Real-Time Exposure to 3D-Printing Emissions Elicits Metabolic and Pro-Inflammatory Responses in Human Airway Epithelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. 3D Printer Emission Generation and Particle Characterization
2.3. SEM-EDX—Elemental Analysis of 3D Printer Emissions
2.4. Cell Culture and Air–Liquid Interface (ALI) Protocol
2.5. In Vitro Exposure to 3D Printer Emissions
2.6. Calculation of 3D Printer Emissions Mass Deposition
2.7. Cellular Viability Assay
2.8. Total Glutathione Level Measurement
2.9. Cytokine Analysis
2.10. HPLC-MS Metabolomic Profiling
2.11. Bioinformatics Analysis
2.12. Pathway Enrichment Analysis
2.13. Cytokine–Metabolomics Network Analysis
2.14. Statistical Analysis
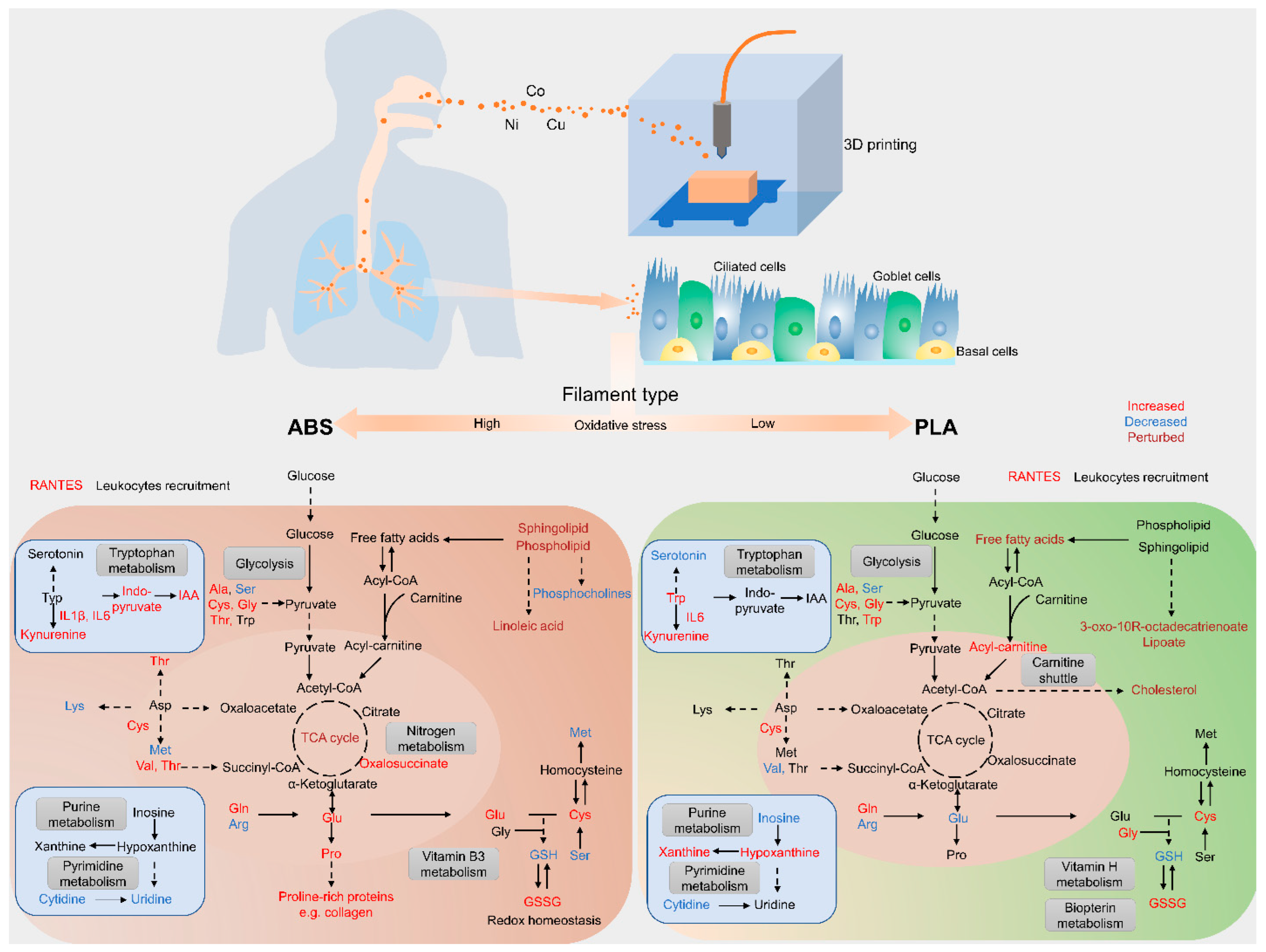
3. Results and Discussion
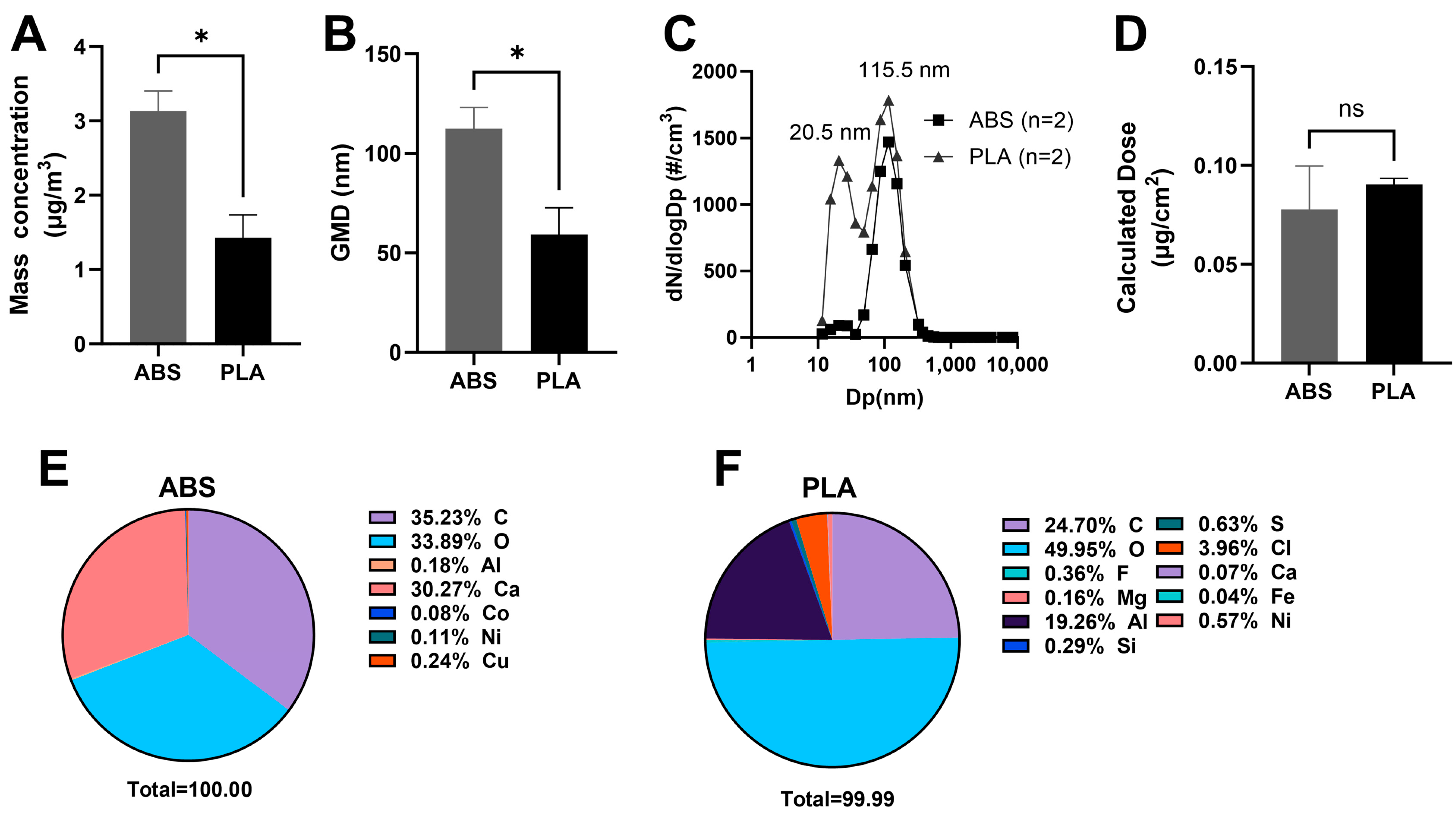
3.1. 3D-Printer-Emitted Aerosol Characterization
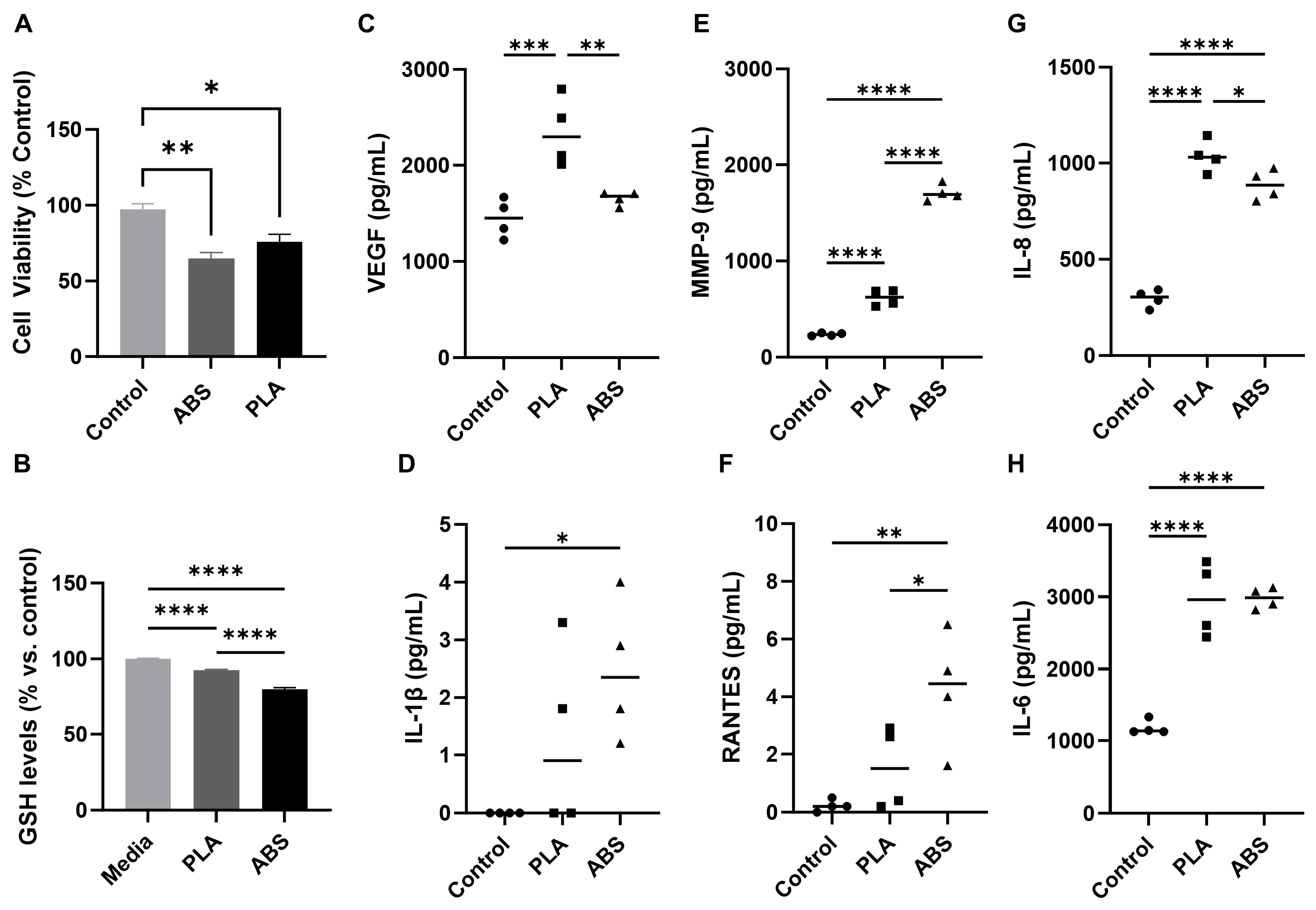
3.2. Effect of ABS and PLA Filament Emissions on Cellular Viability, Oxidative Stress, and Inflammation
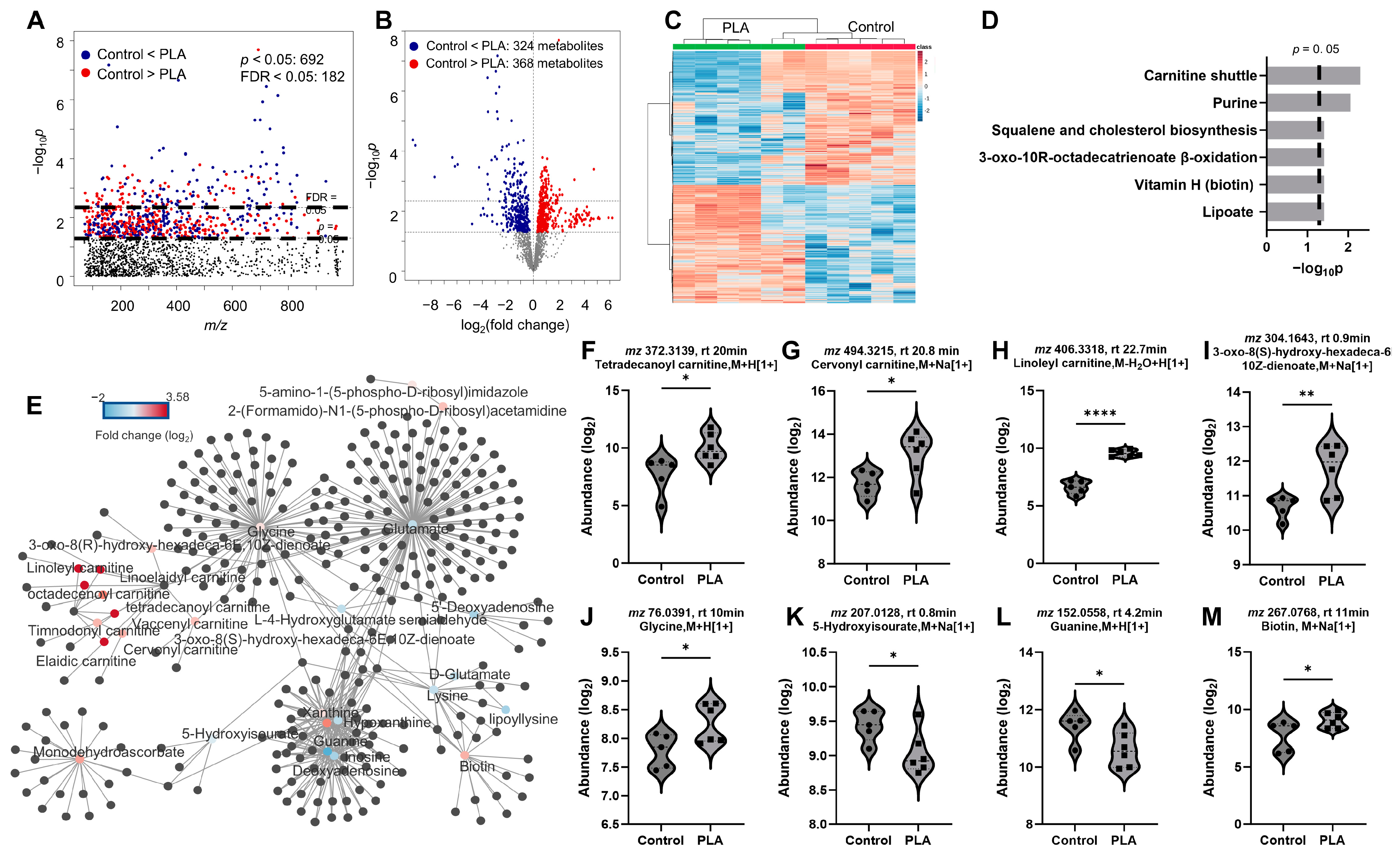
3.3. Metabolomic Responses to ABS and PLA Filament Emissions
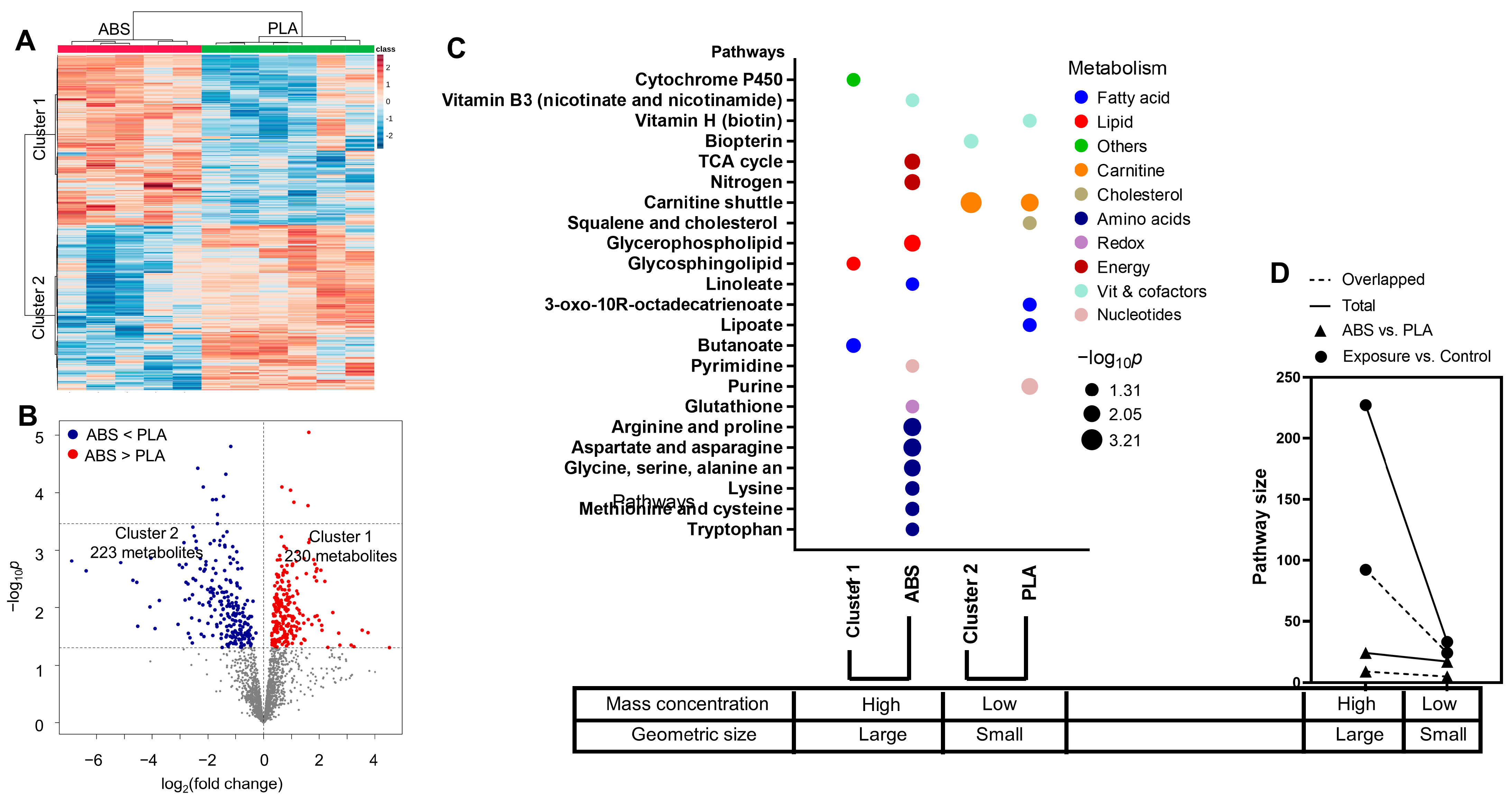
3.4. Impact of Filament Selection on Metabolic Functions
3.5. Cytokine–Metabolomics Network Analysis
3.6. Limitations
4. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Q.; Pardo, M.; Rudich, Y.; Kaplan-Ashiri, I.; Wong, J.P.; Davis, A.Y.; Black, M.S.; Weber, R.J. Chemical composition and toxicity of particles emitted from a consumer-level 3D printer using various materials. Environ. Sci. Technol. 2019, 53, 12054–12061. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Yoon, C.; Ham, S.; Park, J.; Kim, S.; Kwon, O.; Tsai, P.-J. Emissions of nanoparticles and gaseous material from 3D printer operation. Environ. Sci. Technol. 2015, 49, 12044–12053. [Google Scholar] [CrossRef] [PubMed]
- Poikkimäki, M.; Koljonen, V.; Närhi, M.; Kangasniemi, O.; Kausiala, O.; Leskinen, N.; Dal Maso, M. Nanocluster aerosol emissions of a 3D printer: The effect of nozzle temperature and filament material. In Proceedings of the Physics Days 2019, Helsinki, Finland, 5–7 March 2019. [Google Scholar]
- Stefaniak, A.B.; LeBouf, R.F.; Yi, J.; Ham, J.; Nurkewicz, T.; Schwegler-Berry, D.E.; Chen, B.T.; Wells, J.R.; Duling, M.G.; Lawrence, R.B. Characterization of chemical contaminants generated by a desktop fused deposition modeling 3-dimensional printer. J. Occup. Environ. Hyg. 2017, 14, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Byrley, P.; George, B.J.; Boyes, W.K.; Rogers, K. Particle emissions from fused deposition modeling 3D printers: Evaluation and meta-analysis. Sci. Total Environ. 2019, 655, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Schraufnagel, D.E. The health effects of ultrafine particles. Exp. Mol. Med. 2020, 52, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Azimi, P.; Fazli, T.; Stephens, B. Predicting concentrations of ultrafine particles and volatile organic compounds resulting from desktop 3D printer operation and the impact of potential control strategies. J. Ind. Ecol. 2017, 21, S107–S119. [Google Scholar] [CrossRef]
