Abstract
Emerging contaminants have been increasingly recognized as critical determinants in global public health outcomes. However, the intricate relationship between these contaminants and glucose metabolism remains to be fully elucidated. The paucity of comprehensive clinical data, coupled with the need for in-depth mechanistic investigations, underscores the urgency to decipher the precise molecular and cellular pathways through which these contaminants potentially mediate the initiation and progression of diabetes mellitus. A profound understanding of the epidemiological impact of these emerging contaminants, as well as the elucidation of the underlying mechanistic pathways, is indispensable for the formulation of evidence-based policy and preventive interventions. This review systematically aggregates contemporary findings from epidemiological investigations and delves into the mechanistic correlates that tether exposure to emerging contaminants, including endocrine disruptors, perfluorinated compounds, microplastics, and antibiotics, to glycemic dysregulation. A nuanced exploration is undertaken focusing on potential dietary sources and the consequential role of the gut microbiome in their toxic effects. This review endeavors to provide a foundational reference for future investigations into the complex interplay between emerging contaminants and diabetes mellitus.
1. Introduction
According to the International Diabetes Federation, the global number of individuals with diabetes reached 425 million in 2017 [1]. Projections suggest that by 2045, the global diabetic population will escalate to 783 million. Diabetes is understood to be a polygenic hereditary disorder with a pronounced genetic predisposition. Approximately 60% of type II diabetes patients have a familial history of the disease, showcasing a notable familial aggregation [2]. Current genome-wide association studies have pinpointed over 80 susceptibility loci associated with diabetes [3]. Beyond genetic predispositions, environmental factors exert an undeniable influence on diabetes outcomes [4], with emerging pollutants increasingly taken into consideration [5].
With the advent of modern industrial developments and the introduction of novel chemical compounds, the spectrum of environmental pollutants has expanded. Many of these emerging contaminants possess chemical and toxicological properties that remain insufficiently characterized. These pollutants emanate from diverse sources, are numerous in type, and their inherent resistance to degradation leads to their ubiquitous presence and accumulation in environmental matrices [6,7]. Despite their often low concentrations, the biotoxicity of these pollutants, coupled with their persistence and bioaccumulative potential, presents potential detrimental effects on the environment and biota [8]. As the understanding of the environmental and health impacts of chemical substances deepens, and as environmental detection technologies evolve, an increasing number of these pollutants are being identified. However, there is a noticeable absence of regulatory frameworks overseeing their presence and impact, leading to their collective designation as emerging contaminants (ECs) or emerging pollutants (EPs). It is widely acknowledged that these contaminants, even at low concentrations, can enter biological systems through ingestion, inhalation, or dermal contact [9], with ingestion being the predominant exposure route. Once inside an organism, they accumulate in various tissues and organs [10,11], influencing metabolic pathways, including glucose metabolism. For instance, when mice were exposed to 1.25 mg/kg/d PFOA for 28 days, an elevation in blood glucose levels was observed, accompanied by a reduction in hepatic glucose and glycogen content [12]. Moreover, some studies suggest that certain emerging pollutants might influence host glucose metabolism by altering the composition and function of the gut microbiome. For example, Huang et al. found that when mice fed a high-fat diet were exposed to polystyrene microplastics, there was a marked reduction in the richness and diversity of their gut microbiota, an increase in the relative abundance of Gram-negative bacteria, as well as elevated levels of insulin resistance and pro-inflammatory cytokines [13].
The phenomenon of increasing attention to the impacts of emerging environmental pollutants on the incidence, progression, and complications of diabetes has been the subject of extensive research. A survey of various databases reveals that articles have been published reviewing the changes in glucose metabolism induced by these emerging pollutants [14,15]. However, these articles have primarily focused on the impact of individual pollutants on diabetes and its complications, with a comprehensive review of all emerging pollutants, their presence in the environment, biological exposure pathways, and effects on glucose metabolism remaining unexplored. In addition, studies on the effects of exposure to emerging contaminants that cause disturbances in glucose metabolism through alterations in the structure and composition of the gut microbiota have not been reviewed. Therefore, this review summarizes the effects on glucose metabolism after exposure to the four emerging contaminants and the possible mechanisms, especially through the alteration of glucose metabolism after affecting the gut microbiota, by presenting the four new contaminants. The objective is to furnish a foundation for future research on the influence of these pollutants on diabetes, to enhance public awareness of their potential hazards, and to provide empirical support and scientific underpinning for the management of emerging pollutants and relevant environmental policies.
2. Emerging Contaminants (ECs)
Emerging contaminants (ECs) refer to environmental pollutants detectable in the environment and natural ecosystems, posing significant health and environmental risks to both humans and ecological systems. Yet, they remain either unregulated by laws and standards or inadequately addressed [16,17]. When these contaminants are introduced into the environment, their concentrations tend to be low, often rendering their short-term toxic effects unnoticeable. However, their bioaccumulative nature and resistance to degradation result in persistent accumulation. By the time they are detectable, these pollutants have already accrued and posed long-term hazards [18]. The ECs in the environment carry potential dangers to the ecosystem and all living organisms, including humans, such as chronic toxicity, genetic harm, endocrine-disrupting effects, and the “tri-effects” (carcinogenic, teratogenic, and mutagenic effects) [19]. These characteristics have prompted scientists to pay increasing attention to emerging pollutants in the environment.
Surprisingly, over 3000 types of emerging pollutants have been identified globally. Almost every country has detected the presence of these [20]. Their widespread sources and diversity are alarming. China, being a significant producer and consumer of various chemicals, faces challenges as the large-scale production, misuse, and improper management of these chemical substances introduce them into the environment, intensifying environmental pollution concerns [21]. The difficulty in monitoring and the lack of adequate regulatory measures for these contaminants, combined with their persistent and bioaccumulative nature, make them challenging to manage once they enter the environment [18,22,23].
Pharmaceutical factories, plastics, artificial sweeteners, plasticizers, illicit drugs, cleaning products, cosmetics, personal care products, beverages, and packaging are primary sources of these pollutants [24]. They primarily enter biological systems via dietary exposure. Many studies hint that animal-derived food sources are major contributors to many endocrine-disrupting agents. For instance, residues of polychlorinated biphenyl congeners in Crassostrea tulipa (oysters) and Anadara senilis (mussels) were detected at concentrations of 2.95–11.41 mg/kg wet weight and 5.55–6.37 mg/kg wet weight, respectively [25]. The likelihood of exposure to these biphenyls in bivalves is high, with median concentrations exceeding FDA action levels [26]. Wang et al. [27] detected 11 types of perfluorinated compounds in consumer products such as pork tenderloin, pork heart, pork liver, pork kidney, chicken breast, and chicken liver. Every sample contained these compounds, with pork liver having the highest average content of 3.438 ng/g, followed by pork kidney (0.508 ng/g). Additionally, researchers found microplastics in various salts and bottled waters humans consume, with the highest concentrations in sea salt (550–681 particles/kg) and bottled water showing average concentrations of 10.4 particles/L and 325 particles/L for microplastics with sizes >100 μm and <100 μm, respectively [28,29].
Emerging contaminants closely linked with daily human life can be categorized as biological (e.g., resistant genes, algal toxins), chemical (e.g., novel pesticides, endocrine disruptors, flame retardants, antibiotics, perfluorinated compounds), and physical (e.g., microplastics, nanomaterials) [22,30,31]. Based on publicly available regional or site-specific monitoring data, typical emerging pollutants in China mainly include endocrine disruptors, perfluorinated compounds, microplastics, and antibiotics, all of which are causing severe pollution issues in the air, water, and soil environments [22,30]. Numerous published articles have indicated that the presence of emerging pollutants in the environment increases the risk of diabetes in exposed populations and accelerates the onset and progression of the disease. Moreover, it is well known that the mechanisms of type I diabetes and type II diabetes are different. Type I diabetes is mostly related to genetic factors, while for type II diabetes, lifestyle and exposure to emerging contaminants seem to be more important. This article focuses on these four emerging pollutants, briefly discussing their influence on the onset and progression of diabetes, hoping to offer a scientific foundation for the management and prevention of emerging contaminants (Figure 1).
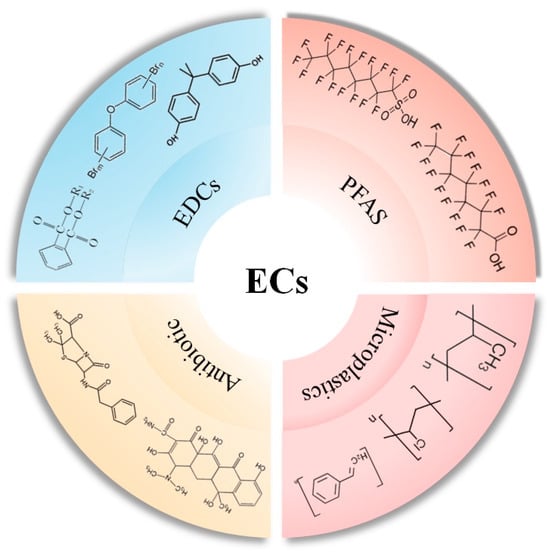
Figure 1.
Typical emerging contaminants and representative compounds.
3. Endocrine-Disrupting Chemicals (EDCs)
Endocrine-disrupting chemicals (EDCs) are defined as “an exogenous substance or mixture that alters function(s) of the endocrine system and consequently causes adverse health effects in an intact organism, or its progeny, or (sub) populations” [32]. The most prevalent EDCs include persistent organic pollutants (POPs), phenolic compounds, insecticides, and flame retardants, among others. Due to the frequent use of endocrine-disrupting chemicals (EDCs) in daily life, they are ubiquitously present in the environment. The detection methods for EDCs in the environment primarily include mass spectroscopy, chromatography-based methods, and advanced sensing approaches [33,34,35], such as electrochemical and colorimetric methods. Owing to their rapid, portable, sensitive, and eco-friendly characteristics, sensing approaches are frequently employed to detect EDCs in environmental and food production systems. Some EDCs are lipophilic, and once ingested by humans through the food chain, they accumulate in adipose tissue [36]. An epidemiological study by Miquel Porta discovered that over 85% of study participants had detectable levels of EDCs such as polychlorinated biphenyls (PCBs), DDE, and DDT in their bloodstream. Additionally, the concentration of EDCs in adipose tissue ranged between 92 ng/g and 399 ng/g [37]. Upon entering organisms, EDCs interfere with the natural synthesis, secretion, and elimination of hormones, leading to endocrine imbalances. Such disturbances can result in endocrine disorders, including obesity and diabetes [38] (Table 1).
