Benzo(a)pyrene and Gut Microbiome Crosstalk: Health Risk Implications
Abstract
1. Introduction
2. Literature Search and Selection Methods
3. B(a)P and Its Health Risks
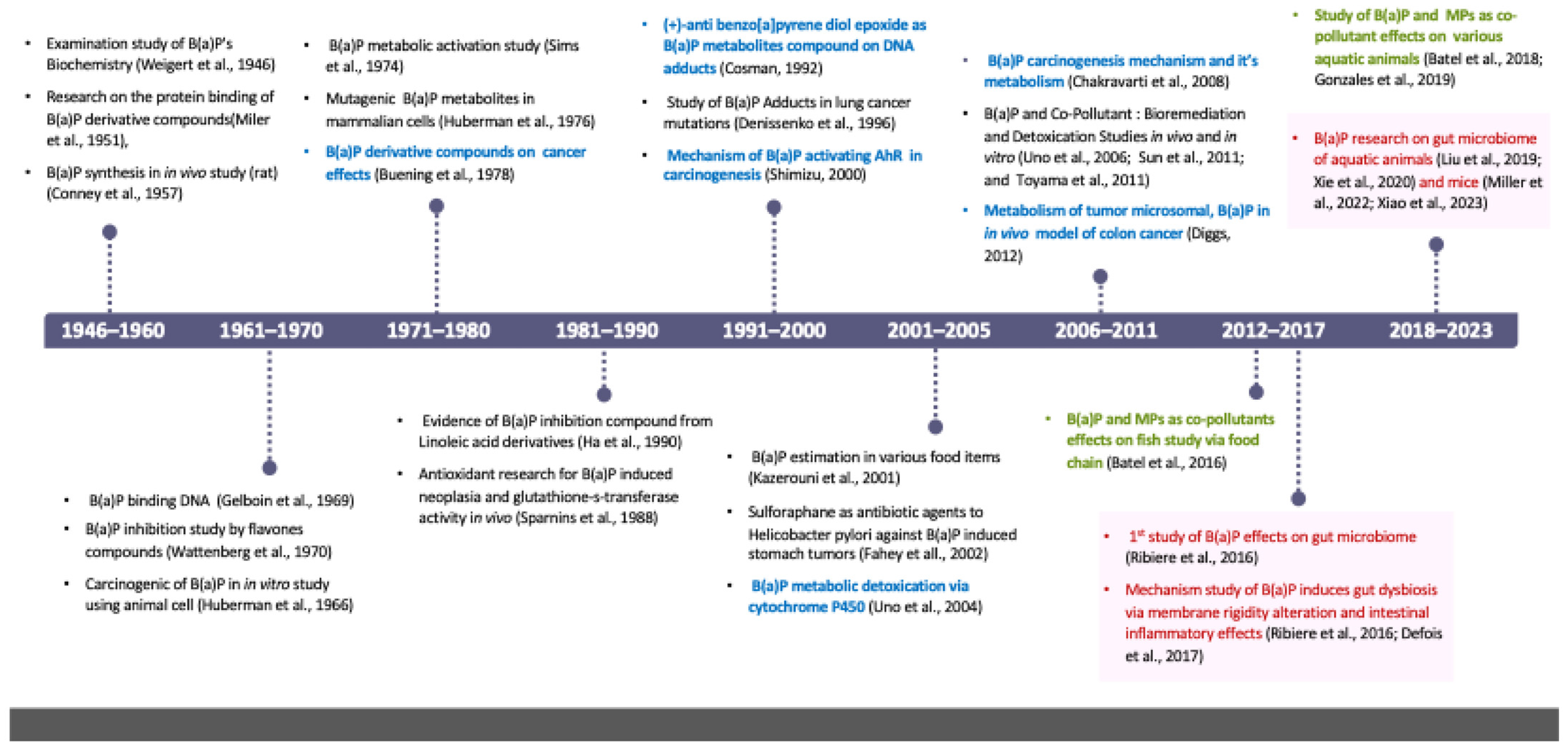
3.1. History of B(a)P Health Risk Study
3.2. Toxic Mode of Action of B(a)P
3.3. Human Exposure and Epidemiological Research of B(a)P
| Pollutant | Structure | Pollutant Sources | Human Daily Exposure Level | Human Adverse Level Concentration 1 | |
|---|---|---|---|---|---|
| Carcinogenic | Other Systems | ||||
| Naphthalene |  | Household and industry | Assumed for 70 kg adult is 1.127 μg/kg per day from air, 0.237 μg/kg per day from food, and 0.235 μg/kg per day from house dust | Probable human carcinogen | Respiratory: 9.3 mg/m3 (LOAEL-HEC) |
| Fluorene | 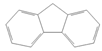 | Industry | Not assessed | Not assessed | Not assessed |
| Anthracene | 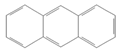 | Industry | 77.4 ng/m3 from the air [72] | Not classifiable as to human carcinogenicity | 1.0 × 103 mg/kg-day (NOAEL) |
| Fluoranthene | 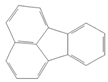 | Industry | Not assessed | Not assessed | Hepatic, urinary: 1.25 × 102 mg/kg-day (NOAEL) |
| Phenanthrene | 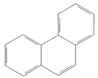 | Industry | Not assessed | Not classifiable as to human carcinogenicity | 0.1–0.2 mg/m3 for airborne exposure limit |
| Chrysene | 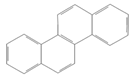 | Industry | Not assessed | Probable human carcinogen | Not assessed |
| Pyrene |  | Industry | Not assessed | Not classifiable as to human carcinogenicity | Urinary: 7.5 × 10 mg/kg-day (NOAEL) |
| Benz(a)anthracene | 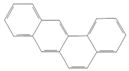 | Industry | Not assessed | Probable human carcinogen | Not assessed |
| 1-Nitropyrene | 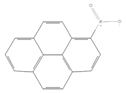 | Industry | Not assessed | Not assessed | Not assessed |
| Benzo(a)pyrene |  | Household and industry | 52 to 95 ng/cigarette, 7.20 ± 1.11 μg/m3 in the air, 4.15 μg/kg in well-done steaks, and 4.00 to 8.33 ng/L in drinking water [63] | 1 per mg/kg-day | Embryo: 4.6 × 103 mg/m3 (LOAEL) |
| Corannulene | 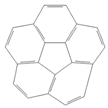 | Lab- synthesized chemical | Not assessed | Not assessed | Not assessed |
| Polychlorinated biphenyl (PCBs) | - | Industry | For adult exposure: 3.04 ng/kg-day indoor inhalation, 3.0 ± 2.2 ng/kg-day for dietary intake [73] | 1 µg/m3 (10-h time-weighted average) | Not assessed |
| Lead (Pb) | - | Household and industry | Human daily intake from food: 0.1 to 0.3 µg/kg body weight/day [74] | Not assessed | Brain–intelligence: Blood lead level of <10 μg/dL in child study [75] |
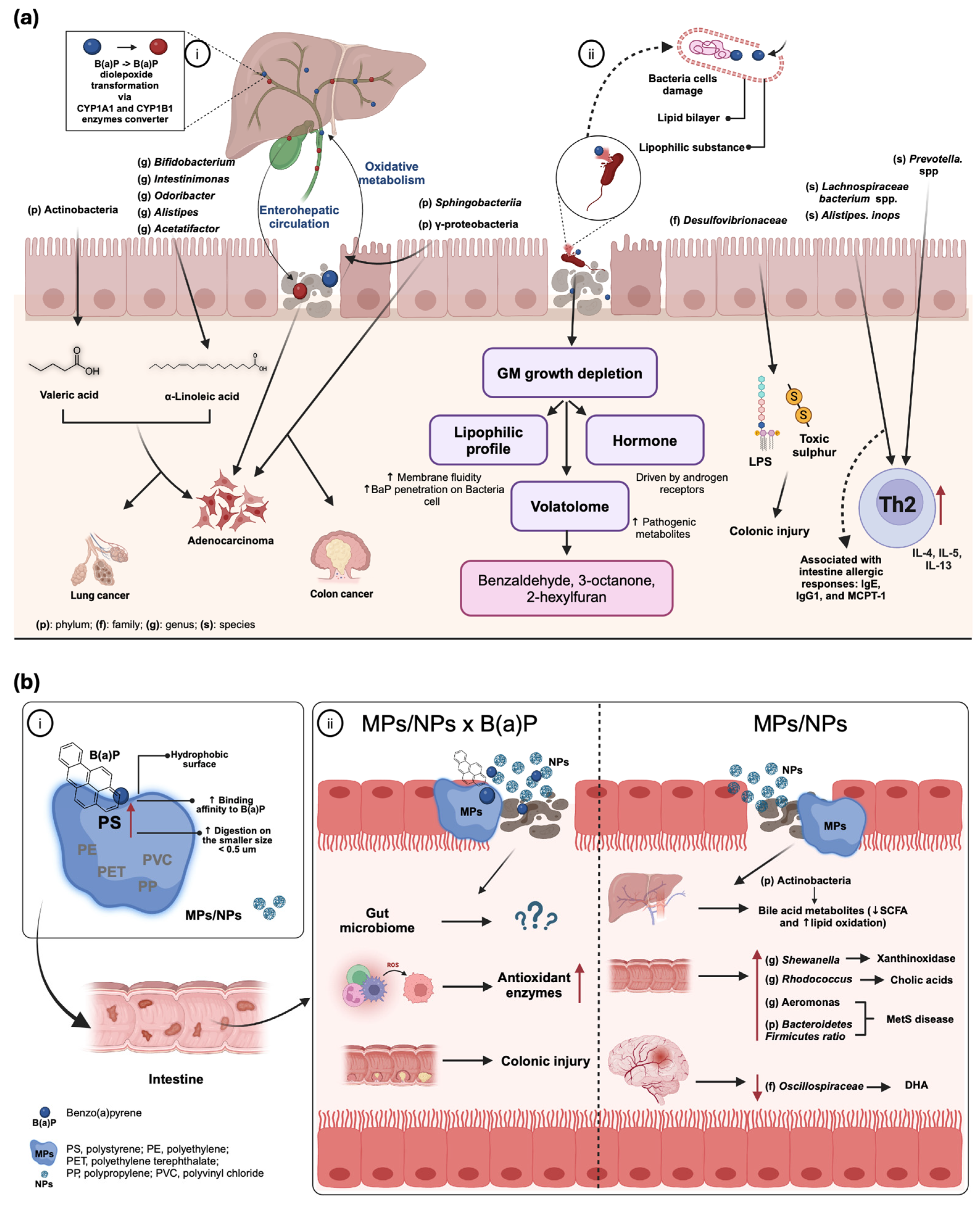
4. B(a)P and Gut Microbiome Interaction
4.1. B(a)P Exposure and Gut Dysbiosis
4.2. Key Influential Factors for B(a)P-Induced Gut Microbiome Change
4.3. Microbiome-Related Health Risks of B(a)P
4.3.1. Colonic Injury and Immunity Impairment
4.3.2. Carcinogenic and Xenobiotic Implications
4.3.3. Metabolic Disturbance
5. Synergistic Toxic Effects of B(a)P and MPs
5.1. MPs as Vectors for B(a)P: Mechanisms, Environmental Fate, and Biological Impacts
5.2. Combined Effects of B(a)P and MPs on Gut Microbiome
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, D.; Chen, L.; Ma, Z.; Zhou, D.; Fu, L.; Liu, M.; Zhang, T.; Yang, J.; Zhen, Q. Source analysis and health risk assessment of polycyclic aromatic hydrocarbon (PAHs) in total suspended particulate matter (TSP) from Bengbu, China. Sci. Rep. 2024, 14, 5080. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.J.; Frickel, S.; Nguyen, D.; Bui, T.; Echsner, S.; Simon, B.R.; Howard, J.L.; Miller, K.; Wickliffe, J.K. A targeted health risk assessment following the Deepwater Horizon oil spill: Polycyclic aromatic hydrocarbon exposure in Vietnamese-American shrimp consumers. Environ. Health Perspect. 2015, 123, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Das, D.N.; Ravi, N. Influences of polycyclic aromatic hydrocarbon on the epigenome toxicity and its applicability in human health risk assessment. Environ. Res. 2022, 213, 113677. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Warshawsky, D. Metabolic activation of polycyclic and heterocyclic aromatic hydrocarbons and DNA damage: A review. Toxicol. Appl. Pharmacol. 2005, 206, 73–93. [Google Scholar] [CrossRef]
- Chakravarti, D.; Venugopal, D.; Mailander, P.C.; Meza, J.L.; Higginbotham, S.; Cavalieri, E.L.; Rogan, E.G. The role of polycyclic aromatic hydrocarbon-DNA adducts in inducing mutations in mouse skin. Mutat. Res. 2008, 649, 161–178. [Google Scholar] [CrossRef]
- Vijay, A.; Valdes, A.M. Role of the gut microbiome in chronic diseases: A narrative review. Eur. J. Clin. Nutr. 2022, 76, 489–501. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Wu, H.J.; Wu, E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 2012, 3, 4–14. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, X.; Li, L.; Zhong, C.; Zhang, Y.; Yang, X.; Li, M.; Yang, C. The role of gut microbiota in intestinal disease: From an oxidative stress perspective. Front. Microbiol. 2024, 15, 1328324. [Google Scholar] [CrossRef]
- Berenblum, I.; Schoental, R.; Brit, J. 3:4-Benzpyrene from Coal Tar. Nature 1945, 156, 601. [Google Scholar] [CrossRef]