- Zhang, Q.; Weber, R.J.; Luxton, T.P.; Peloquin, D.M.; Baumann, E.J.; Black, M.S. Metal compositions of particle emissions from material extrusion 3D printing: Emission sources and indoor exposure modeling. Sci. Total Environ. 2023, 860, 160512. [Google Scholar] [CrossRef]
- Tedla, G.; Jarabek, A.M.; Byrley, P.; Boyes, W.; Rogers, K. Human exposure to metals in consumer-focused fused filament fabrication (FFF)/3D printing processes. Sci. Total Environ. 2022, 814, 152622. [Google Scholar] [CrossRef]
- Azimi, P.; Zhao, D.; Pouzet, C.; Crain, N.E.; Stephens, B. Emissions of ultrafine particles and volatile organic compounds from commercially available desktop three-dimensional printers with multiple filaments. Environ. Sci. Technol. 2016, 50, 1260–1268. [Google Scholar] [CrossRef]
- Yi, J.; LeBouf, R.F.; Duling, M.G.; Nurkiewicz, T.; Chen, B.T.; Schwegler-Berry, D.; Virji, M.A.; Stefaniak, A.B. Emission of particulate matter from a desktop three-dimensional (3D) printer. J. Toxicol. Environ. Health Part A 2016, 79, 453–465. [Google Scholar] [CrossRef]
- Davis, A.; Black, M.; Zhang, Q.; Wong, J.P.; Weber, R. Fine particulate and chemical emissions from desktop 3D printers. In Proceedings of the NIP & Digital Fabrication Conference, Manchester, UK, 12–16 September 2016; Society for Imaging Science and Technology: Virginia, VA, USA, 2016; pp. 121–123. [Google Scholar]
- Davis, A.Y.; Zhang, Q.; Wong, J.P.; Weber, R.J.; Black, M.S. Characterization of volatile organic compound emissions from consumer level material extrusion 3D printers. Build. Environ. 2019, 160, 106209. [Google Scholar] [CrossRef]
- Zhang, Q.; Davis, A.; Black, M.; Weber, R. Particle and chemical emissions from 3D printers and their potential health impacts. ASHRAE Trans. 2019, 125, 106–109. [Google Scholar]
- Vance, M.E.; Pegues, V.; Van Montfrans, S.; Leng, W.; Marr, L.C. Aerosol emissions from fuse-deposition modeling 3D printers in a chamber and in real indoor environments. Environ. Sci. Technol. 2017, 51, 9516–9523. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.; Park, J.; Kim, S.; Park, K.; Yoon, C. Effect of nozzle temperature on the emission rate of ultrafine particles during 3D printing. Indoor Air 2020, 30, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wong, J.P.; Davis, A.Y.; Black, M.S.; Weber, R.J. Characterization of particle emissions from consumer fused deposition modeling 3D printers. Aerosol Sci. Technol. 2017, 51, 1275–1286. [Google Scholar] [CrossRef]
- Farcas, M.T.; McKinney, W.; Coyle, J.; Orandle, M.; Mandler, W.K.; Stefaniak, A.B.; Bowers, L.; Battelli, L.; Richardson, D.; Hammer, M.A. Evaluation of Pulmonary Effects of 3-D Printer Emissions From Acrylonitrile Butadiene Styrene Using an Air-Liquid Interface Model of Primary Normal Human-Derived Bronchial Epithelial Cells. Int. J. Toxicol. 2022, 41, 312–328. [Google Scholar] [CrossRef]
- Farcas, M.T.; Stefaniak, A.B.; Knepp, A.K.; Bowers, L.; Mandler, W.K.; Kashon, M.; Jackson, S.R.; Stueckle, T.A.; Sisler, J.D.; Friend, S.A. Acrylonitrile butadiene styrene (ABS) and polycarbonate (PC) filaments three-dimensional (3-D) printer emissions-induced cell toxicity. Toxicol. Lett. 2019, 317, 1–12. [Google Scholar] [CrossRef]
