Glycyrrhiza Extract and Curcumin Alleviates the Toxicity of Cadmium via Improving the Antioxidant and Immune Functions of Black Goats
Abstract
1. Introduction
2. Materials and Methods
2.1. The Source of GE, CUR, and Cadmium Chloride
2.2. Experimental Design
2.3. Feeding Management
2.4. Sample Collection and Analysis of the Blood and Serum
2.5. Liver Sample Collection and Pathological Section Preparation
2.6. Statistical Analyses
3. Results
3.1. The Effect of GE and CUR on the Pathological Structure in the Liver of the Guizhou Black Goat
3.2. The Effect of GE and CUR on the Minerals in the Organ and Tissues of the Guizhou Black Goat
3.3. The Effect of GE and CUR on the Physiological Indexes in the Blood of the Guizhou Black Goat
3.4. The Effect of GE and CUR on the Antioxidant Capacity in the Serum and Livers of the Guizhou Black Goat
3.5. The Effect of GE and CUR on the Immune Function in the Serum and Livers of the Guizhou Black Goat
4. Discussion
4.1. The Effect of GE and CUR on the Minerals in the Serum and Livers of the Guizhou Black Goat
4.2. The Effect of GE and CUR on the Antioxidant Capacity in the Serum and Livers of the Guizhou Black Goat
4.3. The Effect of GE and CUR on the Immune Function in the Serum and Livers of the Guizhou Black Goat
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Du, J.; Wang, Z.K.; Liu, J.; Zhong, S.Q.; Wei, C.F. Distribution characteristics of soil heavy metals, their source identification and their changes influenced by anthropogenic cultivation activities in purple hilly regions of Sichuan Basin, China. J. Soil Sci. Plant Nut. 2020, 20, 1080–1091. [Google Scholar] [CrossRef]
- Gao, J.Q.; Yu, Y.; Wang, D.H.; Liu, L.J.; Wang, W.; Hao, X.F.; Dai, H.Z. The content and distribution characteristics of heavy metals in root soils in the Jiajika Lithium resource area, Western Sichuan Province. Rock Min. Anal. 2019, 38, 681–692. [Google Scholar]
- Li, H.X.; Ji, H.B.; Shi, C.J.; Gao, Y.; Zhang, Y.; Xu, X.Y.; Ding, H.J.; Tang, L.; Xing, Y.X. Distribution of heavy metals and metalloids in bulk and particle size fractions of soils from coalmine brownfield and implications on human health. Chemosphere 2017, 172, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Heidari, A.H.; Zamiri, M.J.; Nazem, M.N.; Shirazi, M.R.J.; Akhlaghi, A.; Pirsaraei, Z.A. Detrimental effects of long-term exposure to heavy metals on histology, size and trace elements of testes and sperm parameters in Kermani sheep. Ecotox. Environ. Safe. 2021, 207, 111563. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; He, J.; Luo, L.; Wang, Y.C. The combinations of sulfur and molybdenum fertilization improved antioxidant capacity in grazing Nanjiang brown goat. Biol. Trace Elem. Res. 2022, 200, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Shen, X.Y.; Liu, F.Y.; Luo, L.; Wang, Y.C. Molybdenum fertilization improved antioxidant capacity of grazing Nanjiang brown goat on copper-contaminated pasture. Biol. Trace Elem. Res. 2021, 200, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Wang, Y.C.; Shen, X.Y.; Liu, F.Y. The combinations of sulfur and molybdenum fertilizations improved antioxidant capacity of grazing Guizhou semi-fine wool sheep under copper and cadmium stress. Ecotox. Environ. Safe. 2021, 222, 112520. [Google Scholar] [CrossRef] [PubMed]