- Diggs, D.L.; Harris, K.L.; Rekhadevi, P.V.; Ramesh, A. Tumor microsomal metabolism of the food toxicant, benzo(a)pyrene, in ApcMin mouse model of colon cancer. Tumour Biol. 2012, 33, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- Batel, A.; Linti, F.; Scherer, M.; Erdinger, L.; Braunbeck, T. Transfer of benzo[a]pyrene from microplastics to Artemia nauplii and further to zebrafish via a trophic food web experiment: CYP1A induction and visual tracking of persistent organic pollutants. Environ. Toxicol. Chem. 2016, 35, 1656–1666. [Google Scholar] [CrossRef] [PubMed]
- Batel, A.; Borchert, F.; Reinwald, H.; Erdinger, L.; Braunbeck, T. Microplastic accumulation patterns and transfer of benzo[a]pyrene to adult zebrafish (Danio rerio) gills and zebrafish embryos. Environ. Pollut. 2018, 235, 918–930. [Google Scholar] [CrossRef]
- Gonzalez-Soto, N.; Hatfield, J.; Katsumiti, A.; Duroudier, N.; Lacave, J.M.; Bilbao, E.; Orbea, A.; Navarro, E.; Cajaraville, M.P. Impacts of dietary exposure to different sized polystyrene microplastics alone and with sorbed benzo[a]pyrene on biomarkers and whole organism responses in mussels Mytilus galloprovincialis. Sci. Total Environ. 2019, 684, 548–566. [Google Scholar] [CrossRef]
- Buening, M.K.; Wislocki, P.G.; Levin, W.; Yagi, H.; Thakker, D.R.; Akagi, H.; Koreeda, M.; Jerina, D.M.; Conney, A.H. Tumorigenicity of the optical enantiomers of the diastereomeric benzo[a]pyrene 7,8-diol-9,10-epoxides in newborn mice: Exceptional activity of (+)-7beta,8alpha-dihydroxy-9alpha,10alpha-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene. Proc. Natl. Acad. Sci. USA 1978, 75, 5358–5361. [Google Scholar] [CrossRef]
- Conney, A.H.; Miller, E.C.; Miller, J.A. Substrate-induced synthesis and other properties of benzpyrene hydroxylase in rat liver. J. Biol. Chem. 1957, 228, 753–766. [Google Scholar] [CrossRef]
- Defois, C.; Ratel, J.; Denis, S.; Batut, B.; Beugnot, R.; Peyretaillade, E.; Engel, E.; Peyret, P. Environmental Pollutant Benzo[a]Pyrene Impacts the Volatile Metabolome and Transcriptome of the Human Gut Microbiota. Front. Microbiol. 2017, 8, 1562. [Google Scholar] [CrossRef]
- Denissenko, M.F.; Pao, A.; Tang, M.; Pfeifer, G.P. Preferential formation of benzo[a]pyrene adducts at lung cancer mutational hotspots in P53. Science 1996, 274, 430–432. [Google Scholar] [CrossRef]
- Fahey, J.W.; Haristoy, X.; Dolan, P.M.; Kensler, T.W.; Scholtus, I.; Stephenson, K.K.; Talalay, P.; Lozniewski, A. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo[a]pyrene-induced stomach tumors. Proc. Natl. Acad. Sci. USA 2002, 99, 7610–7615. [Google Scholar] [CrossRef]
- Gelboin, H.V. A microsome-dependent binding of benzo[a]pyrene to DNA. Cancer Res. 1969, 29, 1272–1276. [Google Scholar] [PubMed]
- Ha, Y.L.; Storkson, J.; Pariza, M.W. Inhibition of benzo(a)pyrene-induced mouse forestomach neoplasia by conjugated dienoic derivatives of linoleic acid. Cancer Res. 1990, 50, 1097–1101. [Google Scholar] [PubMed]
- Herbstman, J.B.; Tang, D.; Zhu, D.; Qu, L.; Sjodin, A.; Li, Z.; Camann, D.; Perera, F.P. Prenatal exposure to polycyclic aromatic hydrocarbons, benzo[a]pyrene-DNA adducts, and genomic DNA methylation in cord blood. Environ. Health Perspect. 2012, 120, 733–738. [Google Scholar] [CrossRef]
- Huberman, E.; Sachs, L. Cell susceptibility to transformation and cytotoxicity by the carcinogenic hydrocarbon benzo[a]pyrene. Proc. Natl. Acad. Sci. USA 1966, 56, 1123–1129. [Google Scholar] [CrossRef]
- Huberman, E.; Sachs, L.; Yang, S.K.; Gelboin, V. Identification of mutagenic metabolites of benzo(a)pyrene in mammalian cells. Proc. Natl. Acad. Sci. USA 1976, 73, 607–611. [Google Scholar] [CrossRef]
- Schneider, J.; Grosser, R.; Jayasimhulu, K.; Xue, W.; Warshawsky, D. Degradation of Pyrene, Benz[a]anthracene, and Benzo[a]pyrene by Mycobacterium sp. Strain RJGII-135, Isolated from a Former Coal Gasification Site. Appl. Environ. Microbiol. 1996, 62, 13–19. [Google Scholar] [CrossRef]
- Kazerouni, N.; Sinha, R.; Hsu, C.H.; Greenberg, A.; Rothman, N. Analysis of 200 food items for benzo[a]pyrene and estimation of its intake in an epidemiologic study. Food Chem. Toxicol. 2001, 39, 423–436. [Google Scholar] [CrossRef]
- Lu, P.Q.; Jeong, H.; Jankowiak, R.; Small, G.J.; Kim, S.K.; Cosman, M.; Gracintov, N.E. Comparative laser spectroscopic study of DNA and polynucleotide adducts from the (+)-anti-diol epoxide of benzo[a]pyrene. Chem. Res. Toxicol. 1991, 4, 58–69. [Google Scholar] [CrossRef]
- Miller, E.C. Studies on the formation of protein-bound derivatives of 3,4-benzpyrene in the epidermal fraction of mouse skin. Cancer Res. 1951, 11, 100–108. [Google Scholar]
- Cosman, M.; De Los Santos, C.; Fiala, R.; Hingerty, B.E.; Singh, S.B.; Ibanez, V.; Margulis, L.A.; Live, D.; Geacintov, N.E.; Broyde, S. Solution conformation of the major adduct between the carcinogen (+)-anti-benzo[a]pyrene diol epoxide and DNA. Proc. Natl. Acad. Sci. USA 1992, 89, 1914–1918. [Google Scholar] [CrossRef]
- Phillips, D.H. Fifty years of benzo(a)pyrene. Nature 1983, 303, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Hannah, R.; Ramani, P.; Ramanathan, A.; Gheena, S.; Ramasubramanian, A.; Monika, K. CYP2 C9 polymorphism among patients with oral squamous cell carcinoma and its role in altering the metabolism of benzo[a]pyrene. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 130, 306–312. [Google Scholar] [CrossRef]
- Ribiere, C.; Peyret, P.; Parisot, N.; Darcha, C.; Dechelotte, P.J.; Barnich, N.; Peyretaillade, E.; Boucher, D. Oral exposure to environmental pollutant benzo[a]pyrene impacts the intestinal epithelium and induces gut microbial shifts in murine model. Sci. Rep. 2016, 6, 31027. [Google Scholar] [CrossRef]
- Sims, P.; Grover, P.L.; Swaisland, A.; Pal, K.; Hewer, A. Metabolic activation of benzo(a)pyrene proceeds by a diol-epoxide. Nature 1974, 252, 326–328. [Google Scholar] [CrossRef]
