Investigating the Potential Effects of 6PPDQ on Prostate Cancer Through Network Toxicology and Molecular Docking
Abstract
1. Introduction
2. Materials and Methods
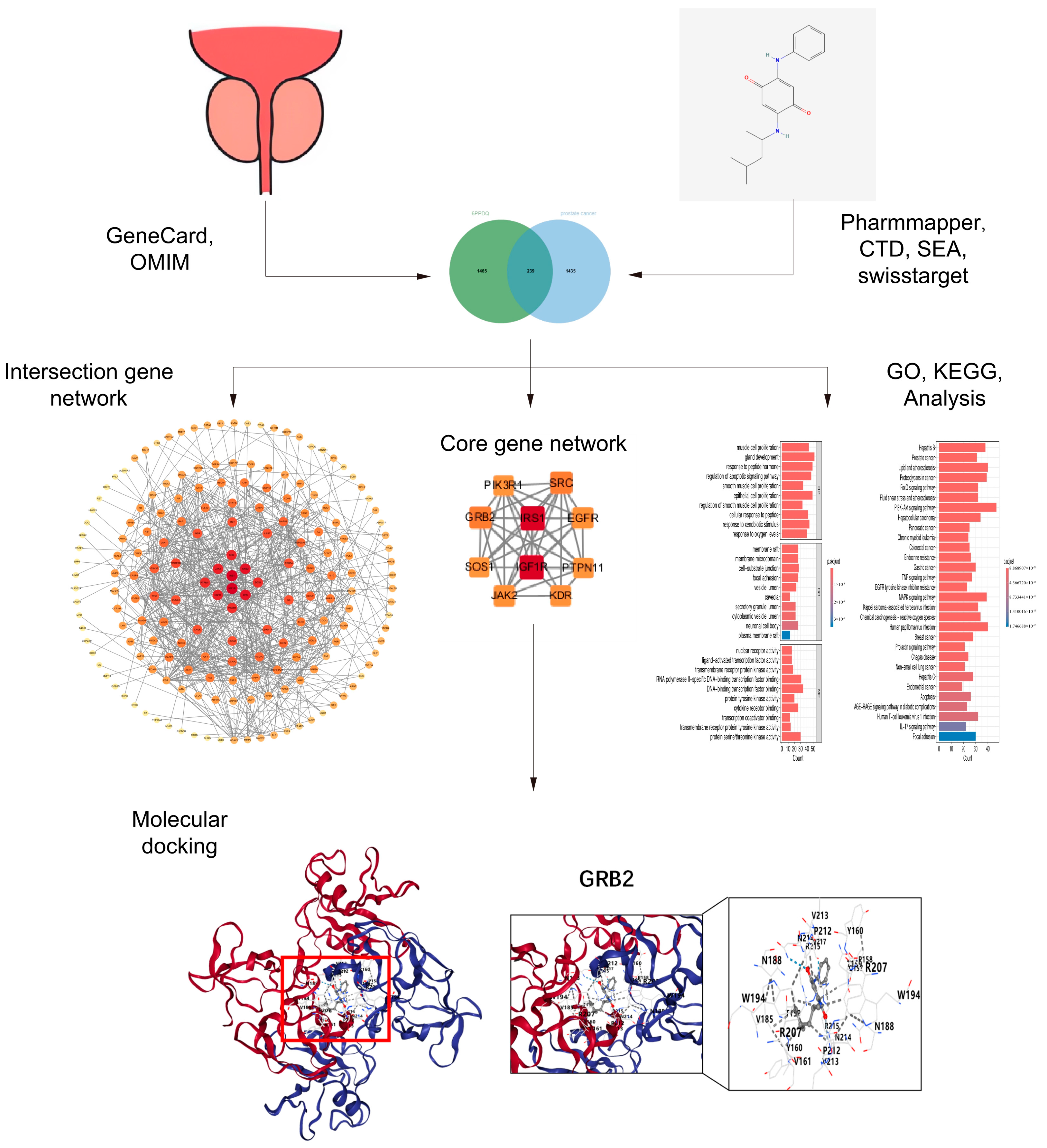
2.1. Predicted Target of 6PPDQ
2.2. Collection of the Gene Targets of Prostate Cancer
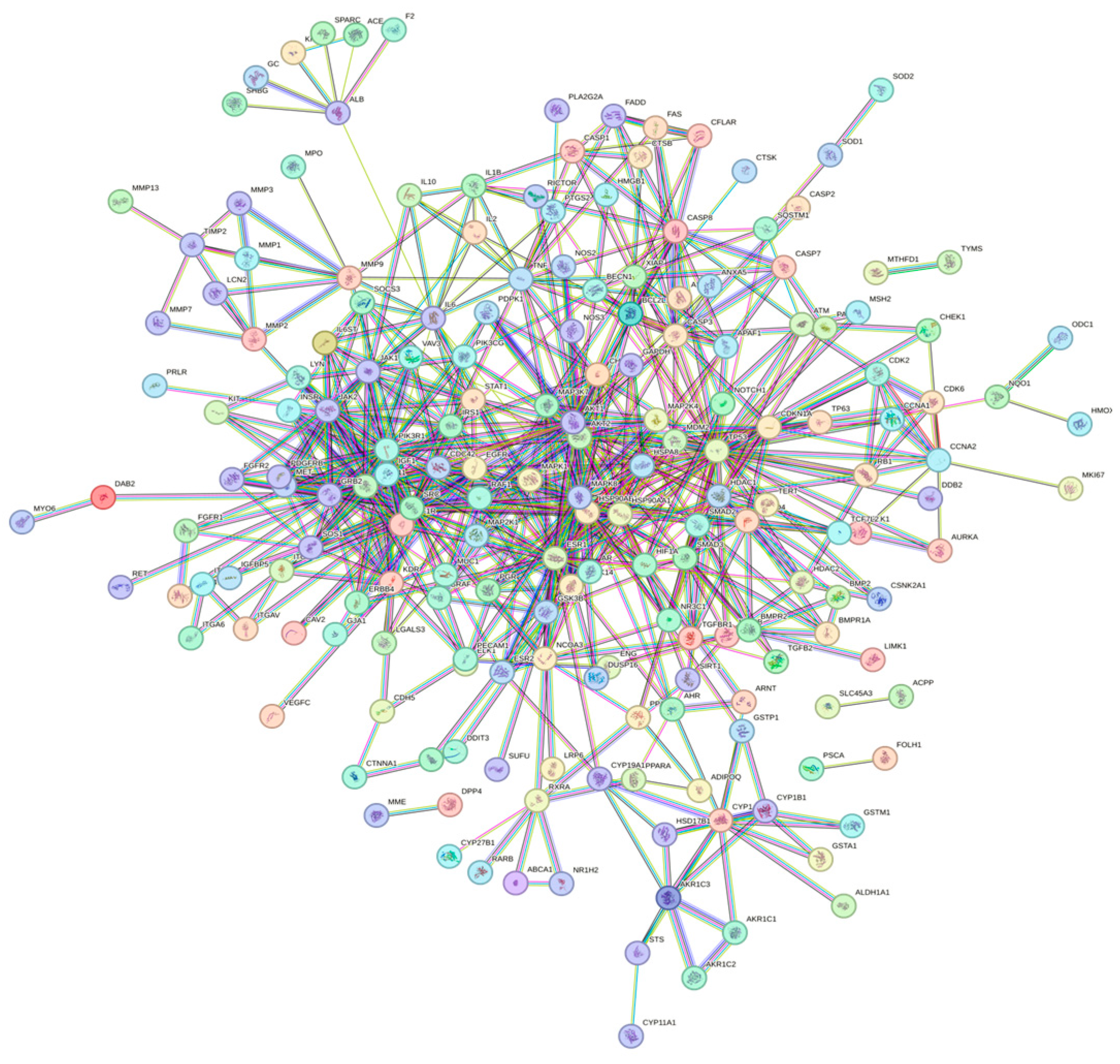
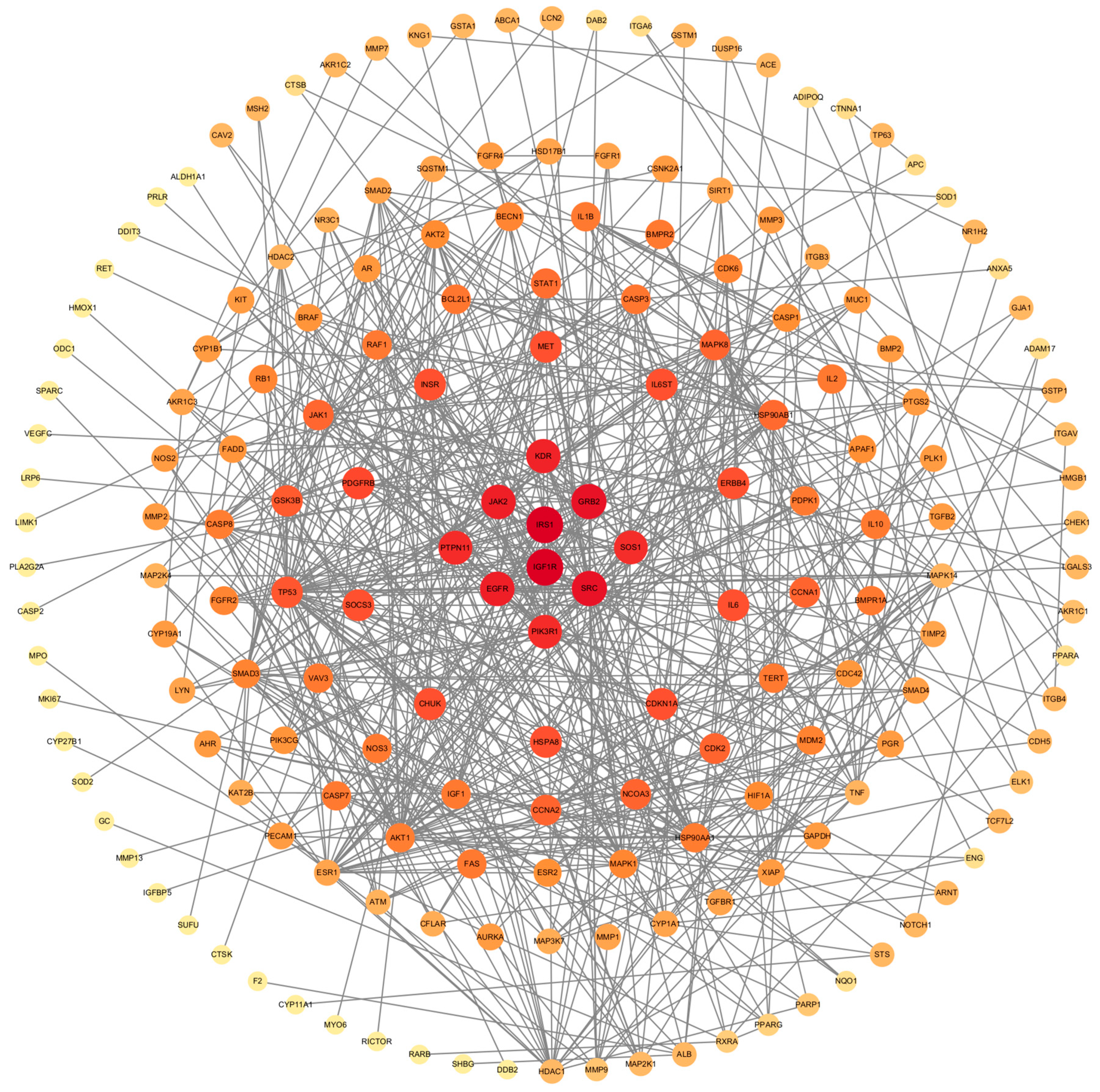
2.3. Construction of PPI Network and Filtration of Core Targets
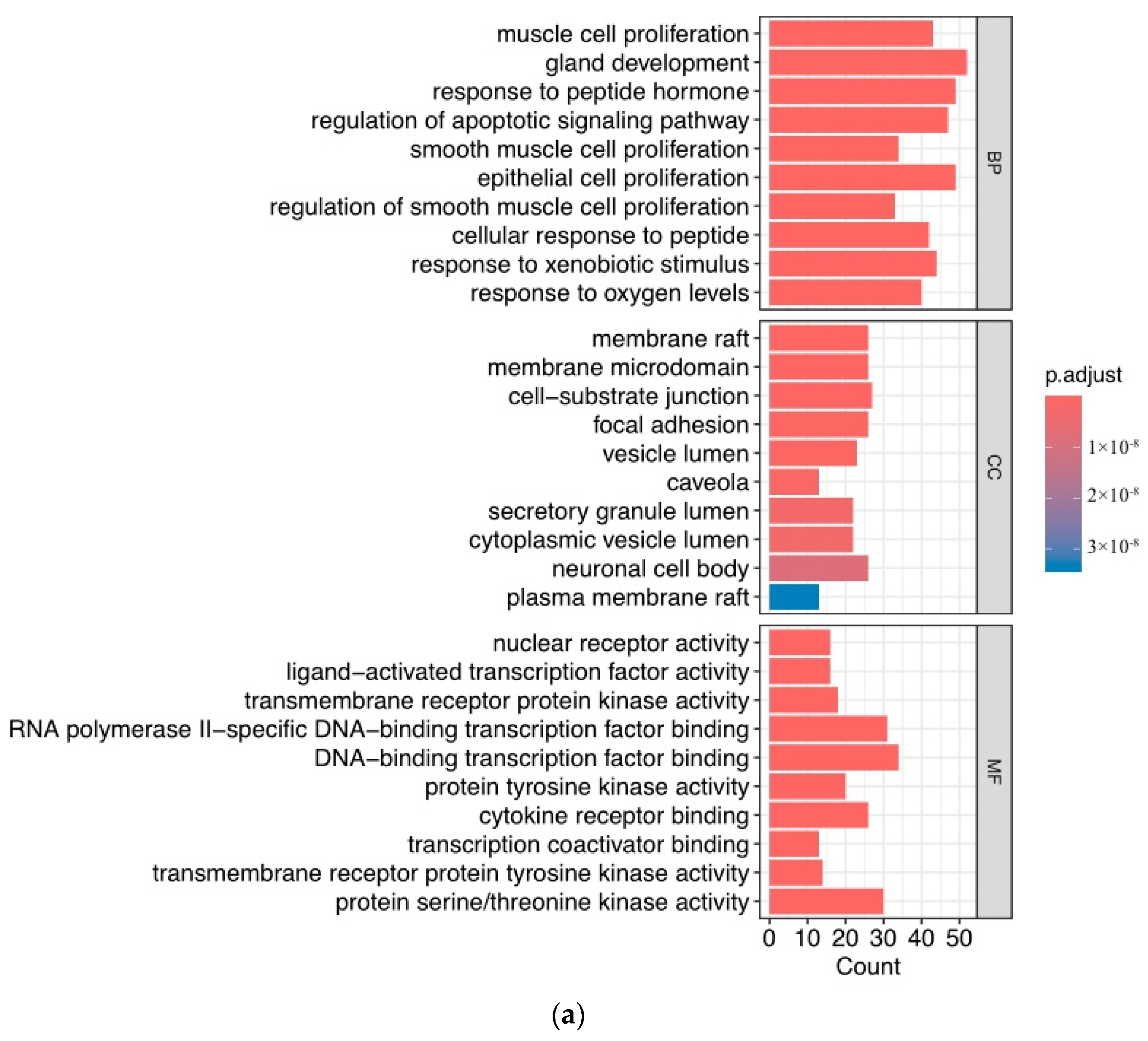
2.4. Gene Ontology and Pathway Enrichment Analysis
2.5. Molecular Docking
3. Results
3.1. Predicted Targets of Prostate Cancer Triggered by 6PPDQ
3.2. Construction of PPI Network and Identification of Core Target
3.3. Function of Target Analysis and Biological Pathway Analysis
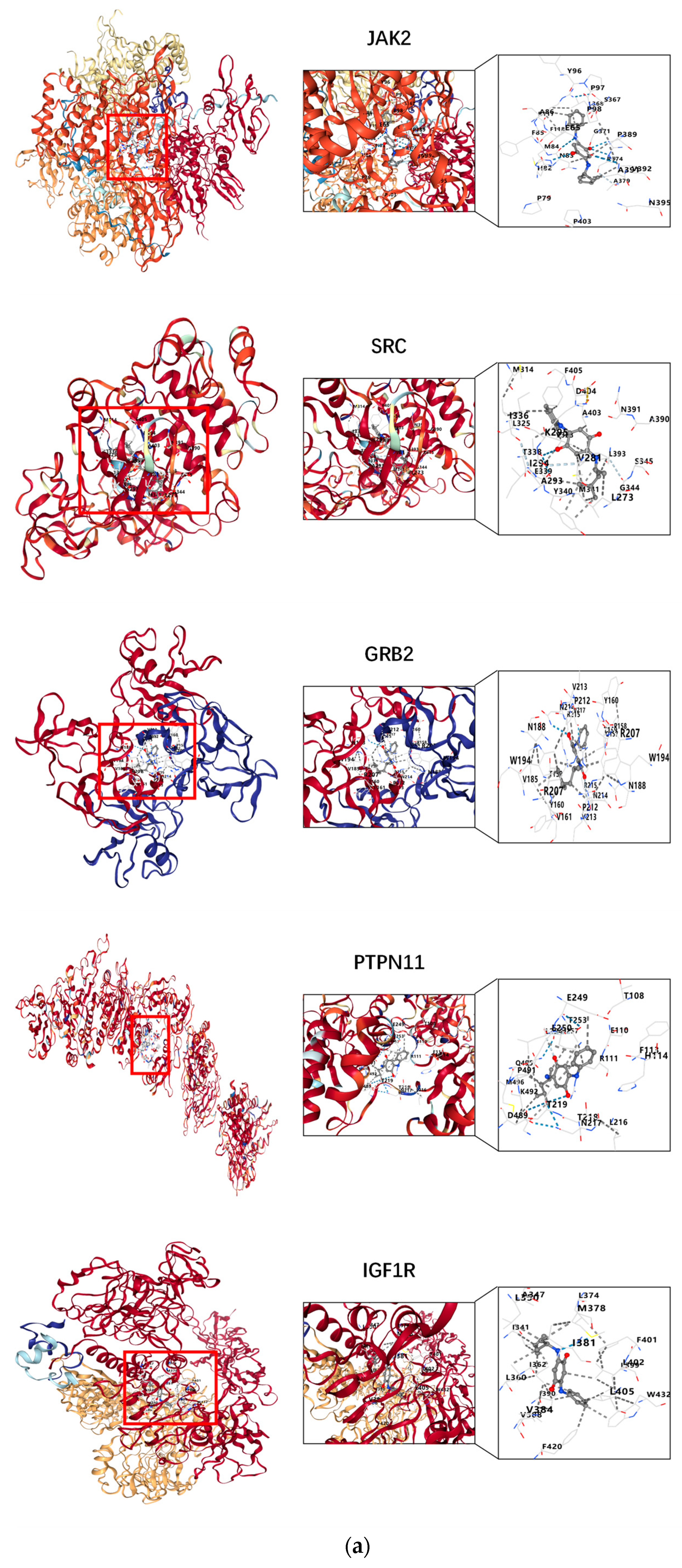
3.4. Results of Molecular Docking
4. Discussion
4.1. Network Toxicology and 6PPDQ
4.2. Core Genes and Prostate Cancer
4.3. Analysis of GO and KEGG Related to Prostate Cancer
4.4. Molecular Docking Between 6PPDQ and Core Proteins
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, G.; Wang, W.; Zhang, J.; Wu, P.; Zhao, X.; Yang, Z.; Hu, D.; Cai, Z. New Evidence of Rubber-Derived Quinones in Water, Air, and Soil. Environ. Sci. Technol. 2022, 56, 4142–4150. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Wang, D. Tire-rubber related pollutant 6-PPD quinone: A review of its transformation, environmental distribution, bioavailability, and toxicity. J. Hazard. Mater. 2023, 459, 132265. [Google Scholar] [CrossRef] [PubMed]
- Seiwert, B.; Nihemaiti, M.; Troussier, M.; Weyrauch, S.; Reemtsma, T. Abiotic oxidative transformation of 6-PPD and 6-PPD quinone from tires and occurrence of their products in snow from urban roads and in municipal wastewater. Water Res. 2022, 212, 118122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-Y.; Gan, X.; Shen, B.; Jiang, J.; Shen, H.; Lei, Y.; Liang, Q.; Bai, C.; Huang, C.; Wu, W.; et al. 6PPD and its metabolite 6PPDQ induce different developmental toxicities and phenotypes in embryonic zebrafish. J. Hazard. Mater. 2023, 455, 131601. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Kang, Q.; Liu, W.; Chen, D.; Wang, L.; Li, S. Chronic exposure to tire rubber-derived contaminant 6PPD-quinone impairs sperm quality and induces the damage of reproductive capacity in male mice. J. Hazard. Mater. 2024, 470, 134165. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Huang, J.-W.; Liu, Y.-H.; Zhang, J.-N.; Huang, Z.; Liu, Y.-S.; Zhao, J.-L.; Ying, G.-G. In vitro metabolism of the emerging contaminant 6PPD-quinone in human and rat liver microsomes: Kinetics, pathways, and mechanism. Environ. Pollut. 2024, 345, 123514. [Google Scholar] [CrossRef]
- Fu, B.; Chen, T.; Jiang, B.; Feng, H.; Zhu, Z.; Li, M.; Zhang, G.; Jiang, Y. 6PPDQ induces cardiomyocyte senescence via AhR/ROS-mediated autophagic flux blockage. Environ. Pollut. 2024, 349, 123872. [Google Scholar] [CrossRef]
- Deng, M.; Ji, X.; Peng, B.; Fang, M. In Vitro and In Vivo Biotransformation Profiling of 6PPD-Quinone toward Their Detection in Human Urine. Environ. Sci. Technol. 2024, 58, 9113–9124. [Google Scholar] [CrossRef]