3.1. Persistent Organic Contaminants (POPs)
Persistent organic contaminants (POPs) are toxic chemicals that are highly resistant to environmental degradation and metabolic degradation [39]. Most POPs are hydrophobic and can accumulate continuously in the fat of animals and humans [40], causing significant biological toxicity, such as developmental defects, metabolic diseases, cancer, and even death [41]. The primary POPs in the environment include phthalates (PAEs), polybrominated diphenyl ethers (PBDEs), polychlorinated biphenyls, and dioxins.
Phthalates (PAEs) are frequently introduced into the environment as plasticizers used in various plastic products [42]. Given China’s role as a major producer of PAEs and its lack of effective pollution control measures, the PAE pollution level in Chinese waters is exceptionally high [43]. Moreover, recent studies have shown that when PAEs enter an organism, they can disrupt glucose metabolism and affect blood glucose levels. A case–control study of volunteers from China found that environmental PAEs can be metabolized within the human body, and their metabolites can be excreted in urine. There is a significant positive correlation between these metabolites and fasting blood glucose and glycated hemoglobin levels, thereby interfering with normal glucose metabolism and affecting the development of type II diabetes [44]. Additionally, metabolic pathways closely related to glucose metabolism, such as galactose metabolism, amino acid metabolism, riboflavin metabolism, and pyridoxal metabolism, can also be affected by PAEs, changing their metabolic marker levels. For instance, in a case–control study of volunteers in Tianjin, China, researchers have observed that the metabolic products involved in galactose metabolism in the serum of patients with type II diabetes were significantly elevated and showed a positive correlation with serum PAE levels. Other metabolites involved in amino acid metabolism, riboflavin metabolism, and pyridoxal metabolism also showed significant changes [45]. Similarly to type II diabetes, gestational diabetes also showed a significant correlation with PAE metabolites [46,47]. While there are a growing number of population studies on the relationship between PAEs and diabetes, research on their specific mechanisms is still relatively limited. When rat insulinoma (INS-1) cells were exposed to dibutyl phthalate (DBP), Yang found that exposure at concentrations of 60 μmol/L and 120 μmol/L led to increased cell apoptosis, significant reductions in mitochondrial membrane potential, increased cellular oxidative stress levels, and decreased superoxide dismutase levels. It is speculated that DBP might reduce INS-1 cell insulin synthesis and secretion through mitochondrial apoptosis and oxidative stress pathways [48].
Polybrominated diphenyl ethers (PBDEs) are brominated flame retardants. Since the 1960s, they have been used as flame retardants in commercial and household products (44). Like PAEs, PBDEs continuously accumulate in organisms, affecting their glucose and lipid metabolism. Data from a nested case–control study indicate a significant correlation between brominated biphenyl ethers (BDEs) and gestational diabetes [49]. Furthermore, BDE-153, BDE-154, and BDE-183 all have odds ratios >1, showing a significant positive and inverted U-shaped correlation with diabetes. Similarly, Ongono and colleagues found in a cohort study that dietary exposure to hexabromocyclododecane is positively correlated with type II diabetes and dietary exposure to PBDEs has a positive non-linear relationship with type II diabetes [50]. Liu used mice orally exposed to BDE-153 to explore the potential mechanisms by which brominated flame retardants might affect glucose metabolism. He found that mouse insulin levels showed a dose-dependent relationship with BDE-153 and the expression of PPARγ and AMPKα was disturbed. It is speculated that BDE-153 might interfere with the expression of adipokines and insulin secretion by affecting the expression of PPARγ and AMPKα, leading to metabolic dysregulation [51].
In addition to PAEs and PBDEs, there are numerous other POPs present in the environment, including polychlorinated biphenyls (PCBs) and dioxins. Organisms living within such environments, especially those on a high-fat diet or at risk for diabetes, can experience altered glucose metabolism when exposed to these POPs. Ibrahim et al. [52] fed C57BL/6J mice a high-fat diet containing POPs and observed that, compared to the unexposed group, both the high-fat-diet mice and those on a Western diet exhibited exacerbated manifestations of insulin resistance, visceral obesity, and abnormalities in glucose tolerance. Additionally, it was found that mice in the low-exposure group exhibited better insulin sensitivity and glucose tolerance than those in the high-exposure group. Furthermore, when C57BL/6J mice on a high-fat diet or those with diabetes were exposed to PCBs, they displayed glucose metabolic abnormalities characterized by glucose intolerance, increased gluconeogenesis, elevated tricarboxylic acid cycle flux, hyperinsulinemia, and intensified systemic insulin resistance [41,53]. Interestingly, a study by Nicki A. Baker revealed that while exposure to PCBs induced lipid inflammation and glucose and insulin tolerance impairment in mice on a low-fat diet, the glycemic equilibrium in obese mice remained unaffected unless they underwent weight reduction [54,55].
The continuous accumulation of dioxins, specifically 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), in the environment and their impact on glucose metabolism is gradually gaining attention from scientists. When mice were exposed to environmental TCDD at a dose of 20 ng/kg, the hyperglycemia induced by a high-fat diet and the reduction in plasma insulin levels induced by glucose were intensified. These mice exhibited significantly elevated blood sugar levels, substantial changes in islet endocrine and metabolic pathways, and increased expression of mRNAs encoding the sodium–glucose transporter 1 and glucose transporter 2 in the intestines [56,57]. Kurita’s research yielded similar conclusions: post-TCDD exposure, there was a notable decrease in plasma insulin concentrations in mice, with insulin secretion levels significantly reduced [58]. Furthermore, researchers discovered that when high-fat-diet mice undergoing mating, pregnancy, or lactation were injected with 20 ng/kg TCDD twice a week, these mice experienced accelerated weight gain, faster onset of hyperglycemia, reduced islet levels, and islet shrinkage [59].
The review identified that phthalates, polybrominated diphenyl ethers, polychlorinated biphenyls, and other persistent organic pollutants are widely present in the environment and can enter biological organisms, including humans, through various pathways. Studies have found a significant positive correlation between the concentration of persistent organic pollutants in human serum or urine and fasting blood glucose levels. This phenomenon has been further substantiated in animal and cell experiments, with discussions on potential mechanisms.
3.2. Bisphenol A (BPA) and Its Structural Analogs
Bisphenol A (BPA) and its structural analogs (BPS, BPF, BPAF) are synthetically produced and ubiquitously present in the environment, serving as endocrine disruptors. The omnipresence of BPA compounds means that human exposure to this contaminant is inevitable [60]. Once introduced to the human body, BPA can instigate a myriad of detrimental effects, disrupting metabolic processes including lipid metabolism and glycemic regulation [61]. To date, numerous epidemiological studies have scrutinized the association between urinary concentrations of BPA and its metabolites and diabetes, not only pinpointing a robust correlation between BPA and type II diabetes but also identifying it as a risk factor for gestational diabetes. Beyond this, to elucidate the specific role of BPA in the onset and progression of diabetes and to understand its potential mechanisms leading to the disease, numerous animal experiments have been conducted. For instance, when mice on a standard diet were exposed to 5, 50, 500, and 5000 μgBPA/kg for 8 months, they manifested clear hyperglycemia and hypercholesterolemia. Male mice exposed to 5000 μg/kg/d BPA displayed pronounced insulin resistance [62]. Similarly, in mice fed a high-fat diet, research indicated that BPA aggravates the pre-diabetic symptoms induced by such a diet. Intriguingly, while male mice exhibited only impaired glucose tolerance, female mice also demonstrated increased weight and elevated serum insulin levels, among other symptoms [63]. Regarding the glucose intolerance induced by BPA, a study by Moon et al. posits that it might be attributed to altered serum adipocytokine levels and skeletal muscle phosphorylation, subsequently inducing glucose tolerance abnormalities [64].
Bisphenol A, as an endocrine disruptor, is prevalent in the environment. Numerous population studies have also discovered its association with the development of diabetes, and animal experiments have been conducted to investigate the potential mechanisms of bisphenol A in disrupting glucose metabolism.

Table 1.
Summary of studies related to the effects of POPs on glucose metabolism.
Table 1.