- Sparnins, V.L.; Barany, G.; Wattenberg, L.W. Effects of organosulfur compounds from garlic and onions on benzo[a]pyrene-induced neoplasia and glutathione S-transferase activity in the mouse. Carcinogenesis 1988, 9, 131–134. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, Q.; Xu, Y.; Wang, L.; Liang, X. Phytoremediation for co-contaminated soils of benzo[a]pyrene (B[a]P) and heavy metals using ornamental plant Tagetes patula. J. Hazard. Mater. 2011, 186, 2075–2082. [Google Scholar] [CrossRef]
- Toyama, T.; Furukawa, T.; Maeda, N.; Inoue, D.; Sei, K.; Mori, K.; Kikuchi, S.; Ike, M. Accelerated biodegradation of pyrene and benzo[a]pyrene in the Phragmites australis rhizosphere by bacteria-root exudate interactions. Water Res. 2011, 45, 1629–1638. [Google Scholar] [CrossRef]
- Uno, S.; Dalton, T.P.; Derkenne, S.; Curran, C.P.; Miller, M.L.; Shertzer, H.G.; Nebert, D.W. Oral exposure to benzo[a]pyrene in the mouse: Detoxication by inducible cytochrome P450 is more important than metabolic activation. Mol. Pharmacol. 2004, 65, 1225–1237. [Google Scholar] [CrossRef]
- Uno, S.; Dalton, T.P.; Dragin, N.; Curran, C.P.; Derkenne, S.; Miller, M.L.; Shertzer, H.G.; Gonzalez, F.J.; Nebert, D.W. Oral benzo[a]pyrene in Cyp1 knockout mouse lines: CYP1A1 important in detoxication, CYP1B1 metabolism required for immune damage independent of total-body burden and clearance rate. Mol. Pharmacol. 2006, 69, 1103–1114. [Google Scholar] [CrossRef]
- Wattenberg, L.W.; Leong, J.L. Inhibition of the carcinogenic action of benzo(a)pyrene by flavones. Cancer Res. 1970, 30, 1922–1925. [Google Scholar]
- Shimizu, Y.; Nakatsuru, Y.; Ichinose, M.; Takahashi, Y.; Kume, H.; Mimura, J.; Fujii-Kuriyama, Y.; Ishikawa, T. Benzo[a]pyrene carcinogenicity is lost in mice lacking the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. USA 2000, 97, 4. [Google Scholar] [CrossRef] [PubMed]
- Weigert, F.; Mottram, J.C. The Biochemistry of Benzpyrene II. The Course of Its Metabolism and the Chemical Nature of the Metabolites. Cancer Res. 1946, 6, 109–120. [Google Scholar] [PubMed]
- Zhao, Y.; Liu, H.; Wang, Q.; Li, B.; Zhang, H.; Pi, Y. The effects of benzo[a]pyrene on the composition of gut microbiota and the gut health of the juvenile sea cucumber Apostichopus japonicus Selenka. Fish Shellfish Immunol. 2019, 93, 369–379. [Google Scholar] [CrossRef] [PubMed]
- DeBofsky, A.; Xie, Y.; Grimard, C.; Alcaraz, A.J.; Brinkmann, M.; Hecker, M.; Giesy, J.P. Differential responses of gut microbiota of male and female fathead minnow (Pimephales promelas) to a short-term environmentally-relevant, aqueous exposure to benzo[a]pyrene. Chemosphere 2020, 252, 126461. [Google Scholar] [CrossRef]
- Du, B.; Xiao, X.; Wang, H.; Li, W.; Xia, Z.; Yang, P.; Huang, S.K.; Yuan, R.; Liu, J.; Han, M.; et al. Evaluation of the Impact of BaP Exposure on the Gut Microbiota and Allergic Responses in an OVA-Sensitized Mouse Model. Environ. Health Perspect. 2023, 131, 67004. [Google Scholar] [CrossRef]
- Motwani, H.V.; Westberg, E.; Tornqvist, M. Interaction of benzo[a]pyrene diol epoxide isomers with human serum albumin: Site specific characterisation of adducts and associated kinetics. Sci. Rep. 2016, 6, 36243. [Google Scholar] [CrossRef]
- Reed, L.; Arlt, V.M.; Phillips, D.H. The role of cytochrome P450 enzymes in carcinogen activation and detoxication: An in vivo-in vitro paradox. Carcinogenesis 2018, 39, 851–859. [Google Scholar] [CrossRef]
- Sulc, M.; Indra, R.; Moserova, M.; Schmeiser, H.H.; Frei, E.; Arlt, V.M.; Stiborova, M. The impact of individual cytochrome P450 enzymes on oxidative metabolism of benzo[a]pyrene in human livers. Environ. Mol. Mutagen. 2016, 57, 229–235. [Google Scholar] [CrossRef]
- Einaudi, L.; Courbiere, B.; Tassistro, V.; Prevot, C.; Sari-Minodier, I.; Orsiere, T.; Perrin, J. In vivo exposure to benzo(a)pyrene induces significant DNA damage in mouse oocytes and cumulus cells. Hum. Reprod. 2014, 29, 548–554. [Google Scholar] [CrossRef]
- Bukowska, B.; Duchnowicz, P. Molecular Mechanisms of Action of Selected Substances Involved in the Reduction of Benzo[a]pyrene-Induced Oxidative Stress. Molecules 2022, 27, 1379. [Google Scholar] [CrossRef]
- Guo, B.; Feng, D.; Xu, Z.; Qi, P.; Yan, X. Acute benzo[a]pyrene exposure induced oxidative stress, neurotoxicity and epigenetic change in blood clam Tegillarca granosa. Sci. Rep. 2021, 11, 18744. [Google Scholar] [CrossRef] [PubMed]
- Shields, H.J.; Traa, A.; Van Raamsdonk, J.M. Beneficial and Detrimental Effects of Reactive Oxygen Species on Lifespan: A Comprehensive Review of Comparative and Experimental Studies. Front. Cell Dev. Biol. 2021, 9, 628157. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.A.; Perez de la Lastra, J.M.; Plou, F.J.; Perez-Lebena, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.L.A.a.J.M. Oxygen Toxicity and Reactive Oxygen Species: The Devil Is in the Details. Pediatr. Res. 2009, 66, 2009. [Google Scholar]
- Shi, Q.; Fijten, R.R.; Spina, D.; Riffo Vasquez, Y.; Arlt, V.M.; Godschalk, R.W.; Van Schooten, F.J. Altered gene expression profiles in the lungs of benzo[a]pyrene-exposed mice in the presence of lipopolysaccharide-induced pulmonary inflammation. Toxicol. Appl. Pharmacol. 2017, 336, 8–19. [Google Scholar] [CrossRef]
- Vazquez-Gomez, G.; Rocha-Zavaleta, L.; Rodriguez-Sosa, M.; Petrosyan, P.; Rubio-Lightbourn, J. Benzo[a]pyrene activates an AhR/Src/ERK axis that contributes to CYP1A1 induction and stable DNA adducts formation in lung cells. Toxicol. Lett. 2018, 289, 54–62. [Google Scholar] [CrossRef]
