Using Four Machine Learning Methods to Analyze the Association Between Polycyclic Aromatic Hydrocarbons and Visual Impairment in American Adults: Evidence from NHANES
Abstract
1. Introduction
2. Methods
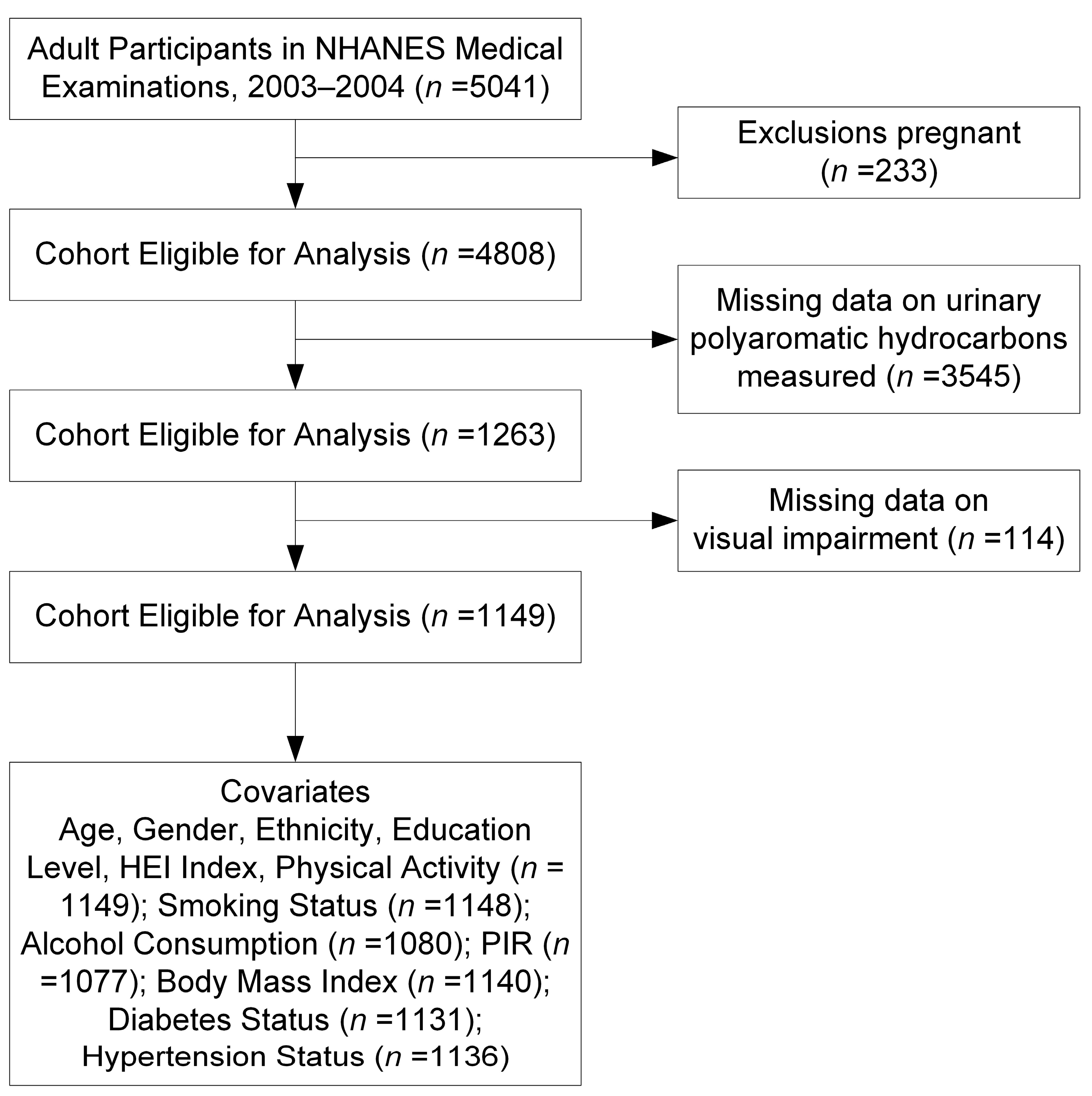
2.1. Study Population
2.2. Analysis of Urinary PAHs in NHANES 2003–2004
2.3. Visual Acuity Assessment Method
2.4. Detection of Inflammatory Parameters
2.5. Covariates
2.6. Statistical Analysis
3. Results
3.1. Characteristics of Participants
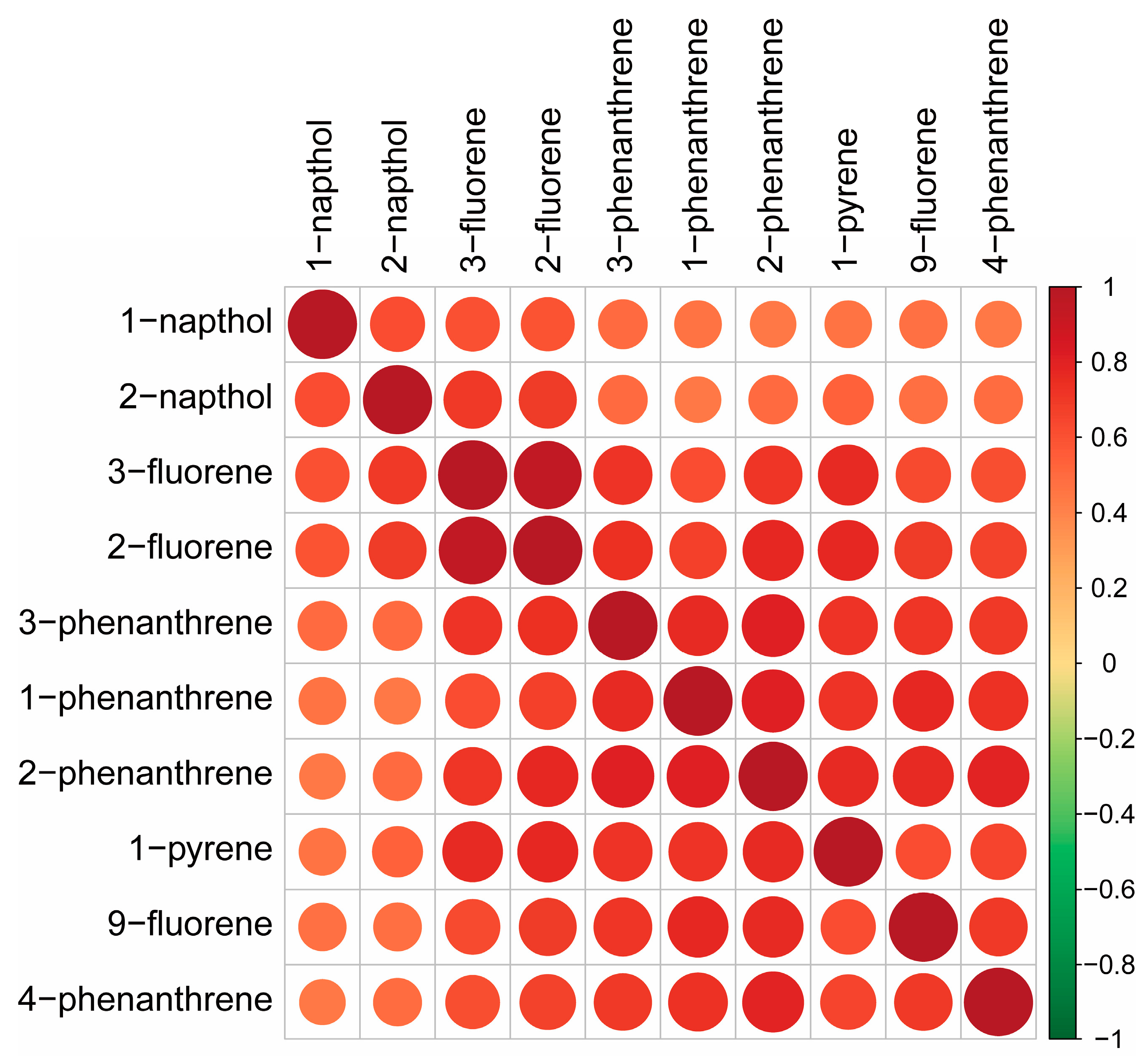
3.2. Supervised Methods
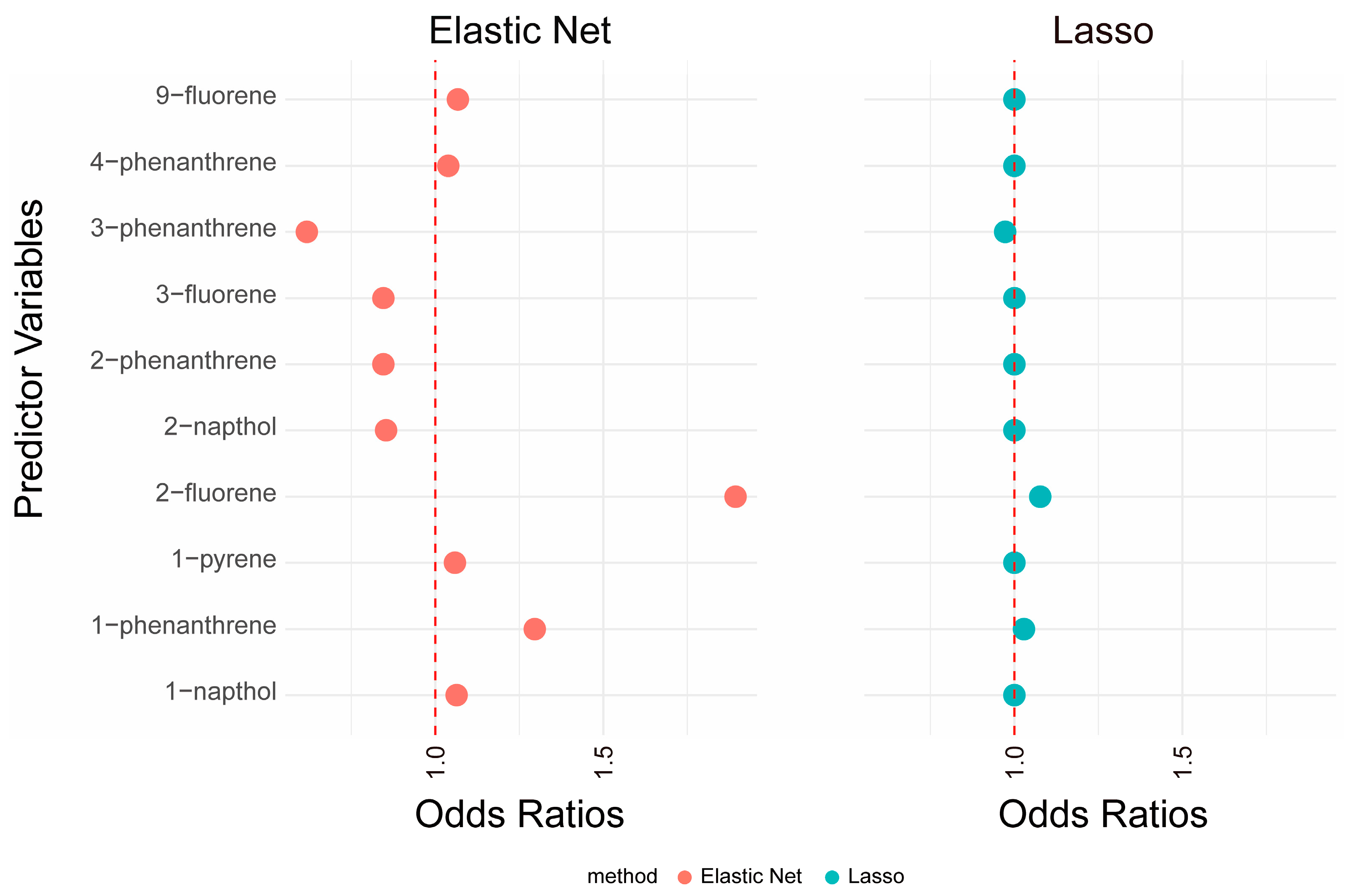
3.2.1. Lasso and Elastic Net
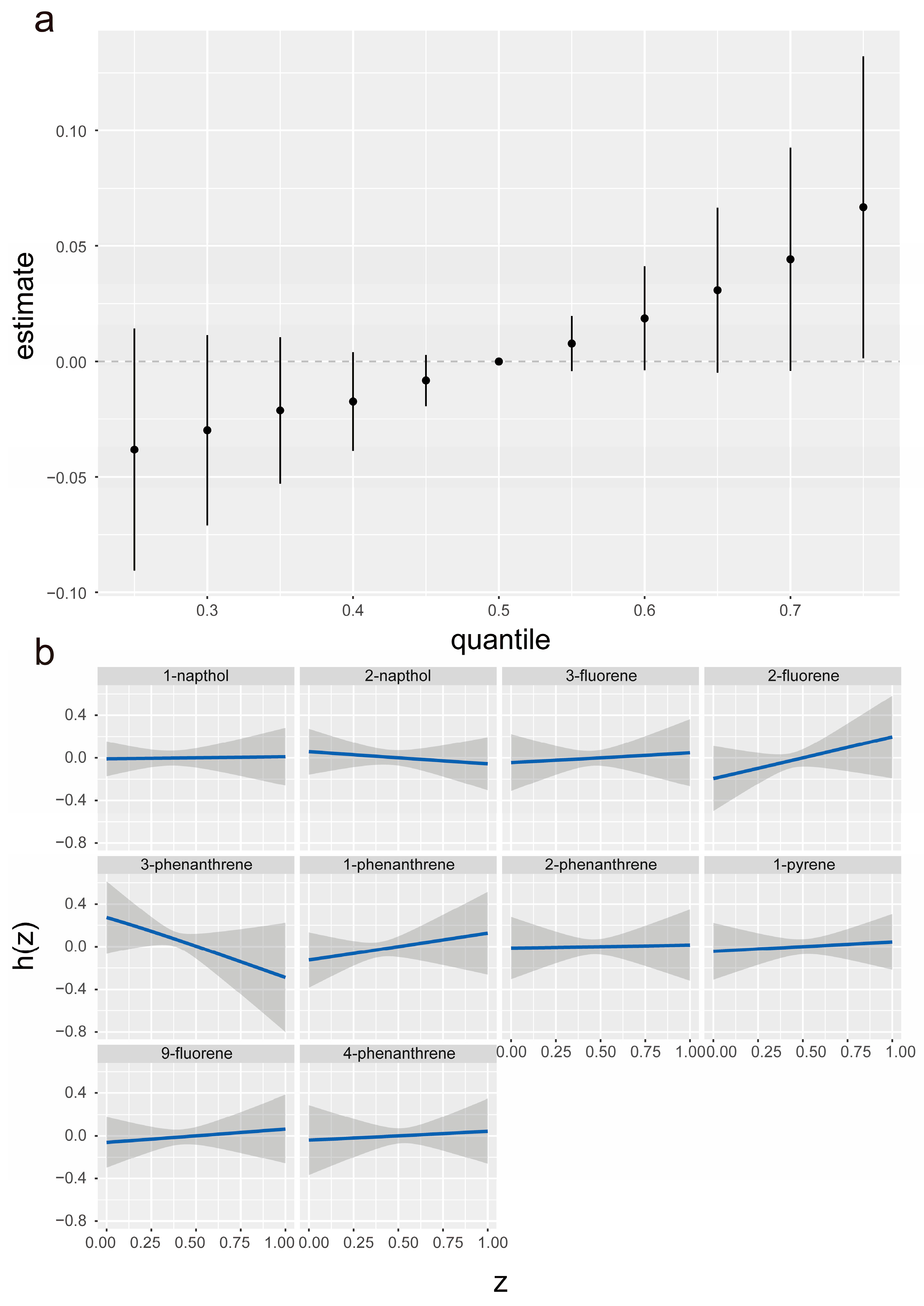
3.2.2. Weighted Quantile Sum Regression
3.2.3. Bayesian Kernel Machine Regression
3.2.4. Mediation Analysis of the Association between 2-Fluorene Exposure and VI
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Congdon, N.G.; Friedman, D.S.; Lietman, T. Important causes of visual impairment in the world today. JAMA 2003, 290, 2057–2060. [Google Scholar] [CrossRef] [PubMed]
- Cumberland, P.M.; Rahi, J.S. Visual Function, Social Position, and Health and Life Chances: The UK Biobank Study. JAMA Ophthalmol. 2016, 134, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Bourne, R.R.A.; Flaxman, S.R.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H.; Leasher, J.; Limburg, H.; et al. Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e888–e897. [Google Scholar] [CrossRef]
- Swenor, B.K.; Lee, M.J.; Varadaraj, V.; Whitson, H.E.; Ramulu, P.Y. Aging With Vision Loss: A Framework for Assessing the Impact of Visual Impairment on Older Adults. Gerontologist 2020, 60, 989–995. [Google Scholar] [CrossRef]
- Scott, A.W.; Bressler, N.M.; Ffolkes, S.; Wittenborn, J.S.; Jorkasky, J. Public Attitudes About Eye and Vision Health. JAMA Ophthalmol. 2016, 134, 1111–1118. [Google Scholar] [CrossRef]
- Xu, T.; Wang, B.; Liu, H.; Wang, H.; Yin, P.; Dong, W.; Li, J.; Wang, Y.X.; Yusufu, M.; Briant, P.; et al. Prevalence and causes of vision loss in China from 1990 to 2019: Findings from the Global Burden of Disease Study 2019. Lancet Public Health 2020, 5, e682–e691. [Google Scholar] [CrossRef]