- Athmouni, K.; Belhaj, D.; El Feki, A.; Ayadi, H. Optimization, antioxidant properties and GC-MS analysis of periploca angustifolia polysaccharides and chelation therapy on cadmium-induced toxicity in human HepG2 cells line and rat liver. Int. J. Biol. Macromol. 2018, 108, 853–862. [Google Scholar] [CrossRef]
- Souza-Arroyo, V.; Fabián, J.J.; Bucio-Ortiz, L.; Miranda-Labra, R.U.; Gomez-Quiroz, L.E.; Gutiérrez-Ruiz, M.C. The mechanism of the cadmium-induced toxicity and cellular response in the liver. Toxicology 2022, 480, 153339. [Google Scholar] [CrossRef]
- Ferramola, M.L.; Antón, R.I.; Anzulovich, A.C.; Giménez, M.S. Myocardial oxidative stress following sub-chronic and chronic oral cadmium exposure in rats. Environ. Toxicol. Pharmacol. 2011, 32, 17–26. [Google Scholar] [CrossRef]
- Robea, M.A.; Ilie, O.D.; Nicoara, M.N.; Solcan, G.; Romila, L.E.; Ureche, D.; Ciobica, A. Vitamin B12 Ameliorates Pesticide-Induced Sociability Impairment in Zebrafish (Danio rerio): A Prospective Controlled Intervention Study. Animals 2024, 14, 405. [Google Scholar] [CrossRef]
- Paduraru, E.; Flocea, E.I.; Lazado, C.C.; Simionov, I.A.; Nicoara, M.; Ciobica, A.; Faggio, C.; Jijie, R. Vitamin C Mitigates Oxidative Stress and Behavioral Impairments Induced by Deltamethrin and Lead Toxicity in Zebrafish. Int. J. Mol. Sci. 2021, 22, 12714. [Google Scholar] [CrossRef] [PubMed]
- Rarinca, V.; Nicoara, M.N.; Ureche, D.; Ciobica, A. Exploitation of Quercetin’s Antioxidative Properties in Potential Alternative Therapeutic Options for Neurodegenerative Diseases. Antioxidants 2023, 12, 1418. [Google Scholar] [CrossRef]
- Flick, D.F.; Kraybill, H.F.; Dimitroff, J.M. Toxic effects of cadmium: A review. Environ Res. 1971, 4, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Pinot, F.; Kreps, S.E.; Bachelet, M.; Hainaut, P.; Bakonyi, M.; Polla, B.S. Cadmium in the environment: Sources, mechanisms of biotoxicity, and biomarkers. Rev. Environ. Health 2000, 15, 299–323. [Google Scholar] [CrossRef]
- Muntau, H.; Baudo, R. Sources of cadmium, its distribution and turnover in the freshwater environment. IARC Sci. Publ. 1992, 118, 133–148. [Google Scholar]
- Kukongviriyapan, U.; Apaijit, K.; Kukongviriyapan, V. Oxidative stress and cardiovascular dysfunction associated with cadmium exposure: Beneficial effects of curcumin and tetrahydrocurcumin. Tohoku J. Exp. Med. 2016, 239, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Noorafshan, A.; Ashkani-Esfahani, S. A review of therapeutic effects of curcumin. Curr. Pharm. Design 2013, 19, 2032–2046. [Google Scholar]
- Zhang, D. Cadmium Poisoning and Mechanism of Zinc on Cadmium Poisoning; Huazhong Agricultural University: Wuhan, China.
- Niu, Y.; He, J.T.; Zhao, Y.W.; Shen, M.M.; Zhang, L.L.; Zhong, X.; Wang, C.; Wang, T. Effect of curcumin on growth performance, inflammation, insulin level, and lipid metabolism in weaned piglets with IUGR. Animals 2019, 9, 1098. [Google Scholar] [CrossRef]
- Yang, R.; Yuan, B.C.; Ma, Y.S.; Zhou, S.; Liu, Y. The anti-inflammatory activity of licorice, a widely used Chinese herb. Pharm. Biol. 2017, 55, 5–18. [Google Scholar] [CrossRef]
- Sun, Z.G.; Zhao, T.T.; Lu, N.; Yang, Y.A.; Zhu, H.L. Research progress of glycyrrhizic acid on antiviral activity. Mini-Rev. Med. Chem. 2019, 19, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.X. Molecular Mechanism of Glycyrrhizic Acid and Probiotics in Alleviating Vomiting Toxin Harm to Growth and Intestinal Health of Weaned Piglets; Henan Agricultural University: Zhengzhou, China.