- Coelho, N.R.; Pimpao, A.B.; Correia, M.J.; Rodrigues, T.C.; Monteiro, E.C.; Morello, J.; Pereira, S.A. Pharmacological blockage of the AHR-CYP1A1 axis: A call for in vivo evidence. J. Mol. Med. 2022, 100, 215–243. [Google Scholar] [CrossRef]
- Larigot, L.; Juricek, L.; Dairou, J.; Coumoul, X. AhR signaling pathways and regulatory functions. Biochim. Open 2018, 7, 1–9. [Google Scholar] [CrossRef]
- Granados, J.C.; Falah, K.; Koo, I.; Morgan, E.W.; Perdew, G.H.; Patterson, A.D.; Jamshidi, N.; Nigam, S.K. AHR is a master regulator of diverse pathways in endogenous metabolism. Sci. Rep. 2022, 12, 16625. [Google Scholar] [CrossRef]
- Uppstad, H.; Ovrebo, S.; Haugen, A.; Mollerup, S. Importance of CYP1A1 and CYP1B1 in bioactivation of benzo[a]pyrene in human lung cell lines. Toxicol. Lett. 2010, 192, 221–228. [Google Scholar] [CrossRef]
- Shiizaki, K.; Kawanishi, M.; Yagi, T. Modulation of benzo[a]pyrene-DNA adduct formation by CYP1 inducer and inhibitor. Genes Environ. 2017, 39, 14. [Google Scholar] [CrossRef] [PubMed]
- Androutsopoulos, V.P.; Tsatsakis, A.M.; Spandidos, D.A. Cytochrome P450 CYP1A1: Wider roles in cancer progression and prevention. BMC Cancer 2009, 9, 187. [Google Scholar] [CrossRef] [PubMed]
- Bukowska, B.; Mokra, K.; Michalowicz, J. Benzo[a]pyrene-Environmental Occurrence, Human Exposure, and Mechanisms of Toxicity. Int. J. Mol. Sci. 2022, 23, 6348. [Google Scholar] [CrossRef]
- Maurice, N.M.; Sadikot, R.T. Mitochondrial Dysfunction in Bacterial Infections. Pathogens 2023, 12, 1005. [Google Scholar] [CrossRef]
- Pellegrino, M.W.; Nargund, A.M.; Kirienko, N.V.; Gillis, R.; Fiorese, C.J.; Haynes, C.M. Mitochondrial UPR-regulated innate immunity provides resistance to pathogen infection. Nature 2014, 516, 414–417. [Google Scholar] [CrossRef]
- Zhu, L.; Zhou, Q.; He, L.; Chen, L. Mitochondrial unfolded protein response: An emerging pathway in human diseases. Free Radic. Biol. Med. 2021, 163, 125–134. [Google Scholar] [CrossRef]
- Montano, L.; Pironti, C.; Pinto, G.; Ricciardi, M.; Buono, A.; Brogna, C.; Venier, M.; Piscopo, M.; Amoresano, A.; Motta, O. Polychlorinated Biphenyls (PCBs) in the Environment: Occupational and Exposure Events, Effects on Human Health and Fertility. Toxics 2022, 10, 365. [Google Scholar] [CrossRef]
- Mallah, M.A.; Mallah, M.A.; Liu, Y.; Xi, H.; Wang, W.; Feng, F.; Zhang, Q. Relationship Between Polycyclic Aromatic Hydrocarbons and Cardiovascular Diseases: A Systematic Review. Front. Public Health 2021, 9, 763706. [Google Scholar] [CrossRef]
- Booc, F.; Thornton, C.; Lister, A.; MacLatchy, D.; Willett, K.L. Benzo[a]pyrene effects on reproductive endpoints in Fundulus heteroclitus. Toxicol. Sci. 2014, 140, 73–82. [Google Scholar] [CrossRef]
- Hu, X.; Geetha, R.V.; Surapaneni, K.M.; Veeraraghavan, V.P.; Chinnathambi, A.; Alahmadi, T.A.; Manikandan, V.; Manokaran, K. Lung cancer induced by Benzo(A)Pyrene: ChemoProtective effect of sinapic acid in swiss albino mice. Saudi J. Biol. Sci. 2021, 28, 7125–7133. [Google Scholar] [CrossRef]
- Qu, Z.; Zhang, L.; Hou, R.; Ma, X.; Yu, J.; Zhang, W.; Zhuang, C. Exposure to a mixture of cigarette smoke carcinogens disturbs gut microbiota and influences metabolic homeostasis in A/J mice. Chem. Biol. Interact. 2021, 344, 109496. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, L.; Zhang, H.; Xing, W.; Wang, Y.; Bai, P.; Zhang, L.; Hayakawa, K.; Toriba, A.; Wei, Y.; et al. Assessing Approaches of Human Inhalation Exposure to Polycyclic Aromatic Hydrocarbons: A Review. Int. J. Environ. Res. Public Health 2021, 18, 3124. [Google Scholar] [CrossRef] [PubMed]
- Weitekamp, C.A.; Phillips, L.J.; Carlson, L.M.; DeLuca, N.M.; Cohen Hubal, E.A.; Lehmann, G.M. A state-of-the-science review of polychlorinated biphenyl exposures at background levels: Relative contributions of exposure routes. Sci. Total Environ. 2021, 776, 145912. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, Y.; Li, W.; Wang, L.; Jiao, Y.; Wang, Y.; Jiang, D.; Gao, X. Occurrence and dietary exposure of heavy metals in marketed vegetables and fruits of Shandong Province, China. Food Sci. Nutr. 2021, 9, 5166–5173. [Google Scholar] [CrossRef] [PubMed]
- Jusko, T.A.; Henderson, C.R.; Lanphear, B.P.; Cory-Slechta, D.A.; Parsons, P.J.; Canfield, R.L. Blood lead concentrations < 10 microg/dL and child intelligence at 6 years of age. Environ. Health Perspect. 2008, 116, 243–248. [Google Scholar] [CrossRef]
- Li, P.; Jiang, Y.; Fei, Y.Y.; Zeng, X.C. Dynamic Variation of Camel Gastrointestinal Bacterial Communities Contributing to Benzo(a)pyrene Degradation. Appl. Biochem. Microbiol. 2022, 58, 796–805. [Google Scholar] [CrossRef]
- He, S.; Li, X.; Li, C.; Deng, H.; Shao, Y.; Yuan, L. Isoorientin attenuates benzo[a]pyrene-induced colonic injury and gut microbiota disorders in mice. Food Res. Int. 2019, 126, 108599. [Google Scholar] [CrossRef]
- Li, G.; Xiong, H.; Saeed, K.; Ma, R.; Xing, Y.; Bi, Y.; Li, C.; Huang, J.; Zhang, Y. Comparative toxicity analysis of corannulene and benzo[a]pyrene in mice. Toxicol Lett 2020, 331, 130–142. [Google Scholar] [CrossRef]
- Garcia, W.L.; Miller, C.J.; Lomas, G.X.; Gaither, K.A.; Tyrrell, K.J.; Smith, J.N.; Brandvold, K.R.; Wright, A.T. Profiling How the Gut Microbiome Modulates Host Xenobiotic Metabolism in Response to Benzo[a]pyrene and 1-Nitropyrene Exposure. Chem. Res. Toxicol. 2022, 35, 585–596. [Google Scholar] [CrossRef]