- Fang, J.; Wang, X.; Cao, G.; Wang, F.; Ru, Y.; Wang, B.; Zhang, Y.; Zhang, D.; Yan, J.; Xu, J.; et al. 6PPD-quinone exposure induces neuronal mitochondrial dysfunction to exacerbate Lewy neurites formation induced by α-synuclein preformed fibrils seeding. J. Hazard. Mater. 2024, 465, 133312. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, X.; Qian, Z.; Zhong, Q.; Wang, Q.; Hylkema, M.N.; Snieder, H.; Huo, X. Association between 6PPD-quinone exposure and BMI, influenza, and diarrhea in children. Environ. Res. 2024, 247, 118201. [Google Scholar] [CrossRef]
- Wan, X.; Liang, G.; Wang, D. Potential human health risk of the emerging environmental contaminant 6-PPD quinone. Sci. Total Environ. 2024, 949, 175057. [Google Scholar] [CrossRef] [PubMed]
- DeBono, N.L.; Logar-Henderson, C.; Warden, H.; Shakik, S.; Dakouo, M.; MacLeod, J.; Demers, P.A. Cancer surveillance among workers in plastics and rubber manufacturing in Ontario, Canada. Occup. Environ. Med. 2020, 77, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Zeegers, M.P.A.; Friesema, I.H.M.; Goldbohm, R.A.; van den Brandt, P.A. A prospective study of occupation and prostate cancer risk. J. Occup. Environ. Med. 2004, 46, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Fan, H.; Li, Y.; Xiao, X. Research Advances on Hepatotoxicity of Herbal Medicines in China. Biomed. Res. Int. 2016, 2016, 7150391. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Hou, X. Aspartame carcinogenic potential revealed through network toxicology and molecular docking insights. Sci. Rep. 2024, 14, 11492. [Google Scholar] [CrossRef]
- Huang, S. A novel strategy for the study on molecular mechanism of prostate injury induced by 4,4’-sulfonyldiphenol based on network toxicology analysis. J. Appl. Toxicol. 2024, 44, 28–40. [Google Scholar] [CrossRef]
- Wang, X.; Shen, Y.; Wang, S.; Li, S.; Zhang, W.; Liu, X.; Lai, L.; Pei, J.; Li, H. PharmMapper 2017 update: A web server for potential drug target identification with a comprehensive target pharmacophore database. Nucleic Acids Res. 2017, 45, W356–W360. [Google Scholar] [CrossRef]
- Davis, A.P.; Wiegers, T.C.; Johnson, R.J.; Sciaky, D.; Wiegers, J.; Mattingly, C.J. Comparative Toxicogenomics Database (CTD): Update 2023. Nucleic Acids Res. 2023, 51, D1257–D1262. [Google Scholar] [CrossRef]
- Keiser, M.J.; Roth, B.L.; Armbruster, B.N.; Ernsberger, P.; Irwin, J.J.; Shoichet, B.K. Relating protein pharmacology by ligand chemistry. Nat. Biotechnol. 2007, 25, 197–206. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef]
- Stelzer, G.; Rosen, N.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Stein, T.I.; Nudel, R.; Lieder, I.; Mazor, Y.; et al. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr. Protoc. Bioinform. 2016, 54, 1–30. [Google Scholar] [CrossRef] [PubMed]
- McKusick, V.A. Mendelian Inheritance in Man and its online version, OMIM. Am. J. Hum. Genet. 2007, 80, 588–604. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef] [PubMed]
- Mobashir, M.; Turunen, S.P.; Izhari, M.A.; Ashankyty, I.M.; Helleday, T.; Lehti, K. An Approach for Systems-Level Understanding of Prostate Cancer from High-Throughput Data Integration to Pathway Modeling and Simulation. Cells 2022, 11, 4121. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Otasek, D.; Morris, J.H.; Bouças, J.; Pico, A.R.; Demchak, B. Cytoscape Automation: Empowering workflow-based network analysis. Genome Biol. 2019, 20, 185. [Google Scholar] [CrossRef]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef]