Summary of studies related to the effects of POPs on glucose metabolism.
| Class | Study Subject | Research Models | Chemical | Toxicity | Reference |
|---|---|---|---|---|---|
| Population Studies | General population (250 T2DM and 250 controls) | Case–control study | PAEs |
| [44] |
| General population (60 T2DM and 60 controls) | Case–control study | mPAEs, bisphenols |
| [45] | |
| First trimester (T1) pregnant women (60 GDM and 90 IGT) | Case–control study | PAEs |
| [46] | |
| First trimester (T1) pregnant women (169 GDM and 169 controls) | Case–control study | PAEs |
| [47] | |
| First trimester (T1) pregnant women (439 pregnant women) | Nested case–control study | PBDEs |
| [49] | |
| General population (71,415 women) | Prospective cohort study | Brominated flame retardants (BFRs) |
| [50] | |
| Cell Experiments | Rat insulinoma (INS-1) cells | Experimental research | DBP |
| [48] |
| Animal Experiments | Adult male C57BL/6J mice | Experimental research | BDE-153 |
| [51] |
| High-fat-diet male mice (C57BL/6J) | Experimental research | POPs |
| [52] | |
| High-fat-diet male mice (C57BL/6J) | Experimental research | TSP, D2O, PCB126 |
| [41] | |
| High-fat-diet female mice (C57BL/6J) | Experimental research | PCBs |
| [53] | |
| Low-fat-diet or high-fat-diet female mice (C57BL/6J) | Experimental research | PCB-77, PCB-126 |
| [54] | |
| Low-fat-diet or high-fat-diet male mice | Experimental research | PCB-77 |
| [55] | |
| High-fat-diet mice (C57BL/6J) | Experimental research | TCDD |
| [56] | |
| Male C57BL/6J mice and DBA/2J mice | Experimental research | TCDD |
| [57] | |
| Male C57BL/6J mice, AhR mice, islets from C57BL/6J mice | Experimental research | TCDD |
| [58] | |
| High-fat-diet female mice (C57BL/6J) | Experimental research | TCDD |
| [59] | |
| Male CD1 mice | Experimental research | BPA |
| [62] | |
| High-fat-diet mice (C57BL/6J) | Experimental research | BPA |
| [63] | |
| High-fat-diet male mice (C57BL/6J) | Experimental research | BPA |
| [64] |
4. Per- and Polyfluoroalkyl Substances (PFASs)
Per- and polyfluoroalkyl substances (PFASs) typically consist of carbon chains ranging from 4 to 14 carbons, complemented by a few functional groups [65]. Due to their intrinsic properties such as thermal stability, hydrophobicity, and oleophobicity, PFASs have found extensive applications in industrial production and consumer goods [66], for instance, non-stick cookware, grease-resistant food packaging, and personal care products [67]. Per- and polyfluoroalkyl substances (PFASs), as novel pollutants, have not been thoroughly researched. Due to their widespread application in consumer products, PFASs are omnipresent in the environment, posing potential threats to both the environment and humans. Perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA), the most frequently detected PFASs [68], persist in the environment because of the stability of their carbon–fluorine bonds [69]. The current method employed by the United States Environmental Protection Agency for detecting PFASs in the environment relies on combinations of liquid chromatography and mass spectroscopy [70]. However, due to the high costs and the need for trained specialized laboratory personnel, recent research efforts have focused on developing rapid, portable, and low-cost detection methods. The precise toxicological profile of PFASs remains elusive, but mounting research underscores the potential health risks they pose, inclusive of metabolic disturbances (Table 2).
4.1. Perfluorooctane Sulfonate (PFOS)
PFOS, a degradation product among many PFASs, emerges as one of the most scrutinized compounds in the PFAS family. Scientists have ascertained that PFOS not only remains persistent in the environment but also has a notably long half-life of approximately 5.4 years in human serum once ingested, with serum concentrations averaging around 0.05 μg/mL [11,71]. Recently, the connection between PFOS and diabetes has gained research traction.
A growing body of epidemiological evidence associates increased PFAS serum concentrations in humans with elevated fasting glucose, fasting insulin levels, changes in the insulin homeostasis model, and enhanced cellular functions. This compound has been identified not only as a risk factor for gestational diabetes but also as an augmenting agent for type II diabetes susceptibility [72,73,74]. Observations from human population studies are progressively being corroborated by animal experiments. For instance, Sant et al. [75] exposed zebrafish embryos in the blastula stage to 16, 32, 64 Mm PFOS, noting congenital anomalies mirroring the increased risk factors for human diabetes. The embryos and larvae exhibited perturbed pancreatic growth, pancreatic islet malformations, and a U-shaped dose–response relationship with respect to islet size and PFOS exposure. Qin et al. [76] discovered through in vivo and in vitro studies that PFOS exposure stimulates the free-fatty-acid-regulated membrane receptor G protein-coupled receptor 40 in pancreatic β-cells, thereby heightening intracellular calcium levels and insulin secretion. Moreover, insulin secretion was augmented in a concentration-dependent manner upon acute PFOS exposure, with a marked increase observed at concentrations exceeding 50 μM [77]. Intriguingly, Duan et al. [78] yielded contrary findings, indicating that prolonged PFOS exposure (48 h) inhibits glucose-stimulated insulin secretion. Furthermore, in specific cohorts such as pregnant and lactating mice, research has showcased the biological effects of elevated fasting glucose and insulin levels in both F1 juvenile and adult mice due to PFOS exposure. However, insulin resistance and glucose intolerance anomalies were conspicuously observed only in adult mice. Notably, a high-fat diet exacerbated these effects [79].
4.2. Perfluorooctanoic Acid (PFOA)
Perfluorooctanoic acid (PFOA) is frequently employed as an emulsifier in the production of polytetrafluoroethylene and fluorinated polymers. Ambient concentrations of PFOA in the air typically range from 0.07–0.9 ng/m3 [80], but can spike to 0.12–0.91 μg/m3 in the vicinity of fluoropolymer-manufacturing plants [81]. PFOA emissions during the manufacturing process are carried by the wind to adjacent agricultural areas, where they settle in the topsoil layer, eventually seeping downward to the water table [82]. Once introduced into organisms via environmental exposure, PFOA accumulates over time. Ehresmanet reported human serum PFOA concentrations spanning from the detection limit (5 or 10 ng/mL) to 7320 ng/mL [83]. Owing to PFOA’s crucial role in metabolic processes and its newfound potential to influence human glucose metabolism, an escalating number of researchers are probing its implications for diabetes and its hypothesized operational mechanisms.
Numerous epidemiological studies have identified a correlation between serum PFOA levels and the proinsulin-to-insulin ratio, after adjusting for confounding factors. Notably, diabetic subjects exhibit significantly elevated lnPFOA levels compared to their non-diabetic counterparts, and these levels can presage the onset of diabetes [84,85]. Analogous findings have emerged from animal studies concerning PFOA and diabetes. For instance, when Zheng and colleagues administered a dose of 1.25 mg/kg/d of PFOA to mice via gavage for 28 days, the mice in the exposed group, although unchanged in weight, manifested conspicuously elevated fasting blood glucose levels, coupled with decreased hepatic glycogen and glucose content [12]. Similarly, Yan et al. observed heightened insulin sensitivity and glucose tolerance in mice exposed to 5 mg/kg/d PFOA. This was attributed to suppressed hepatic gluconeogenesis, leading to diminished liver glycogen synthesis [86]. While mounting research is spotlighting the influence of PFOA on blood glucose levels, the underpinning mechanisms remain only partially elucidated. In an investigation by He [87] on the potential impact of PFOA on the functionality of pancreatic β-cells in mice, it was discerned that at a dose of 500µM, PFOA stimulates β-cell apoptosis. Moreover, even lower doses of PFOA resulted in diminished insulin secretion upon glucose stimulation and a pronounced upregulation of endoplasmic-reticulum-stress-related gene expression.
The review finds that exposure to perfluorinated compounds leads to increased fasting blood glucose levels and disrupted glucose metabolism in mice, along with alterations in the morphology, size, and length of the islets, thereby impacting insulin secretion. However, the underlying mechanisms of these effects have yet to be fully elucidated.

Table 2.
Summary of studies related to the effects of PFAS on glucose metabolism.
Table 2.
Summary of studies related to the effects of PFAS on glucose metabolism.
| Class | Study Population | Research Models | Chemical | Toxicity | Reference |
|---|---|---|---|---|---|
| Population Studies | General population (474 adolescents and 969 Adults) | Cross-sectional study | PFNA, PFOS, PFOA, etc. |
| [72] |
| General population (1045 adults) | Cross-sectional study | PFOS and PFOA |
| [73] | |
| Pregnant women (171 GDM and 169 controls) | Cross-sectional study | PFOS, PFOA, PFNA, etc. |
| [74] | |
| General population (1016 men and women aged 70 years) | Prospective cohort study | PFOA, PFNA |
| [84] | |
| General population (100 participants) | Prospective cohort study | PFOA, PFOS |
| [85] | |
| Cell Experiments | GPR40-KO C57BL/6, C57BL/6 mice and mouse islet β-cells | Experimental research | PFOS |
| [76] |
| Beta-TC-6 pancreatic cells | Experimental research | PFOS |
| [77] | |
| Mouse pancreatic β-cells | Experimental research | PFOS |
| [78] | |
| Mouse pancreatic β-cell line (MIN6 cells) | Experimental research | PFOA |
| [87] | |
| Animal Experiments | Zebrafish (Danio rerio) embryos | Experimental research | PFOS |
| [75] |
| Pregnant mice and offspring | Experimental research | PFOS |
| [79] | |
| Adult male Balb/c mice | Experimental research | PFOA |
| [12] | |
| Male Balb/c | Experimental research | PFOA |
| [86] |
5. Microplastics
Microplastics refer to plastic particles with a diameter of ≤5 mm. Traditional methods for detecting microplastics in the environment include visual identification or microscopic observation, Fourier transform infrared spectroscopy, thermal pyrolysis, and Raman spectroscopy. However, the diverse sources and compositions of environmental microplastics, along with the presence of numerous impurities, render these conventional detection methods inadequate for comprehensive microplastic detection [88]. With increasing interest in the toxicity of microplastics and advancements in detection technology, more sensitive and high-performance detection techniques are being developed, such as a variety of remote sensing techniques including polarized light optical microscopy (PLM), atomic force microscopy, and hybrid combinations of these techniques [89]. Humans and other organisms are exposed to environmental microplastics through ingestion, inhalation, and dermal contact [90]. Furthermore, oral exposure has been reported as the primary route of microplastic exposure. Kumar et al. [91], in their review, mention seafood, beer, table salt, bottled mineral water, and milk as the main pathways for microplastics to enter the human body. Once internalized, microplastics accumulate within tissues and organs, leading to histopathological alterations and cytotoxic responses [6,92]. For instance, a study by Lu et al. on zebrafish found that exposure to microplastics first leads to accumulation in liver tissues, causing inflammation and lipid accumulation, and disrupting lipid and energy metabolism, leading to metabolic changes [93]. Cortés et al.’s cellular experiments also found that microplastics induce the production of a significant amount of ROS in Caco-2 cells, thereby increasing cytotoxicity [94]. The same conclusion was reached in cell experiments with T98G and HeLa [95]. Additionally, immune responses and changes in the structure and composition of the gut microbiota induced by microplastic exposure have also been increasingly identified [91]. It is well-established that gut microbiota dysbiosis, inflammatory reactions, oxidative stress, and changes in innate immune responses—all consequences of microplastic exposure—are major pathophysiological factors for insulin resistance. Consequently, scientists posit a strong link between microplastic exposure and insulin resistance, necessitating comprehensive research and elucidation. However, current investigations in this area remain limited (Table 3).
