Toxicological Effects of Naturally Occurring Endocrine Disruptors on Various Human Health Targets: A Rapid Review
Abstract
1. Introduction
2. Materials and Methods
- Development of a search strategy to identify the studies published in a scientific database over the last five years.
- Strategy for analyzing the results obtained applying a predefined set of eligibility criteria.
- Data extraction.
2.1. Research Strategy
2.2. Analysis Strategy
2.3. Title and Abstract Screening Step
2.4. Full-Text Screening Step
2.5. Data Extraction
- Origin of the N-EDCs
- Class of the N-EDCs
- N-EDCs
- Exposure route
- Target systems affected (including specific exposure windows)
- Affected organ or function
3. Results
3.1. Research Strategy
3.2. Analysis Strategy
3.3. Data Extraction
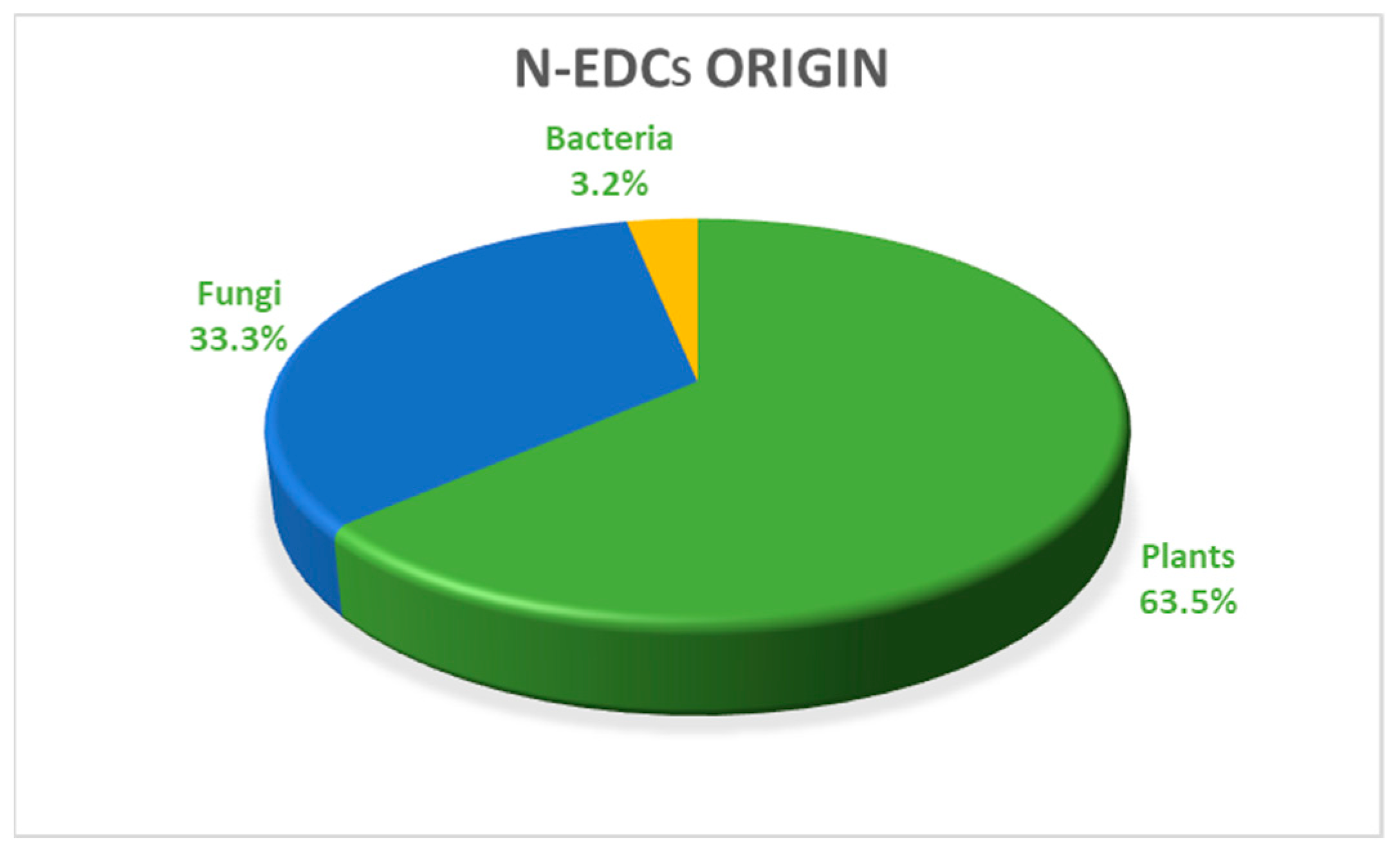
3.3.1. Origin of the N-EDCs
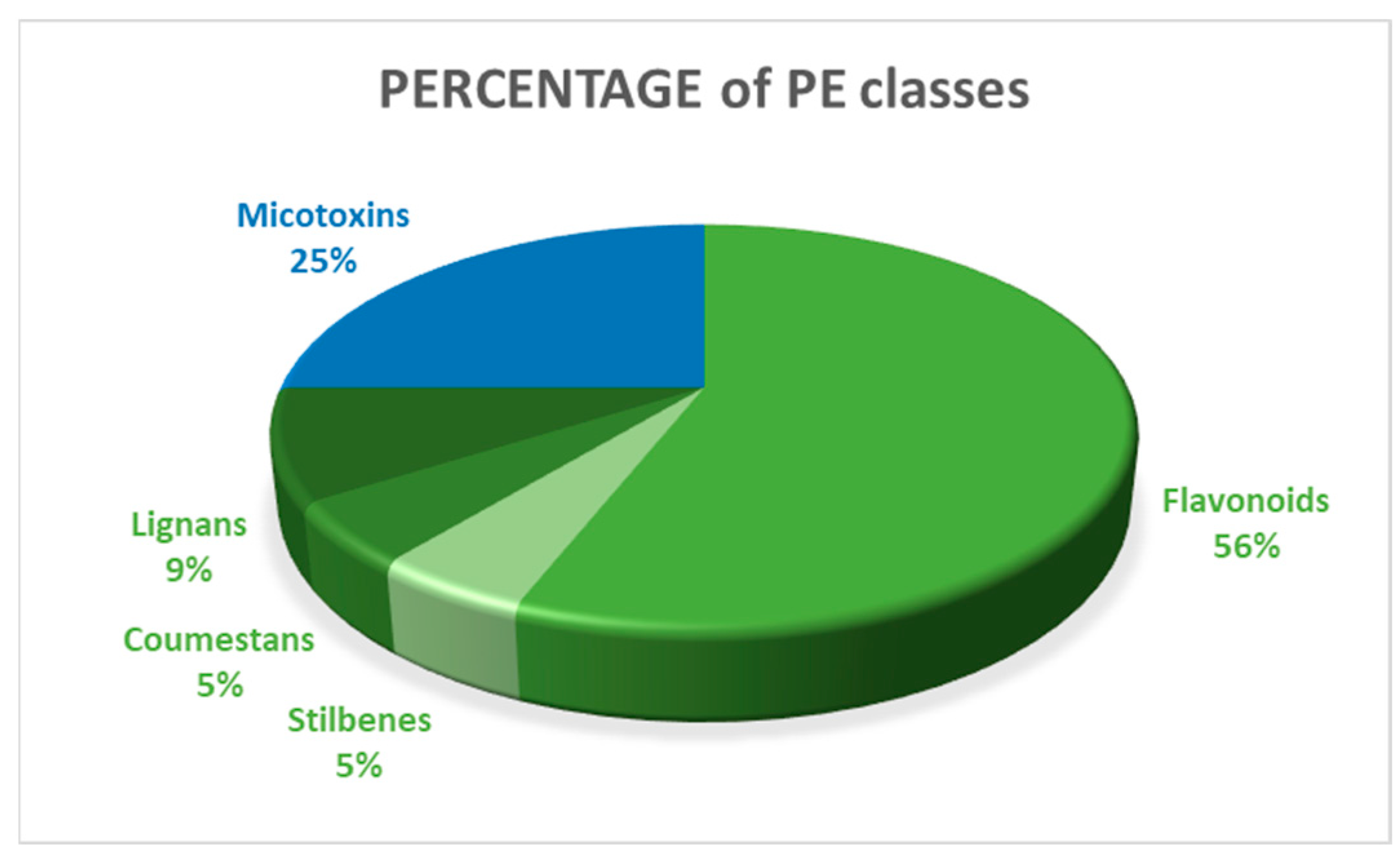
3.3.2. Class of N-EDCs
3.3.3. Natural Endocrine-Disruptor Compounds (N-EDCs)
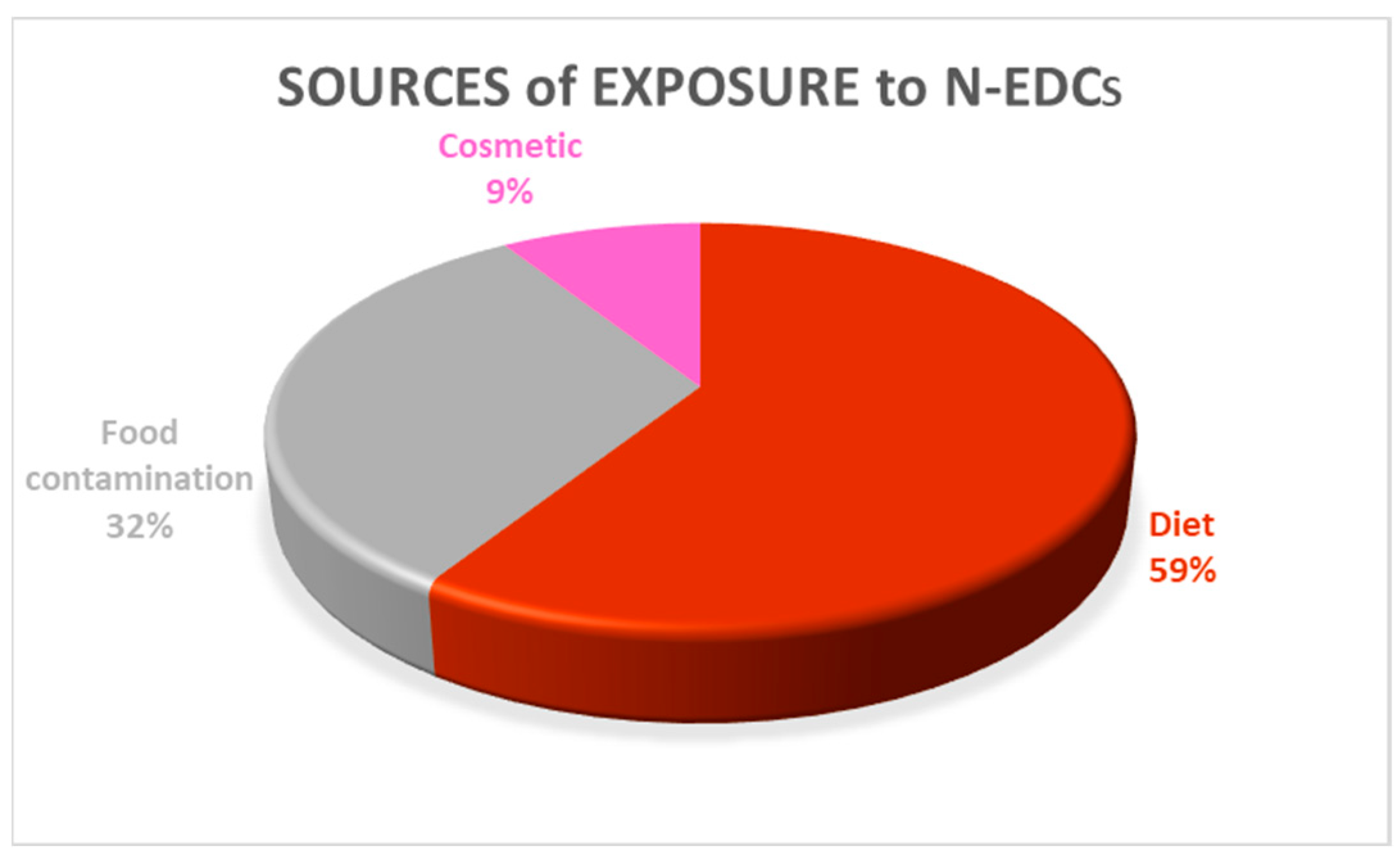
3.3.4. N-EDCs and Human Health
4. Discussion
4.1. The Characterization of N-EDCs
4.2. The Male Reproductive System and N-EDCs
4.3. The Female Reproductive System and N-EDCs
4.4. The Endocrine, Nervous, Cardiovascular and Bone Systems and Adipose Tissue and N-EDCs
4.5. Pregnancy and N-EDCs
4.6. Children and N-EDCs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kabir, E.R.; Rahman, M.S.; Rahman, I. A review on endocrine disruptors and their possible impacts on human health. Environ. Toxicol. Pharmacol. 2015, 40, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Demeneix, B.; Slama, R. Endocrine Disruptors: From Scientific Evidence to Human Health Protection; Policy Department for Citizens’ Rights and Constitutional Affairs European Parliament: Brussels, Belgium, 2019. [Google Scholar]
- Autrup, H.; Barile, F.A.; Berry, S.C.; Blaauboer, B.J.; Boobis, A.; Bolt, H.; Borgert, C.J.; Dekant, W.; Dietrich, D.; Domingo, J.L.; et al. Human exposure to synthetic endocrine disrupting chemicals (S-EDCs) is generally negligible as compared to natural compounds with higher or comparable endocrine activity. How to evaluate the risk of the S-EDCs? Environ. Toxicol. Pharmacol. 2020, 78, 103396. [Google Scholar] [CrossRef] [PubMed]
- Cano, R.; Pérez, J.L.; Dávila, L.A.; Ortega, Á.; Gómez, Y.; Valero-Cedeño, N.J.; Parra, H.; Manzano, A.; Castro, T.I.V.; Díaz Albornoz, M.P.; et al. Role of endocrine-disrupting chemicals in the pathogenesis of non-alcoholic fatty liver disease: A comprehensive review. Int. J. Mol. Sci. 2021, 22, 4807. [Google Scholar] [CrossRef] [PubMed]
- Rami, Y.; Ebrahimpour, K.; Maghami, M.; Shoshtari-Yeganeh, B.; Kelishadi, R. The Association Between Heavy Metals Exposure and Sex Hormones: A Systematic Review on Current Evidence. Biol. Trace Elem. Res. 2022, 200, 3491–3510. [Google Scholar] [CrossRef] [PubMed]
- Messina, M.; Mejia, S.B.; Cassidy, A.; Duncan, A.; Kurzer, M.; Nagato, C.; Ronis, M.; Rowland, I.; Sievenpiper, J.; Barnes, S. Neither soyfoods nor isoflavones warrant classification as endocrine disruptors: A technical review of the observational and clinical data. Crit. Rev. Food Sci. Nutr. 2022, 62, 5824–5885. [Google Scholar] [CrossRef]
