Analytical Method Development and Dermal Absorption of 4-Amino-3-Nitrophenol (4A3NP), a Hair Dye Ingredient under the Oxidative or Non-Oxidative Condition
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. LC-MS/MS Analysis Conditions
2.3. Method Validation
2.4. In Vitro Dermal Absorption
2.5. Statistical Analysis
3. Results
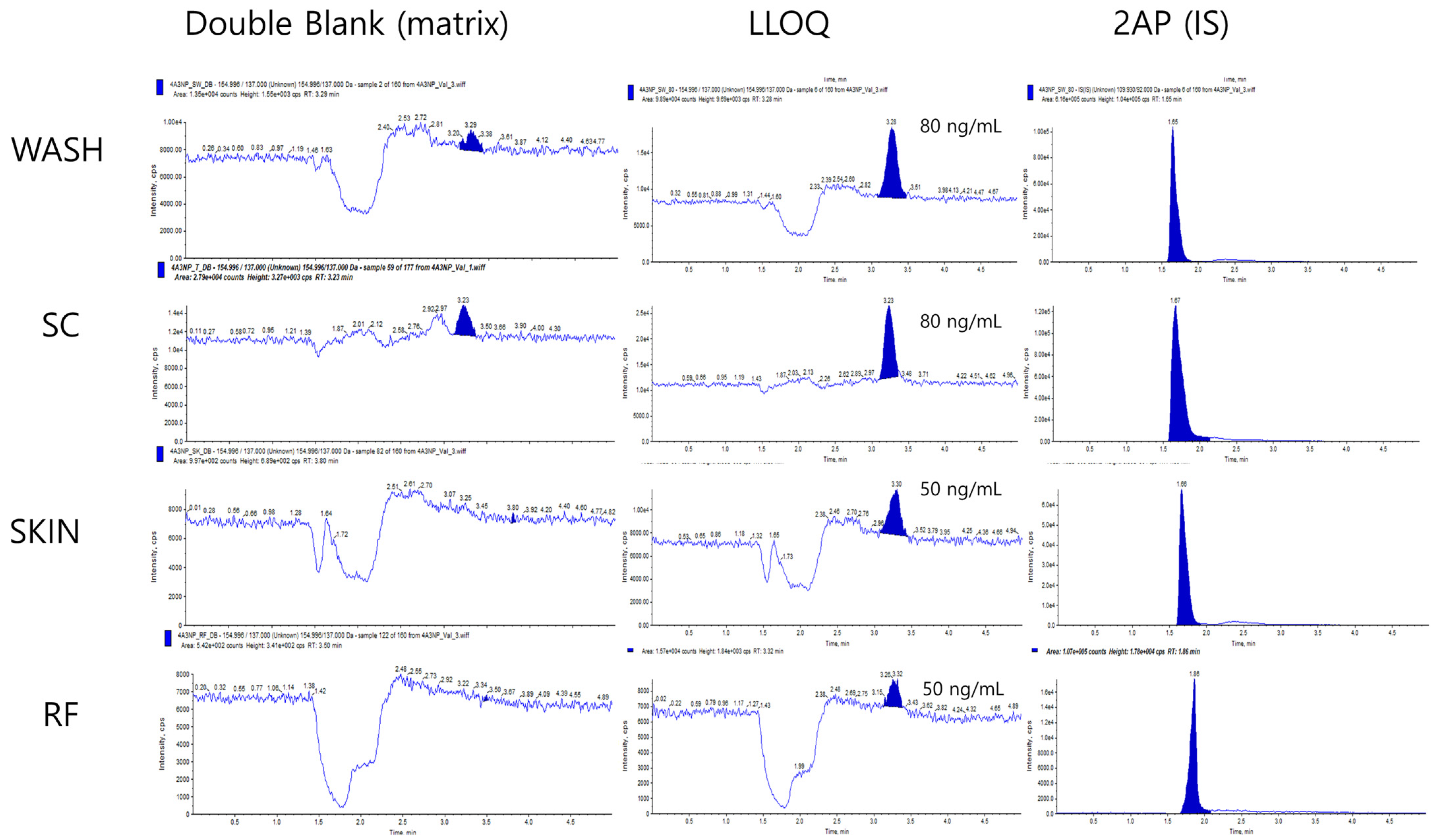
3.1. Validation of Analytical Methodology (Accuracy and Precision)
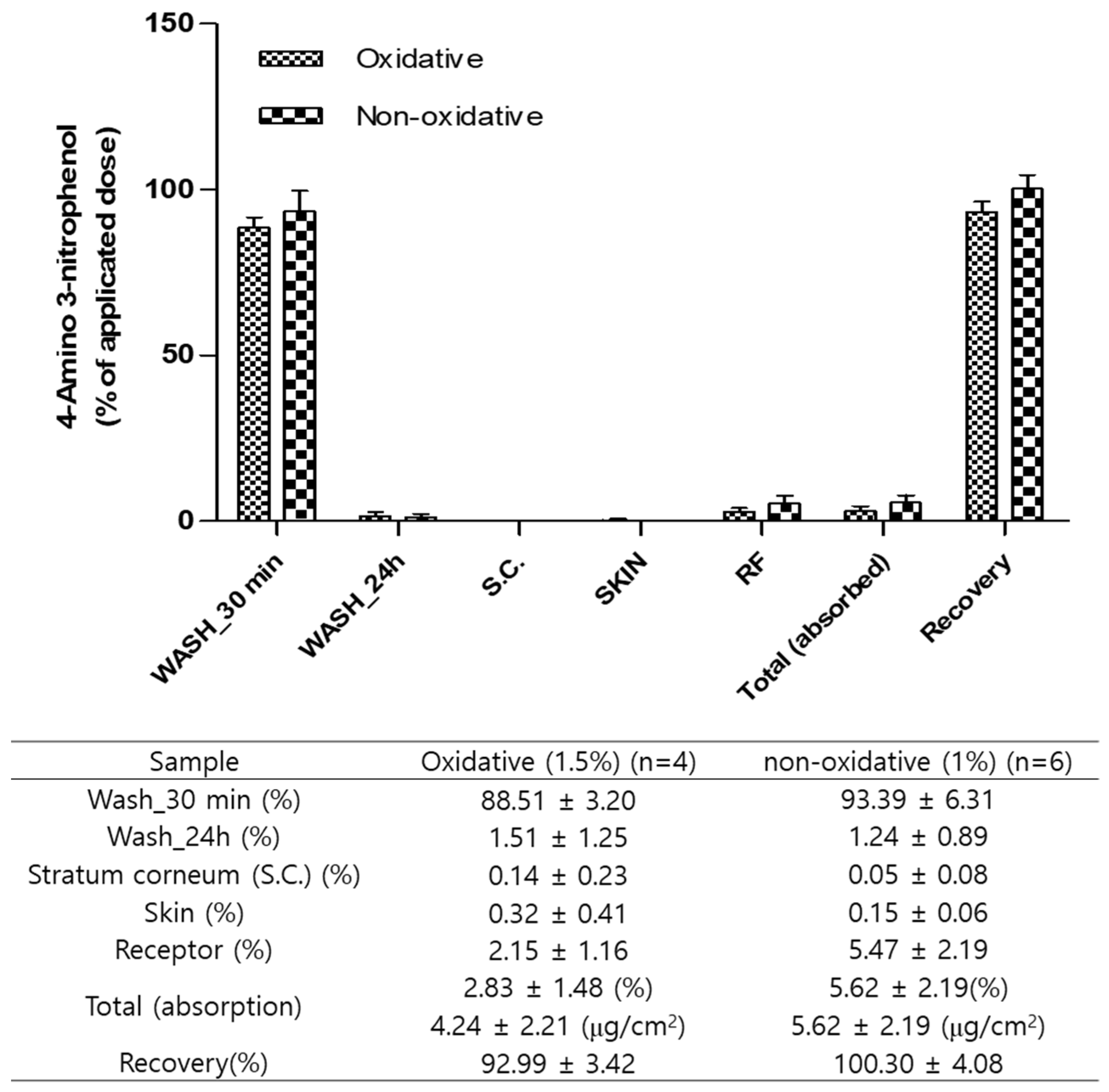
3.2. In Vitro Dermal Absorption
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burnett, C.; Bergfeld, W.; Belsito, D.; Klaassen, C.; Marks, J.; Shank, R.; Slaga, T.; Snyder, P.; Andersen, F. Final Report on the Safety Assessment of Amino Nitrophenols as Used in Hair Dyes. Int. J. Toxicol. 2009, 28 (Suppl. 3), 217S–251S. [Google Scholar] [CrossRef] [PubMed]
- SCCP (Scientific Committee on Consumer Products). Opinion on 4-Amino-3-Nitrophenol; European Commission: Brussels, Belgium, 2007. [Google Scholar]
- Kim, K.B.; Kwack, S.J.; Lee, J.Y.; Kacew, S.; Lee, B.M. Current opinion on risk assessment of cosmetics. J. Toxicol. Environ. Health B 2021, 24, 137–161. [Google Scholar] [CrossRef]
- Toner, F. The In Vitro Percutaneous Absorption of Radio labelled 4-amino-3-nitrophenol through human Skin. Inveresk Study 2005, 774976. [Google Scholar]
- OECD (Organisation for Economic Cooperation and Development). Guideline for the Testing of Chemicals Skin Absorption: In Vitro Method. 2004. Available online: https://ntp.niehs.nih.gov/iccvam/suppdocs/feddocs/oecd/oecdtg428-508.pdf (accessed on 30 April 2024).
