Sustainable Electrochemical Activation of Self-Generated Persulfate for the Degradation of Endocrine Disruptors: Kinetics, Performances, and Mechanisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Experimental Procedures
2.3. Analytical Methods
3. Result and Discussion
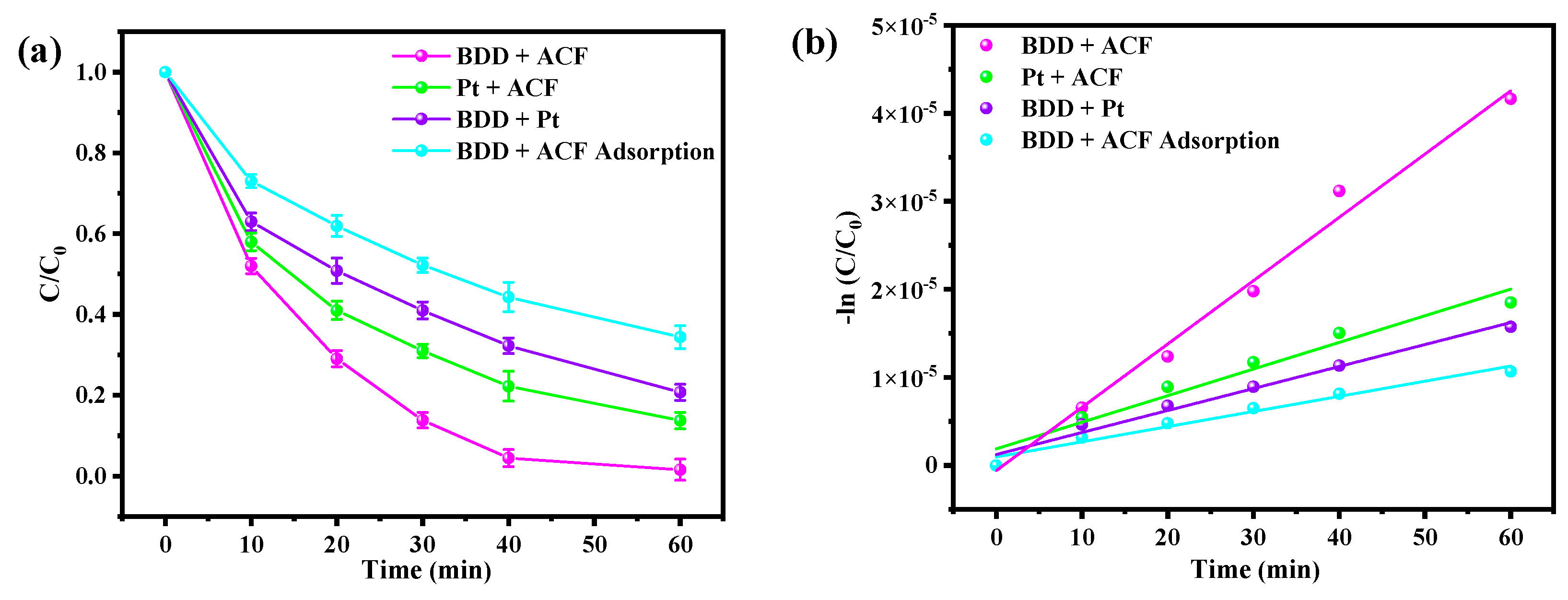
3.1. Comparative Degradation of BPA in Different Electrode Systems
3.2. Process Optimization
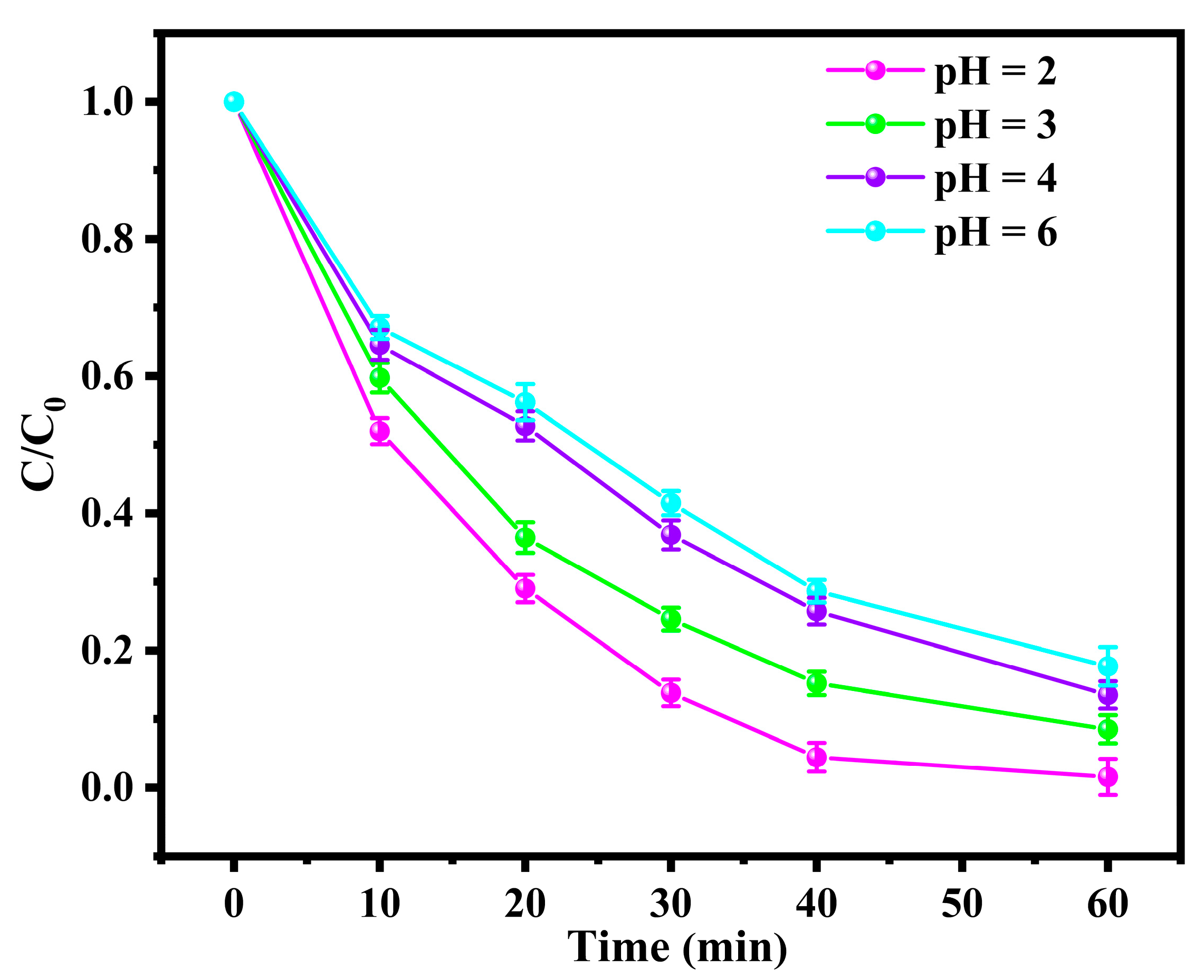
3.2.1. Effect of pH on the Degradation of BPA in the BDD and ACF System
3.2.2. Effect of Current Density on the Degradation of BPA in the BDD and ACF System
3.2.3. Stability and Extensive Applicability in Environmental Remediation of the BDD and ACF System
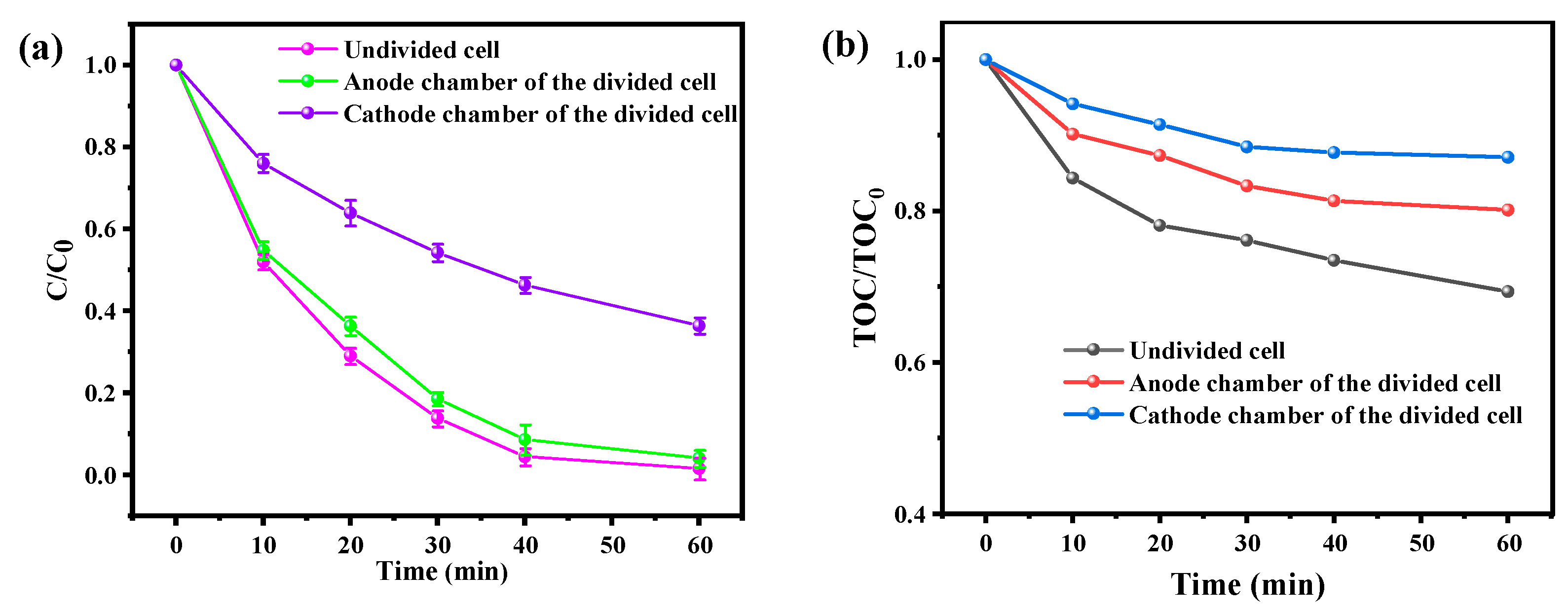
3.3. Comparison of the Degradation of BPA in Divided/Undivided Cell Systems
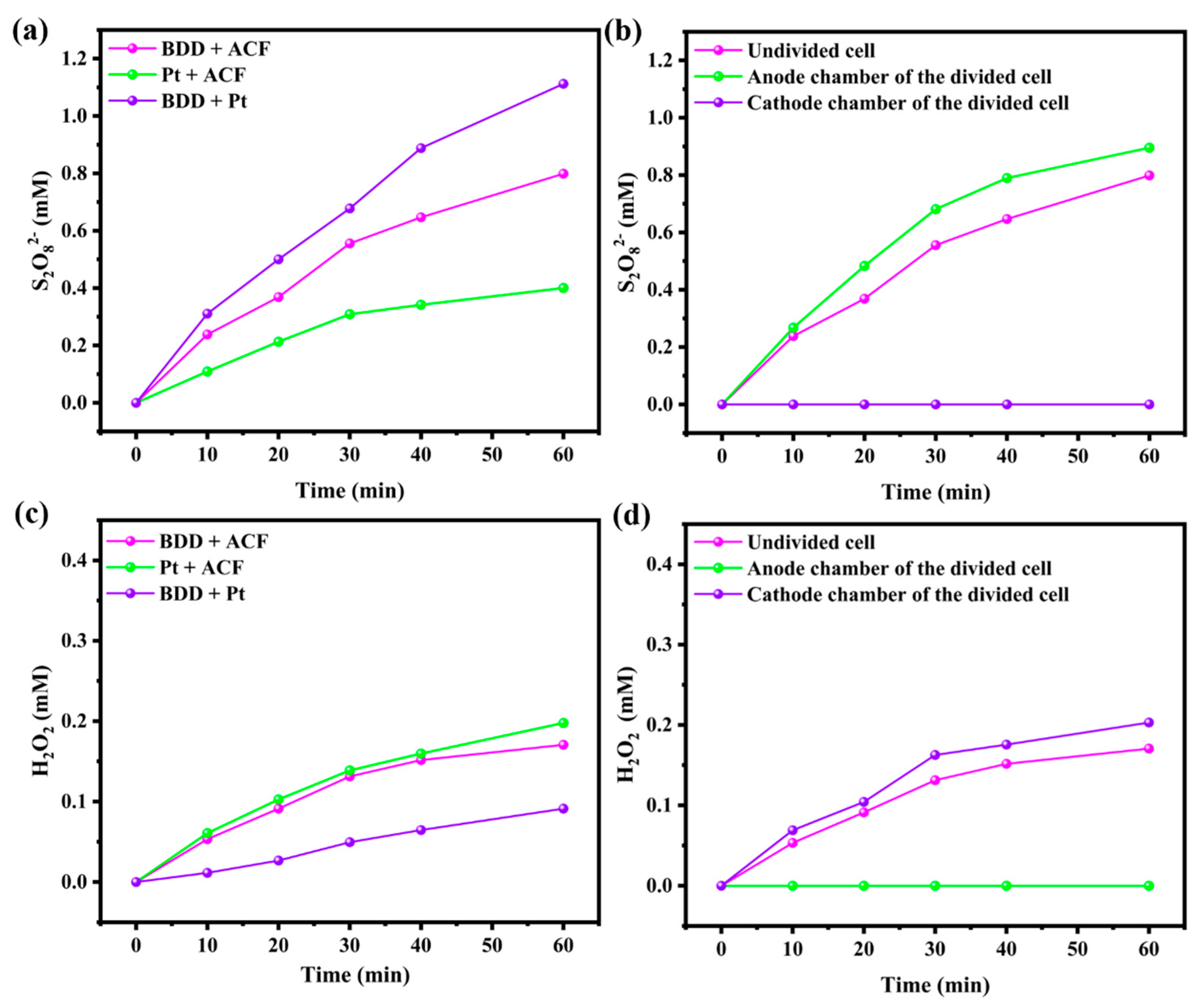
3.4. The Yield of S2O82− and H2O2 in the Electrolysis System
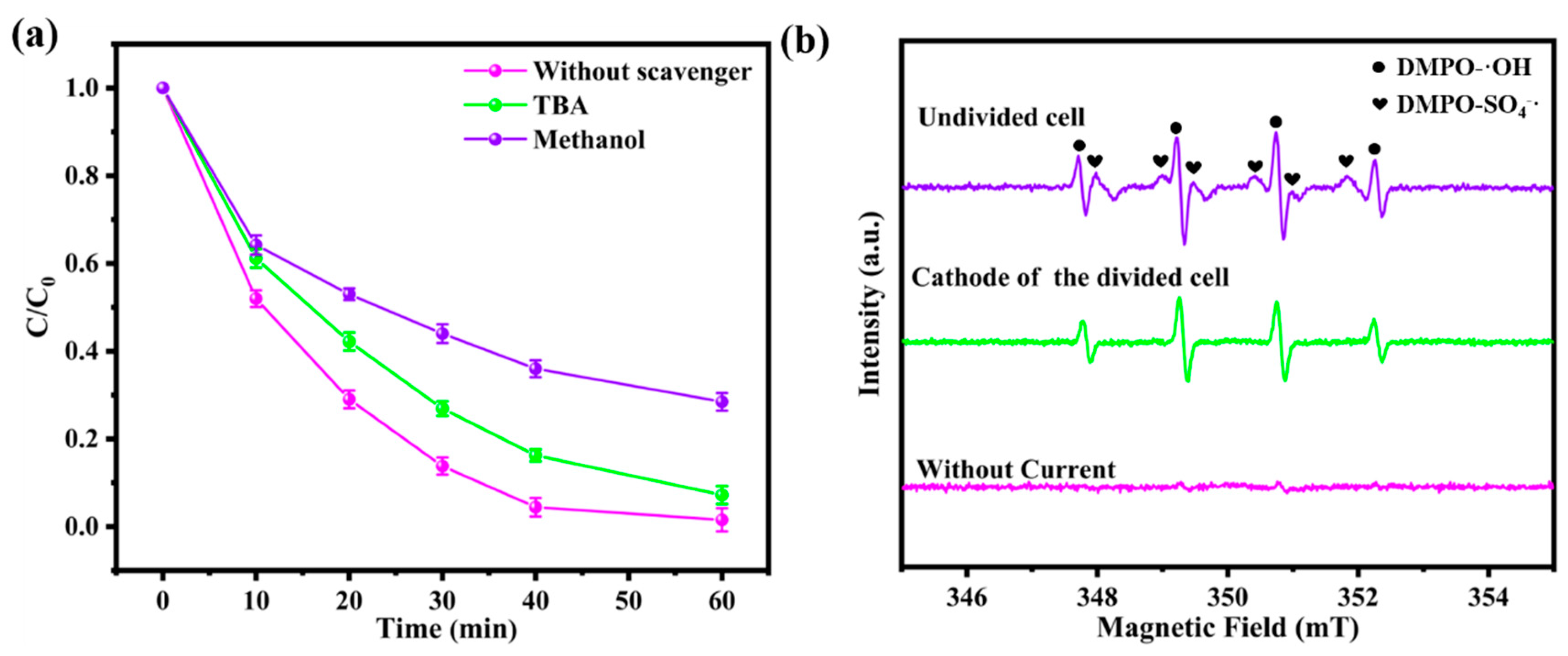
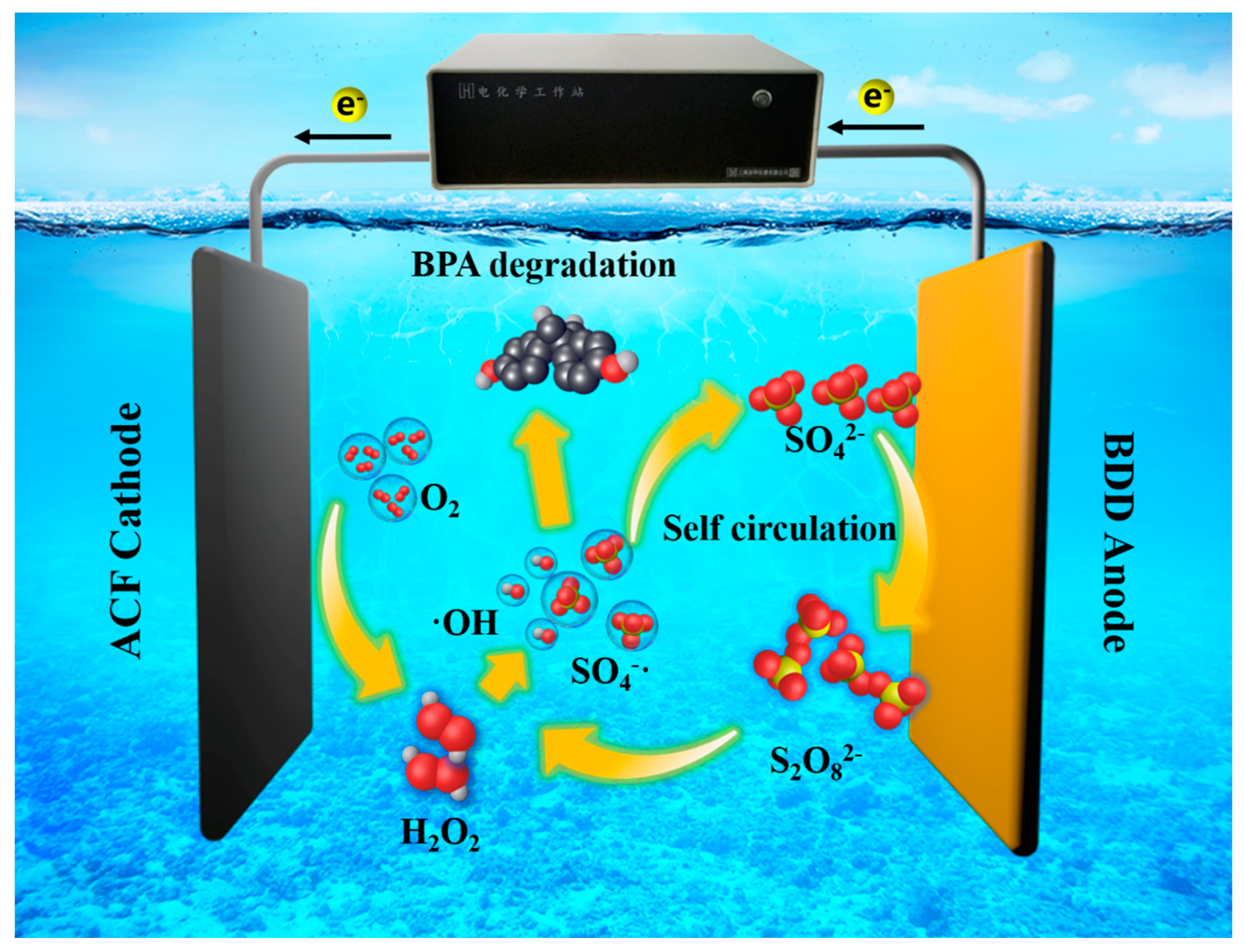
3.5. Mechanism of the Degradation of BPA in Divided/Undivided Cell Systems
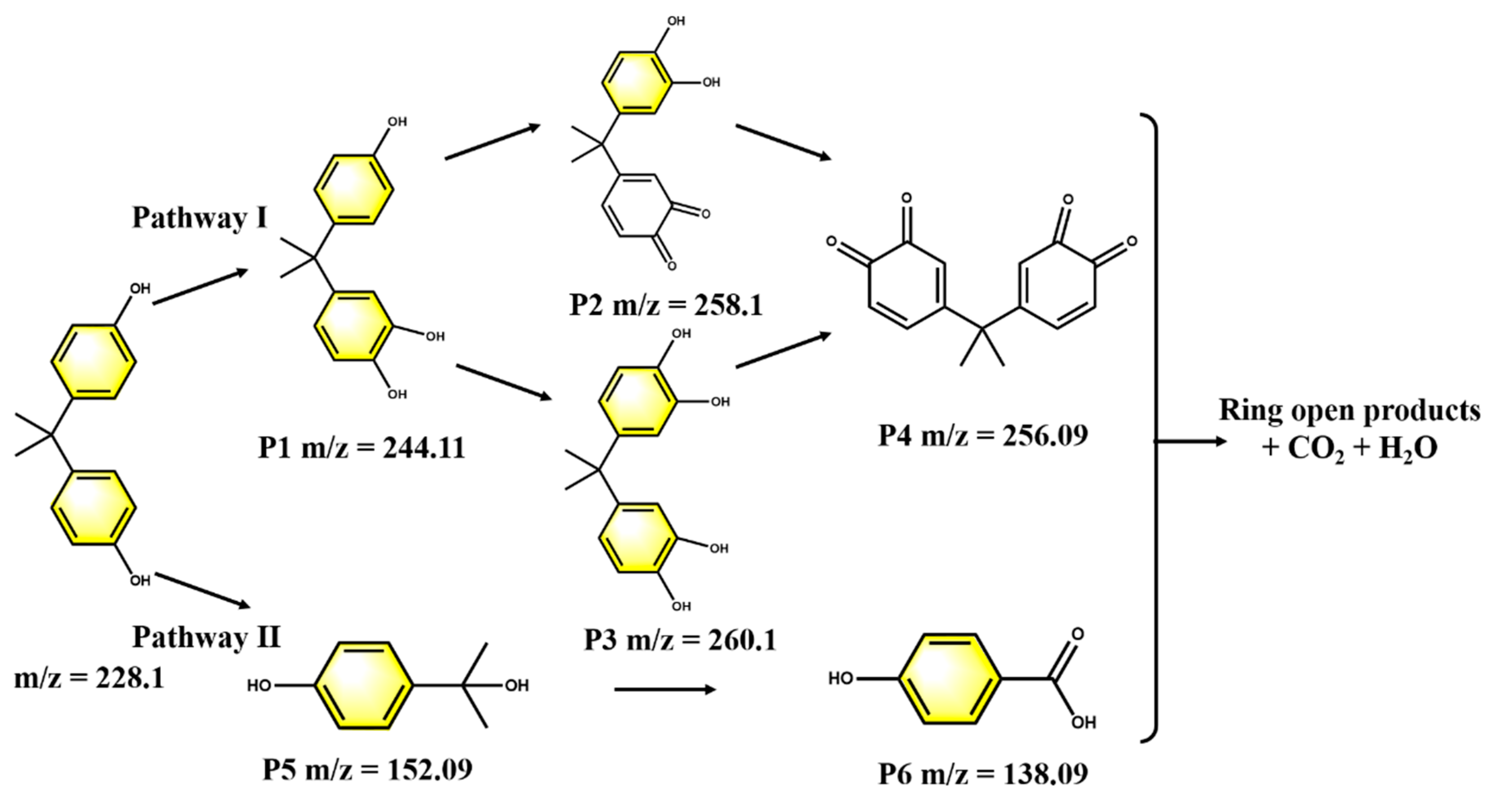
3.6. Proposed Pathways of BPA Degradation
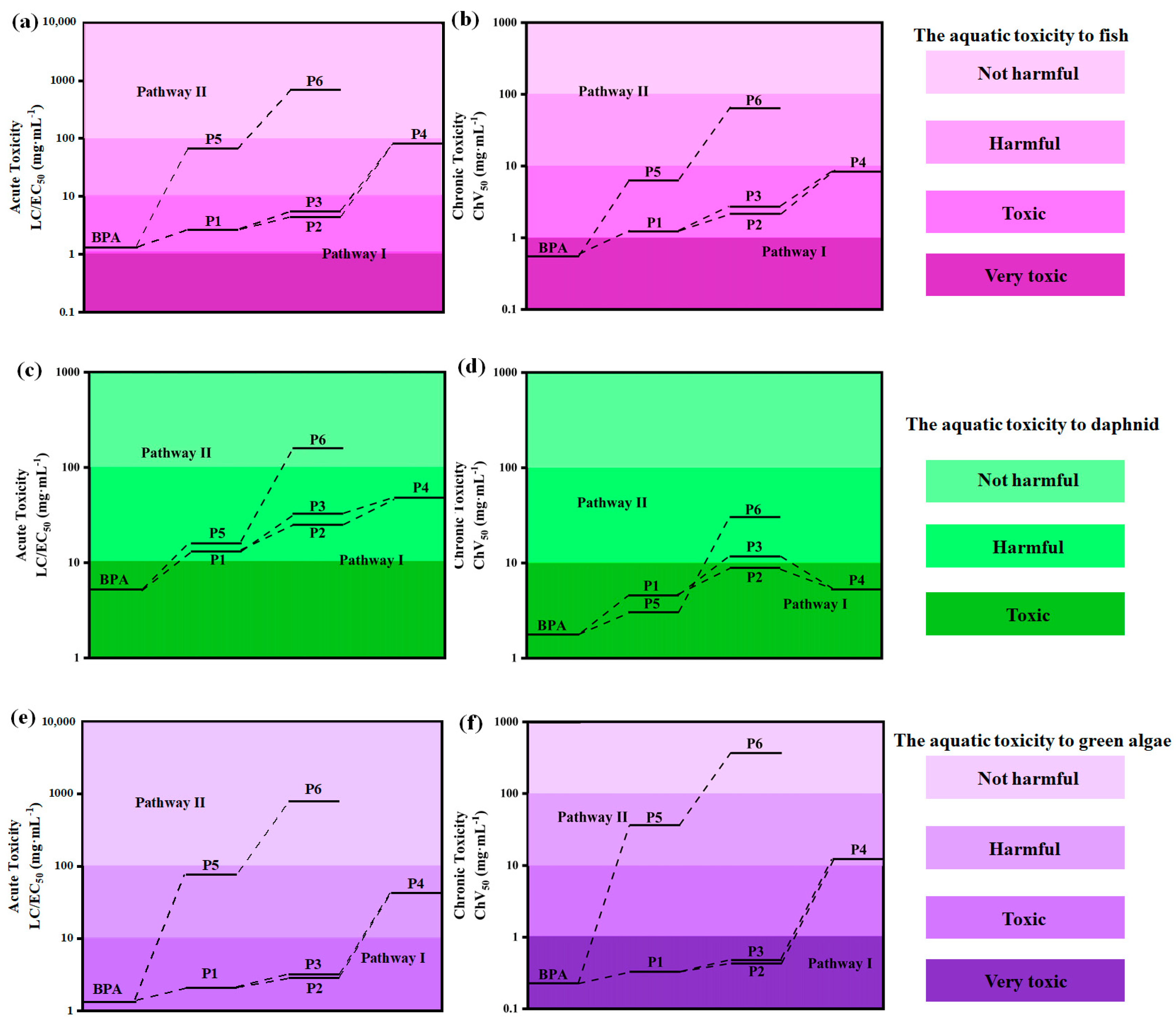
3.7. Toxicity Assessment
3.8. Cost Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, J.; Zhu, Z.; Zhang, H.; Shen, X.; Qiu, Y.; Yin, D.; Wang, S. Persistent free radicals on N-doped hydrochar for degradation of endocrine disrupting compounds. Chem. Eng. J. 2020, 398, 125538. [Google Scholar] [CrossRef]
- Ren, Y.; Chu, Y.; Li, N.; Lai, B.; Zhang, W.; Liu, C.; Li, J. A critical review of environmental remediation via iron-mediated sulfite advanced oxidation processes. Chem. Eng. J. 2023, 455, 140859. [Google Scholar] [CrossRef]