- Szukiewicz, D. Insight into the Potential Mechanisms of Endocrine Disruption by Dietary Phytoestrogens in the Context of the Etiopathogenesis of Endometriosis. Int. J. Mol. Sci. 2023, 24, 12195. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Pal, R.; Srivastava, P.; Misra, G.; Shukla, Y.; Sharma, P.K. Exposure of androgen mimicking environmental chemicals enhances proliferation of prostate cancer (LNCaP) cells by inducing AR expression and epigenetic modifications. Environ. Pollut. 2021, 272, 116397. [Google Scholar] [CrossRef] [PubMed]
- Men, Y.; Zhao, Y.; Zhang, P.; Zhang, H.; Gao, Y.; Liu, J.; Feng, Y.; Li, L.; Shen, W.; Sun, Z.; et al. Gestational exposure to low-dose zearalenone disrupting offspring spermatogenesis might be through epigenetic modifications. Basic Clin. Pharmacol. Toxicol. 2019, 125, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhao, Y.; Zhang, H.; Zhang, P.; Liu, J.; Feng, Y.; Men, Y.; Li, L.; Shen, W.; Sun, Z.; et al. Pubertal exposure to low doses of zearalenone disrupting spermatogenesis through ERα related genetic and epigenetic pathways. Toxicol. Lett. 2019, 315, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Urbanek, K.A.; Kowalska, K.; Habrowska-Górczyńska, D.E.; Kozieł, M.J.; Domińska, K.; Piastowska-Ciesielska, A.W. Revealing the Role of Alternariol in the Local Steroidogenesis in Human Prostate Normal and Cancer Cells. Int. J. Mol. Sci. 2023, 24, 9513. [Google Scholar] [CrossRef] [PubMed]
- Balló, A.; Busznyákné Székvári, K.; Czétány, P.; Márk, L.; Török, A.; Szántó, Á.; Máté, G. Estrogenic and Non-Estrogenic Disruptor Effect of Zearalenone on Male Reproduction: A Review. Int. J. Mol. Sci. 2023, 24, 1578. [Google Scholar] [CrossRef] [PubMed]
- Kinkade, C.W.; Rivera-Núñez, Z.; Gorcyzca, L.; Aleksunes, L.M.; Barrett, E.S. Impact of fusarium-derived mycoestrogens on female reproduction: A systematic review. Toxins 2021, 13, 373. [Google Scholar] [CrossRef] [PubMed]
- Yunes, J.S. Cyanobacterial Toxins. In Cyanobacteria: From Basic Science to Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 443–458. [Google Scholar] [CrossRef]
- de Moraes, A.C.N.; Caires, F.O.; Imperio, G.E.; Nóbrega, R.H.; Ortiga-Carvalho, T.M.; de Magalhães, V.F. Cylindrospermopsin Disrupts Estrous Cycle and Increases Spermatogenesis in Mice. Reprod. Sci. 2022, 29, 2876–2884. [Google Scholar] [CrossRef] [PubMed]
- Barbaud, A.; Lafforgue, C. Risks associated with cosmetic ingredients. Ann. De Dermatol. De Venereol. 2021, 148, 77–93. [Google Scholar] [CrossRef] [PubMed]
- Desmawati, D.; Sulastri, D. Phytoestrogens and their health effect. Open Access Maced. J. Med. Sci. 2019, 7, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Qasem, R.J. The estrogenic activity of resveratrol: A comprehensive review of in vitro and in vivo evidence and the potential for endocrine disruption. Crit. Rev. Toxicol. 2020, 50, 439–462. [Google Scholar] [CrossRef] [PubMed]