- DeBofsky, A.; Xie, Y.; Challis, J.K.; Jain, N.; Brinkmann, M.; Jones, P.D.; Giesy, J.P. Responses of juvenile fathead minnow (Pimephales promelas) gut microbiome to a chronic dietary exposure of benzo[a]pyrene. Environ. Pollut. 2021, 278, 116821. [Google Scholar] [CrossRef]
- Quintanilla-Mena, M.; Vega-Arreguin, J.; Del Rio-Garcia, M.; Patino-Suarez, V.; Peraza-Echeverria, S.; Puch-Hau, C. The effect of benzo[a]pyrene on the gut microbiota of Nile tilapia (Oreochromis niloticus). Appl. Microbiol. Biotechnol. 2021, 105, 7935–7947. [Google Scholar] [CrossRef] [PubMed]
- Stagaman, K.; Kasschau, K.D.; Tanguay, R.L.; Sharpton, T.J. Experimental methods modestly impact interpretation of the effect of environmental exposures on the larval zebrafish gut microbiome. Sci. Rep. 2022, 12, 14538. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Miao, J.; Pan, L.; Zhou, Y.; Gao, Z.; Yang, Y.; Xu, R.; Zhang, X. Impacts of benzo(a)pyrene exposure on scallop (Chlamys farreri) gut health and gut microbiota composition. Sci. Total Environ. 2021, 799, 149471. [Google Scholar] [CrossRef] [PubMed]
- Murinova, S.; Dercova, K. Response mechanisms of bacterial degraders to environmental contaminants on the level of cell walls and cytoplasmic membrane. Int. J. Microbiol. 2014, 2014, 873081. [Google Scholar] [CrossRef]
- Wang, X.; Quinn, P.J. Lipopolysaccharide: Biosynthetic pathway and structure modification. Prog. Lipid Res. 2010, 49, 97–107. [Google Scholar] [CrossRef]
- Hardonniere, K.; Saunier, E.; Lemarie, A.; Fernier, M.; Gallais, I.; Helies-Toussaint, C.; Mograbi, B.; Antonio, S.; Benit, P.; Rustin, P.; et al. The environmental carcinogen benzo[a]pyrene induces a Warburg-like metabolic reprogramming dependent on NHE1 and associated with cell survival. Sci. Rep. 2016, 6, 30776. [Google Scholar] [CrossRef]
- Almeida-da-Silva, C.L.C.; Savio, L.E.B.; Coutinho-Silva, R.; Ojcius, D.M. The role of NOD-like receptors in innate immunity. Front. Immunol. 2023, 14, 1122586. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, G.; Qi, M.; El-Assaad, F.; Wang, Y.; Dong, S.; Chen, L.; Yu, D.; Weaver, J.C.; Beretov, J.; et al. Gram Negative Bacterial Inflammation Ameliorated by the Plasma Protein Beta 2-Glycoprotein I. Sci. Rep. 2016, 6, 33656. [Google Scholar] [CrossRef]
- Defois, C.; Ratel, J.; Garrait, G.; Denis, S.; Le Goff, O.; Talvas, J.; Mosoni, P.; Engel, E.; Peyret, P. Food Chemicals Disrupt Human Gut Microbiota Activity and Impact Intestinal Homeostasis as Revealed by In Vitro Systems. Sci. Rep. 2018, 8, 11006. [Google Scholar] [CrossRef]
- De Preter, V.; Joossens, M.; Ballet, V.; Shkedy, Z.; Rutgeerts, P.; Vermeire, S.; Verbeke Phd, K. Metabolic profiling of the impact of oligofructose-enriched inulin in Crohn’s disease patients: A double-blinded randomized controlled trial. Clin. Transl. Gastroenterol. 2013, 4, e30. [Google Scholar] [CrossRef]
- d’Afflitto, M.; Upadhyaya, A.; Green, A.; Peiris, M. Association Between Sex Hormone Levels and Gut Microbiota Composition and Diversity—A Systematic Review. J. Clin. Gastroenterol. 2022, 56, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, R.; Teixeira, D.; Calhau, C. Estrogen signaling in metabolic inflammation. Mediat. Inflamm. 2014, 2014, 615917. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Mathis, D.; Shoelson, S.E. Immunometabolism: An emerging frontier. Nat. Rev. Immunol. 2011, 11, 81. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Shi, L.; Li, H.; Song, W.; Li, J.; Yuan, L. Selenium-Enriched Black Soybean Protein Prevents Benzo(a)pyrene-Induced Pyroptotic Colon Damage and Gut Dysbacteriosis. J. Agric. Food Chem. 2022, 70, 12629–12640. [Google Scholar] [CrossRef]
- Wu, J.C.; Tsai, M.L.; Lai, C.S.; Lo, C.Y.; Ho, C.T.; Wang, Y.J.; Pan, M.H. Polymethoxyflavones prevent benzo[a]pyrene/dextran sodium sulfate-induced colorectal carcinogenesis through modulating xenobiotic metabolism and ameliorate autophagic defect in ICR mice. Int. J. Cancer 2018, 142, 1689–1701. [Google Scholar] [CrossRef]
- Csak, T.; Ganz, M.; Pespisa, J.; Kodys, K.; Dolganiuc, A.; Szabo, G. Fatty acid and endotoxin activate inflammasomes in mouse hepatocytes that release danger signals to stimulate immune cells. Hepatology 2011, 54, 133–144. [Google Scholar] [CrossRef]
- Ganz, M.; Csak, T.; Nath, B.; Szabo, G. Lipopolysaccharide induces and activates the Nalp3 inflammasome in the liver. World J. Gastroenterol. 2011, 17, 4772–4778. [Google Scholar] [CrossRef]
- Xie, S.; Zhou, A.; Xu, N.; Feng, Y.; Pan, Z.; Junaid, M.; Wang, J.; Zou, J. Benzo[a]pyrene induces microbiome dysbiosis and inflammation in the intestinal tracts of western mosquitofish (Gambusia affinis) and zebrafish (Danio rerio). Fish Shellfish Immunol. 2020, 105, 24–34. [Google Scholar] [CrossRef]
- Mostafavi Abdolmaleky, H.; Zhou, J.R. Gut Microbiota Dysbiosis, Oxidative Stress, Inflammation, and Epigenetic Alterations in Metabolic Diseases. Antioxidants 2024, 13, 985. [Google Scholar] [CrossRef]
- Khan, M.F.; Wang, H. Environmental Exposures and Autoimmune Diseases: Contribution of Gut Microbiome. Front. Immunol. 2019, 10, 3094. [Google Scholar] [CrossRef] [PubMed]
- Sencio, V.; Machado, M.G.; Trottein, F. The lung-gut axis during viral respiratory infections: The impact of gut dysbiosis on secondary disease outcomes. Mucosal. Immunol. 2021, 14, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Cheng, Q.; Wang, Y.; Mu, H.; Zhang, Y. COPD and Gut-Lung Axis: How Microbiota and Host Inflammasome Influence COPD and Related Therapeutics. Front. Microbiol. 2022, 13, 868086. [Google Scholar] [CrossRef] [PubMed]
