Rat Hepatocytes Protect against Lead–Cadmium-Triggered Apoptosis Based on Autophagy Activation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Conditions for Cell Culture and Exposure
2.3. Cell Viability Assay
2.4. Nuclear Morphological Observations
2.5. AnnexinV-FITC/PI Apoptosis Analysis
2.6. Detection of ROS Level
2.7. Mitochondrial Membrane Potential (MMP) Determination
2.8. MDC-Stained Observation of Autophagy Vesicles
2.9. LC3 Immunofluorescence Aggregation Site Study
2.10. Transcriptomics
2.11. Real-Time Quantitative PCR Fluorescence Analysis
2.12. Western Blot Analysis
2.13. Statistical Analysis
3. Results
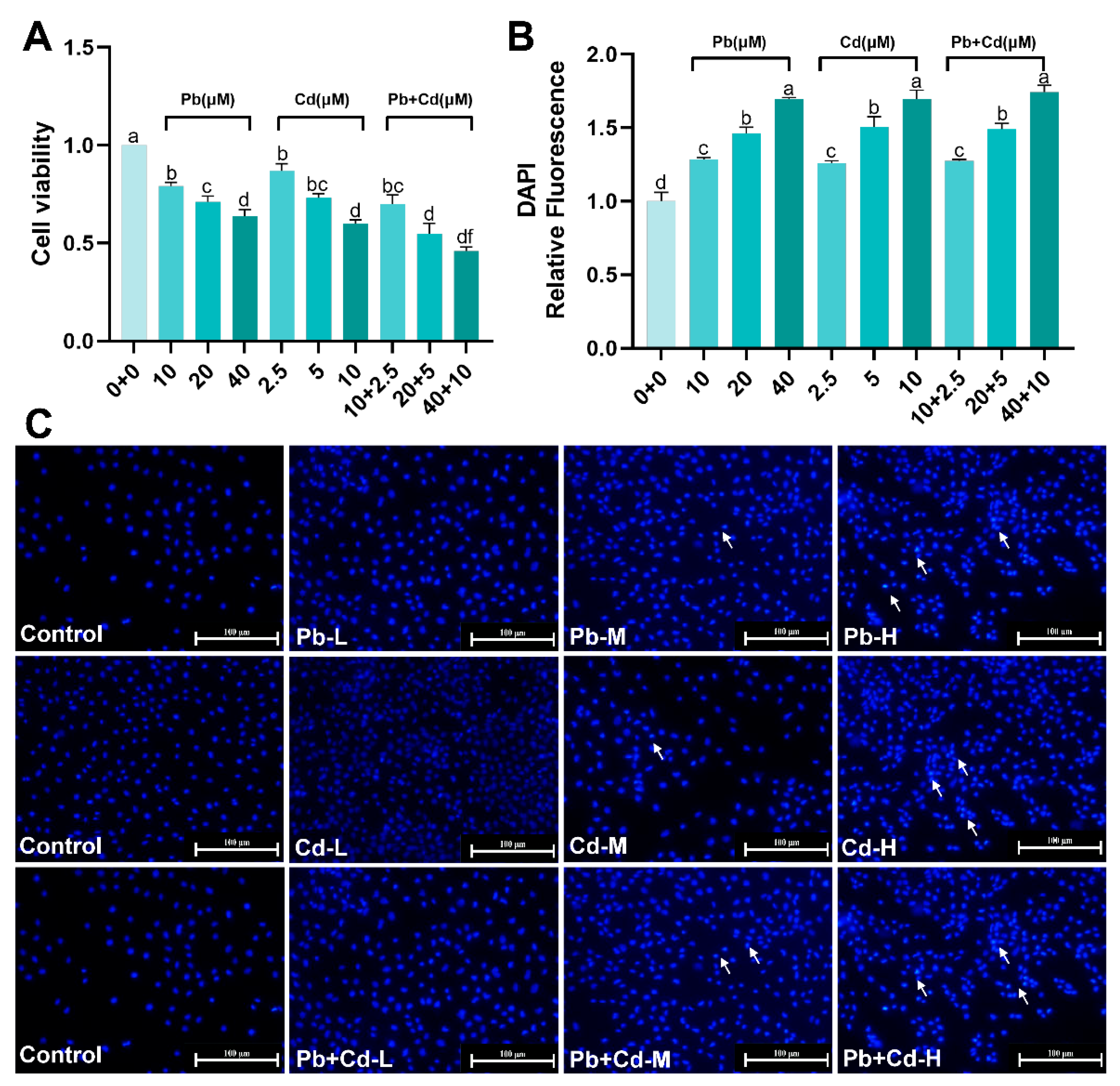
3.1. Cytotoxicity of Pb and Cd in BRL-3A Cells
3.2. Pb and Cd Cause ROS and MMP Changes in BRL-3A Cells
3.3. Pb and Cd Induce Apoptosis in BRL-3A Cells
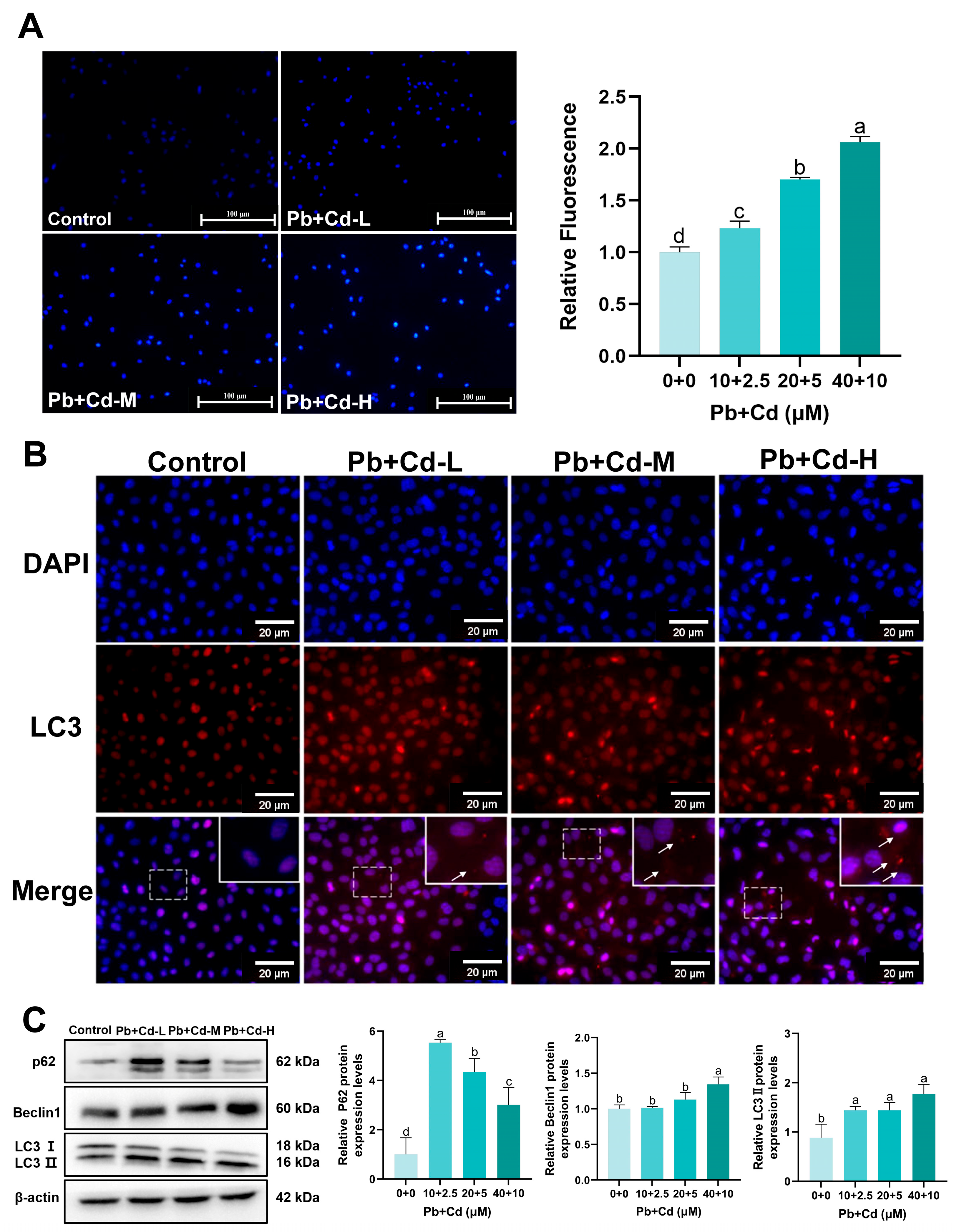
3.4. Pb and Cd Trigger Autophagy in BRL-3A Cells
3.5. GO Enrichment Analysis and Transcriptomic Validation of DEGs
3.6. Autophagy Plays a Protective Role in BRL-3A Cells Exposed to Pb and Cd
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Screening for Pb, Cd, 3-MA, and Rapa Concentrations

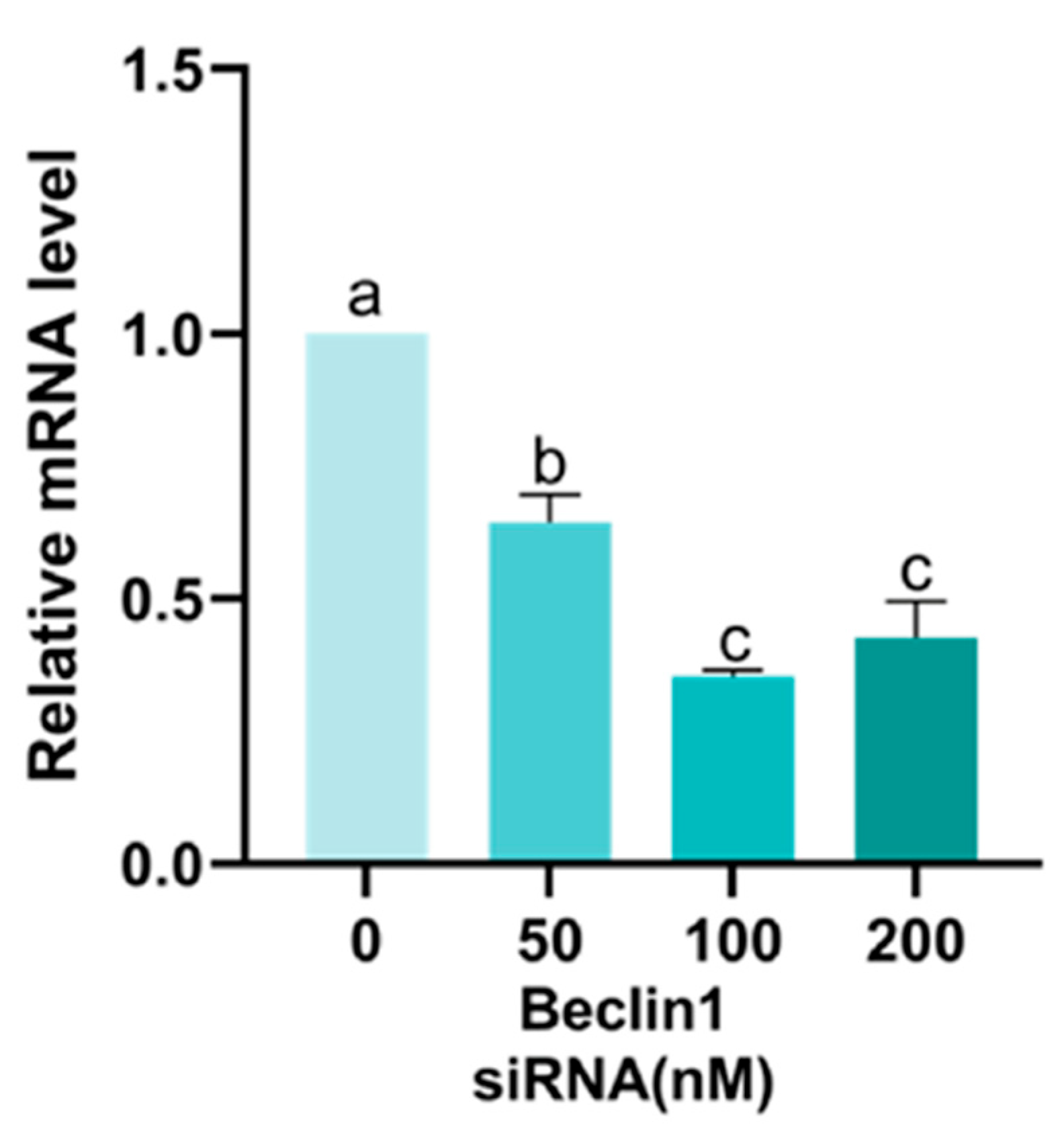
Appendix A.2. Screening of Beclin1 siRNA Concentration
References
- Guo, Y.; Yang, Y.; Li, R.; Liao, X.; Li, Y. Distribution of cadmium and Pb in soil-rice systems and their environmental driving factors at the island scale. Ecotoxicol. Environ. Saf. 2023, 265, 115530. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Jiang, S.; Yan, X.; Qin, Z.; Jia, C.; Li, Z.; Zhang, J.; Huang, R. The mobility of cadmium and Pb in the soil-mulberry-silkworm system. Chemosphere 2020, 242, 125179. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Liu, H.; Banet, T.; Wang, H.; Ippolito, J.A.; Li, L. Cadmium, copper, Pb and zinc accumulation in wild plant species near a Pb smelter. Ecotoxicol. Environ. Saf. 2020, 198, 110683. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Kumar, A.; Gupta, V.K.; Sharma, B. Biochemical and Molecular Bases of Pb-Induced Toxicity in Mammalian Systems and Possible Mitigations. Chem. Res. Toxicol. 