- Grgic, D.; Betschler, A.; Früholz, R.; Novak, B.; Varga, E.; Marko, D. Estrogenic in vitro evaluation of zearalenone and its phase I and II metabolites in combination with soy isoflavones. Arch. Toxicol. 2022, 96, 3385–3402. [Google Scholar] [CrossRef] [PubMed]
- Salsano, S.; Pérez-Debén, S.; Quiñonero, A.; González-Martín, R.; Domínguez, F. Phytoestrogen exposure alters endometrial stromal cells and interferes with decidualization signaling. Fertil. Steril. 2019, 112, 947–958.e3. [Google Scholar] [CrossRef] [PubMed]
- Caserta, D.; De Marco, M.P.; Besharat, A.R.; Costanzi, F. Endocrine Disruptors and Endometrial Cancer: Molecular Mechanisms of Action and Clinical Implications, A Systematic Review. Int. J. Mol. Sci. 2022, 23, 2956. [Google Scholar] [CrossRef]
- Oliveira, J.M.; Oliveira, I.M.; Sleiman, H.K.; Dal Forno, G.O.; Romano, M.A.; Romano, R.M. Consumption of soy isoflavones during the prepubertal phase delays puberty and causes hypergonadotropic hypogonadism with disruption of hypothalamic-pituitary gonadotropins regulation in male rats. Toxicol. Lett. 2022, 369, 1–11. [Google Scholar] [CrossRef] [PubMed]
- An, B.H.; Jeong, H.; Kim, J.H.; Park, S.; Jeong, J.H.; Kim, M.J.; Chang, M. Estrogen receptor-mediated transcriptional activities of spent coffee grounds and spent coffee grounds compost, and their phenolic acid constituents. J. Agric. Food Chem. 2019, 67, 8649–8659. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, J.T.; Li, Y.; Arao, Y.; Naidu, A.; Coons, L.A.; DIaz, A.; Korach, K.S. Lavender Products Associated with Premature Thelarche and Prepubertal Gynecomastia: Case Reports and Endocrine-Disrupting Chemical Activities. J. Clin. Endocrinol. Metab. 2019, 104, 5393–5405. [Google Scholar] [CrossRef] [PubMed]
- Garritty, C.; Gartlehner, G.; Nussbaumer-Streit, B.; King, V.J.; Hamel, C.; Kamel, C.; Affengruber, L.; Stevens, A. Cochrane Rapid Reviews Methods Group offers evidence-informed guidance to conduct rapid reviews. J. Clin. Epidemiol. 2021, 130, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Garritty, C.; Hamel, C.; Hersi, M.; Butler, C.; Monfaredi, Z.; Stevens, A.; Nussbaumer-Streit, B.; Cheng, W.; Moher, D. Assessing how information is packaged in rapid reviews for policy-makers and other stakeholders: A cross-sectional study. Health Res. Policy Syst. 2020, 18, 112. [Google Scholar] [CrossRef] [PubMed]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef] [PubMed]
- Sridevi, V.; Naveen, P.; Karnam Venkat, S.; Reddy, P.R.; Arifullah, M. Beneficiary and Adverse Effects of Phytoestrogens: A Potential Constituent of Plant-based Diet. Curr. Pharm. Des. 2020, 27, 802–815. [Google Scholar] [CrossRef] [PubMed]
- Solano, F.; Hernández, E.; Juárez-Rojas, L.; Rojas-Maya, S.; López, G.; Romero, C.; Casillas, F.; Betancourt, M.; López, A.; Heidari, R.; et al. Reproductive disruption in adult female and male rats prenatally exposed to mesquite pod extract or daidzein. Reprod. Biol. 2022, 22, 100683. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Mo, J.; Wang, Y.; Ni, C.; Li, X.; Zhu, Q.; Ge, R.S. Endocrine disruptors of inhibiting testicular 3β-hydroxysteroid dehydrogenase. Chem.-Biol. Interact. 2019, 303, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.; Boisvert, A.; Malusare, P.; Culty, M. Impact of Fetal Exposure to Endocrine Disrupting Chemical Mixtures on FOXA3 Gene and Protein Expression in Adult Rat Testes. Int. J. Mol. Sci. 2023, 24, 1211. [Google Scholar] [CrossRef] [PubMed]
