Safe Disposal of Accident Wastewater in Chemical Industrial Parks Using Non-Thermal Plasma with ZnO-Fe3O4 Composites
Abstract
1. Introduction
2. Experimental
2.1. Reagents
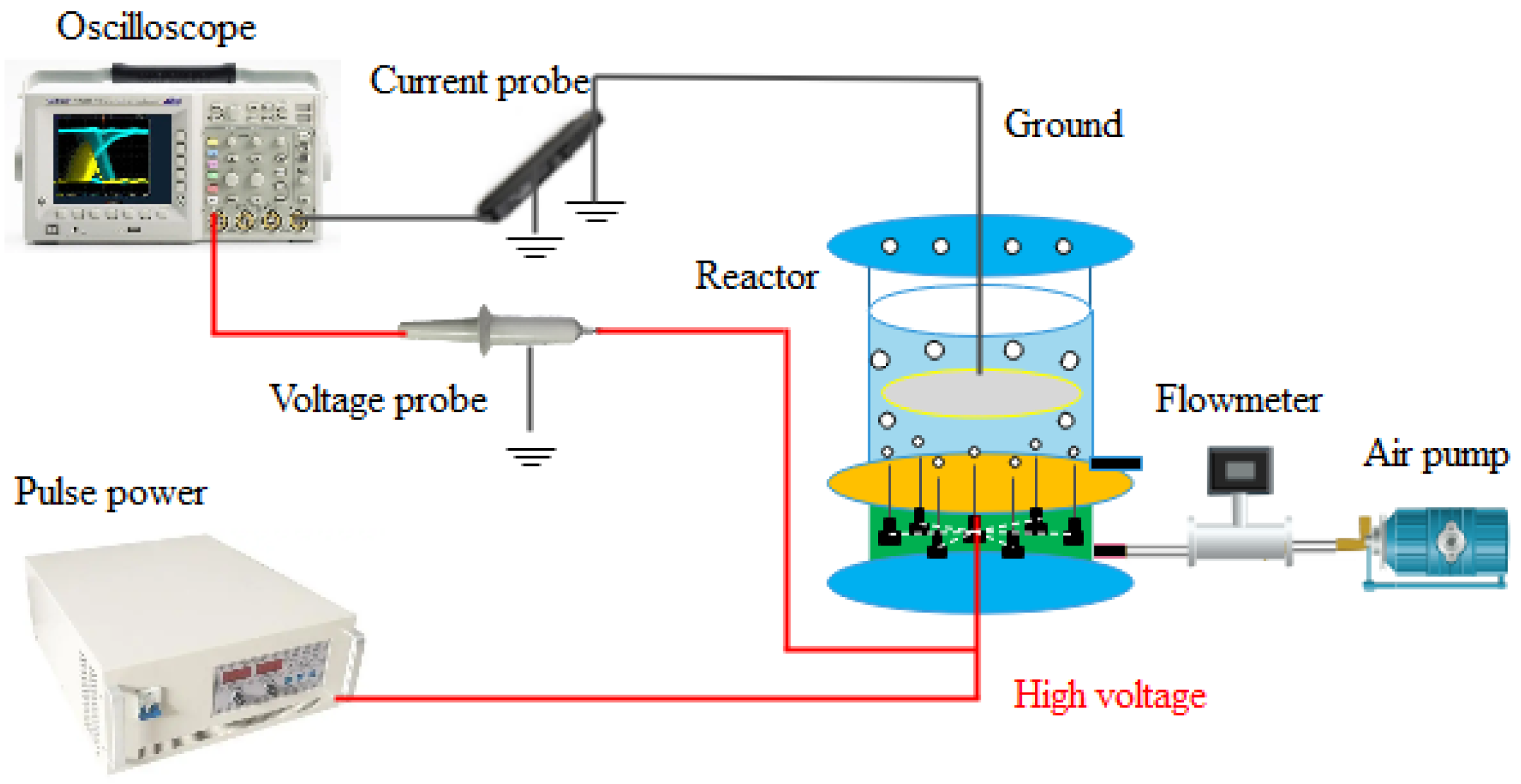
2.2. Experimental Setup and Analysis
2.3. ZnO-Fe3O4 Preparation and Characterization
2.4. Toxicity Analysis Using ECOSAR
3. Results and Discussion
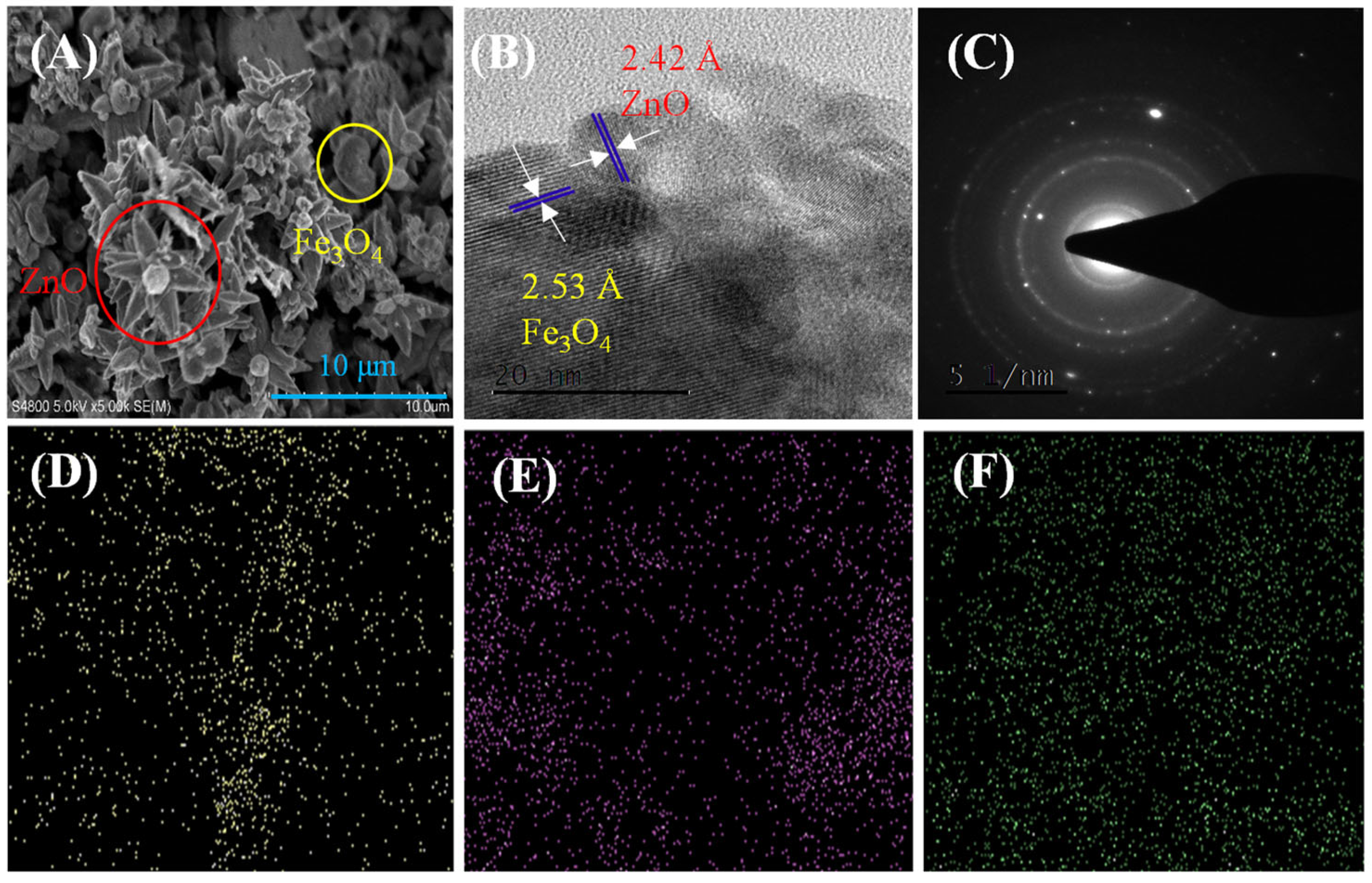
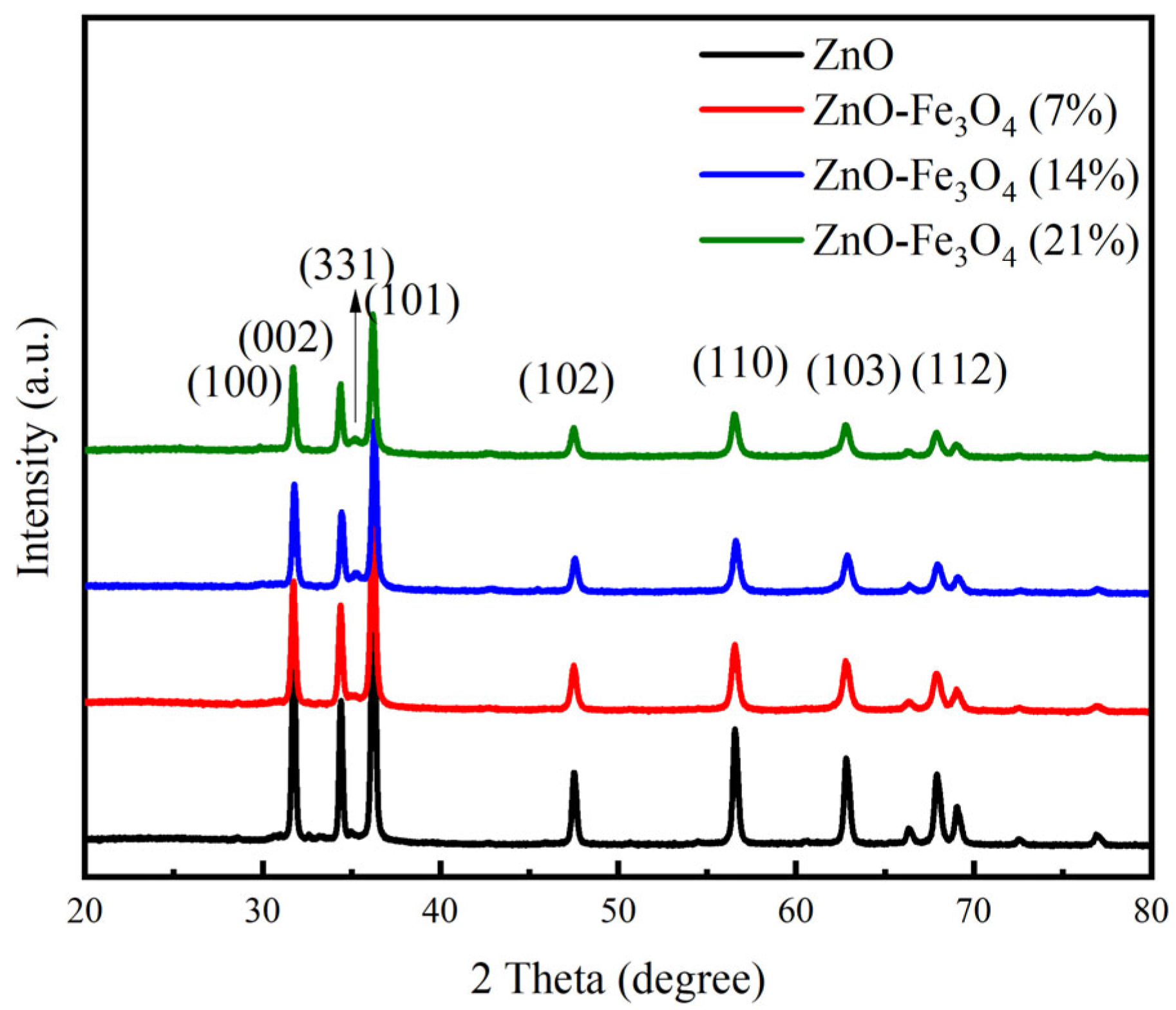
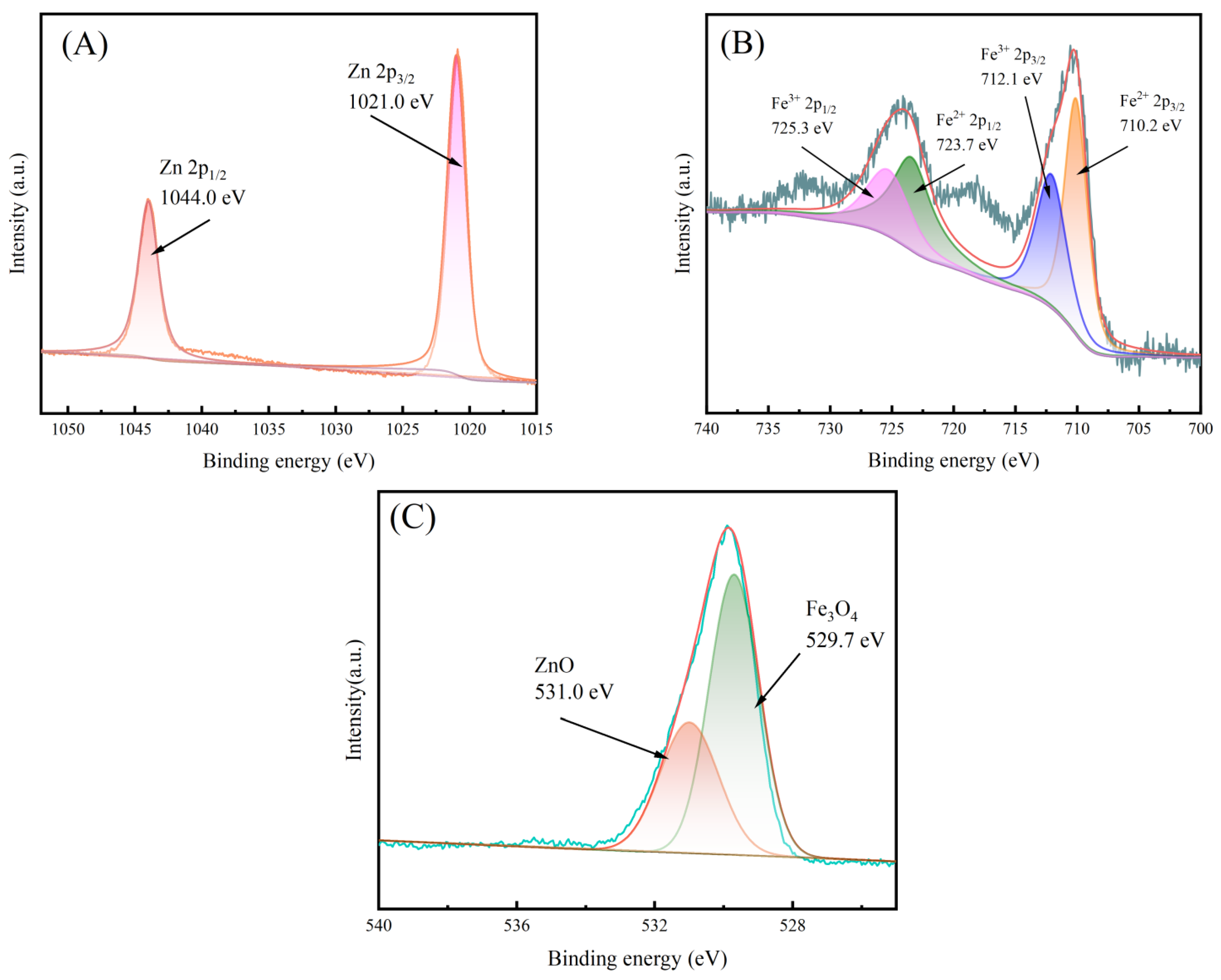
3.1. Characterization
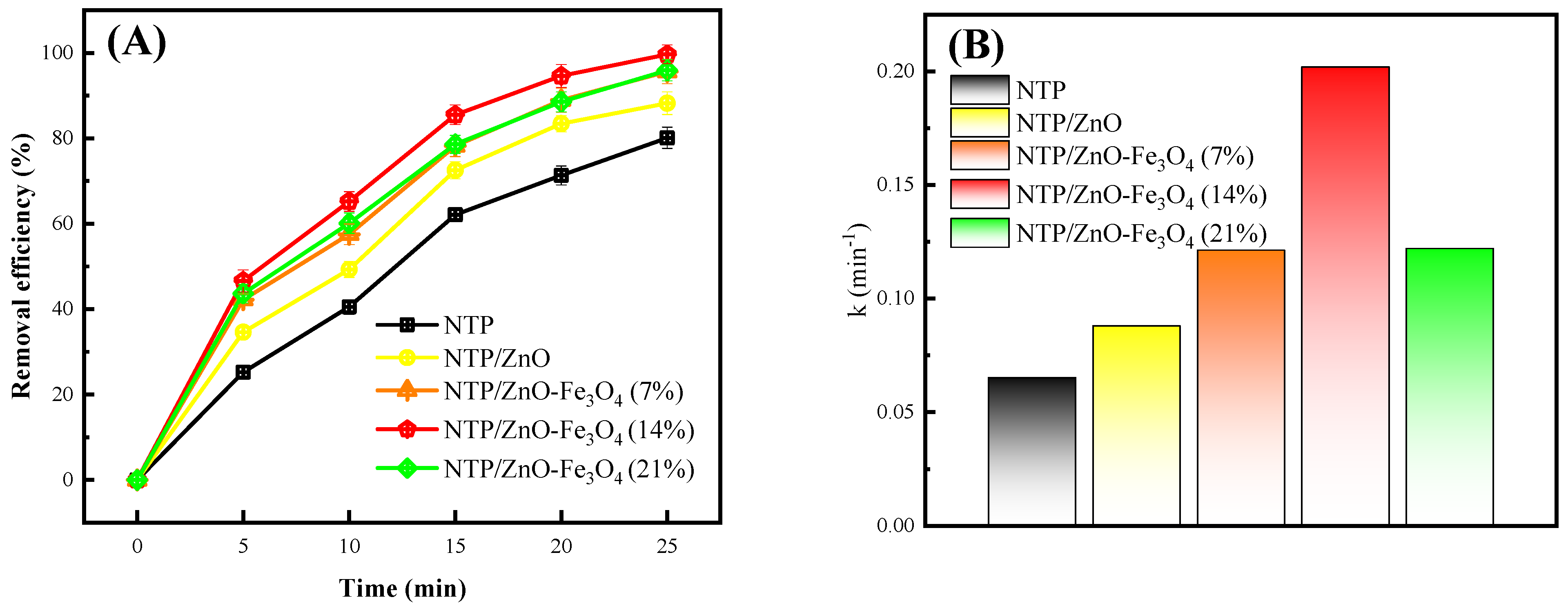
3.2. Catalytic Degradation Performance of ZnO-Fe3O4 Composites
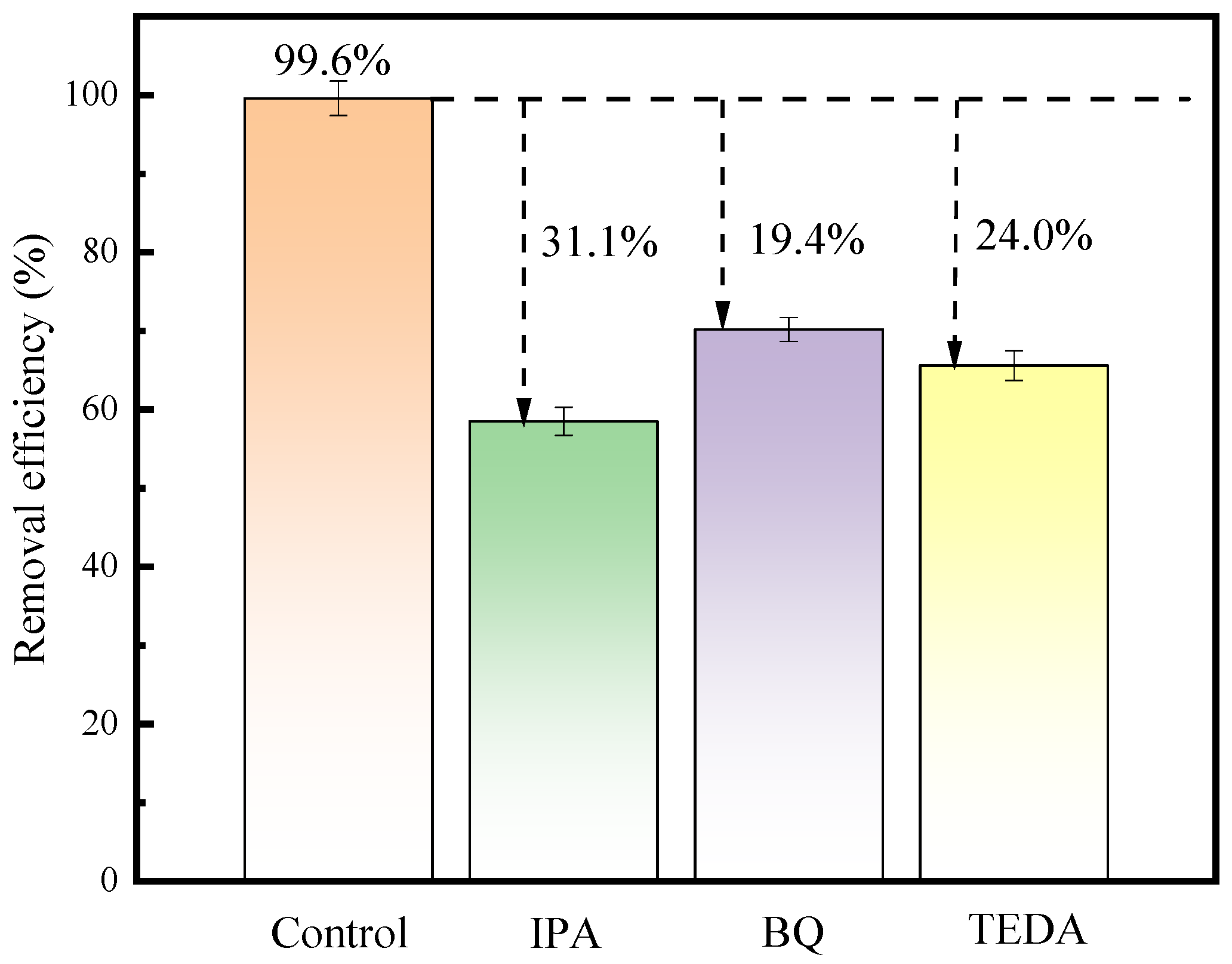
3.3. Role of Active Species
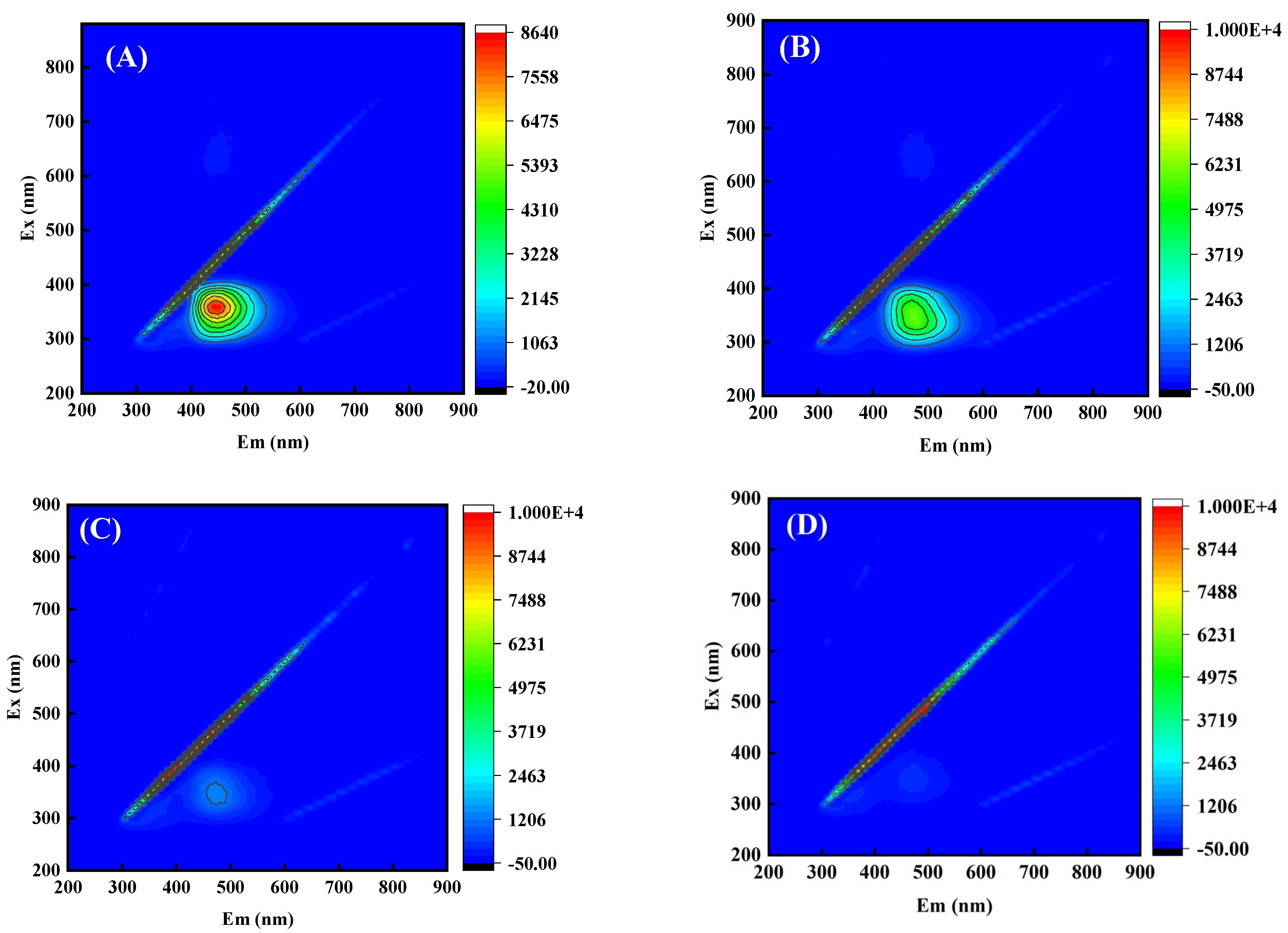
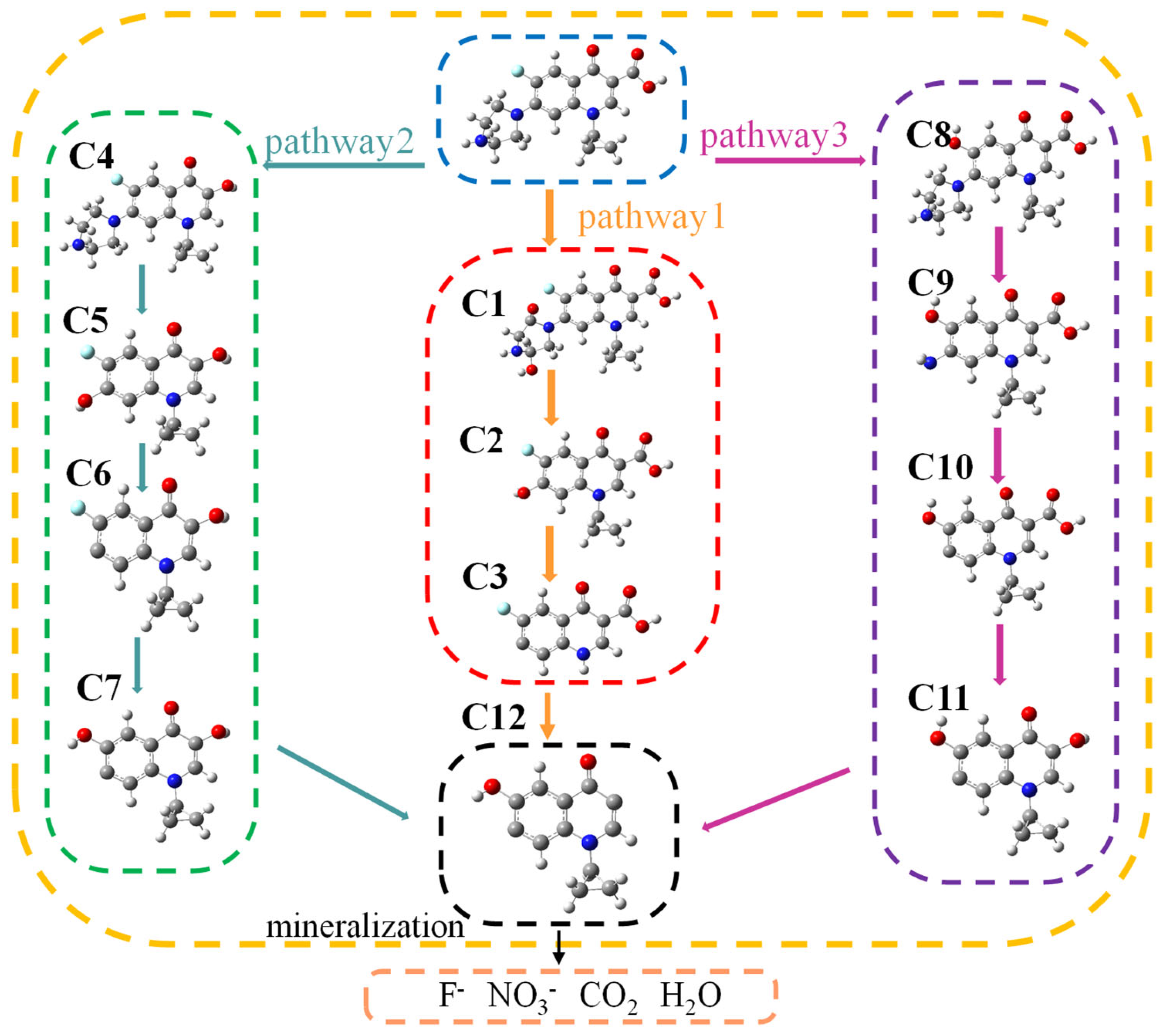
3.4. Degradation Process and Toxicity Variation
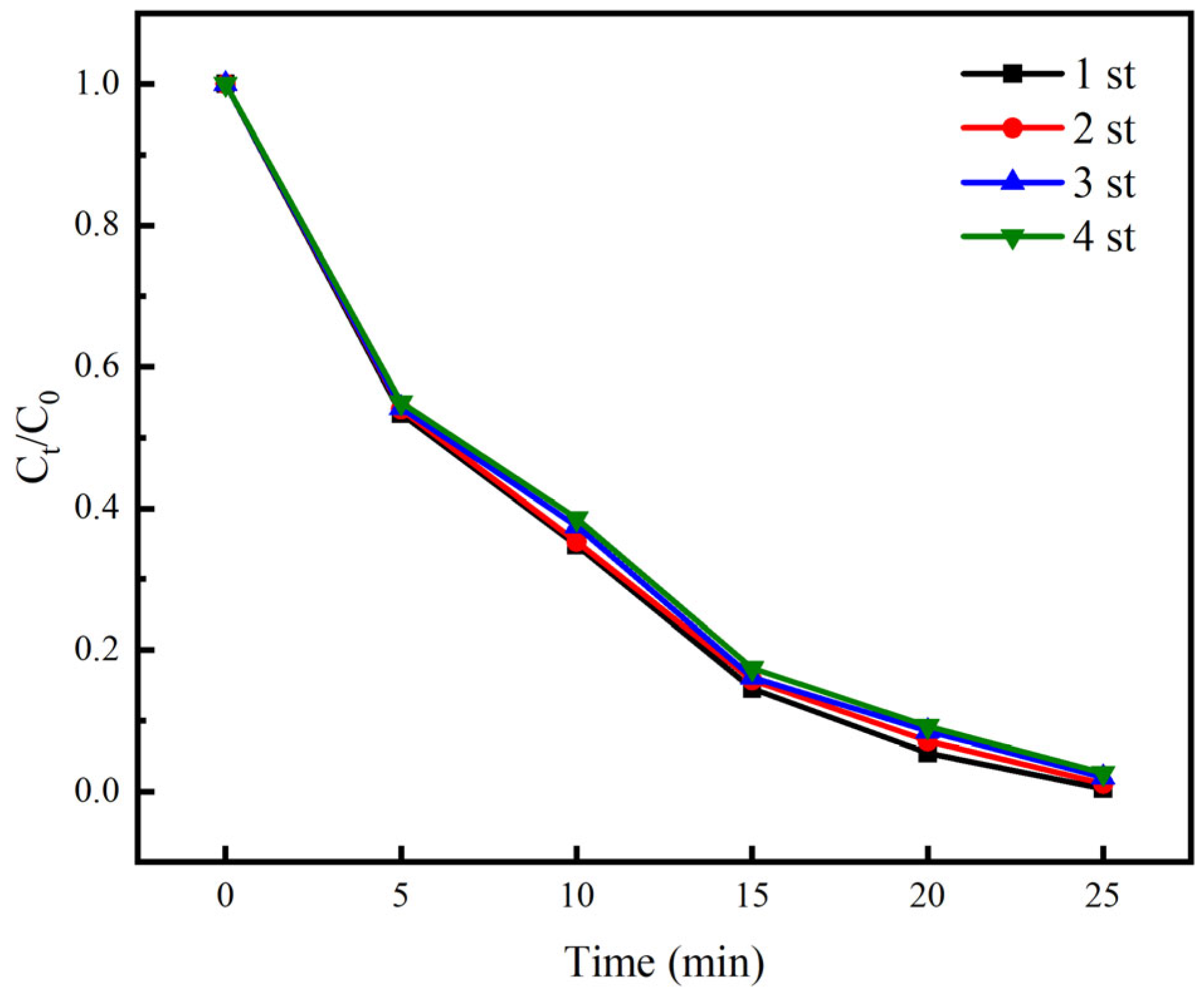
3.5. Reuse Performance and Actual Wastewater Treatment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Q.; Wang, P.L.; Yang, Q.X. Occurrence and diversity of antibiotic resistance in untreated hospital wastewater. Sci. Total Environ. 2018, 621, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Singh, A.K.; Cheng, L.; Hussain, A.; Maiti, A. Occurrence of antibiotics in wastewater: Potential ecological risk and removal through anaerobic–aerobic systems. Environ. Res. 2023, 226, 115678. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.M.; Ni, J.; Zheng, T.L.; Wang, X.N.; Wu, C.F.; Wang, Q.H. Remediation of wastewater contaminated by antibiotics. A review. Environ. Chem. Lett. 2020, 18, 345–360. [Google Scholar] [CrossRef]
- Shanmugavel, S.P.; Kumar, G.; Yogalakshmi, K.N.; Gunasekaran, M.; Banu, R.J. Recent progress in mineralization of emerging contaminants by advanced oxidation process: A review. Environ. Pollut. 2024, 341, 122842. [Google Scholar]
- Liu, W.; Lu, Y.R.; Dong, Y.B.; Jin, Q.; Lin, H. A critical review on reliability of quenching experiment in advanced oxidation processes. Chem. Eng. J. 2023, 466, 143161. [Google Scholar] [CrossRef]
- Priyadarshini, M.; Das, I.; Ghangrekar, M.M.; Blaney, L. Advanced oxidation processes: Performance, advantages, and scale-up of emerging technologies. J. Environ. Manag. 2022, 316, 115295. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Pan, S.J.; Hu, Z.X.; Wang, Y.W.; Jiang, W.X.; Yang, Y.X.; Wang, Y.C.; Han, J.G.; Wu, Y.F.; Wang, T.C. Persulfate activated by non-thermal plasma for organic pollutants degradation: A review. Chem. Eng. J. 2023, 470, 144094. [Google Scholar] [CrossRef]
- Sanito, R.C.; You, S.J.; Wang, Y.F. Degradation of contaminants in plasma technology: An overview. J. Hazard. Mater. 2022, 424, 127390. [Google Scholar] [CrossRef]
