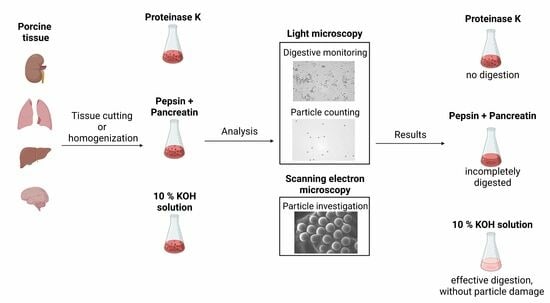
Testing of Different Digestion Solutions on Tissue Samples and the Effects of Used Potassium Hydroxide Solution on Polystyrene Microspheres
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Polystyrene (PS) Polymer Microspheres
2.3. Microscopy
2.4. Digestion
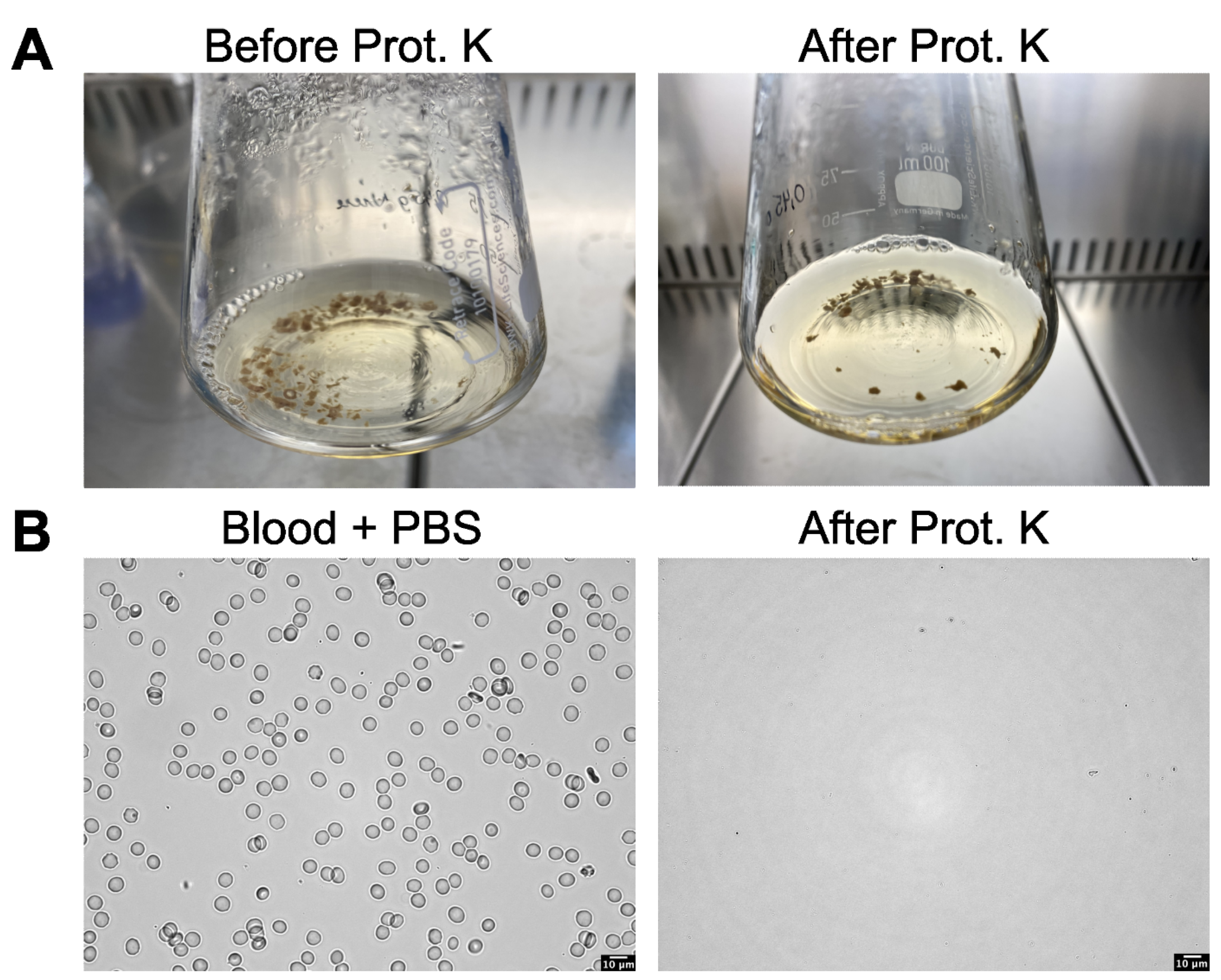
2.4.1. Enzymatic Digestion Method Using Proteinase K
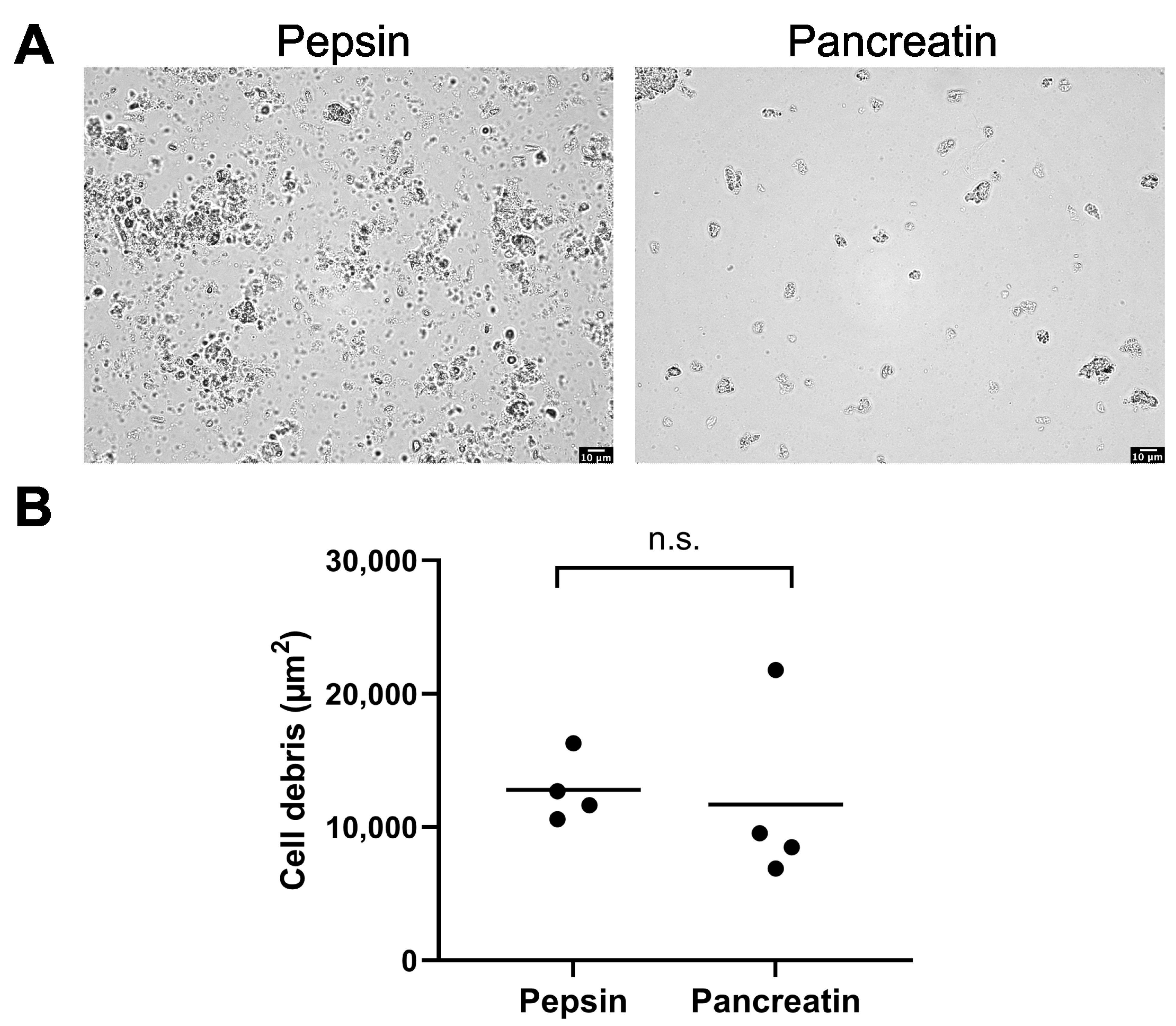