- Chunxi, L.; Haiyue, L.; Yanxia, L.; Jianbing, P.; Jin, S. The Gut Microbiota and Respiratory Diseases: New Evidence. J. Immunol. Res. 2020, 2020, 2340670. [Google Scholar] [CrossRef]
- Carrizales-Sanchez, A.K.; Garcia-Cayuela, T.; Hernandez-Brenes, C.; Senes-Guerrero, C. Gut microbiota associations with metabolic syndrome and relevance of its study in pediatric subjects. Gut Microbes 2021, 13, 1960135. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, L.; Sun, L.; Ye, X.; Ma, M.; Dou, M.; Shi, L. Association Between Intestinal Prevotella copri Abundance and Glycemic Fluctuation in Patients with Brittle Diabetes. Diabetes Metab. Syndr. Obes. 2023, 16, 1613–1621. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Lu, L.; Luo, T.; Zhao, Y.; Cai, C.; Fu, Z.; Jin, Y. Interaction between microplastics and microorganism as well as gut microbiota: A consideration on environmental animal and human health. Sci. Total Environ. 2019, 667, 94–100. [Google Scholar] [CrossRef]
- Liao, X.; Zhao, P.; Hou, L.; Adyari, B.; Xu, E.G.; Huang, Q.; Hu, A. Network analysis reveals significant joint effects of microplastics and tetracycline on the gut than the gill microbiome of marine medaka. J. Hazard. Mater. 2023, 442, 129996. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, W.; Chan, H.; Peng, J.; Zhu, P.; Li, J.; Jiang, X.; Zhang, Z.; Wang, Y.; Tan, Z.; et al. Polystyrene Microplastics Induce Size-Dependent Multi-Organ Damage in Mice: Insights into Gut Microbiota and Fecal Metabolites. J. Hazard. Mater. 2023, 461, 132503. [Google Scholar] [CrossRef] [PubMed]
- Fournier, E.; Ratel, J.; Denis, S.; Leveque, M.; Ruiz, P.; Mazal, C.; Amiard, F.; Edely, M.; Bezirard, V.; Gaultier, E.; et al. Exposure to polyethylene microplastics alters immature gut microbiome in an infant in vitro gut model. J. Hazard. Mater. 2023, 443, 130383. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Lv, M.; Li, J.; Ding, J.; Wang, Y.; Fu, L.; Sun, X.; Han, X.; Chen, L. The distinct toxicity effects between commercial and realistic polystyrene microplastics on microbiome and histopathology of gut in zebrafish. J. Hazard. Mater. 2022, 434, 128874. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G. Microplastics as vectors of contaminants. Mar. Pollut. Bull. 2019, 146, 921–924. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Bakir, A.; Burton, G.A.; Janssen, C.R. Microplastic as a Vector for Chemicals in the Aquatic Environment: Critical Review and Model-Supported Reinterpretation of Empirical Studies. Environ. Sci. Technol. 2016, 50, 3315–3326. [Google Scholar] [CrossRef]
- Fu, L.; Li, J.; Wang, G.; Luan, Y.; Dai, W. Adsorption behavior of organic pollutants on microplastics. Ecotoxicol. Environ. Saf. 2021, 217, 112207. [Google Scholar] [CrossRef]
- Shaoyong, W.; Jin, H.; Jiang, X.; Xu, B.; Liu, Y.; Wang, Y.; Jin, M. Benzo [a] pyrene-loaded aged polystyrene microplastics promote colonic barrier injury via oxidative stress-mediated notch signalling. J. Hazard. Mater. 2023, 457, 131820. [Google Scholar] [CrossRef]
- Katsumiti, A.; Losada-Carrillo, M.P.; Barros, M.; Cajaraville, M.P. Polystyrene nanoplastics and microplastics can act as Trojan horse carriers of benzo(a)pyrene to mussel hemocytes in vitro. Sci. Rep. 2021, 11, 22396. [Google Scholar] [CrossRef]
- Zou, H.; Qu, H.; Bian, Y.; Sun, J.; Wang, T.; Ma, Y.; Yuan, Y.; Gu, J.; Bian, J.; Liu, Z. Polystyrene Microplastics Induce Oxidative Stress in Mouse Hepatocytes in Relation to Their Size. Int. J. Mol. Sci. 2023, 24, 7382. [Google Scholar] [CrossRef]
- Ziani, K.; Ionita-Mindrican, C.B.; Mititelu, M.; Neacsu, S.M.; Negrei, C.; Morosan, E.; Draganescu, D.; Preda, O.T. Microplastics: A Real Global Threat for Environment and Food Safety: A State of the Art Review. Nutrients 2023, 15, 617. [Google Scholar] [CrossRef]
- Amelia, T.S.M.; Khalik, W.M.A.W.M.; Ong, M.C.; Shao, Y.T.; Pan, H.-J.; Bhubalan, K. Marine microplastics as vectors of major ocean pollutants and its hazards to the marine ecosystem and humans. Prog. Earth Planet. Sci. 2021, 8, 12. [Google Scholar] [CrossRef]
- Cao, J.; Yang, Q.; Jiang, J.; Dalu, T.; Kadushkin, A.; Singh, J.; Fakhrullin, R.; Wang, F.; Cai, X.; Li, R. Coronas of micro/nano plastics: A key determinant in their risk assessments. Part. Fibre Toxicol. 2022, 19, 55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, Y.; Li, X.; Zhang, Y.; Gao, W.; Jiang, J.; Mo, A.; He, D. Size/shape-dependent migration of microplastics in agricultural soil under simulative and natural rainfall. Sci. Total Environ. 2022, 815, 152507. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, M.; Ding, G.; Shi, H.; Cong, Y.; Li, Z.; Wang, J. Polystyrene microplastics alleviate adverse effects of benzo[a]pyrene on tissues and cells of the marine mussel, Mytilus galloprovincialis. Aquat. Toxicol. 2023, 256, 106430. [Google Scholar] [CrossRef]
- Bebianno, M.J.; Mendes, V.M.; O’Donovan, S.; Carteny, C.C.; Keiter, S.; Manadas, B. Effects of microplastics alone and with adsorbed benzo(a)pyrene on the gills proteome of Scrobicularia plana. Sci. Total Environ. 2022, 842, 156895. [Google Scholar] [CrossRef]
- Romdhani, I.; De Marco, G.; Cappello, T.; Ibala, S.; Zitouni, N.; Boughattas, I.; Banni, M. Impact of environmental microplastics alone and mixed with benzo[a]pyrene on cellular and molecular responses of Mytilus galloprovincialis. J. Hazard. Mater. 2022, 435, 128952. [Google Scholar] [CrossRef]