- Xiao, K.; Wu, X.M.; Yan, Y.; Xiao, Q. Therapeutic effect of Rhubarb licorice root decoction on liver and kidney injury in rats exposed to cadmium. China Occup. Med. 2019, 46, 331–334. [Google Scholar]
- Liu, Z.P.; Ma, Z.; Li, W.F.; Cheng, X.F.; Hui, T.C. Toxicological study of cadmium on sheep. Acta Veter. Zoot Sin. 1996, 27, 546–553. [Google Scholar]
- Shen, X.Y.; Chi, Y.K.; Xiong, K.N. The effect of heavy metal contamination on humans and animals in the vicinity of a zinc smelting facility. PLoS ONE 2019, 14, e0207423. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; He, J.; Shen, X.Y. Effects of nano-selenium poisoning on immune function in the Wumeng semi-fine wool sheep. Biol. Trace Elem. Res. 2021, 199, 2919–2924. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Wang, Y.C.; Shen, X.Y. Effects of sulfur fertilization on antioxidant capacity of Wumeng semi-fine wool sheep in the Wumeng Prairie. Pol. J. Environ. Stud. 2021, 30, 3919–3926. [Google Scholar] [CrossRef]
- Li, Y.F.; Liu, H.W.; He, J.; Shen, X.Y.; Zhao, K.; Wang, Y.C. The effects of oral administration of molybdenum fertilizers on immune function of Nanjiang brown goat grazing on natural pastures contaminated by mixed heavy metal. Biol. Trace Elem. Res. 2022, 200, 2750–2757. [Google Scholar] [CrossRef]
- Bao, S.D. Soil Agrochemical Analysis; China Agricultural Publishing House: Beijing, China, 2000; pp. 22–42. [Google Scholar]
- Cui, H.M.; Chen, H.T.; Deng, J.L. Pathological study on experimental copper poisoning in Ducklings. Acta Veter. Zoot Sin. 2005, 36, 715–721. [Google Scholar]
- Kan, X.Q.; Dong, Y.Q.; Feng, L.; Zhou, M.; Hou, H.B. Contamination and health risk assessment of heavy metals in China’s lead-zinc mine tailings: A meta-analysis. Chemosphere 2021, 267, 128909. [Google Scholar] [CrossRef]
- Tokumoto, M.; Lee, J.Y.; Fujiwara, Y.; Satoh, M. Long-term exposure to cadmium causes hepatic iron deficiency through the suppression of iron-transport-related gene expression in the proximal duodenum. Toxics 2023, 11, 641. [Google Scholar] [CrossRef]
- Nishito, Y.; Kambe, T. Absorption mechanisms of iron, copper, and zinc: An overview. J. Nutr. Sci. Vitaminol. 2018, 64, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The effects of cadmium toxicity. Int. J. Environ. Res. Public. Health 2020, 17, 3782. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Q.; Zhang, S.; Tong, J.Y.; Teng, X.J.; Zhang, Z.Y.; Li, S.; Teng, X.H. Whole transcriptome-based miRNA-mRNA network analysis revealed the mechanism of inflammation-immunosuppressive damage caused by cadmium in common carp spleens. Sci. Total Environ. 2020, 717, 137081. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Shah, S.W.A.; Zhou, Q.; Yin, X.J.; Teng, X.H. The contributions of miR-25-3p, oxidative stress, and heat shock protein in a complex mechanism of autophagy caused by pollutant cadmium in common carp (Cyprinus carpio L.) hepatopancreas. Environ. Pollut. 2021, 287, 117554. [Google Scholar] [CrossRef]
- Li, Y.F.; Fan, M.D.; Qiu, Q.Y.; Wang, Y.C.; Shen, X.Y.; Zhao, K. Nano-selenium and macleaya cordata extracts improved immune function and reduced oxidative damage of sows and IUGR piglets after heat stress of sows in late gestation. Biol. Trace Elem. Res. 2022, 200, 5081–5090. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P. Nutrients and oxidative stress: Friend or foe? Oxid. Med. Cell Longev. 2018, 2018, 9719584. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.H.; Yang, S.W.; Chen, F.; Guan, W.T.; Zhang, S.A. Nutritional strategies to alleviate oxidative stress in sows. Anim. Nutr. 2022, 9, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Jan, A.T.; Azam, M.; Siddiqui, K.; Ali, A.; Choi, I.; Haq, Q.M.R. Heavy metals and human health: Mechanistic insight into toxicity and counter defense system of antioxidants. Int. J. Mol. Sci. 2015, 16, 29592–29630. [Google Scholar] [CrossRef] [PubMed]
- Hasanzadeh, S.; Read, M.I.; Bland, A.R.; Majeed, M.; Jamialahmadi, T.; Sahebkar, A. Curcumin: An inflammasome silencer. Pharmacol. Res. 2020, 159, 104921. [Google Scholar] [CrossRef]
- You, T. Effects of licorice extract on growth performance, immune function and intestinal health of weaned piglets; Sichuan Agricultural University: Yaan, China.