2018, 31, 1009–1021. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Jing, D.; Huang, X.; Xiao, Y.; Shu, Z.; Luo, D.; Duan, Y.; He, M.; Xiao, S.; Chen, X. Effects of co-exposure to multiple metals on children′s behavior problems in China. Sci. Total Environ. 2022, 826, 154062. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, R.; Bhattacharya, R.; Chatterjee, M. Biochemical, haematological and histopathological study in relation to time-related cadmium-induced hepatotoxicity in mice. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2000, 13, 231. [Google Scholar]
- Renu, K.; Chakraborty, R.; Haritha, M.; Rajeshwari, K.; Abilash, V.G. Molecular mechanism of heavy metals (Pb, Chromium, Arsenic, Mercury, Nickel and Cadmium) induced hepatotoxicity—A review. Chemosphere 2021, 271, 129735. [Google Scholar] [CrossRef]
- Ma, L.; Liu, J.; Dong, J.; Xiao, Q.; Zhao, J.; Jiang, F. Toxicity of Pb2+ on rat liver mitochondria induced by oxidative stress and mitochondrial permeability transition. Toxicol. Res. 2017, 6, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Matović, V.; Buha, A.; Ðukić-Ćosić, D.; Bulat, Z. Insight into the oxidative stress induced by Pb and/or cadmium in blood, liver and kidneys. Food Chem. Toxicol. 2015, 78, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Pandya, C.D.; Pillai, P.P.; Gupta, S.S. Pb and Cadmium Co-exposure Mediated Toxic Insults on Hepatic Steroid Metabolism and Antioxidant System of Adult Male Rats. Biol. Trace Elem. Res. 2010, 134, 307. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Feng, X.; Shen, J.; Wang, Y.; Zhang, Y.; Zhou, R. Pb intoxication-induced exosomes promote autophagy and apoptosis in renal proximal tubule cells by activating the adenosine 5′-monophosphate-activated protein kinase signaling. Environ. Toxicol. 2023, 38, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Jin, X.; Fan, R.; Xing, M.; Guo, J.; Zhang, Z.; Zhang, J.; Xu, S. Cadmium-mediated miR-30a-GRP78 Pbs to JNK-dependent autophagy in chicken kidney. Chemosphere 2019, 215, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Huang, J.; Yang, J.; Cai, J.; Liu, Q.; Zhang, X.; Bao, J.; Zhang, Z. Cadmium induces apoptosis and autophagy in swine small intestine by downregulating the PI3K/Akt pathway. Environ. Sci. Pollut. Res. Int. 2022, 29, 41207–41218. [Google Scholar] [CrossRef] [PubMed]