- Kalungi, F.; Nsubuga, A.; Anywar, G. Network analysis and molecular docking studies of quercetin as a potential treatment for prostate cancer. In Silico Pharmacol. 2023, 11, 24. [Google Scholar] [CrossRef]
- Vikhar Danish Ahmad, A.; Ayaz Ali, S.; Yasar, Q.; Sakle, N.S.; Mukhtar Khan, M. Integrative network pharmacology, molecular docking, and dynamic simulation analysis of a polyherbal formulation for potential therapeutic impact on prostate cancer. Heliyon 2024, 10, e34531. [Google Scholar] [CrossRef]
- Ongaba, T.; Ndekezi, C.; Nakiddu, N. A Molecular Docking Study of Human STEAP2 for the Discovery of New Potential Anti-Prostate Cancer Chemotherapeutic Candidates. Front. Bioinform. 2022, 2, 869375. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, X.; Gan, J.; Chen, S.; Xiao, Z.-X.; Cao, Y. CB-Dock2: Improved protein-ligand blind docking by integrating cavity detection, docking and homologous template fitting. Nucleic Acids Res. 2022, 50, W159–W164. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, Y.; Gan, J.; Xiao, Z.-X.; Cao, Y. FitDock: Protein-ligand docking by template fitting. Brief. Bioinform. 2022, 23, bbac087. [Google Scholar] [CrossRef] [PubMed]
- Nogales, C.; Mamdouh, Z.M.; List, M.; Kiel, C.; Casas, A.I.; Schmidt, H.H.H.W. Network pharmacology: Curing causal mechanisms instead of treating symptoms. Trends Pharmacol. Sci. 2022, 43, 136–150. [Google Scholar] [CrossRef]
- Xie, L.; Huang, B.; Zhao, X.; Zhu, N. Exploring the mechanisms underlying effects of bisphenol a on cardiovascular disease by network toxicology and molecular docking. Heliyon 2024, 10, e31473. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhu, X.; Xu, K.; Li, Y.; Zhou, J. Network toxicological and molecular docking to investigate the mechanisms of toxicity of agricultural chemical Thiabendazole. Chemosphere 2024, 363, 142711. [Google Scholar] [CrossRef] [PubMed]
- Elemam, N.M.; Hotait, H.Y.; Saleh, M.A.; El-Huneidi, W.; Talaat, I.M. Insulin-like growth factor family and prostate cancer: New insights and emerging opportunities. Front. Endocrinol. 2024, 15, 1396192. [Google Scholar] [CrossRef]
- Guerra, F.K.; Eijan, A.M.; Puricelli, L.; Alonso, D.F.; Bal de Kier Joffé, E.; Kornblihgtt, A.R.; Charreau, E.H.; Elizalde, P.V. Varying patterns of expression of insulin-like growth factors I and II and their receptors in murine mammary adenocarcinomas of different metastasizing ability. Int. J. Cancer 1996, 65, 812–820. [Google Scholar] [CrossRef]
- Xiong, W.; Huang, C.; Deng, H.; Jian, C.; Zen, C.; Ye, K.; Zhong, Z.; Zhao, X.; Zhu, L. Oncogenic non-coding RNA NEAT1 promotes the prostate cancer cell growth through the SRC3/IGF1R/AKT pathway. Int. J. Biochem. Cell Biol. 2018, 94, 125–132. [Google Scholar] [CrossRef]
- Capik, O.; Sanli, F.; Kurt, A.; Ceylan, O.; Suer, I.; Kaya, M.; Ittmann, M.; Karatas, O.F. CASC11 promotes aggressiveness of prostate cancer cells through miR-145/IGF1R axis. Prostate Cancer Prostatic Dis. 2021, 24, 891–902. [Google Scholar] [CrossRef]
- Lang, C.; Yin, C.; Lin, K.; Li, Y.; Yang, Q.; Wu, Z.; Du, H.; Ren, D.; Dai, Y.; Peng, X. m6 A modification of lncRNA PCAT6 promotes bone metastasis in prostate cancer through IGF2BP2-mediated IGF1R mRNA stabilization. Clin. Transl. Med. 2021, 11, e426. [Google Scholar] [CrossRef]