- Seid, L.; Lakhdari, D.; Berkani, M.; Belgherbi, O.; Chouder, D.; Vasseghian, Y.; Lakhdari, N. High-efficiency electrochemical degradation of phenol in aqueous solutions using Ni-PPy and Cu-PPy composite materials. J. Hazard. Mater. 2022, 423, 126986. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Li, H.; Liu, C.; Zheng, L.; Liu, C.; Wang, J.O.; Liu, S.; Han, Y.; Gu, L.; Qian, J.; et al. Single-atom Fe catalysts for fenton-like reactions: Roles of different N species. Adv. Mater. 2022, 34, 2110653. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Tian, W.; Guan, Z.; Zhang, H.; Duan, X.; Wang, H.; Sun, H.; Fang, Y.; Huang, Y.; Wang, S. Functional carbon nitride materials in photo-fenton-like catalysis for environmental remediation. Adv. Funct. Mater. 2022, 32, 2201743. [Google Scholar] [CrossRef]
- Mao, D.; Yuan, J.; Qu, X.; Sun, C.; Yang, S.; He, H. Size tunable Bi3O4Br hierarchical hollow spheres assembled with {0 0 1}-facets exposed nanosheets for robust photocatalysis against phenolic pollutants. J. Catal. 2019, 369, 209–221. [Google Scholar] [CrossRef]
- Issaka, E.; Amu-Darko, J.N.; Yakubu, S.; Fapohunda, F.O.; Ali, N.; Bilal, M. Advanced catalytic ozonation for degradation of pharmaceutical pollutants-A review. Chemosphere 2022, 289, 133208. [Google Scholar] [CrossRef]
- Thiam, A.; Brillas, E.; Garrido, J.A.; Rodríguez, R.M.; Sirés, I. Routes for the electrochemical degradation of the artificial food azo-colour Ponceau 4R by advanced oxidation processes. Appl. Catal. B Environ. 2016, 180, 227–236. [Google Scholar] [CrossRef]
- Fabianska, A.; Bialk-Bielinska, A.; Stepnowski, P.; Stolte, S.; Siedlecka, E.M. Electrochemical degradation of sulfonamides at BDD electrode: Kinetics, reaction pathway and eco-toxicity evaluation. J. Hazard. Mater. 2014, 280, 579–587. [Google Scholar] [CrossRef]
