed]
- EPA. Method 533: Determination of Per- and Polyfluoroalkyl Substances in Drinking Water by Isotope Dilution Anion Exchange Solid Phase Extraction and Liquid Chromatography/Tandem Mass Spectrometry; U.S. EPA: Washington, DC, USA, 2019. Available online: https://www.epa.gov/dwanalyticalmethods/method-533-determination-and-polyfluoroalkyl-substances-drinking-water-isotope (accessed on 22 May 2024).
- 2nd Draft Method 1633: Analysis of Per- and Polyfluoroalkyl Substances (PFAS) in Aqueous, Solid, Biosolids, and Tissue Samples by LC-MS/MS; U.S. EPA: Washington, DC, USA, 2022. Available online: https://www.epa.gov/system/files/documents/2022-07/2nd%20Draft%20of%20Method%201633%20June%202022%20508-compliant.pdf (accessed on 22 May 2024).
- Zhou, J.Q.; Baumann, K.; Surratt, J.D.; Turpin, B.J. Legacy and emerging airborne per- and polyfluoroalkyl substances (PFAS) collected on PM filters in close proximity to a fluoropolymer manufacturing facility. Environ. Sci. Process. Impacts 2022, 24, 2272–2283. [Google Scholar] [CrossRef]
- Kotlarz, N.; McCord, J.; Wiecha, N.; Weed, R.A.; Cuffney, M.; Enders, J.R.; Strynar, M.; Knappe, D.R.U.; Reich, B.J.; Hoppin, J.A. Measurement of Hydro-EVE and 6:2 FTS in Blood from Wilmington, North Carolina, Residents, 2017–2018. Environ. Health Persp. 2024, 132, 027702. [Google Scholar] [CrossRef]
- Kotlarz, N.; McCord, J.; Wiecha, N.; Weed, R.A.; Cuffney, M.; Enders, J.R.; Strynar, M.; Knappe, D.R.U.; Reich, B.J.; Hoppin, J.A. Reanalysis of PFO5DoA Levels in Blood from Wilmington, North Carolina, Residents, 2017–2018. Environ. Health Persp. 2024, 132, 027701. [Google Scholar] [CrossRef] [PubMed]
- Kotlarz, N.; Guillette, T.; Critchley, C.; Collier, D.; Lea, C.S.; McCord, J.; Strynar, M.; Cuffney, M.; Hopkins, Z.R.; Knappe, D.R.U.; et al. Per- and polyfluoroalkyl ether acids in well water and blood serum from private well users residing by a fluorochemical facility near Fayetteville, North Carolina. J. Expo. Sci. Environ. Epidemiol. 2024, 34, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Cahoon, L.B. GenX in Cape Fear River Water Was Only One Part of the PFAS Story in North Carolina. In Contaminants in Our Water: Identification and Remediation Methods; American Chemical Society: Washington, DC, USA, 2020; Volume 1352, pp. 91–103. [Google Scholar]
- The North Carolina Department of Health and Human Services. Press Release: NCDHHS Recommends Limiting Fish Consumption from the Middle and Lower Cape Fear River Due to Contamination with “Forever Chemicals”; The North Carolina Department of Health and Human Services: Raleigh, NC, USA, 2023. [Google Scholar]
- Liu, Y.; D’Agostino, L.A.; Qu, G.; Jiang, G.; Martin, J.W. High-resolution mass spectrometry (HRMS) methods for nontarget discovery and characterization of poly- and per-fluoroalkyl substances (PFASs) in environmental and human samples. TrAC Trends Anal. Chem. 2019, 121, 115420. [Google Scholar] [CrossRef]