- Guo, H.; Su, Y.Y.; Yang, X.Y.; Wang, Y.W.; Li, Z.; Wu, Y.F.; Ren, J.Y. Dielectric Barrier Discharge Plasma Coupled with Catalysis for Organic Wastewater Treatment: A Review. Catalysts 2023, 13, 10. [Google Scholar] [CrossRef]
- Yan, X.; Yi, C.W.; Wang, Y.H.; Cao, W.D.; Mao, D.N.; Ou, Q.Q.; Shen, P.Y.; Wang, H.J. Multi-catalysis of nano-zinc oxide for bisphenol A degradation in a dielectric barrier discharge plasma system: Effect and mechanism. Sep. Purif. Technol. 2020, 231, 115897. [Google Scholar] [CrossRef]
- Hamid, S.B.A.; The, S.J.; Lai, C.W. Photocatalytic Water Oxidation on ZnO: A Review. Catalysts 2017, 7, 93. [Google Scholar] [CrossRef]
- Abdel-Fattah, E.; Alotibi, S. Synergistic Effect of Nonthermal Plasma and ZnO Nanoparticles on Organic Dye Degradation. Appl. Sci. 2023, 13, 10045. [Google Scholar] [CrossRef]
- Farzinfar, B.; Qaderi, F. Synergistic degradation of aqueous p-nitrophenol using DBD plasma combined with ZnO photocatalyst. Process Saf. Environ. Prot. 2022, 168, 907–917. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, W.X.; Huang, J.W.; Wang, Y.W.; Guo, H. ZnO Promoted Persulfate Activation in Discharge Plasma System for Ofloxacin Degradation. Catalysts 2023, 13, 847. [Google Scholar] [CrossRef]