- Ji, S.; Li, Z.W.; Song, W.; Wang, Y.R.; Liang, W.F.; Li, K.; Tang, S.N.; Wang, Q.; Qiao, X.; Zhou, D.M.; et al. Bioactive constituents of glycyrrhiza uralensis (licorice): Discovery of the effective components of a traditional herbal medicine. J. Nat. Prod. 2016, 79, 281–292. [Google Scholar] [CrossRef]
- Kostaropoulos, I.; Kalmanti, D.; Theodoropoulou, B.; Loumbourdis, N.S. Effects of exposure to a mixture of cadmium and chromium on detoxification enzyme (GST, P450-MO) activities in the frog Rana ridibunda. Ecotoxicology 2005, 14, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Tamber, S.S.; Bansal, P.; Sharma, S.; Singh, R.B.; Sharma, R. Biomarkers of liver diseases. Mol. Biol. Rep. 2023, 50, 7815–7823. [Google Scholar] [CrossRef] [PubMed]
- Miyake, S. The mechanism of release of hepatic enzymes in various liver diseases. II. Altered activity ratios of GOT to GPT in serum and liver of patients with liver diseases. Acta Med. Okayama 1979, 33, 343–358. [Google Scholar] [PubMed]
- Vojdani, A. Elevated IgG antibody to aluminum bound to human serum albumin in patients with Crohn’s, Celiac and Alzheimer’s disease. Toxics 2021, 9, 212. [Google Scholar] [CrossRef] [PubMed]
- Ehrenstein, M.R.; Notley, C.A. The importance of natural IgM: Scavenger, protector and regulator. Nat. Rev. Immunol. 2010, 10, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Chen, W.; Xia, B.; Xiang, Y.; Shen, Z.; Han, Y.; Xue, S. Ammonia toxicity in the Bighead Carp (Aristichthys nobilis): Hematology, antioxidation, immunity, inflammation and stress. Toxics 2023, 11, 243. [Google Scholar] [CrossRef]