Studies have identified correlations between changes in blood glucose levels and insulin resistance caused by microplastic exposure, specifically noting connections to gut microbiota disruption, inflammation, and oxidative stress. Huang et al. [13] exposed mice on a high-fat diet to polystyrene microplastics of sizes 5, 50, 100, and 200 μm. The mice displayed insulin resistance accompanied by elevated levels of plasma lipopolysaccharides and pro-inflammatory cytokines (tumor necrosis factor and interleukin-1β). A reduction in gut microbiota richness and diversity was also observed, particularly with an increased relative abundance of Gram-negative bacteria. Based on these findings, scientists hypothesize that insulin resistance triggered by microplastics might be due to tissue accumulation and microbiota-induced inflammatory responses, thereby inhibiting the insulin signaling pathway. Takuro Okamura also demonstrated that mice exposed to microplastics showed elevated blood glucose levels and deposition of microplastics in the gut mucosa, resulting in an increase in intrinsic inflammatory cells and a reduction in anti-inflammatory cells [96]. Additionally, the insulin resistance and elevated blood glucose levels induced by microplastics might be associated with high levels of reactive oxygen species (ROS) in mice exposed to 5 mg/kg and 15 mg/kg, which potentially disrupt the PI3K/Art pathway related to glucose metabolism [97]. In another study, beyond increasing oxidative stress, glucose tolerance, and insulin resistance, 30 mg/kg/d microplastic exposure also led to decreased phosphorylation levels of AKT and GSK3β [98]. AKT agonists can effectively alleviate oxidative stress, elevated blood glucose levels, and insulin resistance, suggesting that part of the diabetes mechanism induced by microplastics might be related to AKT/GSK3β phosphorylation. Furthermore, research has identified that reduced cortisol levels in mice exposed to 55 μg/d microplastics might interfere with insulin secretion, thereby inducing insulin resistance [99]. A review of microplastics reveals that current research on the relationship between microplastics and glucose metabolism is relatively scarce. Studies have found that exposure to microplastics can affect an organism’s glucose metabolism and the development of diabetes through inflammatory responses, oxidative stress, and disruption of the composition and structure of the gut microbiota.

Table 3.
Summary of studies related to the effects of MPs on glucose metabolism.
Table 3.
Summary of studies related to the effects of MPs on glucose metabolism.
| Class | Study Population | Microplastics | Toxicity | Reference |
|---|---|---|---|---|
| Animal Experiments | Five-week-old high -fat-diet male mice (Mus musculus, ICR) | Polystyrene (5, 50, 100, and 200 μm) |
| [13] |
| High-fat-diet male mice (C57BL/6) | Polystyrene (0.45–0.53 μm) |
| [96] | |
| Mice | Polystyrene nanoplastics |
| [97] | |
| High-fat-diet male mice (C57BL/6) | Polystyrene (80 nm) |
| [98] | |
| ICR mice | Polystyrene (1 μm) |
| [99] |
6. Antibiotics
Besides the aforementioned emerging pollutants, antibiotics have also been identified as a significant new class of pollutants, extensively present in the environment and water bodies. By the late 1990s, antibiotics had become widely used in medicine and established as pillars of modern medical practice. The consumption of antibiotics has seen a steady increase, with global consumption growing by 39% between 2000 to 2015. Particularly, antibiotic consumption in low-income countries surged by 77% during the same period [100]. In 2011, the global human utilization of antibiotics was estimated at 70 billion, equivalent to an annual consumption rate of 10 per individual [101]. Subsequently, these antibiotics, or their metabolites, enter the environment through human and animal urine and feces, ultimately persisting in soil and aquatic environments [102]. Current detection of antibiotics mainly relies on instrumental analysis, which is highly sensitive. However, due to high costs and laborious pre-treatment, traditional instrumental analysis methods are no longer sufficient for the growing number of samples. Therefore, the development of rapid, high-throughput, and low-cost detection methods is essential. Current methods for detecting antibiotic residues include the microbial method, electrochemical method, high-performance liquid chromatography, liquid mass spectrometry, fluorescence method, Raman spectroscopy, etc. [103]. Scientists have detected various antibiotics, such as amoxicillin, clindamycin, and ciprofloxacin, in the inlet and outlet water of wastewater treatment plants. Furthermore, the highest concentrations of triclocarban and triclosan detected in Indian aquatic environments have reached 5860 ng/L [104], indicating the non-negligible potential hazards of residual antibiotics in the environment. It is widely recognized that antibiotic intake can impact the structure, composition, and function of gut microbiota. Moreover, alterations in the gut microbiota have been closely linked with the onset and progression of diabetes [105]. Consequently, there is mounting concern within the scientific community regarding the relationship between antibiotic consumption and diabetes.
A significant body of research has been conducted to investigate the association between antibiotics and diabetes. For instance, several studies have shown that mice on a high-fat diet or those modeled for diabetes, when treated with antibiotics, exhibited reduced levels of endotoxins in plasma and inflammatory factors in adipose tissue. Healthy mice, on the other hand, displayed beneficial effects on glucose metabolism, including reduced fasting blood glucose and decreased area under the glucose tolerance curve [106,107]. Interestingly, another prospective cohort study revealed that patients treated with antibiotics for durations ranging from twenty-five days to two months, or more than two months, saw their risks for type II diabetes increase by 23% and 20%, respectively [108]. In light of this intriguing observation, Fu et al. [109] studied the impact of antibiotic treatment on blood glucose changes in db/db mice. They found that the effects of antibiotics on blood glucose exhibit both immediate and delayed responses: compared to the control group, mice treated with antibiotics for 12 days showed significant declines in body weight and blood glucose levels. However, 24 days post-treatment, these mice experienced weight gains that even surpassed those of the control group, along with elevated levels of plasma and liver total cholesterol and an increase in liver weight. Research on the relationship between antibiotics and diabetes is already quite abundant, with a relatively comprehensive understanding of the relationship between antibiotics and the development of diabetes. It should not be neglected that the timing of antibiotic exposure is also very important and should not be overlooked, such as in pregnant mothers, infants, and adulthood. A comprehensive review of the relationship between antibiotics and diabetes has been presented in an article by Fenneman and will not be further elaborated upon here [110].
7. Role of Gut Microbiota
In recent years, the gut microbiota has garnered unprecedented attention. An increasing corpus of evidence underscores its fundamental role in the digestion of polysaccharides, the biosynthesis of vitamins, and other essential nutrients. This microbial community is inextricably linked with human health [111,112,113]. Undoubtedly, acting as a novel organ, the gut microbiota functions optimally. However, disruptions in its composition and structure due to external substances can have implications for disease onset and progression [114,115,116]. Particularly noteworthy are recent studies highlighting how exposure to emergent environmental contaminants can destabilize the gut microbiota, leading to adverse health effects, including disorders in glucose metabolism [99,117,118,119] (Table 4).
The current scientific discourse is replete with research focusing on the implications of perfluorinated compound exposure on diabetes via its perturbative effects on gut microbiota. Lai et al. [120] embarked on a study exploring the impacts of dietary PFOS exposure on the gut microbiota of adult mice and scrutinized the consequent changes in induced metabolic functions. Their findings delineated a marked increase in the abundance of Turicibacterales and Allobaculum in the exposed group of mice, juxtaposed with a significant decline in B. acidifaciens. Moreover, the researchers discerned that mice exposed to 3 μg/g/day PFOS exhibited a precocious decline in blood glucose levels after oral glucose ingestion. The area under the curve manifested a conspicuous reduction, and after intraperitoneal insulin injection, these mice’s blood glucose levels were markedly lower than those in the control group. Delving deeper into another emergent pollutant, microplastics, it has been discerned that, post-exposure, it can influence the onset and trajectory of diabetes via various mechanisms, with the resultant disruption in gut microbiota being non-trivial. Using mice as model organisms, investigations into the aftermath of microplastics exposure on the gut microbiota were conducted. The outcomes indicated that, post-exposure, there was a perturbation in the gut–liver axis of the mice, a pronounced reduction in gut microbiota diversity, diminished richness of Bacteroidetes and Verrucomicrobia, and an increased abundance of Firmicute, Deferribacteres, and Actinobacteria. Concurrently, scientists observed that microplastic-exposed mice presented elevated fasting blood glucose and insulin levels [99]. These findings echo the results from Huang et al., who, in addition to observing reduced microbial richness, also noted an increased relative abundance of Gram-negative bacteria within the mice [13]. It is common knowledge that antibiotics have had a longstanding history of use, targeting pathogenic strains within the microbiota. Yet, their administration might also inadvertently impact other microbial communities, resulting in a decrease in the host’s short-chain fatty acid content. This, in turn, might disrupt metabolic processes and energy assimilation, potentially influencing the onset and progression of diabetes [117,121]. Currently, articles published on the topic of new pollutants and their impact on glucose metabolism through the influence on the structure and composition of the gut microbiota are relatively few, with most studies focusing on perfluorinated compounds and microplastics. Additionally, research in this area remains significantly under-developed. The changes in the composition and structure of the gut microbiota after exposure to new pollutants, alterations in their metabolic pathways and metabolites, and the specific mechanisms of their impact on blood sugar still require more in-depth investigation.

Table 4.
Summary of studies related to the effects of emerging contaminants on glucose-metabolism-associated gut microbiota.
Table 4.