- Getzinger, G.J.; Higgins, C.P.; Ferguson, P.L. Structure Database and In Silico Spectral Library for Comprehensive Suspect Screening of Per- and Polyfluoroalkyl Substances (PFASs) in Environmental Media by High-resolution Mass Spectrometry. Anal. Chem. 2021, 93, 2820–2827. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Li, J.; Zhao, Y.; Wu, Y. Human Exposure of Fipronil Insecticide and the Associated Health Risk. J. Agric. Food Chem. 2022, 70, 63–71. [Google Scholar] [CrossRef]
- Singh, N.S.; Sharma, R.; Singh, S.K.; Singh, D.K. A comprehensive review of environmental fate and degradation of fipronil and its toxic metabolites. Environ. Res. 2021, 199, 111316. [Google Scholar] [CrossRef]
- Kim, Y.A.; Yoon, Y.S.; Kim, H.S.; Jeon, S.J.; Cole, E.; Lee, J.; Kho, Y.; Cho, Y.H. Distribution of fipronil in humans, and adverse health outcomes of fipronil sulfone exposure in newborns. Int. J. Hyg. Environ. Health 2019, 222, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.W.; Zheng, Y.X.; Tang, X.W.; Zhao, N.; Wang, B.; Lin, H.; Lin, Y.F. Nontarget Identification of Novel Per- and Polyfluoroalkyl Substances in Cord Blood Samples. Environ. Sci. Technol. 2022, 56, 17061–17069. [Google Scholar] [CrossRef]
- Gunasekara, A.S.; Truong, T.; Goh, K.S.; Spurlock, F.; Tjeerdema, R.S. Environmental fate and toxicology of fipronil. J. Pestic. Sci. 2007, 32, 189–199. [Google Scholar] [CrossRef]
- McMahen, R.L.; Strynar, M.J.; McMillan, L.; DeRose, E.; Lindstrom, A.B. Comparison of fipronil sources in North Carolina surface water and identification of a novel fipronil transformation product in recycled wastewater. Sci. Total Environ. 2016, 569, 880–887. [Google Scholar] [CrossRef]
- Zhao, W.X.; Sun, J.W. Triflimide (HNTf2) in Organic Synthesis. Chem. Rev. 2018, 118, 10349–10392. [Google Scholar] [CrossRef] [PubMed]
- Allanore, A.; Sadoway, D.R. Extraction of Liquid Elements by Electrolysis of Oxides. U.S. Patent US8764962B2, 1 July 2014. [Google Scholar]
- Elabd, Y.A.; Winey, K.I.; Ye, Y.; Choi, J.-H.; Tsen-Shan, S.S. Polymerized Ionic Liquid Block Copolymers as Battery Membranes. U.S. Patent US8853286B2, 7 October 2014. [Google Scholar]
- Barola, C.; Bucaletti, E.; Moretti, S.; Buiarelli, F.; Simonetti, G.; Lucarelli, F.; Goracci, L.; Lorenzetti, S.; Di Filippo, P.; Pomata, D.; et al. Untargeted Screening of Per- and Polyfluoroalkyl Substances (PFASs) in Airborne Particulate of Three Italian E-Waste Recycling Facilities. Separations 2023, 10, 547. [Google Scholar] [CrossRef]
- Zhao, M.S.; Yao, Y.M.; Dong, X.Y.; Baqar, M.; Fang, B.; Chen, H.; Sun, H.W. Nontarget Identification of Novel Per- and Polyfluoroalkyl Substances (PFAS) in Soils from an Oil Refinery in Southwestern China: A Combined Approach with TOP Assay. Environ. Sci. Technol. 2023, 57, 20194–20205. [Google Scholar] [CrossRef]