- Zou, M.; Chen, A.; Liu, Z.; Jin, L.; Zheng, D.; Congdon, N.; Jin, G. The burden, causes, and determinants of blindness and vision impairment in Asia: An analysis of the Global Burden of Disease Study. J. Glob. Health 2024, 14, 04100. [Google Scholar] [CrossRef]
- Jiang, Q.; Wang, S.; Zhang, H.; Guo, Y.; Lou, Y.; Huang, S.; You, Q.; Cao, S. The Association Between Solid Fuel Use and Visual Impairment Among Middle-Aged and Older Chinese Adults: Nationwide Population-Based Cohort Study. JMIR Public Health Surveill. 2023, 9, e43914. [Google Scholar] [CrossRef]
- Dumanoir, M.; Leroy, A.; Burel, D.; Laquerrière, A.; Janin, F.; Lebon, A.; Valet, M.; Godefroy, D.; Przegralek, L.; Lecointre, M.; et al. In Utero Alcohol Exposure Impairs Retinal Angiogenesis and the Microvessel-Associated Positioning of Calretinin Interneurons. Eneuro 2023, 10, ENEURO.0295-22.2022. [Google Scholar] [CrossRef]
- Baumann, L.; Ros, A.; Rehberger, K.; Neuhauss, S.C.; Segner, H. Thyroid disruption in zebrafish (Danio rerio) larvae: Different molecular response patterns lead to impaired eye development and visual functions. Aquat. Toxicol. 2016, 172, 44–55. [Google Scholar] [CrossRef]
- Chen, L.; Wei, J.; Ma, T.; Gao, D.; Wang, X.; Wen, B.; Chen, M.; Li, Y.; Jiang, J.; Wu, L.; et al. Ambient gaseous pollutant exposure and incidence of visual impairment among children and adolescents: Findings from a longitudinal, two-center cohort study in China. Environ. Sci. Pollut. Res. Int. 2022, 29, 73262–73270. [Google Scholar] [CrossRef] [PubMed]
- Farzan, S.F.; Chen, Y.; Trachtman, H.; Trasande, L. Urinary polycyclic aromatic hydrocarbons and measures of oxidative stress, inflammation and renal function in adolescents: NHANES 2003–2008. Environ. Res. 2016, 144, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Li, X.; Dai, Y.; Guo, C.; Peng, R.; Qin, P.; Tan, L. Association between polycyclic aromatic hydrocarbon exposure and blood lipid levels: The indirect effects of inflammation and oxidative stress. Environ. Sci. Pollut. Res. Int. 2023, 30, 123148–123163. [Google Scholar] [CrossRef] [PubMed]