2.4.2. Enzymatic Digestion Method Using Pepsin and Pancreatin
2.4.3. Alkaline Digestion Method
2.5. Effects of Digestion Protocols on Plastic Particles
2.6. Statistical Analysis
3. Results
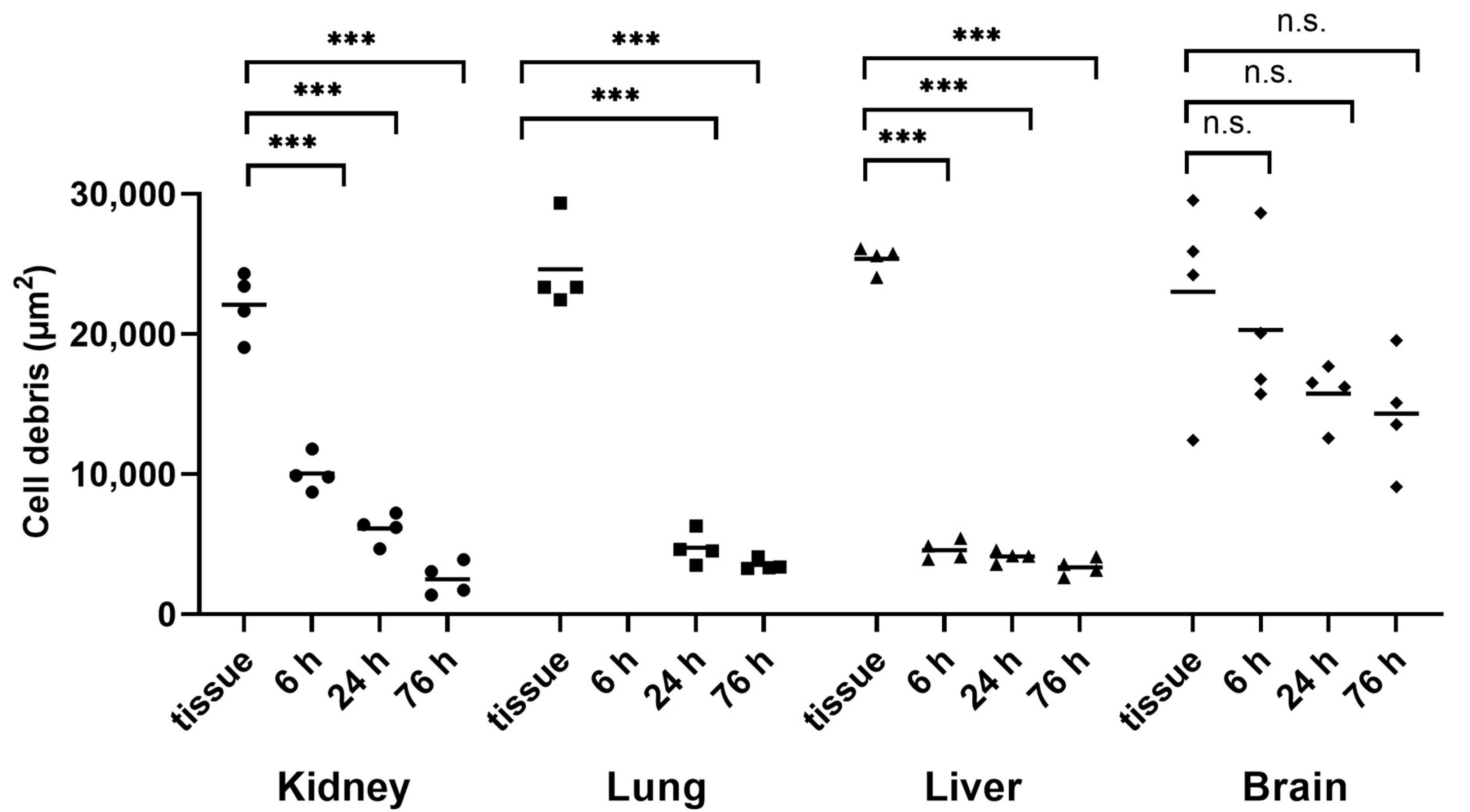