- Feng, C.; Lin, Y.J.; Le, S.Y.; Ji, J.Y.; Chen, Y.H.; Wang, G.Q.; Xiao, P.; Zhao, Y.F.; Lu, D.S. Suspect, Nontarget Screening, and Toxicity Prediction of Per- and Polyfluoroalkyl Substances in the Landfill Leachate. Environ. Sci. Technol. 2024, 58, 4737–4750. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.R.; Lyu, Y.; Chen, H.; Cai, L.L.; Li, J.; Cao, X.Q.; Sun, W.L. Integration of target, suspect, and nontarget screening with risk modeling for per- and polyfluoroalkyl substances prioritization in surface waters. Water Res. 2023, 233, 119735. [Google Scholar] [CrossRef] [PubMed]
- Neuwald, I.; Muschket, M.; Zahn, D.; Berger, U.; Seiwert, B.; Meier, T.; Kuckelkorn, J.; Strobel, C.; Knepper, T.P.; Reemtsma, T. Filling the knowledge gap: A suspect screening study for 1310 potentially persistent and mobile chemicals with SFC- and HILIC-HRMS in two German river systems. Water Res. 2021, 204, 117645. [Google Scholar] [CrossRef] [PubMed]
- Development OfEC-oa. Reconciling Terminology of the Universe of Per-and Polyfluoroalkyl Substances: Recommendations and Practical Guidance. Ser. Risk Manag. 2021, 61, 1–43. [Google Scholar]
- Rodenstein, M.; Zürcher, S.; Tosatti, S.G.P.; Spencer, N.D. Fabricating Chemical Gradients on Oxide Surfaces by Means of Fluorinated, Catechol-Based, Self-Assembled Monolayers. Langmuir 2010, 26, 16211–16220. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Pazos, D.J.; Lasso, J.D.; Li, C.J. Modern methods for the synthesis of perfluoroalkylated aromatics. Org. Biomol. Chem. 2021, 19, 7116–7128. [Google Scholar] [CrossRef]
- Li, N.N.; Noro, J.; Su, J.; Wang, H.B.; Silva, C.; Cavaco-Paulo, A. Enhancing laccase-assisted polymerization reactions with perfluorinated compounds. Biochem. Eng. J. 2022, 189, 108736. [Google Scholar] [CrossRef]
- Jana Semanova (HighChem, B., Slovakia), Thermo Fisher Scientific. Catechol. Repository: MzCloud. Available online: https://beta.mzcloud.org/dataviewer#/app/dataviewer/library/reference?query=myCloudId%253D2984 (accessed on 26 April 2024).
- Manfrin, A.; Hänggli, A.; van den Wildenberg, J.; McNeill, K. Substituent Effects on the Direct Photolysis of Benzotrifluoride Derivatives. Environ. Sci. Technol. 2020, 54, 11109–11117. [Google Scholar] [CrossRef] [PubMed]
- Leitis, E.; Crosby, D.G. Photodecomposition of Trifluralin. J. Agric. Food Chem. 1974, 22, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Lerch, R.N.; Ferrer, I.; Thurman, E.M.; Zablotowicz, R.M. Identification of trifluralin metabolites in soil using ion-trap LC/MS/MS. ACS Symt. Ser. 2003, 850, 291–310. [Google Scholar]


| Pittsboro | Fayetteville | Wilmington | |
|---|---|---|---|
| Total Sample Number | 8 | 10 | 27 |
| Recreation Sample Number | 0 | 3 | 0 |
| Drinking Sample Number | 8 | 7 | 27 |
| Well | n/a | 6 | 20 |
| Municipal | n/a | 1 | 3 |
| Unknown | 8 | 0 | 4 |
| Confidence Level | Confidence | MS2 Data (Number; Type) * | Predicted Molecular Formula and Isotope Pattern Matching | Retention Time | Kendrick Mass Defect (CF2) | Mass Accuracy (ppm) |