Summary of studies related to the effects of emerging contaminants on glucose-metabolism-associated gut microbiota.
| Class | Species | Chemical | Changes in Intestinal Microbiota | Reference | |
|---|---|---|---|---|---|
| Animal Experiments | Female CD-1 mice | PFOS |
|
| [120] |
| ICR mice | Polystyrene microplastics (1 μm) |
|
| [99] | |
| Male ICR mice | Polystyrene microplastics (5, 50, 100, 200 μm) |
|
| [13] | |
| NOD/Shiltj mice | Antibiotics |
|
| [117] |
8. Conclusions
Emerging pollutants, characteristically under-monitored and under-regulated in the environmental sphere, harbor potential, both known and speculative, adverse implications for ecological systems and human health. However, the corpus of research delineating the toxicological nexus between emerging contaminants and glucose metabolic processes remains markedly under-developed. This review aims to intricately weave together four distinct classes of emerging pollutants with glucose metabolism. It methodically dissects and analyzes the toxicological profiles and underlying mechanisms of persistent organic pollutants, perfluorinated compounds, microplastics, and antibiotics, drawing upon a synthesis of empirical evidence from animal model studies and epidemiological research (Figure 2). Overall, EDCs, PFAS, microplastics, and antibiotics cause disturbances in glucose metabolism and accelerate diabetes mellitus. Most of the descriptions of the mechanisms in the currently published articles focus on the effects on glucose metabolism through inflammatory responses, oxidative stress, and disturbances in the gut microbiota. The elucidation provided herein seeks not only to augment the current understanding of the deleterious effects of these emerging pollutants on glucose metabolism but also to catalyze a paradigm shift in the toxicological examination of emerging environmental contaminants.
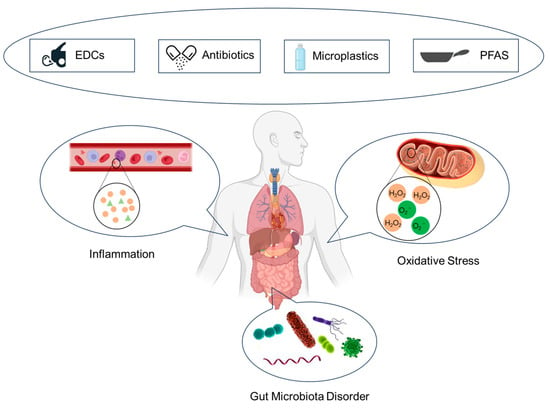
Figure 2.
Mechanisms of glucose metabolism disturbance induced by emerging contaminants.
9. Future Research Directions
With the continual evolution of technology and the deepening understanding of contaminants by scientists, an increasing number of novel environmental contaminants are being detected. The persistent accumulation of these contaminants in the environment, coupled with their multifarious exposure pathways, endangers ecosystems and the organisms residing therein, thus progressively capturing the attention of the scientific community. The repercussions of these environmental contaminants, particularly on populations with diabetes or those on high-fat diets, are of heightened concern. Nonetheless, our comprehension in this domain remains somewhat limited:
- i.
- The majority of current epidemiological studies focus on the relationship between the concentration of new pollutants in the serum or urine of the general population and fasting blood sugar, insulin, and glycated hemoglobin concentrations, with some studies involving changes in metabolic pathways related to glucose metabolism and their metabolites. However, studies on the impact of occupational exposure on diabetes, the specific exposure situations of new pollutants in the diet, the correlation with diabetes, and the impact of different geographical locations are relatively scarce. Diet is likely to be a very important exposure, yet rarely assessed in human studies, or when assessed, by questionnaires, often inaccurate. Quantifying the contribution of the human diet by multitargeted metabolomics of food and microbiota-derived metabolites may provide some clues. Therefore, more in-depth and targeted research is still needed to explore the impact of different factors on the development of diabetes.
- ii.
- The current research primarily focuses on the effects of novel environmental contaminants and their exposure on glucose metabolism in human populations, with fewer studies being directed towards animal models. Thus, there remains a pressing need for comprehensive studies to elucidate the specific mechanisms underlying the impact of these contaminants on diabetic or high-fat-diet populations, as well as the potential health outcomes from long-term low exposure.
- iii.
- Additionally, factors influencing the toxic effects of these novel contaminants, such as dose–response relationships, exposure frequency, gender disparities, and attributes like the type and size of the contaminants, have yet to be thoroughly investigated. Hence, there is an urgent need for more in-depth research into the toxicity of these new contaminants, factors modulating their toxicity levels, and their potential hazards. Such insights would furnish policymakers with a robust scientific foundation, aiding in the resolution of environmental challenges and the safeguarding of human health.
- iv.
- It is widely acknowledged that diabetes is influenced not only by genetic, environmental, and lifestyle factors but also by the structure and composition of the gut microbiota. However, current research on the interrelation between novel contaminants, gut microbiota, and diabetes is relatively scant. Consequently, determining whether exposure to these new contaminants might influence glucose metabolism by altering the gut microbiota’s structure and composition calls for relentless effort and exploration by researchers.
Author Contributions
Conceptualization: H.N., P.T., L.W. and S.S.; Literature search: H.N., Y.X., M.X. (Manjin Xu) and X.L. (Xueqing Li); Writing—original draft: H.N., M.X. (Mingluan Xing), Z.C., X.W. and X.L. (Xiaoming Lou); Writing—review and editing: All authors; Funding acquisition L.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Zhejiang Provincial Project for Medical Research and Health Sciences: 2024KY898 & 2024KY910.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhao, Y.; Li, Y.; Zhuang, Z.; Song, Z.; Wang, W.; Huang, N.; Dong, X.; Xiao, W.; Jia, J.; Liu, Z.; et al. Associations of polysocial risk score, lifestyle and genetic factors with incident type 2 diabetes: A prospective cohort study. Diabetologia 2022, 65, 2056–2065. [Google Scholar] [CrossRef] [PubMed]
- Papazafiropoulou, A.K.; Papanas, N.; Melidonis, A.; Maltezos, E. Family History of Type 2 Diabetes: Does Having a Diabetic Parent Increase the Risk? Curr. Diabetes Rev. 2017, 13, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Qi, H.; Liu, J.; Zhang, G.; Liu, J.; Liu, N.; Zhu, M.; Zhao, X.; Song, C.; Zhou, Z.; et al. Deubiquitinase OTUD3 regulates metabolism homeostasis in response to nutritional stresses. Cell Metab. 2022, 34, 1023–1041. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.; Martin, S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med. 2017, 15, 131. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.W.; Novak, R.F.; Anderson, H.A.; Birnbaum, L.S.; Blystone, C.; Devito, M.; Jacobs, D.; Köhrle, J.; Lee, D.-H.; Rylander, L.; et al. Evaluation of the association between persistent organic pollutants (POPs) and diabetes in epidemiological studies: A national toxicology program workshop review. Environ. Health Perspect. 2013, 121, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef]
- Han, D.; Currell, M.J. Persistent organic pollutants in China’s surface water systems. Sci. Total Environ. 2017, 580, 602–625. [Google Scholar] [CrossRef]
- Ouda, M.; Kadadou, D.; Swaidan, B.; Al-Othman, A.; Al-Asheh, S.; Banat, F.; Hasan, S.W. Emerging contaminants in the water bodies of the Middle East and North Africa (MENA): A critical review. Sci. Total Environ. 2021, 754, 142177. [Google Scholar] [CrossRef]
- Niu, H.; Liu, S.; Jiang, Y.; Hu, Y.; Li, Y.; He, L.; Xing, M.; Li, X.; Wu, L.; Chen, Z.; et al. Are Microplastics Toxic? A Review from Eco-Toxicity to Effects on the Gut Microbiota. Metabolites 2023, 13, 739. [Google Scholar] [CrossRef]
- Li, X.; Yin Yeung, L.W.; Xu, M.; Taniyasu, S.; Lam, P.K.S.; Yamashita, N.; Dai, J. Perfluorooctane sulfonate (PFOS) and other fluorochemicals in fish blood collected near the outfall of wastewater treatment plant (WWTP) in Beijing. Environ. Pollut. 2008, 156, 1298–1303. [Google Scholar] [CrossRef]
- Yeung, L.W.Y.; So, M.K.; Jiang, G.; Taniyasu, S.; Yamashita, N.; Song, M.; Wu, Y.; Li, J.; Giesy, J.P.; Guruge, K.S.; et al. Perfluorooctanesulfonate and related fluorochemicals in human blood samples from China. Environ. Sci. Technol. 2006, 40, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Sheng, N.; Zhang, H.; Yan, S.; Zhang, J.; Wang, J. Perfluorooctanoic acid exposure disturbs glucose metabolism in mouse liver. Toxicol. Appl. Pharmacol. 2017, 335, 41–48. [Google Scholar] [CrossRef]
- Huang, D.; Zhang, Y.; Long, J.; Yang, X.; Bao, L.; Yang, Z.; Wu, B.; Si, R.; Zhao, W.; Peng, C.; et al. Polystyrene microplastic exposure induces insulin resistance in mice via dysbacteriosis and pro-inflammation. Sci. Total Environ. 2022, 838, 155937. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Ding, K.; Huang, W.; Xu, F.; Lei, M.; Yue, R. Potential effects of bisphenol A on diabetes mellitus and its chronic complications: A narrative review. Heliyon 2023, 9, e16340. [Google Scholar] [CrossRef] [PubMed]
- Mariana, M.; Cairrao, E. The Relationship between Phthalates and Diabetes: A Review. Metabolites 2023, 13, 746. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.-J.; Wei, Y.-L.; Yao, Y.; Ruan, Q.-Q.; Zeng, E.Y. Global trends of research on emerging contaminants in the environment and humans: A literature assimilation. Environ. Sci. Pollut. Res. Int. 2015, 22, 1635–1643. [Google Scholar] [CrossRef] [PubMed]
- Naidu, R.; Arias Espana, V.A.; Liu, Y.; Jit, J. Emerging contaminants in the environment: Risk-based analysis for better management. Chemosphere 2016, 154, 350–357. [Google Scholar] [CrossRef]
- Khan, S.; Naushad, M.; Govarthanan, M.; Iqbal, J.; Alfadul, S.M. Emerging contaminants of high concern for the environment: Current trends and future research. Environ. Res. 2022, 207, 112609. [Google Scholar] [CrossRef]
- Mohammadi, A.; Dobaradaran, S.; Schmidt, T.C.; Malakootian, M.; Spitz, J. Emerging contaminants migration from pipes used in drinking water distribution systems: A review of the scientific literature. Environ. Sci. Pollut. Res. Int. 2022, 29, 75134–75160. [Google Scholar] [CrossRef]
- Puri, M.; Gandhi, K.; Kumar, M.S. Emerging environmental contaminants: A global perspective on policies and regulations. J. Environ. Manag. 2023, 332, 117344. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, M.; Zhuang, D. Wastewater treatment and emerging contaminants: Bibliometric analysis. Chemosphere 2022, 297, 133932. [Google Scholar] [CrossRef] [PubMed]
- Bodus, B.; O’Malley, K.; Dieter, G.; Gunawardana, C.; McDonald, W. Review of emerging contaminants in green stormwater infrastructure: Antibiotic resistance genes, microplastics, tire wear particles, PFAS, and temperature. Sci. Total Environ. 2023, 906, 167195. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Shukla, P. Microalgal-based bioremediation of emerging contaminants: Mechanisms and challenges. Environ. Pollut. 2023, 337, 122591. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Mohapatra, S.; Zhang, J.; Tran, N.H.; You, L.; He, Y.; Gin, K.Y.-H. Source, fate, transport and modelling of selected emerging contaminants in the aquatic environment: Current status and future perspectives. Water Res. 2022, 217, 118418. [Google Scholar] [CrossRef] [PubMed]
- Dodoo, D.K.; Essumang, D.K.; Jonathan, J.W.A. Accumulation profile and seasonal variations of polychlorinated biphenyls (PCBs) in bivalves Crassostrea tulipa (oysters) and Anadara senilis (mussels) at three different aquatic habitats in two seasons in Ghana. Ecotoxicol. Environ. Saf. 2013, 88, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Bigler, J.; Greene, A. Guidance for Assessing Chemical Contaminant Data for Use in Fish Advisories; US EPA Office of Water, Office of Science and Technology: Washington, DC, USA, 1993; Volume 2.