- Vaux, D.L. Apoptosis and toxicology—What relevance? Toxicology 2002, 181–182, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Ma, T.; Lv, L.; Yu, Y.; Zhao, M.; Chen, H.; Gao, L. Endoplasmic reticulum stress mediated by ROS participates in cadmium exposure-induced MC3T3-E1 cell apoptosis. Ecotoxicol. Environ. Saf. 2023, 251, 114517. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Liao, J.; Hu, Z.; Li, Q.; Hu, L.; Guo, J.; Li, Y.; Zhang, H.; Pan, J.; Tang, Z. Mitochondria-mediated apoptosis and endoplasmic reticulum stress are involved in the toxicity induced by copper in the porcine spleen. Environ. Sci. Pollut. Res. Int. 2023, 30, 94928–94939. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.B.; Wang, T.; Pan, T.T.; Huang, M.; Ren, W.H.; Xu, G.L.; Amin, H.K.; Kassab, R.B.; Moneim, A.E.A. Senna alexandrina extract supplementation reverses hepatic oxidative, inflammatory, and apoptotic effects of cadmium chloride administration in rats. Environ. Sci. Pollut. Res. 2020, 27, 5981–5992. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; He, Y.; Wang, H.; Zhang, Q. Protective effect of melatonin against chronic cadmium-induced hepatotoxicity by suppressing oxidative stress, inflammation, and apoptosis in mice. Ecotoxicol. Environ. Saf. 2021, 228, 112947. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Kroemer, G. Autophagy in the Pathogenesis of Disease. Cell 2008, 132, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Kroemer, G. Biological Functions of Autophagy Genes: A Disease Perspective. Cell 2019, 176, 11–42. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wang, Y.; An, Y.; Jiao, W.; Xu, Y.; Han, Q.; Teng, X.; Teng, X. Selenium alleviates oxidative stress and autophagy in Pb-treated chicken testes. Theriogenology 2019, 131, 146–152. [Google Scholar] [CrossRef]