- Vallejo-Díaz, J.; Chagoyen, M.; Olazabal-Morán, M.; González-García, A.; Carrera, A.C. The Opposing Roles of PIK3R1/p85α and PIK3R2/p85β in Cancer. Trends Cancer 2019, 5, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, D.; Li, Z.; Li, X.; Jin, M.; Jia, N.; Cui, X.; Hu, G.; Tang, T.; Yu, Q. Pan-cancer analysis on the role of PIK3R1 and PIK3R2 in human tumors. Sci. Rep. 2022, 12, 5924. [Google Scholar] [CrossRef] [PubMed]
- Munkley, J.; Livermore, K.E.; McClurg, U.L.; Kalna, G.; Knight, B.; McCullagh, P.; McGrath, J.; Crundwell, M.; Leung, H.Y.; Robson, C.N.; et al. The PI3K regulatory subunit gene PIK3R1 is under direct control of androgens and repressed in prostate cancer cells. Oncoscience 2015, 2, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, F.; Niu, R. Functions of Shp2 in cancer. J. Cell. Mol. Med. 2015, 19, 2075–2083. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, S.; Sridaran, D.; Weimholt, C.; Luo, J.; Li, T.; Hodgson, M.C.; Santos, L.N.; Le Sommer, S.; Fang, B.; Koomen, J.M.; et al. SHP2 as a primordial epigenetic enzyme expunges histone H3 pTyr-54 to amend androgen receptor homeostasis. Nat. Commun. 2024, 15, 5629. [Google Scholar] [CrossRef]
- Day, K.C.; Lorenzatti Hiles, G.; Kozminsky, M.; Dawsey, S.J.; Paul, A.; Broses, L.J.; Shah, R.; Kunja, L.P.; Hall, C.; Palanisamy, N.; et al. HER2 and EGFR Overexpression Support Metastatic Progression of Prostate Cancer to Bone. Cancer Res. 2017, 77, 74–85. [Google Scholar] [CrossRef]
- Rossini, A.; Giussani, M.; Ripamonti, F.; Aiello, P.; Regondi, V.; Balsari, A.; Triulzi, T.; Tagliabue, E. Combined targeting of EGFR and HER2 against prostate cancer stem cells. Cancer Biol. Ther. 2020, 21, 463–475. [Google Scholar] [CrossRef]
- Nastały, P.; Stoupiec, S.; Popęda, M.; Smentoch, J.; Schlomm, T.; Morrissey, C.; Żaczek, A.J.; Beyer, B.; Tennstedt, P.; Graefen, M.; et al. EGFR as a stable marker of prostate cancer dissemination to bones. Br. J. Cancer 2020, 123, 1767–1774. [Google Scholar] [CrossRef]
- Cai, H.; Babic, I.; Wei, X.; Huang, J.; Witte, O.N. Invasive prostate carcinoma driven by c-Src and androgen receptor synergy. Cancer Res. 2011, 71, 862–872. [Google Scholar] [CrossRef]
- Gelman, I.H.; Peresie, J.; Eng, K.H.; Foster, B.A. Differential requirement for Src family tyrosine kinases in the initiation, progression, and metastasis of prostate cancer. Mol. Cancer Res. 2014, 12, 1470–1479. [Google Scholar] [CrossRef]
- Saad, F.; Lipton, A. SRC kinase inhibition: Targeting bone metastases and tumor growth in prostate and breast cancer. Cancer Treat. Rev. 2010, 36, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.L.; Krop, I.E. Advances in targeting SRC in the treatment of breast cancer and other solid malignancies. Clin. Cancer Res. 2010, 16, 3526–3532. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z.; Timsah, Z.; Suen, K.M.; Cook, N.P.; Lee, G.R.; Lin, C.-C.; Gagea, M.; Marti, A.A.; Ladbury, J.E. Grb2 monomer-dimer equilibrium determines normal versus oncogenic function. Nat. Commun. 2015, 6, 7354. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.-R.; Zhang, X.; Mu, L.; Tian, J.; Du, Y. GRB2-associated binding protein 2 regulates multiple pathways associated with the development of prostate cancer. Oncol. Lett. 2020, 20, 99. [Google Scholar] [CrossRef] [PubMed]