- Alshaarawy, O.; Elbaz, H.A.; Andrew, M.E. The association of urinary polycyclic aromatic hydrocarbon biomarkers and cardiovascular disease in the US population. Environ. Int. 2016, 89, 174–178. [Google Scholar] [CrossRef]
- Humphreys, J.; Valdés Hernández, M.D.C. Impact of polycyclic aromatic hydrocarbon exposure on cognitive function and neurodegeneration in humans: A systematic review and meta-analysis. Front. Neurol. 2022, 13, 1052333. [Google Scholar] [CrossRef]
- Edwards, S.C.; Jedrychowski, W.; Butscher, M.; Camann, D.; Kieltyka, A.; Mroz, E.; Flak, E.; Li, Z.; Wang, S.; Rauh, V.; et al. Prenatal exposure to airborne polycyclic aromatic hydrocarbons and children’s intelligence at 5 years of age in a prospective cohort study in Poland. Environ. Health Perspect. 2010, 118, 1326–1331. [Google Scholar] [CrossRef]
- Perera, F.; Phillips, D.H.; Wang, Y.; Roen, E.; Herbstman, J.; Rauh, V.; Wang, S.; Tang, D. Prenatal exposure to polycyclic aromatic hydrocarbons/aromatics, BDNF and child development. Environ. Res. 2015, 142, 602–608. [Google Scholar] [CrossRef]
- Cho, J.; Sohn, J.; Noh, J.; Jang, H.; Kim, W.; Cho, S.K.; Seo, H.; Seo, G.; Lee, S.K.; Noh, Y.; et al. Association between exposure to polycyclic aromatic hydrocarbons and brain cortical thinning: The Environmental Pollution-Induced Neurological EFfects (EPINEF) study. Sci. Total Environ. 2020, 737, 140097. [Google Scholar] [CrossRef]
- Mallah, M.A.; Changxing, L.; Mallah, M.A.; Noreen, S.; Liu, Y.; Saeed, M.; Xi, H.; Ahmed, B.; Feng, F.; Mirjat, A.A.; et al. Polycyclic aromatic hydrocarbon and its effects on human health: An overeview. Chemosphere 2022, 296, 133948. [Google Scholar] [CrossRef]
- Olasehinde, T.A.; Olaniran, A.O. Neurotoxicity of Polycyclic Aromatic Hydrocarbons: A Systematic Mapping and Review of Neuropathological Mechanisms. Toxics 2022, 10, 417. [Google Scholar] [CrossRef]