- Tauchert, E.; Schneider, S.; de Morais, J.L.; Peralta-Zamora, P. Photochemically-assisted electrochemical degradation of landfill leachate. Chemosphere 2006, 64, 1458–1463. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Liao, D.; Lin, Y.; Hu, C.; Ju, J.; Chen, Y.; Feng, D. Electrochemical degradation of reverse osmosis concentrate (ROC) using the electrodeposited Ti/TiO2-NTs/PbO2 electrode. Sep. Purif. Technol. 2021, 258, 118056. [Google Scholar] [CrossRef]
- Park, S.; Shao, Y.; Liu, J.; Wang, Y. Oxygen electrocatalysts for water electrolyzers and reversible fuel cells: Status and perspective. Energy Environ. Sci. 2012, 5, 9331. [Google Scholar] [CrossRef]
- Yang, N.; Yu, S.; Macpherson, J.V.; Einaga, Y.; Zhao, H.; Zhao, G.; Swain, G.M.; Jiang, X. Conductive diamond: Synthesis, properties, and electrochemical applications. Chem. Soc. Rev. 2019, 48, 157–204. [Google Scholar] [CrossRef] [PubMed]
- Wachter, N.; Aquino, J.M.; Denadai, M.; Barreiro, J.C.; Silva, A.J.; Cass, Q.B.; Bocchi, N.; Rocha-Filho, R.C. Electrochemical degradation of the antibiotic ciprofloxacin in a flow reactor using distinct BDD anodes: Reaction kinetics, identification and toxicity of the degradation products. Chemosphere 2019, 234, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Farhat, A.; Keller, J.; Tait, S.; Radjenovic, J. Removal of persistent organic contaminants by electrochemically activated sulfate. Environ. Sci. Technol. 2015, 49, 14326–14333. [Google Scholar] [CrossRef] [PubMed]
- Hippauf, F.; Dörfler, S.; Zedlitz, R.; Vater, A.; Kaskel, S. Continuous electrooxdiation of sulfuric acid on boron-doped diamond electrodes. Electrochim. Acta 2014, 147, 589–595. [Google Scholar] [CrossRef]