- Zou, H.; Sun, J.; Wu, B.; Yuan, Y.; Gu, J.; Bian, J.; Liu, X.; Liu, Z. Effects of Cadmium and/or Pb on Autophagy and Liver Injury in Rats. Biol. Trace Elem. Res. 2020, 198, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Niture, S.; Lin, M.; Qi, Q.; Moore, J.T.; Levine, K.E.; Ferno, R.A.; Kumar, D. Role of Autophagy in Cadmium-Induced Hepatotoxicity and Liver Diseases. J. Toxicol. 2021, 2021, 9564297. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Zhuo, L.; Han, T.; Hu, D.; Yang, X.; Wang, Y.; Yuan, Y.; Gu, J.; Bian, J.; Liu, X.; et al. Autophagy and gap junctional intercellular communication inhibition are involved in cadmium-induced apoptosis in rat liver cells. Biochem. Biophys. Res. Commun. 2015, 459, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Lamark, T.; Sjottem, E.; Bowitz Larsen, K.; Atesoh Awuh, J.; Overvatn, A.; Mcmahon, M.; Hayes, J.D.; Johansen, T. p62/SQSTM1 Is a Target Gene for Transcription Factor NRF2 and Creates a Positive Feedback Loop by Inducing Antioxidant Response Element-driven Gene Transcription. J. Biol. Chem. 2010, 285, 22576–22591. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, S.; Ma, B.; Wang, J.; Chen, J. Di-isononyl phthalate induces apoptosis and autophagy of mouse ovarian granulosa cells via oxidative stress. Ecotoxicol. Environ. Saf. 2022, 242, 113898. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wen, X.; Lin, P.; Chen, H.; Wang, A.; Jin, Y. HERP depletion inhibits zearalenone-induced apoptosis through autophagy activation in mouse ovarian granulosa cells. Toxicol. Lett. 2018, 301, 1–10. [Google Scholar] [CrossRef]
- Yuan, G.; Dai, S.; Yin, Z.; Lu, H.; Zhang, M. Sub-chronic Pb and cadmium co-induce apoptosis protein expression in liver and kidney of rats. Int. J. Clin. Exp. Pathol. 2014, 7, 2905. [Google Scholar] [PubMed]
- Hu, D.; Zou, H.; Han, T.; Xie, J.Z.; Dai, N.N.; Zhuo, L.L.; Gu, J.H.; Bian, J.C.; Yuan, Y.; Liu, X.Z.; et al. Gap junction blockage promotes cadmium-induced apoptosis in BRL 3A derived from Buffalo rat liver cells. J. Vet. Sci. 2016, 17, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.D.; Fergusson, J.E. The concentrations, distribution and sources of cadmium, copper, Pb and zinc in the atmosphere of an urban environment. Sci. Total Environ. 1994, 144, 179. [Google Scholar] [CrossRef]