- Wang, X.P.; Chen, Y.X.; Fu, M.; Chen, Z.H.; Huang, Q.H. Effect of high-voltage discharge non-thermal plasma on g-C3N4 in a plasma-photocatalyst system. Chin. J. Catal. 2018, 39, 1672–1682. [Google Scholar] [CrossRef]
- Elshypany, R.; Selim, H.; Zakaria, K.; Moustafa, A.H.; Sadeek, S.A.; Sharaa, S.I.; Raynaud, P.; Nada, A.A. Elaboration of Fe3O4/ZnO nanocomposite with highly performance photocatalytic activity for degradation methylene blue under visible light irradiation. Environ. Technol. Innov. 2021, 23, 101710. [Google Scholar] [CrossRef]
- Długosz, O.; Szostak, K.; Krupiński, M.; Banach, M. Synthesis of Fe3O4/ZnO nanoparticles and their application for the photodegradation of anionic and cationic dyes. Int. J. Environ. Sci. Technol. 2021, 18, 561–574. [Google Scholar] [CrossRef]
- Faramawy, A.; Elsayed, H.; Scian, C.; Mattei, G. Structural, Optical, Magnetic and Electrical Properties of Sputtered ZnO and ZnO:Fe Thin Films: The Role of Deposition Power. Ceramics 2022, 5, 1128–1153. [Google Scholar] [CrossRef]
- Li, F.F.; Sun, G.H.; Fan, T.J.; Zhang, N.; Zhao, L.J.; Zhong, R.G.; Peng, Y.Z. Ecotoxicological QSAR modelling of the acute toxicity of fused and non-fused polycyclic aromatic hydrocarbons (FNFPAHs) against two aquatic organisms: Consensus modelling and comparison with ECOSAR. Aquat. Toxicol. 2023, 255, 106393. [Google Scholar] [CrossRef]