|---|---|---|---|---|---|---|
| 1 | Confirmed structure | Matched to library reference Standard | Match to standard | Match to standard | −0.116 to 0.268 ‡ | ≤5 ppm |
| 2a | Probable structure | Library Match to mzCloud | Full match for mono-isotopic and M + 1 peak | Consistent with PFAS elution time patterns † | −0.116 to 0.268 ‡ | ≤5 ppm |
| 2b | Probable structure | ≥3; diagnostic | Full match for mono-isotopic and M + 1 peak | Consistent with PFAS elution time patterns † | −0.116 to 0.268 ‡ | ≤5 ppm |
| 2c | Probable structure | ≥1; diagnostic | Full match for mono-isotopic and M + 1 peak | Consistent with PFAS elution time patterns † | −0.116 to 0.268 ‡ | ≤5 ppm |
| 3 | Tentative structure | ≥1; Subclass Aligned | Full match for mono-isotopic and M + 1 peak | Consistent with PFAS elution time patterns † | −0.116 to 0.268 ‡ | ≤5 ppm |
| 4a | Unequivocal molecular formula | None or structurally inconclusive | Full match for mono-isotopic and M + 1 peak | Consistent with PFAS elution time patterns † | −0.116 to 0.268 ‡ | ≤5 ppm |
| 4b | Putative molecular formula | None or structurally inconclusive | No library match, Predicted Formula Sfit > 50% § | Consistent with PFAS elution time patterns † | −0.116 to 0.268 ‡ | ≤5 ppm |
| 5a | Suspect screening exact mass match to mzCloud or EPA CompTox | None | No library match, Predicted Formula Sfit > 50% § | Consistent with PFAS elution time patterns † | −0.116 to 0.268 ‡ | ≤5 ppm |
| 5b | Exact mass with mass accuracy < 5 ppm | ≥1 subclass hit to in-silico libraries from NIST or Fluoromatch ¶ | No match | Consistent with PFAS elution time patterns † | −0.116 to 0.268 ‡ | ≤5 ppm |
| 5c | Exact mass with mass accuracy < 5 ppm | None | No match | Consistent with PFAS elution time patterns † | −0.116 to 0.268 ‡ | ≤5 ppm |
| * MS2 Fragment types: Diagnostic: structurally informative and or headgroup present; Subclass aligned (in silico): structurally informative and matches in silico database like Fluoromatch or NIST, and one or more of the fragment peak abundances must be 3× greater than background noise (~5000 intensity counts) | ||||||
| † Retention times are consistent with typical PFAS elution times, e.g., low molecular weight and hydrophilic compounds eluted earlier | ||||||
| ‡ This range is representative of 98% of compounds in EPA Comptox list; m/z range from 117 to 1189 [40]. | ||||||
| § M + 2 isotope is ignored unless the predicted molecular formula suggests that a diagnostic atom, like Cl, Br, or S is present. If the isotopic pattern is not in alignment with the predicted molecular formula, then the next best formula is chosen based on the SFit (>50%) [SFit is the spectral similarity score between the theoretical and the measured isotope pattern displayed as a percentage in CD] | ||||||