- Cai, J.; Niu, T.; Shi, P.; Zhao, G. Boron-doped diamond for hydroxyl radical and sulfate radical anion electrogeneration, transformation, and voltage-free sustainable oxidation. Small 2019, 15, 1900153. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Segura, S.; Lima, Á.S.; Cavalcanti, E.B.; Brillas, E. Anodic oxidation, electro-Fenton and photoelectro-Fenton degradations of pyridinium- and imidazolium-based ionic liquids in waters using a BDD/air-diffusion cell. Electrochim. Acta 2016, 198, 268–279. [Google Scholar] [CrossRef]
- Guo, Y.; Zeng, Z.; Liu, Y.; Huang, Z.; Cui, Y.; Yang, J. One-pot synthesis of sulfur doped activated carbon as a superior metal-free catalyst for the adsorption and catalytic oxidation of aqueous organics. J. Mater. Chem. A 2018, 6, 4055–4067. [Google Scholar] [CrossRef]
- Bu, L.; Zhu, S.; Zhou, S. Degradation of atrazine by electrochemically activated persulfate using BDD anode: Role of radicals and influencing factors. Chemosphere 2018, 195, 236–244. [Google Scholar] [CrossRef]
- Liu, Z.; Ding, H.; Zhao, C.; Wang, T.; Wang, P.; Dionysiou, D.D. Electrochemical activation of peroxymonosulfate with ACF cathode: Kinetics, influencing factors, mechanism, and application potential. Water Res. 2019, 159, 111–121. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, B.; Ding, H.; He, H.; Deng, H.; Zhao, C.; Wang, P.; Dionysiou, D.D. Simultaneous regeneration of cathodic activated carbon fiber and mineralization of desorbed contaminations by electro-peroxydisulfate process: Advantages and limitations. Water Res. 2020, 171, 115456. [Google Scholar] [CrossRef]
- Rodríguez-Chueca, J.; Amor, C.; Silva, T.; Dionysiou, D.D.; Li Puma, G.; Lucas, M.S.; Peres, J.A. Treatment of winery wastewater by sulphate radicals: HSO5− /transition metal/UV-A LEDs. Chem. Eng. J. 2017, 310, 473–483. [Google Scholar] [CrossRef]
- Zhu, Y.; Qiu, S.; Deng, F.; Ma, F.; Zheng, Y. Degradation of sulfathiazole by electro-Fenton using a nitrogen-doped cathode and a BDD anode: Insight into the H2O2 generation and radical oxidation. Sci. Total Environ. 2020, 722, 137853. [Google Scholar] [CrossRef]