- ElShebiney, S.; Elgohary, R.; El-Shamarka, M.; Mowaad, N.; Abulseoud, O.A. Natural polyphenols-resveratrol, quercetin, magnolol, and β-catechin-block certain aspects of heroin addiction and modulate striatal IL-6 and TNF-α. Toxics 2023, 11, 379. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, P. Cellular and metabolic mechanisms of nutrient actions in immune function. Eur. J. Clin. Nutr. 2021, 75, 1328–1331. [Google Scholar] [CrossRef]
- Zhang, K.X.; Gu, X.D.; Lan, J.; Zhang, Y.M.; Pervez, A.K.; Liu, Z.Y.; Li, S. Selenium-deficient diet induces inflammatory response in the pig adrenal glands by activating TLR4/NF-κB pathway via miR-30d-R_1. Metallomics 2020, 13, mfab037. [Google Scholar]
- Guan, M.C.; Tang, W.H.; Xu, Z.; Sun, J. Effects of selenium-enriched protein from ganoderma lucidum on the levels of IL-1β and TNF-α, oxidative stress, and NF-κB activation in ovalbumin-induced asthmatic mice. Evid. Based Complement. Alt. 2014, 2014, 1–6. [Google Scholar]
- Raphael, T.J.; Kuttan, G. Effect of naturally occurring triterpenoids ursolic acid and glycyrrhizic acid on the cell-mediated immune responses of metastatic tumor-bearing animals. Immunopharm. Immunot. 2008, 30, 243–255. [Google Scholar] [CrossRef] [PubMed]
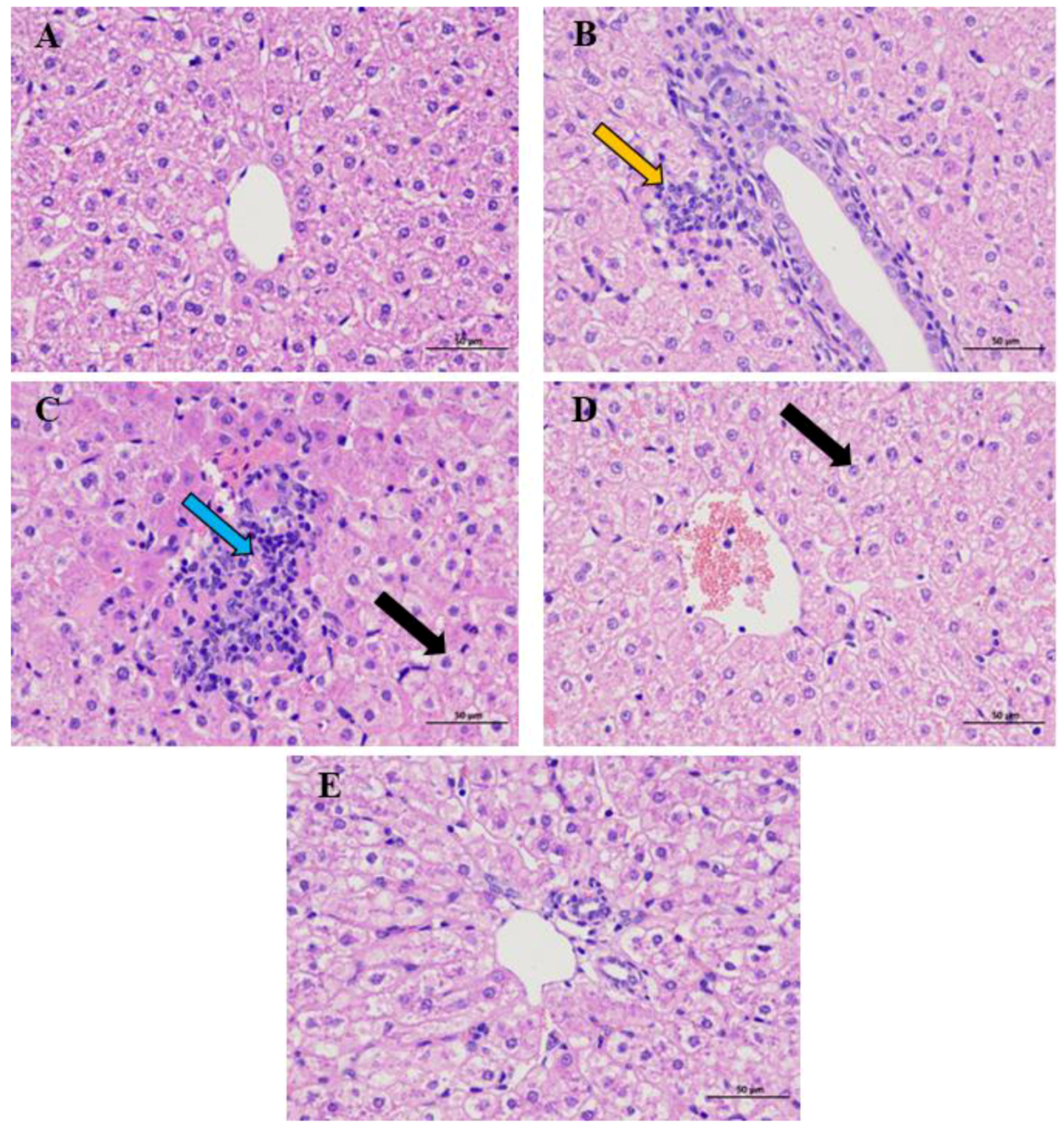
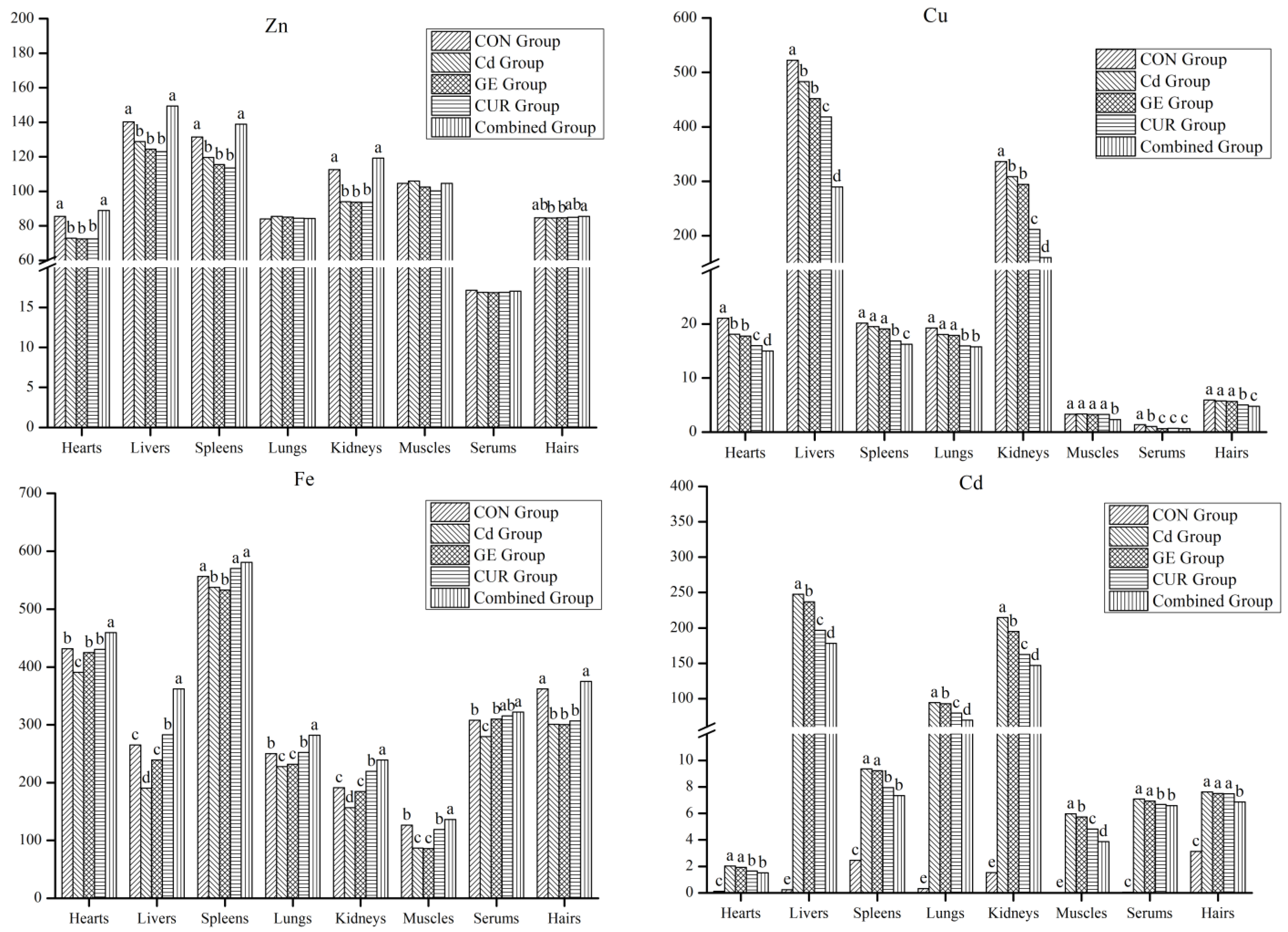
| Items | Serums | Items | Blood |
|---|---|---|---|