- Kim, B.; Shin, J.H.; Kim, H.P.; Jo, M.S.; Kim, H.S.; Lee, J.S.; Lee, H.K.; Kwon, H.C.; Han, S.G.; Kang, N. On-site deployment of an air-liquid-interphase device to assess health hazard potency of airborne workplace contaminants: The case of 3-D printers. Front. Toxicol. 2022, 4, 818942. [Google Scholar] [CrossRef]
- Stefaniak, A.; LeBouf, R.; Duling, M.; Yi, J.; Abukabda, A.; McBride, C.; Nurkiewicz, T. Inhalation exposure to three-dimensional printer emissions stimulates acute hypertension and microvascular dysfunction. Toxicol. Appl. Pharmacol. 2017, 335, 1–5. [Google Scholar] [CrossRef]
- Farcas, M.T.; McKinney, W.; Qi, C.; Mandler, K.W.; Battelli, L.; Friend, S.A.; Stefaniak, A.B.; Jackson, M.; Orandle, M.; Winn, A. Pulmonary and systemic toxicity in rats following inhalation exposure of 3-D printer emissions from acrylonitrile butadiene styrene (ABS) filament. Inhal. Toxicol. 2020, 32, 403–418. [Google Scholar] [CrossRef]
- Mohammadian, Y.; Nasirzadeh, N. Toxicity risks of occupational exposure in 3D printing and bioprinting industries: A systematic review. Toxicol. Ind. Health 2021, 37, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Byrley, P.; Boyes, W.K.; Rogers, K.; Jarabek, A.M. 3D printer particle emissions: Translation to internal dose in adults and children. J. Aerosol Sci. 2021, 154, 105765. [Google Scholar] [CrossRef] [PubMed]
- King, B.J.; Park, E.P.; Christensen, B.J.; Danrad, R. On-site 3-dimensional printing and preoperative adaptation decrease operative time for mandibular fracture repair. J. Oral Maxillofac. Surg. 2018, 76, 1950.e1–1950.e8. [Google Scholar] [CrossRef]
- Bergeron, L.; Bonapace-Potvin, M.; Bergeron, F. In-house 3D model printing for acute cranio-maxillo-facial trauma surgery: Process, time, and costs. Plast. Reconstr. Surg. Glob. Open 2021, 9, e3804. [Google Scholar] [CrossRef]
- Dupret-Bories, A.; Vergez, S.; Meresse, T.; Brouillet, F.; Bertrand, G. Contribution of 3D printing to mandibular reconstruction after cancer. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2018, 135, 133–136. [Google Scholar] [CrossRef]
- Jääskeläinen, T.; Kärkkäinen, O.; Jokkala, J.; Klåvus, A.; Heinonen, S.; Auriola, S.; Lehtonen, M.; FINNPEC Core Investigator Group; Hanhineva, K.; Laivuori, H. A non-targeted LC–MS metabolic profiling of pregnancy: Longitudinal evidence from healthy and pre-eclamptic pregnancies. Metabolomics 2021, 17, 20. [Google Scholar] [CrossRef] [PubMed]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. Metaboanalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res 2018, 46, W486–W494. [Google Scholar] [CrossRef]
- Li, S.; Park, Y.; Duraisingham, S.; Strobel, F.H.; Khan, N.; Soltow, Q.A.; Jones, D.P.; Pulendran, B. Predicting network activity from high throughput metabolomics. PLoS Comput. Biol. 2013, 9, e1003123. [Google Scholar] [CrossRef]
- He, X.; Jarrell, Z.R.; Smith, M.R.; Ly, V.T.; Liang, Y.; Orr, M.; Go, Y.M.; Jones, D.P. Metabolomics of V2O5 nanoparticles and V2O5 nanofibers in human airway epithelial BEAS-2B cells. Toxicol. Appl. Pharmacol. 2023, 459, 116327. [Google Scholar] [CrossRef]