- Ferguson, K.K.; McElrath, T.F.; Pace, G.G.; Weller, D.; Zeng, L.; Pennathur, S.; Cantonwine, D.E.; Meeker, J.D. Urinary Polycyclic Aromatic Hydrocarbon Metabolite Associations with Biomarkers of Inflammation, Angiogenesis, and Oxidative Stress in Pregnant Women. Environ. Sci. Technol. 2017, 51, 4652–4660. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, D.; Peng, Y.; Lu, Z.; Wang, D. Association of polycyclic aromatic hydrocarbons with systemic inflammation and metabolic syndrome and its components. Obesity 2023, 31, 1392–1401. [Google Scholar] [CrossRef] [PubMed]
- Bobb, J.F.; Claus Henn, B.; Valeri, L.; Coull, B.A. Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environ. Health 2018, 17, 67. [Google Scholar] [CrossRef]
- Gibson, E.A.; Nunez, Y.; Abuawad, A.; Zota, A.R.; Renzetti, S.; Devick, K.L.; Gennings, C.; Goldsmith, J.; Coull, B.A.; Kioumourtzoglou, M.-A. An overview of methods to address distinct research questions on environmental mixtures: An application to persistent organic pollutants and leukocyte telomere length. Environ. Health 2019, 18, 76. [Google Scholar] [CrossRef]
- Swenor, B.K.; Lee, M.J.; Tian, J.; Varadaraj, V.; Bandeen-Roche, K. Visual Impairment and Frailty: Examining an Understudied Relationship. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 596–602. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, B.; Rong, S.; Zhang, J.; Du, Y.; Xu, G.; Snetselaar, L.G.; Wallace, R.B.; Lehmler, H.J.; Bao, W. Association of Seafood Consumption and Mercury Exposure With Cardiovascular and All-Cause Mortality Among US Adults. JAMA Netw. Open 2021, 4, e2136367. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. 2008 Physical Activity Guidelines for Americans: Be Active, Healthy, and Happy! U.S. Department of Health and Human Services: Washington, DC, USA, 2008. [Google Scholar]
- Krebs-Smith, S.M.; Pannucci, T.E.; Subar, A.F.; Kirkpatrick, S.I.; Lerman, J.L.; Tooze, J.A.; Wilson, M.M.; Reedy, J. Update of the Healthy Eating Index: HEI-2015. J. Acad. Nutr. Diet. 2018, 118, 1591–1602. [Google Scholar] [CrossRef]