- Li, C.; Shi, L.; Peng, C.; Yu, G.; Zhang, Y.; Du, Z. Pb-induced cardiomyocytes apoptosis by inhibiting gap junction intercellular communication via autophagy activation. Chem. Biol. Interact. 2021, 337, 109331. [Google Scholar] [CrossRef] [PubMed]
- Yin, H. Research on the Role and Mechanism of Autophagy in NiCl2-Induced Apoptosis in Kidney. Ph.D. thesis, Sichuan Agricultural 481 University, Chengdu, China, 2022. [Google Scholar]
- Wang, C.; Nie, G.; Zhuang, Y.; Hu, R.; Zhang, C. Inhibition of autophagy enhances cadmium-induced apoptosis in duck renal tubular epithelial cells. Ecotoxicol. Environ. Saf. 2020, 205, 111188. [Google Scholar] [CrossRef] [PubMed]
- Siraj, M.; Khisroon, M.; Khan, A.; Zaidi, F.; Ullah, A.; Rahman, G. Bio-monitoring of Tissue Accumulation and Genotoxic Effect of Heavy Metals in Cyprinus carpio from River Kabul Khyber Pakhtunkhwa Pakistan. Bull. Environ. Contam. Toxicol. 2018, 100, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Souza-Arroyo, V.; Fabián, J.J.; Bucio-Ortiz, L.; Miranda-Labra, R.U.; Gomez-Quiroz, L.E.; Gutiérrez-Ruiz, M.C. The mechanism of the cadmium-induced toxicity and cellular response in the liver. Toxicology 2022, 480, 153339. [Google Scholar] [CrossRef] [PubMed]
- Chi, Q.; Liu, T.; Sun, Z.; Tan, S.; Li, S.; Li, S. Involvement of mitochondrial pathway in environmental metal pollutant Pb-induced apoptosis of chicken liver: Perspectives from oxidative stress and energy metabolism. Environ. Sci. Pollut. Res. 2017, 24, 28121–28131. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Cheng, J.; Bao, L.; Zhu, X.; Huang, H. Exposure to waterborne cadmium induce oxidative stress, autophagy and mitochondrial dysfunction in the liver of Procypris merus. Ecotoxicol. Environ. Saf. 2020, 204, 111051. [Google Scholar] [CrossRef] [PubMed]
- Palikaras, K.; Tavernarakis, N. Mitochondrial homeostasis: The interplay between mitophagy and mitochondrial biogenesis. Exp. Gerontol. 2014, 56, 182–188. [Google Scholar] [CrossRef]