- Mhemdi, A.; Oturan, M.A.; Oturan, N.; Abdelhédi, R.; Ammar, S. Electrochemical advanced oxidation of 2-chlorobenzoic acid using BDD or Pt anode and carbon felt cathode. J. Electroanal. Chem. 2013, 709, 111–117. [Google Scholar] [CrossRef]
- Zazou, H.; Oturan, N.; Sönmez-Çelebi, M.; Hamdani, M.; Oturan, M.A. Mineralization of chlorobenzene in aqueous medium by anodic oxidation and electro-Fenton processes using Pt or BDD anode and carbon felt cathode. J. Electroanal. Chem. 2016, 774, 22–30. [Google Scholar] [CrossRef]
- Xia, Y.; Dai, Q. Electrochemical degradation of antibiotic levofloxacin by PbO2 electrode: Kinetics, energy demands and reaction pathways. Chemosphere 2018, 205, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Frangos, P.; Shen, W.; Wang, H.; Li, X.; Yu, G.; Deng, S.; Huang, J.; Wang, B.; Wang, Y. Improvement of the degradation of pesticide deethylatrazine by combining UV photolysis with electrochemical generation of hydrogen peroxide. Chem. Eng. J. 2016, 291, 215–224. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, C.; Zhang, M.; Zhang, B.-T.; Yu, Y.-G. The electrochemical degradation of ciprofloxacin using a SnO2-Sb/Ti anode: Influencing factors, reaction pathways and energy demand. Chem. Eng. J. 2016, 296, 79–89. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, M.; Wang, C.; Meng, X.; Zhang, W.; Chen, Z.; Crittenden, J. Electrochemical degradation of methylisothiazolinone by using Ti/SnO2-Sb2O3/α, β-PbO2 electrode: Kinetics, energy efficiency, oxidation mechanism and degradation pathway. Chem. Eng. J. 2019, 374, 626–636. [Google Scholar] [CrossRef]
- Ding, S.; Wan, J.; Wang, Y.; Yan, Z.; Ma, Y. Activation of persulfate by molecularly imprinted Fe-MOF-74@SiO2 for the targeted degradation of dimethyl phthalate: Effects of operating parameters and chlorine. Chem. Eng. J. 2021, 422, 130406. [Google Scholar] [CrossRef]