- Zang, X.; Huang, H.; Zhuang, Z.; Chen, R.; Xie, Z.; Xu, C.; Mo, X. The association between serum copper concentrations and cardiovascular disease risk factors in children and adolescents in NHANES. Environ. Sci. Pollut. Res. Int. 2018, 25, 16951–16958. [Google Scholar] [CrossRef]
- Zang, X.D.; Hu, Q.H.; Liu, X.X.; Da, M.; Yang, Z.C.; Qi, J.R.; Mo, X.M. Serum vitamin E concentration is negatively associated with body mass index change in girls not boys during adolescence. World J. Pediatr. 2021, 17, 517–526. [Google Scholar] [CrossRef]
- Sun, W.; Dai, B.; Hong, L.; Zhang, H. The risk of dental restoration-related lead exposure on renal function. Chemosphere 2023, 337, 139405. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Jin, C.; Fang, R.; Hua, R.; Zang, X.; Zhang, H. The indicative role of inflammatory index in the progression of periodontal attachment loss. Eur. J. Med. Res. 2023, 28, 287. [Google Scholar] [CrossRef] [PubMed]
- Sumner, S.A.; Bowen, D.; Holland, K.; Zwald, M.L.; Vivolo-Kantor, A.; Guy, G.P., Jr.; Heuett, W.J.; Pressley, D.P.; Jones, C.M. Estimating Weekly National Opioid Overdose Deaths in Near Real Time Using Multiple Proxy Data Sources. JAMA Netw. Open 2022, 5, e2223033. [Google Scholar] [CrossRef]
- Ramos, K.J.; Hee Wai, T.; Stephenson, A.L.; Sykes, J.; Stanojevic, S.; Rodriguez, P.J.; Bansal, A.; Mayer-Hamblett, N.; Goss, C.H.; Kapnadak, S.G. Development and Internal Validation of a Prognostic Model of the Probability of Death or Lung Transplantation Within 2 Years for Patients With Cystic Fibrosis and FEV(1) ≤ 50% Predicted. Chest 2022, 162, 757–767. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.; Guan, Q.; Xu, L.; Zhao, S.; Duan, J.; Wang, Y.; Xia, Y.; Xu, Q. Exposure to multiple trace elements and miscarriage during early pregnancy: A mixtures approach. Environ. Int. 2022, 162, 107161. [Google Scholar] [CrossRef]
- Huang, Q.; Wan, J.; Nan, W.; Li, S.; He, B.; Peng, Z. Association between manganese exposure in heavy metals mixtures and the prevalence of sarcopenia in US adults from NHANES 2011–2018. J. Hazard. Mater. 2024, 464, 133005. [Google Scholar] [CrossRef]