| ¶ NIST Compound Class Library: PFAS Fine signature fragment_lib.cLib, Fluoromatch Compound Class Library: PFAS General from FluoroMatch Suite.cLib | ||||||
| Level | Name | Formula | Calculated Molecular Weight | Kendrick Mass Defect [CF2] | Class Coverage: FluoroMatch % | Class Coverage: NIST % |
|---|---|---|---|---|---|---|
| 1 | Perfluoro-1-hexanesulfonic acid (PFHxS) | C6 H F13 O3 S | 398.93685 | −0.030329162 | 0.75 | 18.75 |
| Perfluoro-1-butanesulfonic acid (PFBS) | C4 H F9 O3 S | 298.94319 | −0.03036894 | 0.99 | 31.25 | |
| Perfluoroheptanoic acid (PFHpA) | C7 H F13 O2 | 362.96993 | 0.000458177 | 1.37 | 31.25 | |
| Perfluorooctanoic acid (PFOA) | C8 H F15 O2 | 412.96671 | 0.000515965 | 1.99 | 43.75 | |
| Perfluoro-1-octanesulfonic acid (PFOS) | C8 H F17 O3 S | 498.93051 | −0.030276925 | 3.60 | 62.5 | |
| 2a | Fipronil sulfone | C12 H4 Cl2 F6 N4 O2 S | 451.93405 | −0.037084358 | 0.37 | 12.5 |
| Bistriflimide | C2 H F6 N O4 S2 | 280.92538 | −0.056676962 | 0.12 | 6.25 | |
| 2b | 3-(Tridecafluoroundecyl)catechol | C17 H15 F13 O2 | 498.08725 | 0.119069512 | 1.12 | 18.75 |
| 2-Ethyl-4-nitro-6-(trifluoromethyl)-1H-benzimidazol-1-ol | C10 H8 F3 N3 O3 | 275.05186 | 0.069427657 | 0.37 | 6.25 | |
| 3 | Floctafenine | C20 H17 F3 N2 O4 | 406.1145 | 0.140440239 | 0.12 | 6.25 |
| Ethyl 1,4-dihydro-5-isopropoxy-2-methyl-4-(2-trifluoromethylphenyl)-1,6-naphthyridine-3-carboxylate | C22 H23 F3 N2 O3 | 420.16604 | 0.192881683 | 0.37 | 0 | |
| 2-[2-Imino-6-(trifluoromethoxy)-1,3-benzothiazol-3(2H)-yl]acetamide | C10 H8 F3 N3 O2 S | 291.02921 | 0.047799113 | 0.5 | 18.75 | |
| 2-tert-Butyl-4-(piperazin-1-yl)-6-trifluoromethyl-pyrimidine | C13 H19 F3 N4 | 288.15756 | 0.175964392 | 1.12 | 18.75 | |
| 2,2-Bis(3-amino-4-hydroxyphenyl)hexafluoropropane | C15 H12 F6 N2 O2 | 366.08037 | 0.103751965 | 1.86 | 25 | |
| 3-(Trifluoromethyl)benzyl 3,5-dinitrobenzoate | C15 H9 F3 N2 O6 | 370.04265 | 0.066290222 | 2.36 | 6.25 | |
| 4a | - | C20 H27 F3 O2 | 356.19495 | 0.21770158 | 0 | 0 |
| - | C33 H35 Cl F3 N O3 | 585.22833 | 0.26571272 | 0 | 0 | |
| - | C18 H25 F3 O3 | 346.17581 | 0.197923018 | 0 | 0 | |
| - | C21 H20 F3 N O2 S | 407.11652 | 0.142529987 | 0 | 0 | |
| 4b | - | C14 H17 F3 N4 O2 | 330.13173 | 0.15282143 | 0.37 | 6.25 |
| - | C10 H14 F4 | 210.10223 | 0.115646518 | 0.62 | 0 | |
| 5a | - | C37 H59 F17 O2 S | 890.39997 | 0.456845695 | 0 | 0 |
| - | C22 H15 F7 O | 428.10194 | 0.12928048 | 0 | 0 | |
| - | C14 H19 F13 O3 Si | 510.08959 | 0.12217564 | 0 | 0 | |
| - | C18 H15 F4 N3 O S | 397.08778 | 0.113146866 | 0 | 0 | |
| 5b | - | - | 678.32956 | 0.372887588 | 1.74 | 0 |
| - | - | 556.18405 | 0.21958005 | 3.23 | 6.25 | |
| - | - | 426.11853 | 0.145750621 | 1.99 | 6.25 | |
| - | - | 369.99506 | 0.018690222 | 2.11 | 6.25 | |
| - | - | 412.11908 | 0.145407889 | 0.12 | 0 | |
| - | - | 638.1888 | 0.229560169 | 0.12 | 0 | |
| - | - | 414.2389 | 0.26536011 | 1.99 | 31.25 | |