- Cao, X.; Fu, M.; Bi, R.; Zheng, X.; Liu, J. Cadmium induced BEAS-2B cells apoptosis and mitochondria damage via MAPK signaling pathway. Chemosphere 2021, 263, 128346. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Muneer, P.M.; Chandra, N.; Haorah, J. Interactions of Oxidative Stress and Neurovascular Inflammation in the Pathogenesis of Traumatic Brain Injury. Mol. Neurobiol. 2015, 51, 966. [Google Scholar] [CrossRef] [PubMed]
- Mu, M.; Zhao, H.; Wang, Y.; Liu, J.; Fei, D.; Xing, M. Arsenic trioxide or/and copper sulfate co-exposure induce glandular stomach of chicken injury via destruction of the mitochondrial dynamics and activation of apoptosis as well as autophagy. Ecotoxicol. Environ. Saf. 2019, 185, 109678. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Uson-Lopez, R.A.; Sikder, M.T.; Tan, G.; Hosokawa, T.; Saito, T.; Kurasaki, M. Ameliorative effects of selenium on arsenic-induced cytotoxicity in PC12 cells via modulating autophagy/apoptosis. Chemosphere 2018, 196, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.D.; Manon, S.; Gonçalves, J.; Saraiva, L.; Côrte-Real, M. The importance of humanized yeast to better understand the role of bcl-2 family in apoptosis: Finding of novel therapeutic opportunities. Curr. Pharm. Des. 2011, 17, 246–255. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dai, S.; Yin, Z.; Yuan, G.; Lu, H.; Jia, R.; Xu, J.; Song, X.; Li, L.; Shu, Y.; Liang, X.; et al. Quantification of metallothionein on the liver and kidney of rats by subchronic Pb and cadmium in combination. Environ. Toxicol. Pharmacol. 2013, 36, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.R. Mechanisms of p53-induced apoptosis. Biochem. Pharmacol. 1999, 58, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of Cells and Tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Dai, N.; Wang, Y.; Xu, C.; Liu, Z. Role of autophagy in cadmium-induced apoptosis of primary rat osteoblasts. Sci. Rep. 2016, 6, 20404. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Ma, S.X.; Qi, Y.M.; Wei, X.; Cai, H.; Dong, L.; Lu, Y.F.; Zhang, Y.P.; Guo, Q.J. Quercetin inhibited cadmium-induced autophagy in the mouse kidney via inhibition of oxidative stress. J. Toxicol. Pathol. 2016, 29, 247–252. [Google Scholar] [CrossRef]