- Uppal, K.; Walker, D.I.; Liu, K.; Li, S.; Go, Y.M.; Jones, D.P. Computational metabolomics: A framework for the million metabolome. Chem. Res. Toxicol. 2016, 29, 1956–1975. [Google Scholar] [CrossRef] [PubMed]
- Uppal, K.; Ma, C.; Go, Y.-M.; Jones, D.P.; Wren, J. xMWAS: A data-driven integration and differential network analysis tool. Bioinformatics 2018, 34, 701–702. [Google Scholar] [CrossRef] [PubMed]
- Steinle, P. Characterization of emissions from a desktop 3D printer and indoor air measurements in office settings. J. Occup. Environ. Hyg. 2016, 13, 121–132. [Google Scholar] [CrossRef]
- Zontek, T.L.; Ogle, B.R.; Jankovic, J.T.; Hollenbeck, S.M. An exposure assessment of desktop 3D printing. J. Chem. Health Saf. 2017, 24, 15–25. [Google Scholar] [CrossRef]
- Stefaniak, A.B.; Bowers, L.N.; Cottrell, G.; Erdem, E.; Knepp, A.K.; Martin, S.; Pretty, J.; Duling, M.G.; Arnold, E.D.; Wilson, Z.; et al. Use of 3-dimensional printers in educational settings: The need for awareness of the effects of printer temperature and filament type on contaminant releases. ACS Chem. Health Saf. 2021, 28, 444–456. [Google Scholar] [CrossRef] [PubMed]
- Brinsko-Bechert, K.M.S.; Palenik, C.S. The analysis of 3D printer dust for forensic applications. J. Forensic Sci. 2020, 65, 1480–1496. [Google Scholar] [CrossRef] [PubMed]
- Aquilano, K.; Baldelli, S.; Ciriolo, M.R. Glutathione: New roles in redox signaling for an old antioxidant. Front Pharmacol 2014, 5, 196. [Google Scholar] [CrossRef]
- Jones, D.P. Redefining oxidative stress. Antioxid. Redox Signal. 2006, 8, 1865–1879. [Google Scholar] [CrossRef]
- Pranzini, E.; Pardella, E.; Muccillo, L.; Leo, A.; Nesi, I.; Santi, A.; Parri, M.; Zhang, T.; Uribe, A.H.; Lottini, T.; et al. SHMT2-mediated mitochondrial serine metabolism drives 5-FU resistance by fueling nucleotide biosynthesis. Cell Rep. 2022, 40, 111233. [Google Scholar] [CrossRef]
- Scott, E.M.; Pillus, L. Homocitrate synthase connects amino acid metabolism to chromatin functions through Esa1 and DNA damage. Genes Dev. 2010, 24, 1903–1913. [Google Scholar] [CrossRef]
- Milligan, J.R.; Aguilera, J.A.; Ly, A.; Tran, N.Q.; Hoang, O.; Ward, J.F. Repair of oxidative DNA damage by amino acids. Nucleic Acids Res. 2003, 31, 6258–6263. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Hoppe, T. Role of amino acid metabolism in mitochondrial homeostasis. Front. Cell Dev. Biol. 2023, 11, 2023. [Google Scholar] [CrossRef] [PubMed]
- Parmar, P.; Daya, S. The effect of copper on (3H)-tryptophan metabolism in organ cultures of rat pineal glands. Metab. Brain Dis. 2001, 16, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xia, W.; Liu, H.; Liu, F.; Li, H.; Chang, H.; Sun, J.; Liu, W.; Sun, X.; Jiang, Y.; et al. Urinary metabolomics reveals novel interactions between metal exposure and amino acid metabolic stress during pregnancy. Toxicol. Res. 2018, 7, 1164–1172. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhai, Q.; Wang, G.; Liu, X.; Zhao, J.; Tian, F.; Zhang, H.; Chen, W. Metabolomics analysis reveals heavy metal copper-induced cytotoxicity in HT-29 human colon cancer cells. RSC Adv. 2016, 6, 78445–78456. [Google Scholar] [CrossRef]