- MFDS (Ministry of Food and Drug Safety). Guideline for In Vitro Skin Absorption Method. 2009, B1-2009. Available online: https://www.mfds.go.kr/brd/m_1060/view.do?seq=15205&srchFr=&srchTo=&srchWord=%EB%8F%99%EB%AC%BC%EB%8C%80%EC%B2%B4&srchTp=0&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&Data_stts_gubun=C9999&page=1 (accessed on 30 April 2024).
- Iliopoulos, F.; Caspers, P.J.; Puppels, G.J.; Lane, M.E. Franz Cell Diffusion Testing and Quantitative Confocal Raman Spectroscopy: In Vitro-In Vivo Correlation. Pharmaceutics 2020, 12, 887. [Google Scholar] [CrossRef] [PubMed]
- Franz, T.J.; Lehman, P.A.; Raney, S.G. Use of Excised Human Skin to Assess the Bioequivalence of Topical Products. Skin Pharmacol. Physiol. 2009, 22, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Clowes, H.M.; Scott, R.C.; Heylings, J.R. Skin absorption: Flow-through or static diffusion cells. Toxicol. In Vitro 1994, 8, 827–830. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Chen, B.; Liu, A.; Zhu, W.; Yao, S. Liquid chromatography-mass spectrometric multiple reaction monitoring-based strategies for expanding targeted profiling towards quantitative metabolomics. Curr. Drug Metab. 2012, 13, 1226–1243. [Google Scholar] [CrossRef] [PubMed]
- Szpot, P.; Wachełko, O.; Zawadzki, M. Determination of Prostaglandins (Carboprost, Cloprostenol, Dinoprost, Dinoprostone, Misoprostol, Sulprostone) by UHPLC-MS/MS in Toxicological Investigations. Toxics 2023, 11, 802. [Google Scholar] [CrossRef] [PubMed]
- Hooper, S.E.; Amelon, S.K.; Lin, C.-H. Development of an LC-MS/MS Method for Non-Invasive Biomonitoring of Neonicotinoid and Systemic Herbicide Pesticide Residues in Bat Hair. Toxics 2022, 10, 73. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Cao, M.; Wang, Y. Fast Analysis of Hair Dyes Using an Agilent Poroshell 120 Bouns-RP Column by UHPLC and LC/MS/MS; Application Note; Agilent Technologies Inc.: Santa Clara, CA, USA, 2012; Publication number 5991-1289EN. [Google Scholar]
- Zuo, X.; Di, Z.; Du, Y.; Yang, L.; Zhang, R.; Wu, G. Determination of 40 dyes in oxidative hair dye products by high performance liquid chromatography. Se Pu 2021, 39, 1222–1229. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- MFDS (Ministry of Food and Drug Safety). Guidelines for Validation of Bio-Sampling Methods. Ministry of Food and Drug Safety. 2013. Available online: https://www.mfds.go.kr/brd/m_1060/view.do?seq=13054&srchFr=&srchTo=&srchWord=&srchTp=&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&page=1 (accessed on 30 April 2024).