- Imai, H.; Nishikawa, H.; Suzuki, A.; Kodama, E.; Iida, T.; Mikura, K.; Hashizume, M.; Kigawa, Y.; Tadokoro, R.; Sugisawa, C.; et al. Secondary Hypogonadism due to Excessive Ingestion of Isoflavone in a Man. Intern. Med. 2022, 61, 2899–2903. [Google Scholar] [CrossRef]
- Czarnywojtek, A.; Jaz, K.; Ochmańska, A.; Zgorzalewicz-Stachowiak, M.; Czarnocka, B.; Sawicka-Gutaj, N.; Ziółkowska, P.; Krela-Kaźmierczak, I.; Gut, P.; Florek, E.; et al. The effect of endocrine disruptors on the reproductive system—Current knowledge. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 26450. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Rios, E.; Castro, L.; Liu, J.; Yan, Y.; Dixon, D. Genistein: Dual role in women’s health. Nutrients 2021, 13, 3048. [Google Scholar] [CrossRef] [PubMed]
- Sleiman, H.K.; de Oliveira, J.M.; Langoni de Freitas, G.B. Isoflavones alter male and female fertility in different development windows. Biomed. Pharmacother. 2021, 140, 111448. [Google Scholar] [CrossRef] [PubMed]
- Eustache, F.; Bennani Smires, B.; Moison, D.; Bergès, R.; Canivenc-Lavier, M.C.; Vaiman, D.; Auger, J. Different exposure windows to low doses of genistein and/or vinclozolin result in contrasted disorders of testis function and gene expression of exposed rats and their unexposed progeny. Environ. Res. 2020, 190, 109975. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Liu, Y.; Liu, G.; Wei, L.; Wen, Y.; Huang, S.; Guo, Y.; Zou, F.; Cheng, J. Associations between semen phytoestrogens concentrations and semen quality in Chinese men. Environ. Int. 2019, 129, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Patisaul, H.B. Endocrine disrupting chemicals (EDCs) and the neuroendocrine system: Beyond estrogen, androgen, and thyroid. Adv. Pharmacol. 2021, 92, 101–150. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.; Garza, S.; Papadopoulos, V.; Culty, M. Impact of endocrine-disrupting chemicals on steroidogenesis and consequences on testicular function. Mol. Cell. Endocrinol. 2021, 527, 111215. [Google Scholar] [CrossRef] [PubMed]
- Erguc, E.I.; Tascioglu-Aliyev, A.; Entezari, B.; Gurer-Orhan, H. The Role of Biotransformation in the Activity of Endocrine Disruptors. Curr. Drug Metab. 2021, 22, 628–644. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.W.; Poynor, V.; McEligot, A.J. Urinary Phytoestrogen Levels Are Associated with Female Hormonal Cancers: An Analysis of NHANES Data from 1999 to 2010. Nutr. Cancer 2022, 74, 2748–2756. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.L.Y.; Co, V.A.; El-Nezami, H. Endocrine disrupting chemicals and breast cancer: A systematic review of epidemiological studies. Crit. Rev. Food Sci. Nutr. 2022, 62, 6549–6576. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.H.; Chen, L.R.; Chen, K.H. In vitro and vivo identification, metabolism and action of xenoestrogens: An overview. Int. J. Mol. Sci. 2021, 22, 4013. [Google Scholar] [CrossRef] [PubMed]