- Lerner, C.A.; Rutagarama, P.; Ahmad, T.; Sundar, I.K.; Elder, A.; Rahman, I. Electronic cigarette aerosols and copper nanoparticles induce mitochondrial stress and promote DNA fragmentation in lung fibroblasts. Biochem. Biophys. Res. Commun. 2016, 477, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Edelmann, M.J.; Shack, L.A.; Naske, C.D.; Walters, K.B.; Nanduri, B. SILAC-based quantitative proteomic analysis of human lung cell response to copper oxide nanoparticles. PLoS ONE 2014, 9, e114390. [Google Scholar] [CrossRef]
- Tacu, I.; Kokalari, I.; Abollino, O.; Albrecht, C.; Malandrino, M.; Ferretti, A.M.; Schins, R.P.; Fenoglio, I. Mechanistic insights into the role of iron, copper, and carbonaceous component on the oxidative potential of ultrafine particulate matter. Chem. Res. Toxicol. 2021, 34, 767–779. [Google Scholar] [CrossRef]
- Kumar, S.; Khaliq, F.; Singh, S.; Ahmed, R.; Kumar, R.; Deshmukh, P.S.; Banerjee, B.D. Pulmonary functions, oxidative stress and DNA damage in workers of a copper processing industry. Int. J. Occup. Environ. Med. 2016, 7, 107. [Google Scholar] [CrossRef]
- Agier, L.; Basagaña, X.; Maitre, L.; Granum, B.; Bird, P.K.; Casas, M.; Oftedal, B.; Wright, J.; Andrusaityte, S.; de Castro, M.; et al. Early-life exposome and lung function in children in Europe: An analysis of data from the longitudinal, population-based HELIX cohort. Lancet Planet. Health 2019, 3, e81–e92. [Google Scholar] [CrossRef]
- Maitre, L.; Julvez, J.; López-Vicente, M.; Warembourg, C.; Tamayo-Uria, I.; Philippat, C.; Gützkow, K.B.; Guxens, M.; Andrusaityte, S.; Basagaña, X.; et al. Early-life environmental exposure determinants of child behavior in Europe: A longitudinal, population-based study. Environ. Int. 2021, 153, 106523. [Google Scholar] [CrossRef]
- Warembourg, C.; Maitre, L.; Tamayo-Uria, I.; Fossati, S.; Roumeliotaki, T.; Aasvang, G.M.; Andrusaityte, S.; Casas, M.; Cequier, E.; Chatzi, L.; et al. Early-life environmental exposures and blood pressure in children. J. Am. Coll. Cardiol. 2019, 74, 1317–1328. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.M.; Xiao, C.; Finley, L.W.; Lahusen, T.; Souza, A.L.; Pierce, K.; Li, Y.H.; Wang, X.; Laurent, G.; German, N.J.; et al. SIRT4 has tumor-suppressive activity and regulates the cellular metabolic response to DNA damage by inhibiting mitochondrial glutamine metabolism. Cancer Cell 2013, 23, 450–463. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Yang, S.; Kim, M.; Hong, Y.; Kim, B.; Lee, E.K.; Jeong, S.M. Mitochondrial glutamine metabolism regulates sensitivity of cancer cells after chemotherapy via amphiregulin. Cell Death Discov. 2021, 7, 395. [Google Scholar] [CrossRef] [PubMed]
- Surjana, D.; Halliday, G.M.; Damian, D.L. Role of nicotinamide in DNA damage, mutagenesis, and DNA repair. J. Nucleic Acids 2010, 2010, 157591. [Google Scholar] [CrossRef] [PubMed]