- MFDS (The Ministry of Food and Drug Safety). Regulations on Cosmetic Safety Standards. Ministry of Food and Drug Safety, 2014, 2014–2118 issue. Available online: http://www.mfds.go.kr/index.do?mid=687andpageNo=2andseq=8003andcmd=v (accessed on 30 April 2024).
- Madmon, M.; Shamai Yamin, T.; Pitel, S.; Belay, C.; Segula, Y.; Toister, E.; Hindi, A.; Cherry, L.; Ophir, Y.; Zichel, R.; et al. Development and Validation of an Innovative Analytical Approach for the Quantitation of Tris(Hydroxymethyl)Aminomethane (TRIS) in Pharmaceutical Formulations by Liquid Chromatography Tandem Mass Spectrometry. Molecules 2022, 28, 73. [Google Scholar] [CrossRef] [PubMed]
- Im, J.E.; Kim, H.Y.; Lee, J.D.; Park, J.J.; Kang, K.S.; Kim, K.B. Effect of Application Amounts on In Vitro Dermal Absorption Test Using Caffeine and Testosterone. Pharmaceutics 2021, 13, 641. [Google Scholar] [CrossRef] [PubMed]
- Jewell, C.; Heylings, J.; Clowes, H.M.; Williams, F.M. Percutaneous absorption and metabolism of dinitrochlorobenzene in vitro. Arch. Toxicol. 2000, 74, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Barbero, A.M.; Frasch, H.F. Pig and guinea pig skin as surrogates for human in vitro penetration studies: A quantitative review. Toxicol. In Vitro 2009, 23, 1–13. [Google Scholar] [CrossRef]
- Qvist, M.H.; Hoeck, U.; Kreilgaard, B.; Madsen, F.; Frokjaer, S. Evaluation of Gottingen minipig skin for transdermal in vitro permeation studies. Eur. J. Pharm. Sci. 2000, 11, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.C.; Friedman, A.J. Hydrogen peroxide and cutaneous biology: Translational applications, benefits, and risks. J. Am. Acad. Dermatol. 2019, 81, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- Steiling, W.; Kreutz, J.; Hofer, H. Percutaneous penetration/dermal absorption of hair dyes in vitro. Toxicol. In Vitro 2001, 15, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kim, H.Y.; Lee, J.D.; Kim, H.Y.; Im, J.E.; Kim, K.B. Analytical method development and dermal absorption of 2-amino-5-nitrophenol (2A5NP), a hair dye ingredient under oxidative condition. Toxicol. Res. 2022, 39, 231–238. [Google Scholar] [CrossRef]
- Bartnik, F.G.; Reddy, A.K.; Klecak, G.; Zimmermann, V.; Hostynek, J.J.; Ku¨nstler, K. Percutaneous absorption, metabolism and haemolytic activity of n-butoxyethanol. Fundam. Appl. Toxicol. 1987, 8, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.P.; Chen, C.C.; Huang, C.W.; Chang, Y.C. Evaluating Molecular Properties Involved in Transport of Small Molecules in Stratum Corneum: A Quantitative Structure-Activity Relationship for Skin Permeability. Molecules 2018, 23, 911. [Google Scholar] [CrossRef] [PubMed]
- ECETOC (European Centre for Ecotoxicology and Toxicology of Chemicals). Percutaneous Absorption. Monograph No. 20. 1993. Available online: https://www.ecetoc.org/wp-content/uploads/2014/08/MON-020.pdf (accessed on 30 April 2024).