- Chu, B.-X.; Fan, R.-F.; Lin, S.-Q.; Yang, D.-B.; Wang, Z.-Y.; Wang, L. Interplay between autophagy and apoptosis in Pb(II)-induced cytotoxicity of primary rat proximal tubular cells. J. Inorg. Biochem. Interdiscip. J. 2018, 182, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Pu, W.J.; Chu, X.S.; Guo, H.L.; Huang, G.; Cui, T.; Huang, B.Y.; Dai, X.Y.; Zhang, C.Y. The activated ATM/AMPK/mTOR axis promotes autophagy in response to oxidative stress-mediated DNA damage co-induced by molybdenum and cadmium in duck testes. Environ. Pollut. 2023, 316, 11. [Google Scholar] [CrossRef]
- Lv, X.H.; Zhao, D.H.; Cai, S.Z.; Luo, S.Y.; You, T.; Xu, B.L.; Chen, K. Autophagy plays a protective role in cell death of osteoblasts exposure to Pb chloride. Toxicol. Lett. 2015, 239, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Xin-Yu, W.; Heng, Y.; Min-Ge, W.; Du-Bao, Y.; Zhen-Yong, W.; Lin, W. Trehalose protects against cadmium-induced cytotoxicity in primary rat proximal tubular cells via inhibiting apoptosis and restoring autophagic flux. Cell Death Dis. 2017, 8, e3099. [Google Scholar]
- Maiuri, M.C.; Zalckvar, E.; Kimchi, A.; Kroemer, G. Self-eating and self-killing: Crosstalk between autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2007, 8, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.X.; Zhu, H.L.; Shi, X.T.; Nan, Y.; Wang, H. Autophagy in Sertoli cell protects against environmental cadmium-induced germ cell apoptosis in mouse testes. Environ. Pollut. 2021, 270, 116241. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Hu, D.; Han, T.; Zhao, H.; Xie, J.; Liu, X.; Wang, Y.; Gu, J.; Yuan, Y.; Bian, J.; et al. Salidroside ameliorates Cd-induced calcium overload and gap junction dysfunction in BRL 3A rat liver cells. Biol. Trace Elem. Res. 2015, 164, 90–98. [Google Scholar] [CrossRef]