- Wang, J.; Shi, Y.; Pan, Y.; Cai, Y. Perfluorooctane sulfonate (PFOS) and other fluorochemicals in viscera and muscle of farmed pigs and chickens in Beijing, China. Chin. Sci. Bull. 2010, 55, 3550–3555. [Google Scholar] [CrossRef]
- Yang, D.; Shi, H.; Li, L.; Li, J.; Jabeen, K.; Kolandhasamy, P. Microplastic Pollution in Table Salts from China. Environ. Sci. Technol. 2015, 49, 13622–13627. [Google Scholar] [CrossRef]
- Mason, S.A.; Welch, V.G.; Neratko, J. Synthetic Polymer Contamination in Bottled Water. Front. Chem. 2018, 6, 407. [Google Scholar] [CrossRef]
- Chen, X.; Wang, S.; Mao, X.; Xiang, X.; Ye, S.; Chen, J.; Zhu, A.; Meng, Y.; Yang, X.; Peng, S.; et al. Adverse health effects of emerging contaminants on inflammatory bowel disease. Front. Public. Health 2023, 11, 1140786. [Google Scholar] [CrossRef]
- Snow, D.D.; Cassada, D.A.; Biswas, S.; Malakar, A.; D’Alessio, M.; Marshall, A.H.L.; Sallach, J.B. Detection, occurrence, and fate of emerging contaminants in agricultural environments (2020). Water Environ. Res. 2020, 92, 1741–1750. [Google Scholar] [CrossRef]
- Alonso-Magdalena, P.; Quesada, I.; Nadal, A. Endocrine disruptors in the etiology of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2011, 7, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, C.D.; Bayen, S.; Desrosiers, M.; Muñoz, G.; Sauvé, S.; Yargeau, V. Methods for the analysis of endocrine disrupting chemicals in selected environmental matrixes. Environ. Res. 2022, 206, 112616. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Sha, J.; Li, Z.; Wang, W.; Zhang, H. High affinity truncated aptamers for ultra-sensitive colorimetric detection of bisphenol A with label-free aptasensor. Food Chem. 2020, 317, 126459. [Google Scholar] [CrossRef] [PubMed]
- Jebril, S.; Cubillana-Aguilera, L.; Palacios-Santander, J.M.; Dridi, C. A novel electrochemical sensor modified with green gold sononanoparticles and carbon black nanocomposite for bisphenol A detection. Mater. Sci. Eng. B 2021, 264, 114951. [Google Scholar] [CrossRef]
- Lind, P.M.; Lind, L. Endocrine-disrupting chemicals and risk of diabetes: An evidence-based review. Diabetologia 2018, 61, 1495–1502. [Google Scholar] [CrossRef] [PubMed]
- Porta, M.; Gasull, M.; Puigdomènech, E.; Garí, M.; Bosch de Basea, M.; Guillén, M.; López, T.; Bigas, E.; Pumarega, J.; Llebaria, X.; et al. Distribution of blood concentrations of persistent organic pollutants in a representative sample of the population of Catalonia. Environ. Int. 2010, 36, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Kassotis, C.D.; Vandenberg, L.N.; Demeneix, B.A.; Porta, M.; Slama, R.; Trasande, L. Endocrine-disrupting chemicals: Economic, regulatory, and policy implications. Lancet Diabetes Endocrinol. 2020, 8, 719–730. [Google Scholar] [CrossRef]
- Fisher, B.E. Most unwanted. Environ. Health Perspect. 1999, 107, A18–A23. [Google Scholar] [CrossRef]
- Lee, Y.M.; Kim, K.S.; Jacobs, D.R.; Lee, D.H. Persistent organic pollutants in adipose tissue should be considered in obesity research. Obes. Rev. 2017, 18, 129–139. [Google Scholar] [CrossRef]
- Tian, Y.; Rimal, B.; Gui, W.; Koo, I.; Smith, P.B.; Yokoyama, S.; Patterson, A.D. Early Life Polychlorinated Biphenyl 126 Exposure Disrupts Gut Microbiota and Metabolic Homeostasis in Mice Fed with High-Fat Diet in Adulthood. Metabolites 2022, 12, 894. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiao, Y.; Li, Z.; Tao, Y.; Yang, Y. Hazards of phthalates (PAEs) exposure: A review of aquatic animal toxicology studies. Sci. Total Environ. 2021, 771, 145418. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Li, Z.; Wang, H.; Liang, H. An overview of phthalate acid ester pollution in China over the last decade: Environmental occurrence and human exposure. Sci. Total Environ. 2018, 645, 1400–1409. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Sun, H.; Han, L.; Chen, L. Association between phthalate exposure and glycosylated hemoglobin, fasting glucose, and type 2 diabetes mellitus: A case-control study in China. Sci. Total Environ. 2019, 670, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Sun, H.; Yao, Y.; Han, L.; Chen, L. Perturbation of serum metabolome in relation to type 2 diabetes mellitus and urinary levels of phthalate metabolites and bisphenols. Environ. Int. 2021, 155, 106609. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, R.M.; Ferguson, K.K.; Sheppard, L.; James-Todd, T.; Butts, S.; Chandrasekaran, S.; Swan, S.H.; Barrett, E.S.; Nguyen, R.; Bush, N.; et al. Maternal urinary phthalate metabolites in relation to gestational diabetes and glucose intolerance during pregnancy. Environ. Int. 2019, 123, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; He, C.; Liu, X.; An, S.; Wang, X.; Tao, L.; Zhang, H.; Tian, Y.; Wu, N.; Xu, P.; et al. Effects of exposure to phthalate during early pregnancy on gestational diabetes mellitus: A nested case-control study with propensity score matching. Environ. Sci. Pollut. Res. Int. 2023, 30, 33555–33566. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Zheng, J.; Qin, J.; Liu, S.; Liu, X.; Gu, Y.; Yang, S.; Du, J.; Li, S.; Chen, B.; et al. Dibutyl phthalate affects insulin synthesis and secretion by regulating the mitochondrial apoptotic pathway and oxidative stress in rat insulinoma cells. Ecotoxicol. Environ. Saf. 2023, 249, 114396. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L.; Li, J.; Meng, G.; Chi, M.; Li, T.; Zhao, Y.; Wu, Y. A nested case-control study of the association between exposure to polybrominated diphenyl ethers and the risk of gestational diabetes mellitus. Environ. Int. 2018, 119, 232–238. [Google Scholar] [CrossRef]
- Ongono, J.S.; Dow, C.; Gambaretti, J.; Severi, G.; Boutron-Ruault, M.-C.; Bonnet, F.; Fagherazzi, G.; Mancini, F.R. Dietary exposure to brominated flame retardants and risk of type 2 diabetes in the French E3N cohort. Environ. Int. 2019, 123, 54–60. [Google Scholar] [CrossRef]
- Liu, Z.-L.; Jiang, S.-R.; Fan, Y.; Wang, J.-S.; Wang, M.-L.; Li, M.-Y. 2,2’,4,4’,5,5’-Hexabromophenyl ether (BDE-153) causes abnormal insulin secretion and disorders of glucose and lipid metabolism in mice. J. Chin. Med. Assoc. 2023, 86, 388–398. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Fjære, E.; Lock, E.-J.; Naville, D.; Amlund, H.; Meugnier, E.; Le Magueresse Battistoni, B.; Frøyland, L.; Madsen, L.; Jessen, N.; et al. Chronic consumption of farmed salmon containing persistent organic pollutants causes insulin resistance and obesity in mice. PLoS ONE 2011, 6, e25170. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.L.; Shaw, A.C.; Gagne, A.X.; Chan, H.M. Chronic exposure to PCBs (Aroclor 1254) exacerbates obesity-induced insulin resistance and hyperinsulinemia in mice. J. Toxicol. Environ. Health A 2013, 76, 701–715. [Google Scholar] [CrossRef] [PubMed]
- Baker, N.A.; Karounos, M.; English, V.; Fang, J.; Wei, Y.; Stromberg, A.; Sunkara, M.; Morris, A.J.; Swanson, H.I.; Cassis, L.A. Coplanar polychlorinated biphenyls impair glucose homeostasis in lean C57BL/6 mice and mitigate beneficial effects of weight loss on glucose homeostasis in obese mice. Environ. Health Perspect. 2013, 121, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Baker, N.A.; Shoemaker, R.; English, V.; Larian, N.; Sunkara, M.; Morris, A.J.; Walker, M.; Yiannikouris, F.; Cassis, L.A. Effects of Adipocyte Aryl Hydrocarbon Receptor Deficiency on PCB-Induced Disruption of Glucose Homeostasis in Lean and Obese Mice. Environ. Health Perspect. 2015, 123, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Matteo, G.; Hoyeck, M.P.; Blair, H.L.; Zebarth, J.; Rick, K.R.C.; Williams, A.; Gagné, R.; Buick, J.K.; Yauk, C.L.; Bruin, J.E. Prolonged Low-Dose Dioxin Exposure Impairs Metabolic Adaptability to High-Fat Diet Feeding in Female but Not Male Mice. Endocrinology 2021, 162, bqab050. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Kan-o, S.; Mutoh, J.; Takeda, S.; Ishii, Y.; Hashiguchi, I.; Akamine, A.; Yamada, H. 2,3,7,8-Tetrachlorodibenzo-p-dioxin-induced change in intestinal function and pathology: Evidence for the involvement of arylhydrocarbon receptor-mediated alteration of glucose transportation. Toxicol. Appl. Pharmacol. 2005, 205, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Kurita, H.; Yoshioka, W.; Nishimura, N.; Kubota, N.; Kadowaki, T.; Tohyama, C. Aryl hydrocarbon receptor-mediated effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on glucose-stimulated insulin secretion in mice. J. Appl. Toxicol. 2009, 29, 689–694. [Google Scholar] [CrossRef]