- Liu, J.; Zhong, S.; Song, Y.; Wang, B.; Zhang, F. Degradation of tetracycline hydrochloride by electro-activated persulfate oxidation. J. Electroanal. Chem. 2018, 809, 74–79. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, C.; Wang, P.; Zheng, H.; Sun, Y.; Dionysiou, D.D. Removal of carbamazepine in water by electro-activated carbon fiber-peroxydisulfate: Comparison, optimization, recycle, and mechanism study. Chem. Eng. J. 2018, 343, 28–36. [Google Scholar] [CrossRef]
- Chauhan, D.; Ahn, Y.-H. Alkaline electrolysis of wastewater and low-quality water. J. Clean. Prod. 2023, 397, 136613. [Google Scholar] [CrossRef]
- Lado Ribeiro, A.R.; Moreira, N.F.F.; Li Puma, G.; Silva, A.M.T. Impact of water matrix on the removal of micropollutants by advanced oxidation technologies. Chem. Eng. J. 2019, 363, 155–173. [Google Scholar] [CrossRef]
- Rao, Y.F.; Qu, L.; Yang, H.; Chu, W. Degradation of carbamazepine by Fe(II)-activated persulfate process. J. Hazard. Mater. 2014, 268, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Li, L.; Cheng, S.; Huang, L.; Yang, L.; Liu, Y.; Yao, H.; Liu, C.; Liu, W.; Ji, X. Fabrication of a cation exchange membrane with largely reduced anion permeability for advanced aqueous K-ion batery in an alkaline-neutral electrolyte decoupling system. Small 2023, 19, 2205970. [Google Scholar] [CrossRef] [PubMed]
- Irkham; Watanabe, T.; Fiorani, A.; Valenti, G.; Paolucci, F.; Einaga, Y. Co-reactant-on-demand ECL: Electrogenerated chemiluminescence by the in situ production of S2O82− at Boron-doped diamond electrodes. J. Am. Chem. Soc. 2016, 138, 15636–15641. [Google Scholar] [CrossRef]