- Nasri, A.; Pohjanvirta, R. In vitro estrogenic, cytotoxic, and genotoxic profiles of the xenoestrogens 8-prenylnaringenine, genistein and tartrazine. Environ. Sci. Pollut. Res. 2021, 28, 27988–27997. [Google Scholar] [CrossRef] [PubMed]
- Rashid, H.; Alqahtani, S.S.; Alshahrani, S. Diet: A Source of Endocrine Disruptors. Endocr. Metab. Immune Disord. Drug Targets 2019, 20, 633–645. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Qian, H.; Wu, Z.; Li, Z.; Li, X.; Zhang, Y.; Xu, Q.; Lu, C.; Wang, X. Exploratory analysis of the associations between urinary phytoestrogens and thyroid hormones among adolescents and adults in the United States: National Health and Nutrition Examination Survey 2007–2010. Environ. Sci. Pollut. Res. 2022, 29, 2974–2984. [Google Scholar] [CrossRef]
- Fucic, A.; Mantovani, A.; Vena, J.; Bloom, M.S.; Sincic, N.; Vazquez, M.; Aguado-Sierra, J. Impact of endocrine disruptors from mother’s diet on immuno-hormonal orchestration of brain development and introduction of the virtual human twin tool. Reprod. Toxicol. 2023, 117, 108357. [Google Scholar] [CrossRef] [PubMed]
- Heras-González, L.; Latorre, J.A.; Martinez-Bebia, M.; Espino, D.; Olea-Serrano, F.; Mariscal-Arcas, M. The relationship of obesity with lifestyle and dietary exposure to endocrine-disrupting chemicals. Food Chem. Toxicol. 2020, 136, 110983. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, T.; Ji, H.; Wang, X.; Sun, X.; Miao, M.; Wang, Y.; Wu, Q.; Liang, H.; Yuan, W. Associations of maternal soy product consumption and urinary isoflavone concentrations with neonatal anthropometry: A prospective cohort study. Environ. Pollut. 2021, 274, 115752. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Tao, C.; Li, Z.; Huang, Y.; Yan, W.; Zhao, S.; Gao, B.; Xu, Q.; Qin, Y.; Wang, X.; et al. Association of Endocrine-Disrupting Chemicals with All-Cause and Cause-Specific Mortality in the U.S.: A Prospective Cohort Study. Environ. Sci. Technol. 2023, 57, 2877–2886. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, J.; Hires, C.; Dunne, E.; Baker, C. The relationship between lavender and tea tree essential oils and pediatric endocrine disorders: A systematic review of the literature. Complement. Ther. Med. 2020, 49, 102288. [Google Scholar] [CrossRef]
- Lephart, E.D. Phytoestrogens (Resveratrol and equol) for estrogen-deficient skin—Controversies/misinformation versus anti-aging in vitro and clinical evidence via nutraceutical-cosmetics. Int. J. Mol. Sci. 2021, 22, 11218. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Wei, W.; Cao, R.; Lu, L.; Liang, S.; Xiong, M.; Zhang, C.; Liang, X.; Ma, Y. Resveratrol alleviates zea-induced decidualization disturbance in human endometrial stromal cells. Ecotoxicol. Environ. Saf. 2021, 207, 111511. [Google Scholar] [CrossRef] [PubMed]