- Guo, H.; Ouyang, Y.; Yin, H.; Cui, H.; Deng, H.; Liu, H.; Jian, Z.; Fang, J.; Zuo, Z.; Wang, X.; et al. Induction of autophagy via the ROS-dependent AMPK-mTOR pathway protects copper-induced spermatogenesis disorder. Redox Biol. 2022, 49, 102227. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zuo, Z.; Zeng, Y.; Ouyang, Y.; Cui, H.; Deng, H.; Zhu, Y.; Deng, J.; Geng, Y.; Ouyang, P.; et al. Autophagy-mediated ferroptosis involved in nickel-induced nephrotoxicity in the mice. Ecotoxicol. Environ. Saf. 2023, 259, 115049. [Google Scholar] [CrossRef] [PubMed]




| Gene Name | Primer Sequence (5′ to 3′) |
|---|---|
| Beclin1 | Forward: AGGAGTTGCCGTTGTACTGTTCTG Reverse: TGCCTCCAGTGTCTTCAATCTTGC |
| BAX | Forward: CCAGGACGCATCCACCAAGAAGC Reverse: TGCCACACGGAAGAAGACCTCTCG |
| CRAT | Forward: CAAGCAGGACTTCATGGATCTACAG Reverse: GGCAGCGTCTCGTTGTCAATC |
| PRDX5 | Forward: CCACCAGGCAGAAGGCAAGG Reverse: CGATTCCCAAAGAGAGACACCAAAG |
| SIAH1 | Forward: CAAAGTGTCCACCATCCCAGAG Reverse: GGTGGCAATACATAGTCAAAGCAG |
| β-actin | Forward: CTAAGGCCAACCGTGAAAAG Reverse: AACACAGCCTGGATGGCTAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, J.; Liu, H.; Yin, T.; Zhou, X.; Song, X.; Zou, Y.; Li, L.; Jia, R.; Fu, Y.; Zhao, X.; et al. Rat Hepatocytes Protect against Lead–Cadmium-Triggered Apoptosis Based on Autophagy Activation. Toxics 2024, 12, 285. https://doi.org/10.3390/toxics12040285
Xue J, Liu H, Yin T, Zhou X, Song X, Zou Y, Li L, Jia R, Fu Y, Zhao X, et al. Rat Hepatocytes Protect against Lead–Cadmium-Triggered Apoptosis Based on Autophagy Activation. Toxics. 2024; 12(4):285. https://doi.org/10.3390/toxics12040285
Chicago/Turabian StyleXue, Junshu, Huimao Liu, Tianyi Yin, Xun Zhou, Xu Song, Yuanfeng Zou, Lixia Li, Renyong Jia, Yuping Fu, Xinghong Zhao, and et al. 2024. "Rat Hepatocytes Protect against Lead–Cadmium-Triggered Apoptosis Based on Autophagy Activation" Toxics 12, no. 4: 285. https://doi.org/10.3390/toxics12040285
APA StyleXue, J., Liu, H., Yin, T., Zhou, X., Song, X., Zou, Y., Li, L., Jia, R., Fu, Y., Zhao, X., & Yin, Z. (2024). Rat Hepatocytes Protect against Lead–Cadmium-Triggered Apoptosis Based on Autophagy Activation. Toxics, 12(4), 285. https://doi.org/10.3390/toxics12040285