- Perner, F.; Perner, C.; Ernst, T.; Heidel, F.H. Roles of JAK2 in Aging, Inflammation, Hematopoiesis and Malignant Transformation. Cells 2019, 8, 854. [Google Scholar] [CrossRef]
- Gu, L.; Liao, Z.; Hoang, D.T.; Dagvadorj, A.; Gupta, S.; Blackmon, S.; Ellsworth, E.; Talati, P.; Leiby, B.; Zinda, M.; et al. Pharmacologic inhibition of Jak2-Stat5 signaling By Jak2 inhibitor AZD1480 potently suppresses growth of both primary and castrate-resistant prostate cancer. Clin. Cancer Res. 2013, 19, 5658–5674. [Google Scholar] [CrossRef]
- Bao, X.; Zhu, J.; Ren, C.; Zhao, A.; Zhang, M.; Zhu, Z.; Lu, X.; Zhang, Y.; Li, X.; Sima, X.; et al. β-elemonic acid inhibits growth and triggers apoptosis in human castration-resistant prostate cancer cells through the suppression of JAK2/STAT3/MCL-1 and NF-ĸB signal pathways. Chem. Biol. Interact. 2021, 342, 109477. [Google Scholar] [CrossRef]
- Talati, P.G.; Gu, L.; Ellsworth, E.M.; Girondo, M.A.; Trerotola, M.; Hoang, D.T.; Leiby, B.; Dagvadorj, A.; McCue, P.A.; Lallas, C.D.; et al. Jak2-Stat5a/b Signaling Induces Epithelial-to-Mesenchymal Transition and Stem-Like Cell Properties in Prostate Cancer. Am. J. Pathol. 2015, 185, 2505–2522. [Google Scholar] [CrossRef]
- Timofeeva, O.A.; Zhang, X.; Ressom, H.W.; Varghese, R.S.; Kallakury, B.V.S.; Wang, K.; Ji, Y.; Cheema, A.; Jung, M.; Brown, M.L.; et al. Enhanced expression of SOS1 is detected in prostate cancer epithelial cells from African-American men. Int. J. Oncol. 2009, 35, 751–760. [Google Scholar]
- Zheng, L.; Zhang, Y.; Mei, S.; Xie, T.; Zou, Y.; Wang, Y.; Jing, H.; Xu, S.; Dramou, P.; Xu, Z.; et al. Discovery of a Potent Dual Son of Sevenless 1 (SOS1) and Epidermal Growth Factor Receptor (EGFR) Inhibitor for the Treatment of Prostate Cancer. J. Med. Chem. 2024, 67, 7130–7145. [Google Scholar] [CrossRef]
- Poon, R.T.; Fan, S.T.; Wong, J. Clinical implications of circulating angiogenic factors in cancer patients. J. Clin. Oncol. 2001, 19, 1207–1225. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Song, Y.; Guo, W.; Jia, J.; Jin, Y.; Bai, A. Regulatory roles of KDR antisense oligonucleotide on the proliferation of human prostate cancer cell line PC-3. J. BUON 2014, 19, 770–774. [Google Scholar] [PubMed]
- Dearth, R.K.; Cui, X.; Kim, H.-J.; Hadsell, D.L.; Lee, A.V. Oncogenic transformation by the signaling adaptor proteins insulin receptor substrate (IRS)-1 and IRS-2. Cell Cycle 2007, 6, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Hu, X.; Li, S.; Zeng, X.; Qiu, L.; Wei, M.; Wang, Z.; Han, J. miR-203 inhibits cell proliferation and ERK pathway in prostate cancer by targeting IRS-1. BMC Cancer 2020, 20, 1028. [Google Scholar] [CrossRef] [PubMed]
- Kanagasabai, T.; Li, G.; Shen, T.H.; Gladoun, N.; Castillo-Martin, M.; Celada, S.I.; Xie, Y.; Brown, L.K.; Mark, Z.A.; Ochieng, J.; et al. MicroRNA-21 deficiency suppresses prostate cancer progression through downregulation of the IRS1-SREBP-1 signaling pathway. Cancer Lett. 2022, 525, 46–54. [Google Scholar] [CrossRef]
- Shan, Z.; Li, Y.; Yu, S.; Wu, J.; Zhang, C.; Ma, Y.; Zhuang, G.; Wang, J.; Gao, Z.; Liu, D. CTCF regulates the FoxO signaling pathway to affect the progression of prostate cancer. J. Cell. Mol. Med. 2019, 23, 3130–3139. [Google Scholar] [CrossRef]
- Liu, Y.; Ao, X.; Ding, W.; Ponnusamy, M.; Wu, W.; Hao, X.; Yu, W.; Wang, Y.; Li, P.; Wang, J. Critical role of FOXO3a in carcinogenesis. Mol. Cancer 2018, 17, 104. [Google Scholar] [CrossRef]