- Benavente, C.A.; Jacobson, M.K.; Jacobson, E.L. NAD in skin: Therapeutic approaches for niacin. Curr. Pharm. Des. 2009, 15, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Liu, Y.; Dai, H.; Wang, C. Fatty acid metabolism and idiopathic pulmonary fibrosis. Front. Physiol. 2022, 12, 2494. [Google Scholar] [CrossRef]
- Pamungkas, A.D.; Medriano, C.A.; Sim, E.; Lee, S.; Park, Y.H. A pilot study identifying a potential plasma biomarker for determining EGFR mutations in exons 19 or 21 in lung cancer patients. Mol. Med. Rep. 2017, 15, 4155–4161. [Google Scholar] [CrossRef][Green Version]
- Singh, A.V.; Maharjan, R.S.; Jungnickel, H.; Romanowski, H.; Hachenberger, Y.U.; Reichardt, P.; Bierkandt, F.; Siewert, K.; Gadicherla, A.; Laux, P.; et al. Evaluating particle emissions and toxicity of 3D pen printed filaments with metal nanoparticles as additives: In vitro and in silico discriminant function analysis. ACS Sustain. Chem. Eng. 2021, 9, 11724–11737. [Google Scholar] [CrossRef]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human health and environmental toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef]
- Chen, C.H.S.; Kuo, T.C.; Kuo, H.C.; Tseng, Y.J.; Kuo, C.H.; Yuan, T.H.; Chan, C.C. Metabolomics of children and adolescents exposed to industrial carcinogenic pollutants. Environ. Sci. Technol. 2019, 53, 5454–5465. [Google Scholar] [CrossRef] [PubMed]
- Uppala, R.; McKinney, R.W.; Brant, K.A.; Fabisiak, J.P.; Goetzman, E.S. Nickel inhibits mitochondrial fatty acid oxidation. Biochem. Biophys. Res. Commun. 2015, 463, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Gao, X.; Zhu, J.; Cheng, K.; Tang, M. Mechanisms involved in reproductive toxicity caused by nickel nanoparticle in female rats. Environ. Toxicol. 2016, 31, 1674–1683. [Google Scholar] [CrossRef] [PubMed]
- Kotlyarov, S.; Kotlyarova, A. Anti-Inflammatory function of fatty acids and involvement of their metabolites in the resolution of inflammation in chronic obstructive pulmonary disease. Int. J. Mol. Sci. 2021, 22, 12803. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Knudsen, N.H.; Wang, G.; Qiu, W.; Naing, Z.Z.C.; Bai, Y.; Ai, X.; Lee, C.H.; Zhou, X. Genetic control of fatty acid β-oxidation in chronic obstructive pulmonary disease. Am. J. Respir. Cell Mol. Biol. 2017, 56, 738–748. [Google Scholar] [CrossRef]
- Xu, W.; Janocha, A.J.; Erzurum, S.C. Metabolism in pulmonary hypertension. Annu. Rev. Physiol. 2021, 83, 551–576. [Google Scholar] [CrossRef]
- Hoppstädter, J.; Dembek, A.; Höring, M.; Schymik, H.S.; Dahlem, C.; Sultan, A.; Wirth, N.; Al-Fityan, S.; Diesel, B.; Gasparoni, G.; et al. Dysregulation of cholesterol homeostasis in human lung cancer tissue and tumour-associated macrophages. EBioMedicine 2021, 72, 103578. [Google Scholar] [CrossRef]
- Flanagan, J.L.; Simmons, P.A.; Vehige, J.; Willcox, M.D.; Garrett, Q. Role of carnitine in disease. Nutr. Metab. 2010, 7, 30. [Google Scholar] [CrossRef]
- Gowdy, K.M.; Fessler, M.B. Emerging roles for cholesterol and lipoproteins in lung disease. Pulm. Pharmacol. Ther. 2013, 26, 430–437. [Google Scholar] [CrossRef]