- Guo, H.; Li, Z.; Zhang, Y.; Jiang, N.; Wang, H.J.; Li, J. Degradation of chloramphenicol by pulsed discharge plasma with heterogeneous Fenton process using Fe3O4 nanocomposites. Sep. Purif. Technol. 2020, 253, 117540. [Google Scholar] [CrossRef]
- Guo, H.; Yang, H.; Huang, J.W.; Tong, J.; Liu, X.Y.; Wang, Y.W.; Qiao, W.C.; Han, J.G. Theoretical and experimental insight into plasma-catalytic degradation of aqueous p-nitrophenol with graphene-ZnO nanoparticles. Sep. Purif. Technol. 2022, 295, 121362. [Google Scholar] [CrossRef]
- Nekouei, S.; Nekouei, F.; Kargarzadeh, H. Synthesis of ZnO photocatalyst modified with activated carbon for a perfect degradation of ciprofloxacin and its secondary pollutants. Appl. Organomet. Chem. 2018, 32, e4198. [Google Scholar] [CrossRef]
- Wan, Z.; Wang, J. Degradation of sulfamethazine using Fe3O4-Mn3O4/reduced graphene oxide hybrid as fenton-like catalyst. J. Hazard. Mater. 2017, 324, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Erim, B.; Ciğeroğlu, Z.; Şahin, S.; Vasseghian, Y. Photocatalytic degradation of cefixime in aqueous solutions using functionalized SWCNT/ZnO/Fe3O4 under UV-A irradiation. Chemosphere 2022, 291, 132929. [Google Scholar] [CrossRef] [PubMed]