- Hoyeck, M.P.; Merhi, R.C.; Blair, H.L.; Spencer, C.D.; Payant, M.A.; Martin Alfonso, D.I.; Zhang, M.; Matteo, G.; Chee, M.J.; Bruin, J.E. Female mice exposed to low doses of dioxin during pregnancy and lactation have increased susceptibility to diet-induced obesity and diabetes. Mol. Metab. 2020, 42, 101104. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Hauser, R.; Marcus, M.; Olea, N.; Welshons, W.V. Human exposure to bisphenol A (BPA). Reprod. Toxicol. 2007, 24, 139–177. [Google Scholar] [CrossRef]
- Akash, M.S.H.; Sabir, S.; Rehman, K. Bisphenol A-induced metabolic disorders: From exposure to mechanism of action. Environ. Toxicol. Pharmacol. 2020, 77, 103373. [Google Scholar] [CrossRef]
- Marmugi, A.; Lasserre, F.; Beuzelin, D.; Ducheix, S.; Huc, L.; Polizzi, A.; Chetivaux, M.; Pineau, T.; Martin, P.; Guillou, H.; et al. Adverse effects of long-term exposure to bisphenol A during adulthood leading to hyperglycaemia and hypercholesterolemia in mice. Toxicology 2014, 325, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Deng, P.; Lin, M.; Yang, L.; Li, L.; Guo, L.; Zhang, L.; He, M.; Lu, Y.; Pi, H.; et al. Long-term bisphenol A exposure exacerbates diet-induced prediabetes via TLR4-dependent hypothalamic inflammation. J. Hazard. Mater. 2021, 402, 123926. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.K.; Jeong, I.-K.; Jung Oh, T.; Ahn, H.Y.; Kim, H.H.; Park, Y.J.; Jang, H.C.; Park, K.S. Long-term oral exposure to bisphenol A induces glucose intolerance and insulin resistance. J. Endocrinol. 2015, 226, 35–42. [Google Scholar] [CrossRef]
- Lau, C.; Anitole, K.; Hodes, C.; Lai, D.; Pfahles-Hutchens, A.; Seed, J. Perfluoroalkyl acids: A review of monitoring and toxicological findings. Toxicol. Sci. 2007, 99, 366–394. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; DeWitt, J.C.; Higgins, C.P.; Cousins, I.T. A Never-Ending Story of Per- and Polyfluoroalkyl Substances (PFASs)? Environ. Sci. Technol. 2017, 51, 2508–2518. [Google Scholar] [CrossRef] [PubMed]
- Domingo, J.L.; Nadal, M. Human exposure to per- and polyfluoroalkyl substances (PFAS) through drinking water: A review of the recent scientific literature. Environ. Res. 2019, 177, 108648. [Google Scholar] [CrossRef]
- Lee, J.-W.; Lee, H.-K.; Lim, J.-E.; Moon, H.-B. Legacy and emerging per- and polyfluoroalkyl substances (PFASs) in the coastal environment of Korea: Occurrence, spatial distribution, and bioaccumulation potential. Chemosphere 2020, 251, 126633. [Google Scholar] [CrossRef]
- Wan, H.T.; Zhao, Y.G.; Wei, X.; Hui, K.Y.; Giesy, J.P.; Wong, C.K.C. PFOS-induced hepatic steatosis, the mechanistic actions on β-oxidation and lipid transport. Biochim. Biophys. Acta 2012, 1820, 1092–1101. [Google Scholar] [CrossRef]
- Sanan, T.; Magnuson, M. Analysis of per- and polyfluorinated alkyl substances in sub-sampled water matrices with online solid phase extraction/isotope dilution tandem mass spectrometry. J. Chromatogr. A 2020, 1626, 461324. [Google Scholar] [CrossRef]
- Olsen, G.W.; Burris, J.M.; Ehresman, D.J.; Froehlich, J.W.; Seacat, A.M.; Butenhoff, J.L.; Zobel, L.R. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ. Health Perspect. 2007, 115, 1298–1305. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Chen, P.-C.; Lin, Y.-C.; Lin, L.-Y. Association among serum perfluoroalkyl chemicals, glucose homeostasis, and metabolic syndrome in adolescents and adults. Diabetes Care 2009, 32, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Zeeshan, M.; Zhang, Y.-T.; Yu, S.; Huang, W.-Z.; Zhou, Y.; Vinothkumar, R.; Chu, C.; Li, Q.-Q.; Wu, Q.-Z.; Ye, W.-L.; et al. Exposure to isomers of per- and polyfluoroalkyl substances increases the risk of diabetes and impairs glucose-homeostasis in Chinese adults: Isomers of C8 health project. Chemosphere 2021, 278, 130486. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhang, L.; Zhou, Q.; Ding, J.; Yin, S.; Shang, X.; Tian, Y. Exposure to per- and polyfluoroalkyl substances as a risk factor for gestational diabetes mellitus through interference with glucose homeostasis. Sci. Total Environ. 2022, 838, 156561. [Google Scholar] [CrossRef] [PubMed]
- Sant, K.E.; Jacobs, H.M.; Borofski, K.A.; Moss, J.B.; Timme-Laragy, A.R. Embryonic exposures to perfluorooctanesulfonic acid (PFOS) disrupt pancreatic organogenesis in the zebrafish, Danio rerio. Environ. Pollut. 2017, 220, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.-P.; Cao, L.-Y.; Li, C.-H.; Guo, L.-H.; Colbourne, J.; Ren, X.-M. Perfluoroalkyl Substances Stimulate Insulin Secretion by Islet β Cells via G Protein-Coupled Receptor 40. Environ. Sci. Technol. 2020, 54, 3428–3436. [Google Scholar] [CrossRef]
- Zhang, L.; Duan, X.; Sun, W.; Sun, H. Perfluorooctane sulfonate acute exposure stimulates insulin secretion via GPR40 pathway. Sci. Total Environ. 2020, 726, 138498. [Google Scholar] [CrossRef]
- Duan, X.; Sun, W.; Sun, H.; Zhang, L. Perfluorooctane sulfonate continual exposure impairs glucose-stimulated insulin secretion via SIRT1-induced upregulation of UCP2 expression. Environ. Pollut. 2021, 278, 116840. [Google Scholar] [CrossRef]
- Wan, H.T.; Zhao, Y.G.; Leung, P.Y.; Wong, C.K.C. Perinatal exposure to perfluorooctane sulfonate affects glucose metabolism in adult offspring. PLoS ONE 2014, 9, e87137. [Google Scholar] [CrossRef]
- Harada, K.; Nakanishi, S.; Sasaki, K.; Furuyama, K.; Nakayama, S.; Saito, N.; Yamakawa, K.; Koizumi, A. Particle size distribution and respiratory deposition estimates of airborne perfluorooctanoate and perfluorooctanesulfonate in Kyoto area, Japan. Bull. Environ. Contam. Toxicol. 2006, 76, 306–310. [Google Scholar] [CrossRef]
- Barton, C.A.; Butler, L.E.; Zarzecki, C.J.; Flaherty, J.; Kaiser, M. Characterizing perfluorooctanoate in ambient air near the fence line of a manufacturing facility: Comparing modeled and monitored values. J. Air Waste Manag. Assoc. 2006, 56, 48–55. [Google Scholar] [CrossRef]
- Davis, K.L.; Aucoin, M.D.; Larsen, B.S.; Kaiser, M.A.; Hartten, A.S. Transport of ammonium perfluorooctanoate in environmental media near a fluoropolymer manufacturing facility. Chemosphere 2007, 67, 2011–2019. [Google Scholar] [CrossRef] [PubMed]
- Ehresman, D.J.; Froehlich, J.W.; Olsen, G.W.; Chang, S.-C.; Butenhoff, J.L. Comparison of human whole blood, plasma, and serum matrices for the determination of perfluorooctanesulfonate (PFOS), perfluorooctanoate (PFOA), and other fluorochemicals. Environ. Res. 2007, 103, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Lind, L.; Zethelius, B.; Salihovic, S.; van Bavel, B.; Lind, P.M. Circulating levels of perfluoroalkyl substances and prevalent diabetes in the elderly. Diabetologia 2014, 57, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.M.; Heo, D.-G.; Kim, J.-H.; Yoon, J.S.; Lee, H.W.; Kim, J.-Y.; Moon, J.S.; Won, K.C. Perfluorinated compounds in adults and their association with fasting glucose and incident diabetes: A prospective cohort study. Environ. Health 2022, 21, 101. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Zhang, H.; Zheng, F.; Sheng, N.; Guo, X.; Dai, J. Perfluorooctanoic acid exposure for 28 days affects glucose homeostasis and induces insulin hypersensitivity in mice. Sci. Rep. 2015, 5, 11029. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wu, D.; Xu, Y.; Zhang, Y.; Sun, Y.; Chang, X.; Zhu, Y.; Tang, W. Perfluorooctanoic acid promotes pancreatic β cell dysfunction and apoptosis through ER stress and the ATF4/CHOP/TRIB3 pathway. Environ. Sci. Pollut. Res. Int. 2022, 29, 84532–84545. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Yu, S.; Zhu, X.; Liao, R.; Zhuo, Z.; He, Y.; Ma, H. In-situ Detection Method for Microplastics in Water by Polarized Light Scattering. Front. Mar. Sci. 2021, 8, 739683. [Google Scholar] [CrossRef]