- Bobb, J.F.; Valeri, L.; Claus Henn, B.; Christiani, D.C.; Wright, R.O.; Mazumdar, M.; Godleski, J.J.; Coull, B.A. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 2015, 16, 493–508. [Google Scholar] [CrossRef]
- Zang, X.; Qin, W.; Xiong, Y.; Xu, A.; Huang, H.; Fang, T.; Zang, X.; Chen, M. Using three statistical methods to analyze the association between aldehyde exposure and markers of inflammation and oxidative stress. Environ. Sci. Pollut. Res. Int. 2023, 30, 79437–79450. [Google Scholar] [CrossRef]
- Zang, X.; Feng, L.; Qin, W.; Wang, W.; Zang, X. Using machine learning methods to analyze the association between urinary polycyclic aromatic hydrocarbons and chronic bowel disorders in American adults. Chemosphere 2024, 346, 140602. [Google Scholar] [CrossRef]
- Zang, X.; Zhou, X.; Bian, H.; Jin, W.; Pan, X.; Jiang, J.; Koroleva, M.Y.; Shen, R. Prediction and Construction of Energetic Materials Based on Machine Learning Methods. Molecules 2022, 28, 322. [Google Scholar] [CrossRef]
- Gao, P.; da Silva, E.; Hou, L.; Denslow, N.D.; Xiang, P.; Ma, L.Q. Human exposure to polycyclic aromatic hydrocarbons: Metabolomics perspective. Environ. Int. 2018, 119, 466–477. [Google Scholar] [CrossRef]
- Kang, Q.; Yang, C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020, 37, 101799. [Google Scholar] [CrossRef]
- Liu, Y.; Bell, B.A.; Song, Y.; Zhang, K.; Anderson, B.; Axelsen, P.H.; Bohannan, W.; Agbaga, M.P.; Park, H.G.; James, G.; et al. Deuterated docosahexaenoic acid protects against oxidative stress and geographic atrophy-like retinal degeneration in a mouse model with iron overload. Aging Cell 2022, 21, e13579. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Liu, Q.; Liang, J.; Weng, Z.; Xu, J.; Jiang, Z.; Gu, A. Urinary biomarkers of polycyclic aromatic hydrocarbons and their associations with liver function in adolescents. Environ. Pollut. 2021, 278, 116842. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, C.; Jiang, Z.Y.; Gu, A. Association of polycyclic aromatic hydrocarbons and asthma among children 6-19 years: NHANES 2001–2008 and NHANES 2011–2012. Respir. Med. 2016, 110, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Lim, Z.W.; Chen, W.-L. Exploring the association of Bone Alkaline Phosphatases and Hearing Loss. Sci. Rep. 2020, 10, 4006. [Google Scholar] [CrossRef]
- Rostron, B.L.; Corey, C.G.; Chang, J.T.; van Bemmel, D.M.; Miller, M.E.; Chang, C.M. Changes in Cigarettes per Day and Biomarkers of Exposure Among US Adult Smokers in the Population Assessment of Tobacco and Health Study Waves 1 and 2 (2013–2015). Nicotine Tob. Res. 2020, 22, 1780–1787. [Google Scholar] [CrossRef]
- Tennant, P.W.G.; Murray, E.J.; Arnold, K.F.; Berrie, L.; Fox, M.P.; Gadd, S.C.; Harrison, W.J.; Keeble, C.; Ranker, L.R.; Textor, J.; et al. Use of directed acyclic graphs (DAGs) to identify confounders in applied health research: Review and recommendations. Int. J. Epidemiol. 2021, 50, 620–632. [Google Scholar] [CrossRef]


| Characteristic | Overall |
|---|---|
| Age (years), mean, and sd | 50.07 ± 19.05 |
| Gender, % and n | |
| Male | 572 (49.78%) |
| Female | 577 (50.22%) |
| Ethnicity, % and n | |
| Hispanic | 253 (22.02%) |
| Non-Hispanic White | 604 (52.57%) |
| Non-Hispanic Black | 238 (20.71%) |
| Other race | 54 (4.70%) |
| Education, % and n | |
| Less than high school | 325 (28.29%) |
| High school | 287 (24.98%) |
| More than high school | 537 (46.74%) |
| Poverty index, % and n | |
| ≤1 | 203 (17.67%) |
| >1 | 874 (76.07%) |
| missing | 72 (6.27%) |
| Smoking, % and n | |
| Non-smoker | 559 (48.65%) |
| Ever smoker | 307 (26.72%) |
| Current smoking | 282 (24.54%) |
| missing | 1 (0.09%) |
| Alcohol consumption, % and n | |
| Non-drinker | 821 (71.45%) |
| Moderate | 99 (8.62%) |
| Heavy | 160 (13.93%) |
| Missing | 69 (6.01%) |
| Diabetes, % and n | |
| No | 1003 (87.29%) |
| Yes | 128 (11.14%) |
| Missing | 18 (1.57%) |
| Hypertension, % and n | |
| No | 762 (66.32%) |
| Yes | 374 (32.55%) |
| Missing | 13 (1.13%) |
| HEI Index, mean and sd | 48.54 ± 12.51 |