- Pham, L.H.; Nguyen, V.Q.; Nguyen, H.; Ha, T.H.; Duong, A.T.; Manh, T.T.; Quang, V.T.; Ta, N.B.; Nguyen, T.; Van, D.D. Efficiency enhancement of photocatalytic activity under UV and visible light irradiation using ZnO/Fe3O4 heteronanostructures. Sol. Energy 2023, 249, 712–724. [Google Scholar]
- Zhang, P.; Su, Q.; Han, L.J.; Lin, J.H.; Wei, X.H.; Meng, S.J.; Wang, Y.B. Facile fabrication of magnetic Ag/ZnO/Fe3O4 composite and the photocatalytic performance under simulated sunlight irradiation. Mol. Catal. 2021, 508, 111606. [Google Scholar] [CrossRef]
- Guo, H.; Wang, Y.W.; Yao, X.; Zhang, Y.T.; Li, Z.; Pan, S.J.; Han, J.G.; Xu, L.J.; Qiao, W.C.; Li, J.; et al. A comprehensive insight into plasma-catalytic removal of antibiotic oxytetracycline based on graphene-TiO2-Fe3O4 nanocomposites. Chem. Eng. J. 2021, 425, 130614. [Google Scholar] [CrossRef]
- Zhang, G.Y.; Sun, Y.; Zhang, C.; Yu, Z. Decomposition of acetaminophen in water by a gas phase dielectric barrier discharge plasma combined with TiO2-rGO nanocomposite: Mechanism and degradation pathway. J. Hazard. Mater. 2017, 323, 719–729. [Google Scholar] [CrossRef]
- Wang, Y.W.; Xiang, L.R.; Li, Z.; Han, J.G.; Guo, H. Sulfite activation by water film dielectric barrier discharge plasma for ibuprofen degradation: Efficiency, comparison of persulfate, mechanism, active substances dominant to pathway, and toxicity evaluation. Sep. Purif. Technol. 2024, 330, 125531. [Google Scholar] [CrossRef]