- Rodrigues, A.R.; Mestre, N.C.C.; Fonseca, T.G.D.; Pedro, P.Z.; Carteny, C.C.; Cormier, B.; Keiter, S.; Bebianno, M.J. Influence of Particle Size on Ecotoxicity of Low-Density Polyethylene Microplastics, with and without Adsorbed Benzo-a-Pyrene, in Clam Scrobicularia plana. Biomolecules 2022, 12, 78. [Google Scholar] [CrossRef]
- von Hellfeld, R.; Zarzuelo, M.; Zaldibar, B.; Cajaraville, M.P.; Orbea, A. Accumulation, Depuration, and Biological Effects of Polystyrene Microplastic Spheres and Adsorbed Cadmium and Benzo(a)pyrene on the Mussel Mytilus galloprovincialis. Toxics 2022, 10, 18. [Google Scholar] [CrossRef]
- Martinez-Alvarez, I.; Le Menach, K.; Devier, M.H.; Cajaraville, M.P.; Budzinski, H.; Orbea, A. Screening of the Toxicity of Polystyrene Nano- and Microplastics Alone and in Combination with Benzo(a)pyrene in Brine Shrimp Larvae and Zebrafish Embryos. Nanomaterials 2022, 12, 941. [Google Scholar] [CrossRef]
- Coffin, S.; Magnuson, J.T.; Vliet, S.M.F.; Volz, D.C.; Schlenk, D. Effects of short-term exposure to environmentally-relevant concentrations of benzo(a)pyrene-sorbed polystyrene to White seabass (Atractoscion nobilis)☆. Environ. Pollut. 2020, 263, 114617. [Google Scholar] [CrossRef]
- Abouda, S.; Missawi, O.; Cappello, T.; Boughattas, I.; De Marco, G.; Maisano, M.; Banni, M. Toxicological impact of environmental microplastics and benzo[a]pyrene in the seaworm Hediste diversicolor under environmentally relevant exposure conditions. Environ. Pollut. 2022, 310, 119856. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Qi, H.; Hou, Y.; Gao, M.; Li, J.; Cai, M.; Zhu, X.; Chen, M.; Ge, C.; Fu, D.; et al. Combined Effects of Microplastics and Benzo[a]pyrene on the Marine Diatom Chaetoceros muelleri. Front. Mar. Sci. 2022, 8, 779321. [Google Scholar] [CrossRef]
- Li, W.; Chen, X.; Li, M.; Cai, Z.; Gong, H.; Yan, M. Microplastics as an aquatic pollutant affect gut microbiota within aquatic animals. J. Hazard. Mater. 2022, 423, 127094. [Google Scholar] [CrossRef]
- Usman, S.; Razis, A.F.A.; Shaari, K.; Azmai, M.N.A.; Saad, M.Z.; Isa, N.M.; Nazarudin, M.F. Polystyrene microplastics induce gut microbiome and metabolome changes in Javanese medaka fish (Oryzias javanicus Bleeker, 1854). Toxicol. Rep. 2022, 9, 1369–1379. [Google Scholar] [CrossRef]
- Kang, H.; Zhang, W.; Jing, J.; Huang, D.; Zhang, L.; Wang, J.; Han, L.; Liu, Z.; Wang, Z.; Gao, A. The gut-brain axis involved in polystyrene nanoplastics-induced neurotoxicity via reprogramming the circadian rhythm-related pathways. J. Hazard. Mater. 2023, 458, 131949. [Google Scholar] [CrossRef]
- Grodzicki, W.; Dziendzikowska, K.; Gromadzka-Ostrowska, J.; Kruszewski, M. Nanoplastic Impact on the Gut-Brain Axis: Current Knowledge and Future Directions. Int. J. Mol. Sci. 2021, 22, 12795. [Google Scholar] [CrossRef]
- Yang, Q.; Dai, H.; Cheng, Y.; Wang, B.; Xu, J.; Zhang, Y.; Chen, Y.; Xu, F.; Ma, Q.; Lin, F.; et al. Oral feeding of nanoplastics affects brain function of mice by inducing macrophage IL-1 signal in the intestine. Cell Rep. 2023, 42, 112346. [Google Scholar] [CrossRef]
- Zhao, N.; Zhao, M.; Jin, H. Microplastic-induced gut microbiota and serum metabolic disruption in Sprague-Dawley rats. Environ. Pollut. 2023, 320, 121071. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, J.; Chen, Y. Regulation of Neurotransmitters by the Gut Microbiota and Effects on Cognition in Neurological Disorders. Nutrients 2021, 13, 2099. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
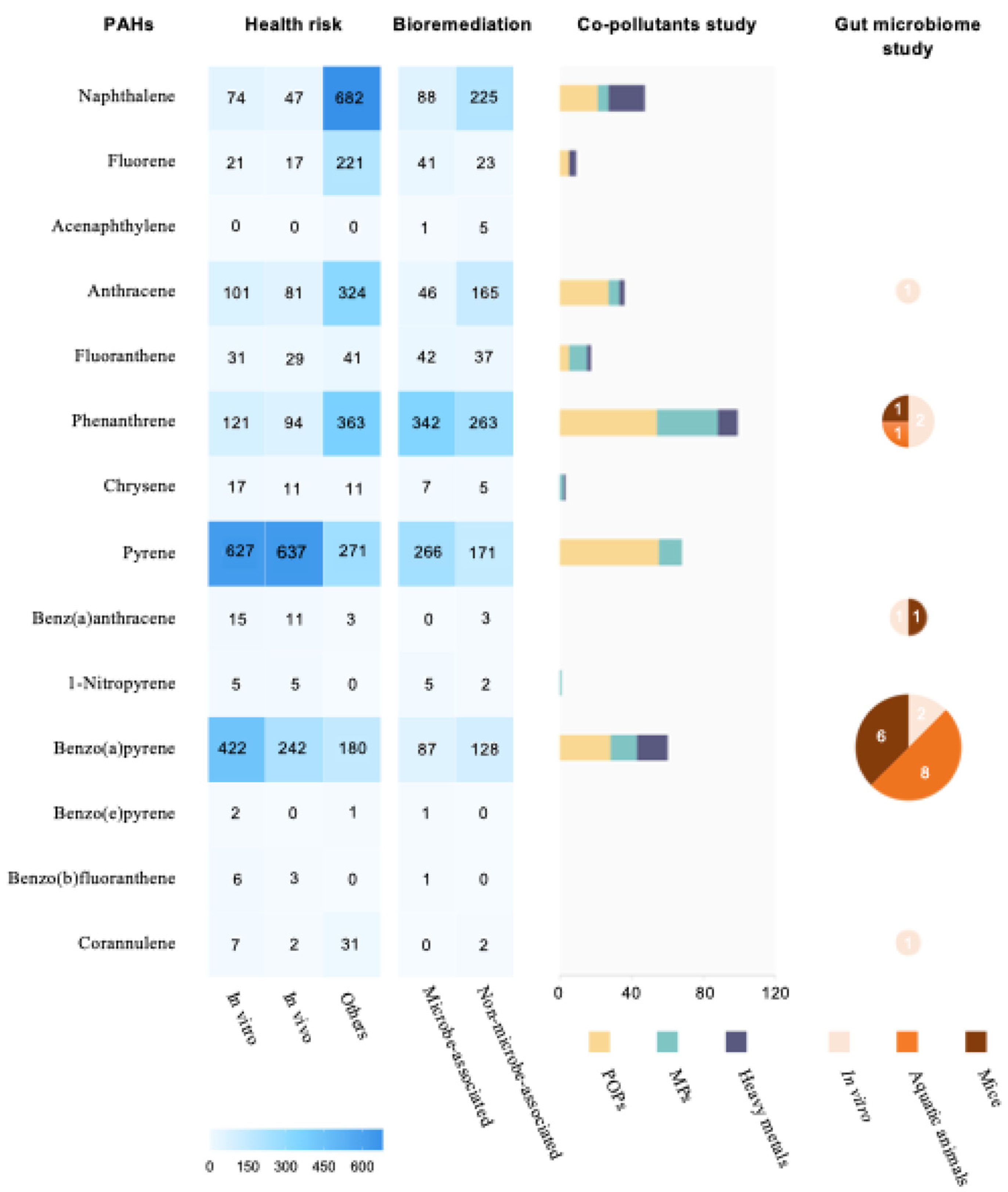
| Species Target | Life Stage | Co-Exposure Pollutant | Pollutants Concentration | Exposure Type | Exposure Period | Intestinal Impacts | Ref. |
|---|---|---|---|---|---|---|---|
| In vitro | |||||||
| Human microbiome | - | - | 0.005, 0.05, and 0.5 mg/mL | Human fecal culture in bio-fermenter | 24 h | ↓ Microbial volatile in a dose-dependent manner ↓ Transcript level of bacterial chemotaxis toward simple carbohydrate pathways | [18] |
| Camel rumen and rectum | - | - | 50 mg/L | Rumen and gut microbes culture | 20 days | Klebsiella sp., Ochrobactrum sp., and Bacillus sp. showed particular function as B(a)P degradation | [76] |
| In vivo (mice) | |||||||
| C57BL/6 mice | 5-week-old (20–25 g) | - | 10 mL/kg B.W. | Oral gavage | 28 days | ↑ Bacteroides, Parabacteroides, and Paraprevotella >27 days ↓ Lactobacillus, A. muciniphila, and Verrumicrobiaceae | [33] |
| BALB/c mice treated with Isoorientin | 5-week-old | - | 50 mg/kg BW | Oral gavage | 42 days | ↑ Desulfovibrio, Acinetobacter, Odoribacter, and Veillonella in B(a)P group ↑ Faecalibaculum and Lactobacillus in B(a)P + ISO group | [77] |
| C57BL/6 mice and male Sprague Dawley (S.D.) rats | 8-week-old mice (22 ± 2 g) and (220–250 g) rats | Corannulene | 100 mg/kg BW COR or B(a)P | Oral gavage and intraperitoneally injection | 3 days | ↑ Bacteroidetes after I.P. injection of B(a)P and COR ↓ Bacteroidetes after oral gavage B(a)P and COR Actinobacteria ↓ by oral COR but ↑ by oral B(a)P | [78] |
| Conventional C57BL/6NTac and germ-free C57Bl/6GFTac mice | 7-week-old | 1-Nitropyrene | 180 mg/kg BW./day B(a)P | Oral gavage | 72 h | Active P450s enzyme in the liver is impacted by the presence of the gut microbiome, which is modified by PAH metabolism | [79] |
| C57BL/6 mice (SPF) treated with Ovalbumin | 5–6 weeks old | - | 50 ug/ mouse/ day | Oral gavage | 23 days | ↑ B. virosa and N. subflava, and ↓ B. uniformis and L. bacterium COE1 in B(a)P group ↑ L. bacterium 3-2, L. bacterium COE1, and Prevotella sp. MGM1 in OVA group | [45] |
| In vivo (Aquatic animals) | |||||||