- Sallese, A.; Suzuki, T.; McCarthy, C.; Bridges, J.; Filuta, A.; Arumugam, P.; Shima, K.; Ma, Y.; Wessendarp, M.; Black, D.; et al. Targeting cholesterol homeostasis in lung diseases. Sci. Rep. 2017, 7, 10211. [Google Scholar] [CrossRef]
- Fessler, M.B. A new frontier in immunometabolism. Cholesterol in lung health and disease. Ann. Am. Thorac. Soc. 2017, 14 (Suppl. S5), S399–S405. [Google Scholar] [CrossRef]
- Esther, C.R., Jr.; O’Neal, W.K.; Anderson, W.H.; Kesimer, M.; Ceppe, A.; Doerschuk, C.M.; Alexis, N.E.; Hastie, A.T.; Barr, R.G.; Bowler, R.P.; et al. Identification of sputum biomarkers predictive of pulmonary exacerbations in COPD. Chest 2022, 161, 1239–1249. [Google Scholar] [CrossRef] [PubMed]
- Esther, C.R., Jr.; Coakley, R.D.; Henderson, A.G.; Zhou, Y.H.; Wright, F.A.; Boucher, R.C. Metabolomic evaluation of neutrophilic airway inflammation in cystic fibrosis. Chest 2015, 148, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Esther, C.R.; Turkovic, L.; Rosenow, T.; Muhlebach, M.S.; Boucher, R.C.; Ranganathan, S.; Stick, S.M. Metabolomic biomarkers predictive of early structural lung disease in cystic fibrosis. Eur. Respir. J. 2016, 48, 1612–1621. [Google Scholar] [CrossRef]
- Quinlan, G.J.; Lamb, N.J.; Tilley, R.; Evans, T.W.; Gutteridge, J.M. Plasma hypoxanthine levels in ARDS: Implications for oxidative stress, morbidity, and mortality. Am. J. Respir. Crit. Care Med. 1997, 155, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Kominsky, D.J.; Campbell, E.L.; Colgan, S.P. Metabolic Shifts in Immunity and Inflammation. J. Immunol. 2010, 184, 4062–4068. [Google Scholar] [CrossRef]
- Jones, D.P. Radical-free biology of oxidative stress. Am. J. Physiol. Cell Physiol. 2008, 295, C849–C868. [Google Scholar] [CrossRef]
- Jones, D.P.; Sies, H. The redox code. Antioxid. Redox Signal. 2015, 23, 734–746. [Google Scholar] [CrossRef]
- Trachootham, D.; Lu, W.; Ogasawara, M.A.; Valle, N.R.D.; Huang, P. Redox regulation of cell survival. Antioxid. Redox Signal. 2008, 10, 1343–1374. [Google Scholar] [CrossRef]
- Go, Y.M.; Chandler, J.D.; Jones, D.P. The cysteine proteome. Free Radic. Biol. Med. 2015, 84, 227–245. [Google Scholar] [CrossRef]
- Liang, M.; Wang, Z.; Li, H.; Cai, L.; Pan, J.; He, H.; Wu, Q.; Tang, Y.; Ma, J.; Yang, L. L-Arginine induces antioxidant response to prevent oxidative stress via stimulation of glutathione synthesis and activation of Nrf2 pathway. Food Chem. Toxicol. 2018, 115, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Mohorko, N.; Petelin, A.; Jurdana, M.; Biolo, G.; Jenko-Pražnikar, Z. Elevated serum levels of cysteine and tyrosine: Early biomarkers in asymptomatic adults at increased risk of developing metabolic syndrome. BioMed Res. Int. 2015, 2015, 418681. [Google Scholar] [CrossRef]
- Rahman, I.; MacNee, W. Oxidative stress and regulation of glutathione in lung inflammation. Eur. Respir. J. 2000, 16, 534–554. [Google Scholar] [CrossRef] [PubMed]
- Hanagata, N.; Zhuang, F.; Connolly, S.; Li, J.; Ogawa, N.; Xu, M. Molecular responses of human lung epithelial cells to the toxicity of copper oxide nanoparticles inferred from whole genome expression analysis. ACS Nano 2011, 5, 9326–9338. [Google Scholar] [CrossRef]