- Tian, S.; Li, Y.; Zeng, H.; Guan, W.; Wang, Y.; Zhao, X. Cyanide oxidation by singlet oxygen generated via reaction between H2O2 from cathodic reduction and OCl− from anodic oxidation. J. Colloid Interface Sci. 2016, 482, 205–211. [Google Scholar] [CrossRef]
- Ding, H.; Zhu, Y.; Wu, Y.; Zhang, J.; Deng, H.; Zheng, H.; Liu, Z.; Zhao, C. In situ regeneration of phenol-saturated activated carbon fiber by an electro-peroxymonosulfate process. Environ. Sci. Technol. 2020, 54, 10944–10953. [Google Scholar] [CrossRef]
- Rastogi, A.; Al-Abed, S.R.; Dionysiou, D.D. Sulfate radical-based ferrous–peroxymonosulfate oxidative system for PCBs degradation in aqueous and sediment systems. Appl. Catal. B Environ. 2009, 85, 171–179. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Y.; Quan, X.; Chen, S. Enhanced oxidation of 4-chlorophenol using sulfate radicals generated from zero-valent iron and peroxydisulfate at ambient temperature. Sep. Purif. Technol. 2010, 71, 302–307. [Google Scholar] [CrossRef]
- Bacha, A.-U.-R.; Nabi, I.; Cheng, H.; Li, K.; Ajmal, S.; Wang, T.; Zhang, L. Photoelectrocatalytic degradation of endocrine-disruptor bisphenol—A with significantly activated peroxymonosulfate by Co-BiVO4 photoanode. Chem. Eng. J. 2020, 389, 124482. [Google Scholar] [CrossRef]
- Xie, Y.; Li, P.; Zeng, Y.; Li, X.; Xiao, Y.; Wang, Y.; Zhang, Y. Thermally treated fungal manganese oxides for bisphenol A degradation using sulfate radicals. Chem. Eng. J. 2018, 335, 728–736. [Google Scholar] [CrossRef]
- Li, W.; Wu, P.-x.; Zhu, Y.; Huang, Z.-j.; Lu, Y.-h.; Li, Y.-w.; Dang, Z.; Zhu, N.-w. Catalytic degradation of bisphenol A by CoMnAl mixed metal oxides catalyzed peroxymonosulfate: Performance and mechanism. Chem. Eng. J. 2015, 279, 93–102. [Google Scholar] [CrossRef]
- Gao, Y.-Q.; Rao, Y.-Y.; Ning, H.; Chen, J.-X.; Zeng, Q.; Tian, F.-X.; Gao, N.-Y. Comparative investigation of diclofenac degradation by Fe2+/chlorine and Fe2+/PMS processes. Sep. Purif. Technol. 2022, 297, 121555. [Google Scholar] [CrossRef]