- Komsky-Elbaz, A.; Kalo, D.; Roth, Z. New evidence for deleterious effects of environmental contaminants on the male gamete. Anim. Reprod. Sci. 2022, 246, 106886. [Google Scholar] [CrossRef]
- Li, F.; Zhao, X.; Jiao, Y.; Duan, X.; Yu, L.; Zheng, F.; Wang, X.; Wang, L.; Wang, J.S.; Zhao, X.; et al. Exposure assessment of aflatoxins and zearalenone in edible vegetable oils in Shandong, China: Health risks posed by mycotoxin immunotoxicity and reproductive toxicity in children. Environ. Sci. Pollut. Res. 2023, 30, 3743–3758. [Google Scholar] [CrossRef] [PubMed]
- Jing, S.; Liu, C.; Zheng, J.; Dong, Z.; Guo, N. Toxicity of zearalenone and its nutritional intervention by natural products. Food Funct. 2022, 13, 10374–10400. [Google Scholar] [CrossRef] [PubMed]
- Warth, B.; Preindl, K.; Manser, P.; Wick, P.; Marko, D.; Buerki-Thurnherr, T. Transfer and metabolism of the xenoestrogen zearalenone in human perfused placenta. Environ. Health Perspect. 2019, 127, 107004. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Núñez, Z.; Barrett, E.S.; Szamreta, E.A.; Shapses, S.A.; Qin, B.; Lin, Y.; Zarbl, H.; Buckley, B.; Bandera, E.V. Urinary mycoestrogens and age and height at menarche in New Jersey girls. Environ. Health A Glob. Access Sci. Source 2019, 18, 24. [Google Scholar] [CrossRef] [PubMed]
- Aichinger, G. Natural dibenzo-α-pyrones: Friends or foes? Int. J. Mol. Sci. 2021, 22, 13063. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Xu, L.L.; Chen, L.; He, J.; Wang, Y.K.; Chen, F.; Chen, Y.; Giesy, J.P.; Wang, Y.T.; Wu, Q.H.; et al. Acute exposure to microcystins affects hypothalamic-pituitary axes of male rats. Environ. Pollut. 2023, 318, 120843. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, J.T.; Shropshire, B.C.; Nagy, T.R.; Chambers, K.D.; Li, Y.; Korach, K.S.; Korach, S. Essential Oils and Health. Yale J. Biol. Med. 2020, 93. [Google Scholar]
- Kiyama, R. Estrogenic activity of coffee constituents. Nutrients 2019, 11, 1401. [Google Scholar] [CrossRef] [PubMed]
- Xue, P.; Wang, Y.; Chen, Y.; Wu, X.; Tang, Y.; Ding, Y.; Wang, X.; Liu, S. A Case of Peripheral Precocious Puberty May Be Caused by a Diet Containing Phytosterols in a 20-Month-Old Boy. Horm. Res. Paediatr. 2022, 95, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Zoller, O.; Brüschweiler, B.J.; Magnin, R.; Reinhard, H.; Rhyn, P.; Rupp, H.; Zeltner, S.; Felleisen, R. Natural occurrence of bisphenol F in mustard. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2015, 33, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Frayn, K.N.; Karpe, F.; Fielding, B.A.; Macdonald, I.A.; Coppack, S.W. Integrative physiology of human adipose tissue. Int. J. Obes. 2003, 27, 875–888. [Google Scholar] [CrossRef] [PubMed]
- Cooke, P.S.; Heine, P.A.; Taylor, J.A.; Lubahn, D.B. The role of estrogen and estrogen receptor-a in male adipose tissue. Mol. Cell. Endocrinol. 2001, 178, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Pallottini, V.; Bulzomi, P.; Galluzzo, P.; Martini, C.; Marino, M. Estrogen Regulation of Adipose Tissue Functions: Involvement of Estrogen Receptor Isoforms. Infect. Disord. Drug Targets 2008, 8, 52–60. [Google Scholar] [CrossRef]