| Zn (µg mL−1) | 16.75 ± 0.42 | Hb (g L−1) | 95.82 ± 4.25 |
| Cu (µg mL−1) | 0.73 ± 0.16 | RBC (1012 L−1) | 9.76 ± 1.18 |
| Fe (µg mL−1) | 287.28 ± 22.35 | PCV (%) | 39.75 ± 3.26 |
| Cd (µg mL−1) | 0.12 ± 0.05 | WBC (109 L−1) | 9.12 ± 0.66 |
| Items | CON Group | Cd Group | GE Group | CUR Group | Combined Group | SEM | p Value | ||
|---|---|---|---|---|---|---|---|---|---|
| GE | CUR | GE × CUR | |||||||
| Hb (g L−1) | 110.38 a | 91.58 b | 109.91 a | 110.20 a | 111.26 a | 1.85 | <0.001 | <0.001 | <0.001 |
| RBC (1012 L−1) | 11.04 a | 9.83 b | 11.09 a | 11.08 a | 11.08 a | 0.12 | <0.001 | <0.001 | <0.001 |
| PCV (%) | 42.71 a | 41.00 c | 42.28 a | 42.05 ab | 41.88 b | 0.11 | <0.001 | 0.001 | <0.001 |
| WBC (109 L−1) | 8.61 b | 8.98 a | 8.63 b | 8.70 b | 8.65 b | 0.03 | <0.001 | 0.002 | 0.001 |
| Items | CON Group | Cd Group | GE Group | CUR Group | Combined Group | SEM | p Value | ||
|---|---|---|---|---|---|---|---|---|---|
| GE | CUR | GE × CUR | |||||||
| Serums | |||||||||
| SOD (IU mL−1) | 78.62 b | 53.47 c | 85.24 b | 87.46 b | 96.66 a | 3.25 | <0.001 | <0.001 | <0.001 |
| GSH-Px (IU mL−1) | 38.90 a | 28.67 c | 30.97 b | 32.07 b | 40.64 a | 1.02 | <0.001 | <0.001 | <0.001 |
| CAT (IU mL−1) | 1.58 a | 0.96 c | 1.35 b | 1.42 b | 1.63 a | 0.05 | <0.001 | <0.001 | 0.038 |
| MDA (nmol mL−1) | 24.28 c | 34.40 a | 27.18 b | 25.91 b | 23.27 c | 0.91 | <0.001 | <0.001 | <0.001 |
| GST (IU mL−1) | 8.92 b | 9.65 a | 8.73 b | 8.58 b | 8.16 c | 0.23 | <0.001 | <0.001 | 0.042 |