| Physical Activity, MET-min/wk, No. (%) | |
| <600 | 536 (46.65%) |
| 600 ≤, <1200 | 178 (15.49%) |
| ≥1200 | 435 (37.86%) |
| BMI Category (kg/m2), % and n | |
| <25 | 354 (30.81%) |
| 25–30 | 385 (33.51%) |
| ≥30 | 401 (34.90%) |
| Missing | 9 (0.78%) |
| Visual impairment, % and n | |
| No | 1044 (90.86%) |
| Yes | 105 (9.14%) |
| Urinary 1-napthol, ng/L | 2621.40 (1125.70–9253.40) |
| Urinary 2-napthol, ng/L | 3133.80 (1340.00–8616.20) |
| Urinary 3-fluorene, ng/L | 97.40 (44.90–340.20) |
| Urinary 2-fluorene, ng/L | 279.2 (130.40–778.30) |
| Urinary 3-phenanthrene, ng/L | 113.40 (55.90–216.40) |
| Urinary 1-phenanthrene, ng/L | 160.6 (83.20–282.50) |
| Urinary 2-phenanthrene, ng/L | 63.60 (30.50–122.60) |
| Urinary 1-pyrene, ng/L | 79.50 (35.60–175.00) |
| Urinary 9-fluorene, ng/L | 282.70 (139.60–565.80) |
| Urinary 4-phenanthrene, ng/L | 25.30 (11.3–53.70) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zang, X.; Zhou, W.; Zhang, H.; Zang, X. Using Four Machine Learning Methods to Analyze the Association Between Polycyclic Aromatic Hydrocarbons and Visual Impairment in American Adults: Evidence from NHANES. Toxics 2024, 12, 789. https://doi.org/10.3390/toxics12110789
Zang X, Zhou W, Zhang H, Zang X. Using Four Machine Learning Methods to Analyze the Association Between Polycyclic Aromatic Hydrocarbons and Visual Impairment in American Adults: Evidence from NHANES. Toxics. 2024; 12(11):789. https://doi.org/10.3390/toxics12110789
Chicago/Turabian StyleZang, Xiaowei, Wei Zhou, Hengguo Zhang, and Xiaodong Zang. 2024. "Using Four Machine Learning Methods to Analyze the Association Between Polycyclic Aromatic Hydrocarbons and Visual Impairment in American Adults: Evidence from NHANES" Toxics 12, no. 11: 789. https://doi.org/10.3390/toxics12110789
APA StyleZang, X., Zhou, W., Zhang, H., & Zang, X. (2024). Using Four Machine Learning Methods to Analyze the Association Between Polycyclic Aromatic Hydrocarbons and Visual Impairment in American Adults: Evidence from NHANES. Toxics, 12(11), 789. https://doi.org/10.3390/toxics12110789