- Edwards, I.J. Proteoglycans in prostate cancer. Nat. Rev. Urol. 2012, 9, 196–206. [Google Scholar] [CrossRef]
- Leimgruber, C.; Quintar, A.A.; Sosa, L.D.V.; García, L.N.; Figueredo, M.; Maldonado, C.A. Dedifferentiation of prostate smooth muscle cells in response to bacterial LPS. Prostate 2011, 71, 1097–1107. [Google Scholar] [CrossRef]
- Tuxhorn, J.A.; Ayala, G.E.; Rowley, D.R. Reactive stroma in prostate cancer progression. J. Urol. 2001, 166, 2472–2483. [Google Scholar] [CrossRef]
- Barron, D.A.; Rowley, D.R. The reactive stroma microenvironment and prostate cancer progression. Endocr. Relat. Cancer 2012, 19, R187–R204. [Google Scholar] [CrossRef] [PubMed]
- Tuxhorn, J.A.; McAlhany, S.J.; Dang, T.D.; Ayala, G.E.; Rowley, D.R. Stromal cells promote angiogenesis and growth of human prostate tumors in a differential reactive stroma (DRS) xenograft model. Cancer Res. 2002, 62, 3298–3307. [Google Scholar] [PubMed]
- Yu, S.; Zhang, C.; Lin, C.-C.; Niu, Y.; Lai, K.-P.; Chang, H.; Yeh, S.-D.; Chang, C.; Yeh, S. Altered prostate epithelial development and IGF-1 signal in mice lacking the androgen receptor in stromal smooth muscle cells. Prostate 2011, 71, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Ørsted, D.D.; Bojesen, S.E. The link between benign prostatic hyperplasia and prostate cancer. Nat. Rev. Urol. 2013, 10, 49–54. [Google Scholar] [CrossRef]
- Powers, G.L.; Marker, P.C. Recent advances in prostate development and links to prostatic diseases. Wiley Interdiscip. Rev. Syst. Biol. Med. 2013, 5, 243–256. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, F. FGF signalling in prostate development, tissue homoeostasis and tumorigenesis. Biosci. Rep. 2010, 30, 285–291. [Google Scholar] [CrossRef]
- Fontana, F.; Marzagalli, M.; Montagnani Marelli, M.; Raimondi, M.; Moretti, R.M.; Limonta, P. Gonadotropin-Releasing Hormone Receptors in Prostate Cancer: Molecular Aspects and Biological Functions. Int. J. Mol. Sci. 2020, 21, 9511. [Google Scholar] [CrossRef]
- Domińska, K.; Okła, P.; Kowalska, K.; Habrowska-Górczyńska, D.E.; Urbanek, K.A.; Ochędalski, T.; Piastowska-Ciesielska, A.W. Angiotensin 1-7 modulates molecular and cellular processes central to the pathogenesis of prostate cancer. Sci. Rep. 2018, 8, 15772. [Google Scholar] [CrossRef]
- Unterberger, C.J.; Maklakova, V.I.; Lazar, M.; Arneson, P.D.; Mcilwain, S.J.; Tsourkas, P.K.; Hu, R.; Kopchick, J.J.; Swanson, S.M.; Marker, P.C. GH Action in Prostate Cancer Cells Promotes Proliferation, Limits Apoptosis, and Regulates Cancer-related Gene Expression. Endocrinology 2022, 163, bqac031. [Google Scholar] [CrossRef]
- Cao, R.; Ke, M.; Wu, Q.; Tian, Q.; Liu, L.; Dai, Z.; Lu, S.; Liu, P. AZGP1 is androgen responsive and involved in AR-induced prostate cancer cell proliferation and metastasis. J. Cell. Physiol. 2019, 234, 17444–17458. [Google Scholar] [CrossRef]