| GPT (IU L−1) | 25.31 b | 28.35 a | 26.28 b | 25.93 b | 25.62 b | 0.19 | <0.001 | <0.001 | 0.368 |
| GLT (IU L−1) | 112.08 b | 125.05 a | 110.68 b | 112.17 b | 108.92 b | 0.22 | <0.001 | <0.001 | 0.273 |
| Livers | |||||||||
| SOD (IU g−1) | 59.08 a | 35.24 c | 48.93 b | 50.51 b | 59.81 a | 2.05 | <0.001 | <0.001 | 0.128 |
| GSH-Px (IU g−1) | 25.27 b | 20.41 c | 25.24 b | 26.36 b | 30.44 a | 0.79 | <0.001 | <0.001 | 0.452 |
| CAT (IU g−1) | 0.65 c | 0.63 c | 1.09 b | 1.20 b | 1.38 a | 0.06 | <0.001 | <0.001 | 0.002 |
| MDA (nmol g−1) | 4.78 b | 6.50 a | 4.95 b | 4.75 b | 4.53 b | 0.19 | 0.001 | <0.001 | 0.008 |
| Items | CON Group | Cd Group | GE Group | CUR Group | Combined Group | SEM | p Value | ||
|---|---|---|---|---|---|---|---|---|---|
| GE | CUR | GE × CUR | |||||||
| Serums | |||||||||
| IgG (g L−1) | 36.19 ab | 33.19 b | 37.83 ab | 38.29 ab | 39.72 a | 0.55 | <0.001 | <0.001 | <0.001 |
| IgM (g L−1) | 1.68 | 1.66 | 1.67 | 1.69 | 1.70 | 0.02 | 0.762 | 0.438 | 0.929 |
| IgA (g L−1) | 2.03 a | 1.65 c | 1.91 ab | 1.85 b | 2.01 a | 0.04 | <0.001 | 0.004 | 0.231 |
| IL-6 (ng L−1) | 78.07 b | 93.07 a | 81.64 b | 79.43 b | 70.11 c | 1.87 | <0.001 | <0.001 | 0.271 |
| IL-1β (ng L−1) | 6.24 c | 7.54 a | 6.63 b | 6.47 bc | 6.21 c | 0.11 | <0.001 | <0.001 | 0.001 |
| TNF-α (ng L−1) | 0.74 b | 0.84 a | 0.77 b | 0.75 b | 0.73 b | 0.01 | 0.001 | 0.007 | 0.106 |
| Livers | |||||||||
| IgG (g kg−1) | 42.65 a | 34.77 c | 41.95 b | 42.09 ab | 42.30 a | 0.68 | <0.001 | <0.001 | <0.001 |