- Chen, W.; Westerhoff, P.; Leenheer, J.A.; Booksh, K. Fluorescence Excitation−Emission Matrix Regional Integration to Quantify Spectra for Dissolved Organic Matter. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar] [CrossRef]
- Fan, G.D.; Cai, C.J.; Yang, S.W.; Du, B.H.; Luo, J.; Chen, Y.F.; Lin, X.; Li, X.; Wang, Y.M. Sonophotocatalytic degradation of ciprofloxacin by Bi2MoO6/FeVO4 heterojunction: Insights into performance, mechanism and pathway. Sep. Purif. Technol. 2022, 303, 122251. [Google Scholar] [CrossRef]
- Yu, X.J.; Zhang, J.; Zhang, J.; Niu, J.F.; Zhao, J.; Wei, Y.C.; Yao, B.H. Photocatalytic degradation of ciprofloxacin using Zn-doped Cu2O particles: Analysis of degradation pathways and intermediates. Chem. Eng. J. 2019, 374, 316–327. [Google Scholar] [CrossRef]
- Hu, X.; Hu, X.J.; Peng, Q.Q.; Zhou, L.; Tan, X.F.; Jiang, L.H.; Tang, C.F.; Wang, H.; Liu, S.H.; Wang, Y.Q.; et al. Mechanisms underlying the photocatalytic degradation pathway of ciprofloxacin with heterogeneous TiO2. Chem. Eng. J. 2020, 380, 122366. [Google Scholar] [CrossRef]
- Anjali, R.; Shanthakumar, S. Simultaneous degradation of amoxicillin, ciprofloxacin and acetaminophen in a mixture by ozonation: Kinetics and mechanisms pathway. J. Clean. Prod. 2022, 378, 134509. [Google Scholar] [CrossRef]