- Zitting, A.; Savolainen, H. Effects of single and repeated exposures to thermo-oxidative degradation products of poly (acrylonitrile-butadiene-styrene)(ABS) on rat lung, liver, kidney, and brain. Arch. Toxicol. 1980, 46, 295–304. [Google Scholar] [CrossRef]
- Zitting, A.; Heinonen, T. Decrease of reduced glutathione in isolated rat hepatocytes caused by acrolein, acrylonitrile, and the thermal degradation products of styrene copolymers. Toxicology 1980, 17, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, B.; Cormier, S.A. Copper oxide nanoparticles induce oxidative stress and cytotoxicity in airway epithelial cells. Toxicol. In Vitro 2009, 23, 1365–1371. [Google Scholar] [CrossRef]
- Jian, Z.; Guo, H.; Liu, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Oxidative stress, apoptosis and inflammatory responses involved in copper-induced pulmonary toxicity in mice. Aging 2020, 12, 16867. [Google Scholar] [CrossRef]
- El-Kersh, K.; Hopkins, C.D.; Wu, X.; Rai, S.N.; Cave, M.C.; Smith, M.R.; Go, Y.M.; Jones, D.P.; Cai, L.; Huang, J. Metallomics in pulmonary arterial hypertension patients. Pulm. Circ. 2023, 13, e12202. [Google Scholar] [CrossRef]
- Zhang, Z.; Weichenthal, S.; Kwong, J.C.; Burnett, R.T.; Hatzopoulou, M.; Jerrett, M.; Donkelaar, A.V.; Bai, L.; Martin, R.V.; Copes, R.; et al. Long-term exposure to iron and copper in fine particulate air pollution and their combined impact on reactive oxygen species concentration in lung fluid: A population-based cohort study of cardiovascular disease incidence and mortality in Toronto, Canada. Int. J. Epidemiol. 2021, 50, 589–601. [Google Scholar] [CrossRef]
- Khaki, S.; Duffy, E.; Smeaton, A.F.; Morrin, A. Monitoring of particulate matter emissions from 3D printing activity in the home setting. Sensors 2021, 21, 3247. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X.; Barnett, L.M.; Jeon, J.; Zhang, Q.; Alqahtani, S.; Black, M.; Shannahan, J.; Wright, C. Real-Time Exposure to 3D-Printing Emissions Elicits Metabolic and Pro-Inflammatory Responses in Human Airway Epithelial Cells. Toxics 2024, 12, 67. https://doi.org/10.3390/toxics12010067
He X, Barnett LM, Jeon J, Zhang Q, Alqahtani S, Black M, Shannahan J, Wright C. Real-Time Exposure to 3D-Printing Emissions Elicits Metabolic and Pro-Inflammatory Responses in Human Airway Epithelial Cells. Toxics. 2024; 12(1):67. https://doi.org/10.3390/toxics12010067
Chicago/Turabian StyleHe, Xiaojia, Lillie Marie Barnett, Jennifer Jeon, Qian Zhang, Saeed Alqahtani, Marilyn Black, Jonathan Shannahan, and Christa Wright. 2024. "Real-Time Exposure to 3D-Printing Emissions Elicits Metabolic and Pro-Inflammatory Responses in Human Airway Epithelial Cells" Toxics 12, no. 1: 67. https://doi.org/10.3390/toxics12010067
APA StyleHe, X., Barnett, L. M., Jeon, J., Zhang, Q., Alqahtani, S., Black, M., Shannahan, J., & Wright, C. (2024). Real-Time Exposure to 3D-Printing Emissions Elicits Metabolic and Pro-Inflammatory Responses in Human Airway Epithelial Cells. Toxics, 12(1), 67. https://doi.org/10.3390/toxics12010067