| IgM (g kg−1) | 18.68 | 18.64 | 18.69 | 18.75 | 18.79 | 0.03 | 0.386 | 0.071 | 0.926 |
| IgA (g kg−1) | 10.85 a | 5.47 c | 9.51 b | 9.97 b | 11.05 a | 0.47 | <0.001 | <0.001 | <0.001 |
| IL-6 (ng kg−1) | 0.67 b | 0.82 a | 0.68 b | 0.67 b | 0.62 b | 0.02 | 0.001 | <0.001 | 0.082 |
| IL-1β (ng kg−1) | 6.62 bc | 7.74 a | 6.96 b | 6.67 bc | 6.49 c | 0.11 | <0.001 | <0.001 | 0.011 |
| TNF-α (ng kg−1) | 5.48 c | 7.05 a | 6.34 b | 6.39 b | 5.24 c | 0.14 | <0.001 | <0.001 | 0.026 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ran, Y.; Shen, X.; Li, Y. Glycyrrhiza Extract and Curcumin Alleviates the Toxicity of Cadmium via Improving the Antioxidant and Immune Functions of Black Goats. Toxics 2024, 12, 284. https://doi.org/10.3390/toxics12040284
Ran Y, Shen X, Li Y. Glycyrrhiza Extract and Curcumin Alleviates the Toxicity of Cadmium via Improving the Antioxidant and Immune Functions of Black Goats. Toxics. 2024; 12(4):284. https://doi.org/10.3390/toxics12040284
Chicago/Turabian StyleRan, Yang, Xiaoyun Shen, and Yuanfeng Li. 2024. "Glycyrrhiza Extract and Curcumin Alleviates the Toxicity of Cadmium via Improving the Antioxidant and Immune Functions of Black Goats" Toxics 12, no. 4: 284. https://doi.org/10.3390/toxics12040284
APA StyleRan, Y., Shen, X., & Li, Y. (2024). Glycyrrhiza Extract and Curcumin Alleviates the Toxicity of Cadmium via Improving the Antioxidant and Immune Functions of Black Goats. Toxics, 12(4), 284. https://doi.org/10.3390/toxics12040284