- Narciso, L.; Catone, T.; Aquilina, G.; Attias, L.; De Angelis, I.; Iuliano, M.G.; Tassinari, R.; Mantovani, A.; Maranghi, F. The juvenile toxicity study as a tool for a science-based risk assessment in the children population group. Reprod. Toxicol. 2017, 72, 136–141. [Google Scholar] [CrossRef] [PubMed]

| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Only articles in English | Articles not in English |
| Period 2019–2023 | Articles not published in the period 2019–2023 |
| Studies including natural endocrine disruptors (N-EDCs) | Studies focusing solely on synthetic endocrine disruptors (S-EDCs) |
| Studies including the toxicological effects of N-EDCs on human health | Studies focusing solely on the therapeutic effects of N-EDCs on human health |
| Studies including N-EDCs as organic compounds | Studies focusing solely on N-EDCs as chemical elements |
| Studies including specific human health target systems | Studies not including specific target systems |
| N-EDC Group | N-EDC Class | N-EDC | No. of Records Referring to the N-EDC | References |
|---|---|---|---|---|
| PEs | Flavonoids | Genistein | 31 | [3,6,7,8,19,20,22,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50] |
| Daidzein | 19 | [6,7,19,20,22,27,28,29,35,37,40,41,45,46,47,48,49,50,51] | ||
| Equol | 12 | [6,19,27,28,37,40,41,46,47,49,50,52] | ||
| Formononetin | 6 | [27,28,37,40,45,48] | ||
| Biochanin A | 6 | [27,28,30,40,45,48] | ||
| Glycitein | 6 | [7,19,32,40,45,49] | ||
| 8-prenylnaringenin | 1 | [44] | ||
| Apigenin | 1 | [30] | ||
| Mycotoxins | Zearalenone | 17 | [7,9,10,12,13,19,21,40,42,45,47,53,54,55,56,57,58] | |
| Alpha-zearalenone | 7 | [13,19,21,40,56,57,58] | ||
| Beta-zearalenone | 3 | [19,40,56] | ||
| Alternariol | 2 | [11,59] | ||
| Aflatoxin | 2 | [54,55] | ||
| Alternariol 9-methyl-ether | 1 | [59] | ||
| Ochratoxin | 1 | [54] | ||
| Patulin | 1 | [54] | ||
| Fumonisin | 1 | [54] | ||
| Nivalenol | 1 | [54] | ||
| Coumestans | Coumestrol | 8 | [3,7,28,33,37,38,45,48] | |
| Stilbenes | Resveratrol | 6 | [6,7,18,30,42,52] | |
| Pterostilbene | 1 | [7] | ||
| Lignans | Matairesinol | 1 | [48] | |
| Secoisoresinol | 2 | [37,48] | ||
| Enterodiol | 6 | [7,37,40,41,48,50] | ||
| Enterolactone | 6 | [37,40,41,46,48,50] | ||
| Cyanotoxins | Cylindrospermopsin | 1 | [15] | |
| Microcystin | 1 | [60] | ||
| NDG | Lavender essential oil | 4 | [24,52,57,61] | |
| Tea tree essential oil | 4 | [16,24,51,61] | ||
| Caffeic acid | 2 | [23,62] | ||
| Ferulic acid) | 1 | [23] | ||
| 5-O-caffeoylquinic acid | 1 | [23] | ||
| Mesquite pod component | 1 | [29] | ||
| Trigonelline | 1 | [62] | ||
| Caffeine | 1 | [62] | ||
| Gossypol | 1 | [30] | ||
| Phytosterol | 1 | [63] |
| N-EDC Target Systems or Exposure Windows | No. of Records |
|---|---|
| Male reproductive system | 35 |
| Female reproductive system | 30 |
| Endocrine system | 9 |
| Nervous system | 9 |
| Adipose tissue | 7 |
| Cardiovascular system | 4 |