- Dey, T.K.; Uddin, M.E.; Jamal, M. Detection and removal of microplastics in wastewater: Evolution and impact. Environ. Sci. Pollut. Res. Int. 2021, 28, 16925–16947. [Google Scholar] [CrossRef]
- Yong, C.Q.Y.; Valiyaveettil, S.; Tang, B.L. Toxicity of Microplastics and Nanoplastics in Mammalian Systems. Int. J. Environ. Res. Public. Health 2020, 17, 1509. [Google Scholar] [CrossRef]
- Kumar, R.; Manna, C.; Padha, S.; Verma, A.; Sharma, P.; Dhar, A.; Ghosh, A.; Bhattacharya, P. Micro(nano)plastics pollution and human health: How plastics can induce carcinogenesis to humans? Chemosphere 2022, 298, 134267. [Google Scholar] [CrossRef]
- Xia, X.; Sun, M.; Zhou, M.; Chang, Z.; Li, L. Polyvinyl chloride microplastics induce growth inhibition and oxidative stress in Cyprinus carpio var. larvae. Sci. Total Environ. 2020, 716, 136479. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, Y.; Deng, Y.; Jiang, W.; Zhao, Y.; Geng, J.; Ding, L.; Ren, H. Uptake and Accumulation of Polystyrene Microplastics in Zebrafish (Danio rerio) and Toxic Effects in Liver. Environ. Sci. Technol. 2016, 50, 4054–4060. [Google Scholar] [CrossRef] [PubMed]
- Cortés, C.; Domenech, J.; Salazar, M.; Pastor, S.; Marcos, R.; Hernández, A. Nanoplastics as a potential environmental health factor: Effects of polystyrene nanoparticles on human intestinal epithelial Caco-2 cells. Environ. Sci. Nano 2020, 7, 272–285. [Google Scholar] [CrossRef]
- Schirinzi, G.F.; Pérez-Pomeda, I.; Sanchís, J.; Rossini, C.; Farré, M.; Barceló, D. Cytotoxic effects of commonly used nanomaterials and microplastics on cerebral and epithelial human cells. Environ. Res. 2017, 159, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Okamura, T.; Hamaguchi, M.; Hasegawa, Y.; Hashimoto, Y.; Majima, S.; Senmaru, T.; Ushigome, E.; Nakanishi, N.; Asano, M.; Yamazaki, M.; et al. Oral Exposure to Polystyrene Microplastics of Mice on a Normal or High-Fat Diet and Intestinal and Metabolic Outcomes. Environ. Health Perspect. 2023, 131, 27006. [Google Scholar] [CrossRef]
- Fan, X.; Wei, X.; Hu, H.; Zhang, B.; Yang, D.; Du, H.; Zhu, R.; Sun, X.; Oh, Y.; Gu, N. Effects of oral administration of polystyrene nanoplastics on plasma glucose metabolism in mice. Chemosphere 2022, 288, 132607. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, Z.; Xu, K.; Wang, X.; Gao, X.; Han, Q.; Wang, S.; Chen, M. The effect and a mechanistic evaluation of polystyrene nanoplastics on a mouse model of type 2 diabetes. Food Chem. Toxicol. 2023, 173, 113642. [Google Scholar] [CrossRef]
- Shi, C.; Han, X.; Guo, W.; Wu, Q.; Yang, X.; Wang, Y.; Tang, G.; Wang, S.; Wang, Z.; Liu, Y.; et al. Disturbed Gut-Liver axis indicating oral exposure to polystyrene microplastic potentially increases the risk of insulin resistance. Environ. Int. 2022, 164, 107273. [Google Scholar] [CrossRef]
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Gandra, S.; Ashok, A.; Caudron, Q.; Grenfell, B.T.; Levin, S.A.; Laxminarayan, R. Global antibiotic consumption 2000 to 2010: An analysis of national pharmaceutical sales data. Lancet Infect. Dis. 2014, 14, 742–750. [Google Scholar] [CrossRef]
- Tran, N.H.; Chen, H.; Reinhard, M.; Mao, F.; Gin, K.Y.-H. Occurrence and removal of multiple classes of antibiotics and antimicrobial agents in biological wastewater treatment processes. Water Res. 2016, 104, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Chen, J.; Rao, Z.; Guo, B.; Xu, Y. Recent Advances of Biosensors for Detection of Multiple Antibiotics. Biosensors 2023, 13, 850. [Google Scholar] [CrossRef] [PubMed]
- Gago-Ferrero, P.; Díaz-Cruz, M.S.; Barceló, D. Occurrence of multiclass UV filters in treated sewage sludge from wastewater treatment plants. Chemosphere 2011, 84, 1158–1165. [Google Scholar] [CrossRef] [PubMed]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes pathophysiology. eBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.R.; Greer, R.L.; Dong, X.; Dsouza, K.N.; Gurung, M.; Wu, J.Y.; Morgun, A.; Shulzhenko, N. Antibiotic-Induced Alterations in Gut Microbiota Are Associated with Changes in Glucose Metabolism in Healthy Mice. Front. Microbiol. 2017, 8, 2306. [Google Scholar] [CrossRef]
- Yuan, J.; Hu, Y.J.; Zheng, J.; Kim, J.H.; Sumerlin, T.; Chen, Y.; He, Y.; Zhang, C.; Tang, J.; Pan, Y.; et al. Long-term use of antibiotics and risk of type 2 diabetes in women: A prospective cohort study. Int. J. Epidemiol. 2020, 49, 1572–1581. [Google Scholar] [CrossRef]
- Fu, L.; Qiu, Y.; Shen, L.; Cui, C.; Wang, S.; Wang, S.; Xie, Y.; Zhao, X.; Gao, X.; Ning, G.; et al. The delayed effects of antibiotics in type 2 diabetes, friend or foe? J. Endocrinol. 2018, 238, 137–149. [Google Scholar] [CrossRef]
- Fenneman, A.C.; Weidner, M.; Chen, L.A.; Nieuwdorp, M.; Blaser, M.J. Antibiotics in the pathogenesis of diabetes and inflammatory diseases of the gastrointestinal tract. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 81–100. [Google Scholar] [CrossRef]
- Buffie, C.G.; Bucci, V.; Stein, R.R.; McKenney, P.T.; Ling, L.; Gobourne, A.; No, D.; Liu, H.; Kinnebrew, M.; Viale, A.; et al. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature 2015, 517, 205–208. [Google Scholar] [CrossRef]
- Sharon, G.; Garg, N.; Debelius, J.; Knight, R.; Dorrestein, P.C.; Mazmanian, S.K. Specialized metabolites from the microbiome in health and disease. Cell Metab. 2014, 20, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Claus, S.P.; Guillou, H.; Ellero-Simatos, S. The gut microbiota: A major player in the toxicity of environmental pollutants? NPJ Biofilms Microbiomes 2016, 2, 16003. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Abo, R.P.; Schlieper, K.A.; Graffam, M.E.; Levine, S.; Wishnok, J.S.; Swenberg, J.A.; Tannenbaum, S.R.; Fox, J.G. Arsenic exposure perturbs the gut microbiome and its metabolic profile in mice: An integrated metagenomics and metabolomics analysis. Environ. Health Perspect. 2014, 122, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Tu, P.; Chi, L.; Gao, B.; Ru, H.; Lu, K. Saccharin induced liver inflammation in mice by altering the gut microbiota and its metabolic functions. Food Chem. Toxicol. 2017, 107, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Livanos, A.E.; Greiner, T.U.; Vangay, P.; Pathmasiri, W.; Stewart, D.; McRitchie, S.; Li, H.; Chung, J.; Sohn, J.; Kim, S.; et al. Antibiotic-mediated gut microbiome perturbation accelerates development of type 1 diabetes in mice. Nat. Microbiol. 2016, 1, 16140. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, S.; Härkönen, T.; Vatanen, T.; Hyötyläinen, T.; Knip, M.; Orešič, M. Impact of exposure to per- and polyfluoroalkyl substances on fecal microbiota composition in mother-infant dyads. Environ. Int. 2023, 176, 107965. [Google Scholar] [CrossRef]
- Potera, C. POPs and Gut Microbiota: Dietary Exposure Alters Ratio of Bacterial Species. Environ. Health Perspect. 2015, 123, A187. [Google Scholar] [CrossRef]
- Lai, K.P.; Ng, A.H.-M.; Wan, H.T.; Wong, A.Y.-M.; Leung, C.C.-T.; Li, R.; Wong, C.K.-C. Dietary Exposure to the Environmental Chemical, PFOS on the Diversity of Gut Microbiota, Associated With the Development of Metabolic Syndrome. Front. Microbiol. 2018, 9, 2552. [Google Scholar] [CrossRef]
- Crawford, P.A.; Crowley, J.R.; Sambandam, N.; Muegge, B.D.; Costello, E.K.; Hamady, M.; Knight, R.; Gordon, J.I. Regulation of myocardial ketone body metabolism by the gut microbiota during nutrient deprivation. Proc. Natl. Acad. Sci. USA 2009, 106, 11276–11281. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).