| Female and male Fathead minnow (Pimephales promelas) | Adult (two years) | - | 1.3, 4.0, and 12.0 mg/L | Water immersion | 4 days | ↑ Alpha diversity in the female group compared to the male group ↓ Vibrionaceae, the only abundant family in the male group | [44] |
| Fathead minnow (Pimephales promelas) | Juvenile (2.5-month-old) | - | 1, 10, 100, or 1000 u µg/g in food (DM) | Feeding | 2 weeks | ↓ Alpha diversity, ↑ pathogenic taxa (Erysipelotrichaceae, Moraxellaceae, and Caulobacteraceae) | [80] |
| Nile tilapia (Oreochromis niloticus) | Juvenile (125.6 ± 41.4 g) | - | 20 mg/kg B.W. | Intraperitoneal injection | 24, 72, and 120 h post-injection | ↑ Fusobacteria and Bacteroidetes in <24 h ↓ Proteobacteria and Spirochaetae in >24 h GM recovered after 72 h and was stable at 120 h post-injection | [81] |
| Zebrafish (Danio rerio) | Embryos (9 days post fertilization) | - | 1, 5, and 10 μM | Embryos Incubation test—with different dissection methods | 9 days | Gut microbiota significantly altered based on dose-dependent | [82] |
| Scallop (Chlamys farreri) | (5.7 ± 0.3 cm in length) | - | 0, 0.4, 2 and 10 μg/L | Water immersion | 7, 14 and 21 days | ↓ Alpha diversity, ↑ pathogenic bacteria Mycoplasma and Tenacibaculum. Hydrocarbon-degrading bacteria were found: Pseudomonas, Polaribacter, Amphritea, and Kordiimonas | [83] |
| Sea cucumbers (Apostichopus japonicus) | Juvenile (5.36 ± 0.14 g) | - | 0, 0.5, 5, and 25 μg/L | Water immersion | 14 days | ↑ Ratio of Bacteroidetes to Firmicutes | [43] |
| Species Target | MPs Type | MPs Size | MPs Binding Affinity 1 | Pollutant Concentrations and Exposure Method | Exposure Period | Toxicity Effects | Ref. |
|---|---|---|---|---|---|---|---|
| Marine mussels (M. galloprovincialis) | Green fluorescent polystyrene | 10 µm | *** | 5.5 µg/L MPs + 0.1 µg/L B(a)P in water immersion | 5 days | ↓ mRNA expression of NF- κB in gills ↑ strong affinity adsorption of B(a)P to PS-MPs ↓ The uptake and toxicity of B(a)P | [124] |
| Clam (Scrobicularia plana) | Low-density polyethylene | 11–13 µm | - | MPs with B(a)P adsorbed at one mg/L in water immersion | 14 days | Changes in protein expression of the cytoskeleton, cell structure, oxidative stress, energy metabolism, and DNA binding also induce changes in glucose metabolism, RNA binding, and apoptosis | [125] |
| Marine mussels (M. galloprovincialis) | Environmental mixture MPs: polyethylene, polyethylene terephthalate, polypropylene, polyethylene vinyl acetate, and high-density polyethylene | <100 µm | - | 50 µg/L MP + 1 µg/L B(a)P in water immersion | 1 and 3 days | Induced the apoptosis process: ↑ DNA ligase on day 1 ↑ Bax, Cas-3, and P53 and on day 3 ↓ Bcl-2 and DNA ligase on day 3 | [126] |
| Clam (Scrobicularia plana) | Low-density polyethylene | 4–6 μm and 20–25 μm | - | 1 mg/L MP + 16.64 ± 87 µg/g B(a)P in water immersion | 7 and 14 days | 4–6 μm-sized MPs resulted in more significant alterations in oxidative stress biomarkers | [127] |
| Marine mussels (M. galloprovincialis) | Polystyrene pristine | 4.5 and 45 µm | *** | (0.05, 5, 50 µg/L MPs) + 252.3 µg/L B(a)P and Cd | 3 days | ↑ PS shows a higher affinity to B(a)P than Cd MPs and B(a)P group induced histological alteration in digestive glands | [128] |
| Brine shrimp larvae and zebrafish embryos | Polystyrene | 50 and 500 nm | *** | 0.069–6.87 mg/L PS + 0.1–10 mg/L B(a)P | 24–48 h | ↑ malformation prevalence in the highest concentration of MPs and B(a)P groups in zebrafish; meanwhile, NPs were successful vectors to B(a)P in brine shrimp | [129] |
| White seabass | Polystyrene | 2.00–2.83 mm | *** | 100 g/2 L PS + 1µg/L B(a)P and single dose of 1 µg/L B(a)P and 252 µg/ B(a)P in water immersion | 5 days | Single dose of 252 µg/ B(a)P group; fish exposed to polystyrene B(a)P-absorbed polystyrene show significant variations in the observed cellular or behavioral parameters compared to the control group | [130] |
| Seaworm (Hediste diversicolor) | Environmental mixture MPs: polyethylene, polyethylene terephthalate, polypropylene, polyethylene vinyl acetate, and high-density polyethylene | >3, 3.0–1.22, 1.22–0.45 µm | - | 1 mg/kg (sediment) of environmental MPs + 1 μg/kg (sediment) B[a]P | 3 and 7 days | ↑ cytotoxic and genotoxic damage in the co-exposure and single groups after 7 days. | [131] |
| Marine diatom (Chaetoceros muelleri) | Polyethylene terephthalate | - | - | 200 mg/L PET + (10, 150 µg/L B(a)P) in medium culture | 1, 5, 15 days | ↑ SOD and MDA on day 1 and 5 ↓ SOD and MDA on day 15 MPs and 10 µg/L B(a)P group showed higher antagonistic effects to the marine diatom. | [132] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mauliasari, I.R.; Lee, H.J.; Koo, S.Y.; Hitayezu, E.; Kieu, A.N.T.; Lee, S.-M.; Cha, K.H. Benzo(a)pyrene and Gut Microbiome Crosstalk: Health Risk Implications. Toxics 2024, 12, 938. https://doi.org/10.3390/toxics12120938
Mauliasari IR, Lee HJ, Koo SY, Hitayezu E, Kieu ANT, Lee S-M, Cha KH. Benzo(a)pyrene and Gut Microbiome Crosstalk: Health Risk Implications. Toxics. 2024; 12(12):938. https://doi.org/10.3390/toxics12120938
Chicago/Turabian StyleMauliasari, Intan Rizki, Hee Ju Lee, Song Yi Koo, Emmanuel Hitayezu, Anh Nguyen Thi Kieu, Sang-Min Lee, and Kwang Hyun Cha. 2024. "Benzo(a)pyrene and Gut Microbiome Crosstalk: Health Risk Implications" Toxics 12, no. 12: 938. https://doi.org/10.3390/toxics12120938
APA StyleMauliasari, I. R., Lee, H. J., Koo, S. Y., Hitayezu, E., Kieu, A. N. T., Lee, S.-M., & Cha, K. H. (2024). Benzo(a)pyrene and Gut Microbiome Crosstalk: Health Risk Implications. Toxics, 12(12), 938. https://doi.org/10.3390/toxics12120938