| Bone system | 4 |
| Pregnancy | 9 |
| Childhood * | 21 |
| Toxicology Studies (No.) | References | ||
|---|---|---|---|
| Male Reproductive System | Penile disorders | 6 | [32,33,34,42,52,63] |
| Testicular dysfunction | 18 | [7,9,10,12,18,22,27,28,29,31,32,34,35,36,39,45,54,56] | |
| Prostate disorders | 4 | [27,29,52,54] | |
| Fertility disorders (sperm) | 18 | [6,7,9,10,12,15,28,29,30,34,35,36,37,39,45,52,54,56] | |
| Feminization/ hormone levels (androgens, testosterone, luteinizing hormone, estradiol) | 20 | [6,7,11,15,18,22,28,30,31,32,35,36,38,39,51,52,54,56,60,63] | |
| Premature puberty/other | 14 | [6,8,24,28,32,35,36,42,47,51,52,54,55,63] |
| Female Reproductive System | Toxicology Studies (No.) | References | |
| Uterine/endometrial conditions | 16 | [6,7,13,18,19,20,21,23,27,28,34,35,40,41,53,59] | |
| Ovarian dysfunction | 7 | [13,18,27,28,34,35,41] | |
| Breast cancer | 13 | [6,18,27,28,34,40,41,42,43,44,45,52,59] | |
| Fertility disorders (oocyte) | 8 | [6,13,18,27,28,29,35,56] | |
| Menstrual discomfort | 10 | [6,13,15,18,27,28,29,34,40,42] | |
| Hormone levels (estrogen, progesterone, 17β-hydroxysteroid dehydrogenase, steroids) | 10 | [6,7,15,18,28,29,34,39,45,59] | |
| Premature puberty | 9 | [6,24,29,34,40,51,55,58,61] |
| Nervous System | Toxicology Studies (n°) | References | |
| Hypothalamus | 5 | [18,22,28,38,56] | |
| Pituitary gland | 5 | [18,22,28,56,63] | |
| Brain/neurons | 4 | [18,38,47,56] | |
| Behavioral disorders | 4 | [18,43,45,47] |
| Other Target System | Toxicology Studies (No.) | References | |
| Endocrine system | 9 | [6,7,27,28,38,42,46,52,60] | |
| Cardiovascular system | 4 | [6,18,27,34] | |
| Bone | 4 | [18,27,34,62] | |
| Adipose tissue | 7 | [10,28,29,34,42,48,49] |
| Toxicology Studies (No.) | References | |
|---|---|---|
| Pregnancy | 9 | [6,7,13,28,33,40,53,56,57] |
| Childhood * | 21 | [6,10,12,13,16,18,22,24,27,34,35,36,38,40,45,48,51,52,55,58,61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Virtuoso, S.; Raggi, C.; Maugliani, A.; Baldi, F.; Gentili, D.; Narciso, L. Toxicological Effects of Naturally Occurring Endocrine Disruptors on Various Human Health Targets: A Rapid Review. Toxics 2024, 12, 256. https://doi.org/10.3390/toxics12040256
Virtuoso S, Raggi C, Maugliani A, Baldi F, Gentili D, Narciso L. Toxicological Effects of Naturally Occurring Endocrine Disruptors on Various Human Health Targets: A Rapid Review. Toxics. 2024; 12(4):256. https://doi.org/10.3390/toxics12040256
Chicago/Turabian StyleVirtuoso, Sara, Carla Raggi, Antonella Maugliani, Francesca Baldi, Donatella Gentili, and Laura Narciso. 2024. "Toxicological Effects of Naturally Occurring Endocrine Disruptors on Various Human Health Targets: A Rapid Review" Toxics 12, no. 4: 256. https://doi.org/10.3390/toxics12040256
APA StyleVirtuoso, S., Raggi, C., Maugliani, A., Baldi, F., Gentili, D., & Narciso, L. (2024). Toxicological Effects of Naturally Occurring Endocrine Disruptors on Various Human Health Targets: A Rapid Review. Toxics, 12(4), 256. https://doi.org/10.3390/toxics12040256