- Hryniewicz-Jankowska, A.; Augoff, K.; Sikorski, A.F. The role of cholesterol and cholesterol-driven membrane raft domains in prostate cancer. Exp. Biol. Med. 2019, 244, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.Y.; Lee, E.J.; Yoon, S.; Chung, B.H.; Cho, K.S.; Hong, S.J. Cholesterol level of lipid raft microdomains regulates apoptotic cell death in prostate cancer cells through EGFR-mediated Akt and ERK signal transduction. Prostate 2007, 67, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Kim, J.; Adam, R.M.; Solomon, K.R.; Freeman, M.R. Cholesterol targeting alters lipid raft composition and cell survival in prostate cancer cells and xenografts. J. Clin. Investig. 2005, 115, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Lin, J.; Lu, M.L.; Solomon, K.R.; Freeman, M.R. Cholesterol-rich lipid rafts mediate akt-regulated survival in prostate cancer cells. Cancer Res 2002, 62, 2227–2231. [Google Scholar]
- Kim, J.; Adam, R.M.; Solomon, K.R.; Freeman, M.R. Involvement of cholesterol-rich lipid rafts in interleukin-6-induced neuroendocrine differentiation of LNCaP prostate cancer cells. Endocrinology 2004, 145, 613–619. [Google Scholar] [CrossRef]
- Lisanti, M.P.; Scherer, P.E.; Tang, Z.; Sargiacomo, M. Caveolae, caveolin and caveolin-rich membrane domains: A signalling hypothesis. Trends Cell Biol. 1994, 4, 231–235. [Google Scholar] [CrossRef]
- Yang, G.; Truong, L.D.; Timme, T.L.; Ren, C.; Wheeler, T.M.; Park, S.H.; Nasu, Y.; Bangma, C.H.; Kattan, M.W.; Scardino, P.T.; et al. Elevated expression of caveolin is associated with prostate and breast cancer. Clin. Cancer Res. 1998, 4, 1873–1880. [Google Scholar]
- Satoh, T.; Yang, G.; Egawa, S.; Addai, J.; Frolov, A.; Kuwao, S.; Timme, T.L.; Baba, S.; Thompson, T.C. Caveolin-1 expression is a predictor of recurrence-free survival in pT2N0 prostate carcinoma diagnosed in Japanese patients. Cancer 2003, 97, 1225–1233. [Google Scholar] [CrossRef]
- Goetz, J.G.; Lajoie, P.; Wiseman, S.M.; Nabi, I.R. Caveolin-1 in tumor progression: The good, the bad and the ugly. Cancer Metastasis Rev. 2008, 27, 715–735. [Google Scholar] [CrossRef]
- Agoulnik, I.U.; Vaid, A.; Nakka, M.; Alvarado, M.; Bingman, W.E.; Erdem, H.; Frolov, A.; Smith, C.L.; Ayala, G.E.; Ittmann, M.M.; et al. Androgens modulate expression of transcription intermediary factor 2, an androgen receptor coactivator whose expression level correlates with early biochemical recurrence in prostate cancer. Cancer Res. 2006, 66, 10594–10602. [Google Scholar] [CrossRef]
- Mills, I.G. Maintaining and reprogramming genomic androgen receptor activity in prostate cancer. Nat. Rev. Cancer 2014, 14, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Dou, M.; Zhou, X.; Fan, Z.; Ding, X.; Li, L.; Wang, S.; Xue, W.; Wang, H.; Suo, Z.; Deng, X. Clinical Significance of Retinoic Acid Receptor Beta Promoter Methylation in Prostate Cancer: A Meta-Analysis. Cell Physiol. Biochem. 2018, 45, 2497–2505. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, R.; Park, H.D.; Cusick, J.; Vessella, R.L.; Fournier, G.; Narayan, P. Inhibition of tumorigenic potential and prostate-specific antigen expression in LNCaP human prostate cancer cell line by 13-cis-retinoic acid. Int. J. Cancer 1994, 59, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zou, J.X.; Xue, X.; Cai, D.; Zhang, Y.; Duan, Z.; Xiang, Q.; Yang, J.C.; Louie, M.C.; Borowsky, A.D.; et al. ROR-γ drives androgen receptor expression and represents a therapeutic target in castration-resistant prostate cancer. Nat. Med. 2016, 22, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, X.; Xue, X.; Li, C.; Wang, J.; Wang, R.; Zhang, C.; Wang, C.; Shi, Y.; Zou, L.; et al. Discovery and Characterization of XY101, a Potent, Selective, and Orally Bioavailable RORγ Inverse Agonist for Treatment of Castration-Resistant Prostate Cancer. J. Med. Chem. 2019, 62, 4716–4730. [Google Scholar] [CrossRef]