- Magdy, M.; Gar Alalm, M.; El-Etriby, H.K. Comparative life cycle assessment of five chemical methods for removal of phenol and its transformation products. J. Clean. Prod. 2021, 291, 125923. [Google Scholar] [CrossRef]
- Radwan, M.; Gar Alalm, M.; El-Etriby, H.K. Application of electro-Fenton process for treatment of water contaminated with benzene, toluene, and p-xylene (BTX) using affordable electrodes. J. Water Process Eng. 2019, 31, 100837. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, X.; Jin, Z.; Zou, R.; Zhu, Y.; Yao, X.; Li, M.; Song, S.; Liu, S.; Zeng, T. Sustainable Electrochemical Activation of Self-Generated Persulfate for the Degradation of Endocrine Disruptors: Kinetics, Performances, and Mechanisms. Toxics 2024, 12, 156. https://doi.org/10.3390/toxics12020156
Tang X, Jin Z, Zou R, Zhu Y, Yao X, Li M, Song S, Liu S, Zeng T. Sustainable Electrochemical Activation of Self-Generated Persulfate for the Degradation of Endocrine Disruptors: Kinetics, Performances, and Mechanisms. Toxics. 2024; 12(2):156. https://doi.org/10.3390/toxics12020156
Chicago/Turabian StyleTang, Xiaofeng, Zhiquan Jin, Rui Zou, Yi Zhu, Xia Yao, Mengxuan Li, Shuang Song, Shuangliu Liu, and Tao Zeng. 2024. "Sustainable Electrochemical Activation of Self-Generated Persulfate for the Degradation of Endocrine Disruptors: Kinetics, Performances, and Mechanisms" Toxics 12, no. 2: 156. https://doi.org/10.3390/toxics12020156
APA StyleTang, X., Jin, Z., Zou, R., Zhu, Y., Yao, X., Li, M., Song, S., Liu, S., & Zeng, T. (2024). Sustainable Electrochemical Activation of Self-Generated Persulfate for the Degradation of Endocrine Disruptors: Kinetics, Performances, and Mechanisms. Toxics, 12(2), 156. https://doi.org/10.3390/toxics12020156