| Name | MW | CAS No. | Chemical Composition | Log Pow | Structure | MS Transition (Positive) | ||
|---|---|---|---|---|---|---|---|---|
| Q1 | Q3 | CE | ||||||
| 4-amino-3-nitrophenol (4A3NP) | 154.12 | 610-81-1 | C6H6N2O3 | 0.41 | 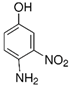 | 154.9 | 137 | 15 |
| 2-aminophenol (2AP) | 109.13 | 95-55-6 | C6H7NO | 0.62 |  | 109.9 | 92 | 23 |
| Compound | Conc. (ng/mL) | Intraday (%) | Interday (%) | ||||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Accuracy | Precision | Mean ± SD | Accuracy (%) | Precision (%) | ||
| WASH (R2 = 0.9987) | 80 | 79.74 ± 1.77 | 99.68 | 2.23 | 79.89 ± 4.92 | 99.68 | 2.23 |
| 240 | 237.40 ± 4.04 | 98.92 | 1.70 | 238.60 ± 16.80 | 98.92 | 1.70 | |
| 400 | 413.20 ± 16.22 | 103.30 | 3.93 | 411.33 ± 36.16 | 103.30 | 3.93 | |
| 750 | 722.20 ± 54.27 | 96.29 | 7.51 | 734.2 ± 77.25 | 96.29 | 7.51 | |
| SC (R2 = 0.9993) | 80 | 74.80 ± 5.19 | 93.50 | 6.94 | 77.83 ± 4.57 | 97.29 | 5.88 |
| 240 | 224.40 ± 7.67 | 93.50 | 3.42 | 240.86 ± 18.38 | 100.36 | 7.63 | |
| 400 | 389.80 ± 26.36 | 97.45 | 6.76 | 405.53 ± 23.78 | 101.38 | 5.86 | |
| 750 | 744.00 ± 31.01 | 99.20 | 4.17 | 780.13 ± 48.17 | 104.02 | 6.17 | |
| SKIN (R2 = 0.9962) | 50 | 51.20 ± 2.18 | 102.40 | 4.25 | 50.08 ± 1.42 | 100.17 | 2.85 |
| 150 | 154.80 ± 11.56 | 103.20 | 7.47 | 151.06 ± 9.21 | 100.71 | 6.10 | |
| 400 | 445.40 ± 10.78 | 111.35 | 2.42 | 410.46 ± 27.57 | 102.62 | 6.72 | |
| 750 | 838.00 ± 20.17 | 111.73 | 2.41 | 752.80 ± 63.38 | 100.37 | 8.42 | |
| RF (R2 = 0.9991) | 50 | 52.32 ± 7.56 | 104.64 | 14.46 | 50.19 ± 5.18 | 100.39 | 10.32 |
| 150 | 150.00 ± 5.10 | 100.00 | 3.40 | 153.26 ± 7.91 | 102.18 | 5.16 | |
| 400 | 407.60 ± 9.79 | 101.90 | 2.40 | 409.46 ± 7.54 | 102.37 | 1.84 | |
| 750 | 761.60 ± 24.93 | 101.55 | 3.27 | 752.60 ± 22.41 | 100.35 | 2.98 | |
| Substance | Conditions | Permeation Parameter Js (Equilibrium Flux, μg/cm2/h) |
|---|---|---|
| 4A3NP | Oxidative (1%) | 0.16 ± 0.07 |
| Non-oxidative (1.5%) | 0.23 ± 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.Y.; Kim, Y.J.; Lee, J.D.; Kim, H.R.; Seo, D.-W. Analytical Method Development and Dermal Absorption of 4-Amino-3-Nitrophenol (4A3NP), a Hair Dye Ingredient under the Oxidative or Non-Oxidative Condition. Toxics 2024, 12, 340. https://doi.org/10.3390/toxics12050340
Kim HY, Kim YJ, Lee JD, Kim HR, Seo D-W. Analytical Method Development and Dermal Absorption of 4-Amino-3-Nitrophenol (4A3NP), a Hair Dye Ingredient under the Oxidative or Non-Oxidative Condition. Toxics. 2024; 12(5):340. https://doi.org/10.3390/toxics12050340
Chicago/Turabian StyleKim, Hyang Yeon, Yu Jin Kim, Jung Dae Lee, Hak Rim Kim, and Dong-Wan Seo. 2024. "Analytical Method Development and Dermal Absorption of 4-Amino-3-Nitrophenol (4A3NP), a Hair Dye Ingredient under the Oxidative or Non-Oxidative Condition" Toxics 12, no. 5: 340. https://doi.org/10.3390/toxics12050340
APA StyleKim, H. Y., Kim, Y. J., Lee, J. D., Kim, H. R., & Seo, D.-W. (2024). Analytical Method Development and Dermal Absorption of 4-Amino-3-Nitrophenol (4A3NP), a Hair Dye Ingredient under the Oxidative or Non-Oxidative Condition. Toxics, 12(5), 340. https://doi.org/10.3390/toxics12050340