- Zhong, C.; Yang, S.; Huang, J.; Cohen, M.B.; Roy-Burman, P. Aberration in the expression of the retinoid receptor, RXRalpha, in prostate cancer. Cancer Biol. Ther. 2003, 2, 179–184. [Google Scholar] [CrossRef]
- Ban, K.; Feng, S.; Shao, L.; Ittmann, M. RET Signaling in Prostate Cancer. Clin. Cancer Res. 2017, 23, 4885–4896. [Google Scholar] [CrossRef]
- Cohen, P.; Alessi, D.R. Kinase drug discovery—What’s next in the field? ACS Chem. Biol. 2013, 8, 96–104. [Google Scholar] [CrossRef]




| Receptors | Degree | Average Shortest Pathlength | Betweenness Centrality | Closeness Centrality | Neighborhood Connectivity | Topological Coefficient |
|---|---|---|---|---|---|---|
| SRC | 38 | 2.288888889 | 0.167929308 | 0.436893204 | 13.02631579 | 0.117354196 |
| PIK3R1 | 27 | 2.544444444 | 0.028902605 | 0.3930131 | 13.96296296 | 0.164270153 |
| EGFR | 26 | 2.355555556 | 0.057176398 | 0.424528302 | 16 | 0.148148148 |
| GRB2 | 25 | 2.627777778 | 0.056225201 | 0.380549683 | 12.96 | 0.161 |
| PTPN11 | 21 | 2.711111111 | 0.011173159 | 0.368852459 | 14.33333333 | 0.188596491 |
| IRS1 | 18 | 2.55 | 0.01546598 | 0.392156863 | 18.5 | 0.203296703 |
| JAK2 | 18 | 2.777777778 | 0.017949836 | 0.36 | 15.05555556 | 0.217391304 |
| KDR | 15 | 2.688888889 | 0.030385955 | 0.371900826 | 14.73333333 | 0.187179487 |
| IGF1R | 13 | 2.727777778 | 0.006617419 | 0.366598778 | 19.61538462 | 0.254745255 |
| SOS1 | 11 | 2.894444444 | 0.004922684 | 0.345489443 | 16.81818182 | 0.274217586 |
| Receptors | PDB ID | Vina Score | Cavity Size | Center | Size | ||||
|---|---|---|---|---|---|---|---|---|---|
| X | Y | Z | X | Y | Z | ||||
| JAK2 | 6e2q | −8.8 | 1731 | 251 | −45 | 59 | 22 | 22 | 22 |
| SRC | 2h8h | −8.7 | 699 | 18 | 25 | 57 | 22 | 22 | 22 |
| GRB2 | 1gri | −8.7 | 865 | 29 | 74 | 22 | 22 | 22 | 22 |
| PTPN11 | 7vxg | −8.5 | 914 | 41 | 29 | 44 | 22 | 22 | 22 |
| IGFR1 | 6jk8 | −8.4 | 1956 | 112 | 104 | 155 | 22 | 22 | 22 |
| EGFR | 7syd | −8 | 10,952 | 162 | 172 | 164 | 22 | 35 | 35 |
| IRS1 | 1qqg | −7.9 | 1317 | 12 | 50 | 14 | 22 | 22 | 22 |
| PIK3R1 | 2v1y | −6.5 | 168 | 73 | 44 | −25 | 22 | 22 | 35 |
| SOS1 | 3ksy | −7.6 | 1849 | 45 | 43 | 69 | 31 | 31 | 22 |
| KDR | 1vr2 | −6.7 | 539 | 35 | 32 | 17 | 22 | 22 | 22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Weng, W.; Wu, S. Investigating the Potential Effects of 6PPDQ on Prostate Cancer Through Network Toxicology and Molecular Docking. Toxics 2024, 12, 891. https://doi.org/10.3390/toxics12120891
Song Y, Weng W, Wu S. Investigating the Potential Effects of 6PPDQ on Prostate Cancer Through Network Toxicology and Molecular Docking. Toxics. 2024; 12(12):891. https://doi.org/10.3390/toxics12120891
Chicago/Turabian StyleSong, Yuanzhi, Wuhong Weng, and Shengde Wu. 2024. "Investigating the Potential Effects of 6PPDQ on Prostate Cancer Through Network Toxicology and Molecular Docking" Toxics 12, no. 12: 891. https://doi.org/10.3390/toxics12120891
APA StyleSong, Y., Weng, W., & Wu, S. (2024). Investigating the Potential Effects of 6PPDQ on Prostate Cancer Through Network Toxicology and Molecular Docking. Toxics, 12(12), 891. https://doi.org/10.3390/toxics12120891