- Guo, H.; Jiang, N.; Wang, H.J.; Shang, K.F.; Lu, N.; Li, J.; Wu, Y. Pulsed discharge plasma induced WO3 catalysis for synergetic degradation of ciprofloxacin in water: Synergetic mechanism and degradation pathway. Chemosphere 2019, 230, 190–200. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, A.; Wang, C.; Qian, C.; Wen, J.; Guo, H. Safe Disposal of Accident Wastewater in Chemical Industrial Parks Using Non-Thermal Plasma with ZnO-Fe3O4 Composites. Toxics 2024, 12, 40. https://doi.org/10.3390/toxics12010040
Li A, Wang C, Qian C, Wen J, Guo H. Safe Disposal of Accident Wastewater in Chemical Industrial Parks Using Non-Thermal Plasma with ZnO-Fe3O4 Composites. Toxics. 2024; 12(1):40. https://doi.org/10.3390/toxics12010040
Chicago/Turabian StyleLi, Aihua, Chaofei Wang, Chengjiang Qian, Jinfeng Wen, and He Guo. 2024. "Safe Disposal of Accident Wastewater in Chemical Industrial Parks Using Non-Thermal Plasma with ZnO-Fe3O4 Composites" Toxics 12, no. 1: 40. https://doi.org/10.3390/toxics12010040
APA StyleLi, A., Wang, C., Qian, C., Wen, J., & Guo, H. (2024). Safe Disposal of Accident Wastewater in Chemical Industrial Parks Using Non-Thermal Plasma with ZnO-Fe3O4 Composites. Toxics, 12(1), 40. https://doi.org/10.3390/toxics12010040