| - | - | 355.19509 | 0.225123167 | 12.5 | 0 | |
| - | - | 309.15351 | 0.180598046 | 12.5 | 0 | |
| - | - | 391.12726 | 0.159582519 | 25 | 0 | |
| - | - | 333.13567 | 0.164291382 | 12.5 | 0 | |
| - | - | 305.17175 | 0.19858475 | 6.25 | 0 | |
| - | - | 393.17118 | 0.203630226 | 6.25 | 0 | |
| - | - | 359.20117 | 0.231450618 | 12.5 | 0 | |
| - | - | 377.15836 | 0.189841783 | 0 | 0 | |
| - | - | 491.15454 | 0.193257638 | 0 | 0 | |
| - | - | 492.16475 | 0.196191042 | 0.5 | 6.25 | |
| 5c | - | - | 355.02762 | 0.050299003 | 0 | 0 |
| - | - | 446.15818 | 0.186676221 | 0 | 0 | |
| - | - | 285.14944 | 0.167651451 | 0 | 0 | |
| - | - | 389.1504 | 0.17526076 | 0 | 0 | |
| - | - | 473.22699 | 0.257217681 | 0 | 0 | |
| - | - | 520.10386 | 0.137083129 | 0 | 0 | |
| - | - | 438.14485 | 0.172840803 | 0 | 0 | |
| - | - | 330.15979 | 0.180881971 | 0 | 0 | |
| - | - | 394.17843 | 0.203611898 | 0 | 0 | |
| - | - | 461.10495 | 0.134406375 | 0 | 0 | |
| - | - | 522.15917 | 0.19252665 | 0 | 0 | |
| - | - | 553.15088 | 0.186216128 | 0 | 0 | |
| - | - | 437.12711 | 0.155033109 | 0 | 0 | |
| - | - | 443.10162 | 0.129920627 | 0 | 0 | |
| - | - | 447.09619 | 0.124745676 | 0 | 0 | |
| - | - | 496.10795 | 0.139637305 | 0 | 0 | |
| - | - | 409.15927 | 0.185403207 | 0 | 0 | |
| - | - | 588.20877 | 0.246341597 | 0 | 0 | |
| - | - | 477.1642 | 0.194684137 | 0 | 0 | |
| - | - | 389.06437 | 0.089225706 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weed, R.A.; Campbell, G.; Brown, L.; May, K.; Sargent, D.; Sutton, E.; Burdette, K.; Rider, W.; Baker, E.S.; Enders, J.R. Non-Targeted PFAS Suspect Screening and Quantification of Drinking Water Samples Collected through Community Engaged Research in North Carolina’s Cape Fear River Basin. Toxics 2024, 12, 403. https://doi.org/10.3390/toxics12060403
Weed RA, Campbell G, Brown L, May K, Sargent D, Sutton E, Burdette K, Rider W, Baker ES, Enders JR. Non-Targeted PFAS Suspect Screening and Quantification of Drinking Water Samples Collected through Community Engaged Research in North Carolina’s Cape Fear River Basin. Toxics. 2024; 12(6):403. https://doi.org/10.3390/toxics12060403
Chicago/Turabian StyleWeed, Rebecca A., Grace Campbell, Lacey Brown, Katlyn May, Dana Sargent, Emily Sutton, Kemp Burdette, Wayne Rider, Erin S. Baker, and Jeffrey R. Enders. 2024. "Non-Targeted PFAS Suspect Screening and Quantification of Drinking Water Samples Collected through Community Engaged Research in North Carolina’s Cape Fear River Basin" Toxics 12, no. 6: 403. https://doi.org/10.3390/toxics12060403
APA StyleWeed, R. A., Campbell, G., Brown, L., May, K., Sargent, D., Sutton, E., Burdette, K., Rider, W., Baker, E. S., & Enders, J. R. (2024). Non-Targeted PFAS Suspect Screening and Quantification of Drinking Water Samples Collected through Community Engaged Research in North Carolina’s Cape Fear River Basin. Toxics, 12(6), 403. https://doi.org/10.3390/toxics12060403