3.1. Kidney, Lung, Liver, and Brain Tissue Digestion Using Proteinase K
3.2. Kidney, Lung, Liver, and Brain Tissue Digestion Using Pepsin and Pancreatin
3.3. Kidney, Lung, Liver, and Brain Tissue Digestion Using KOH
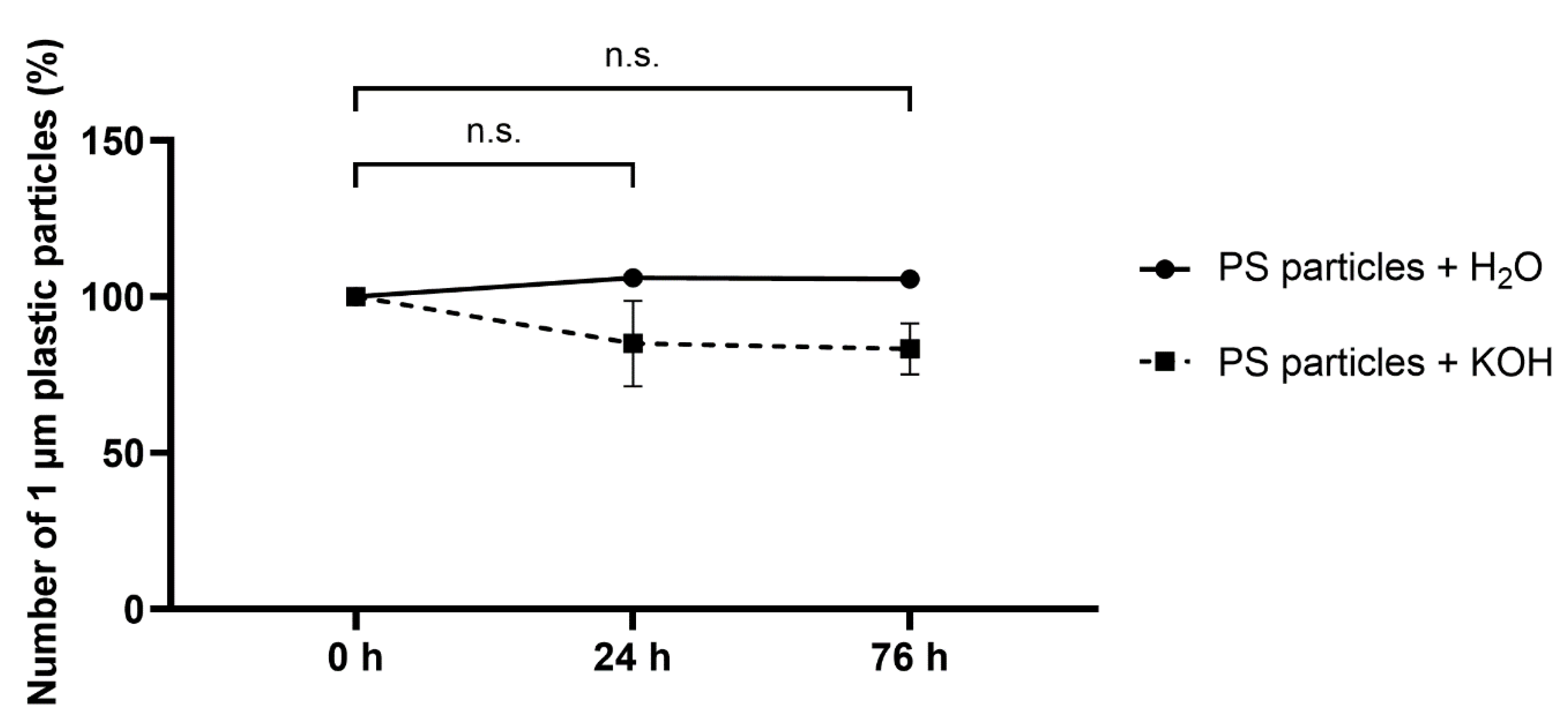
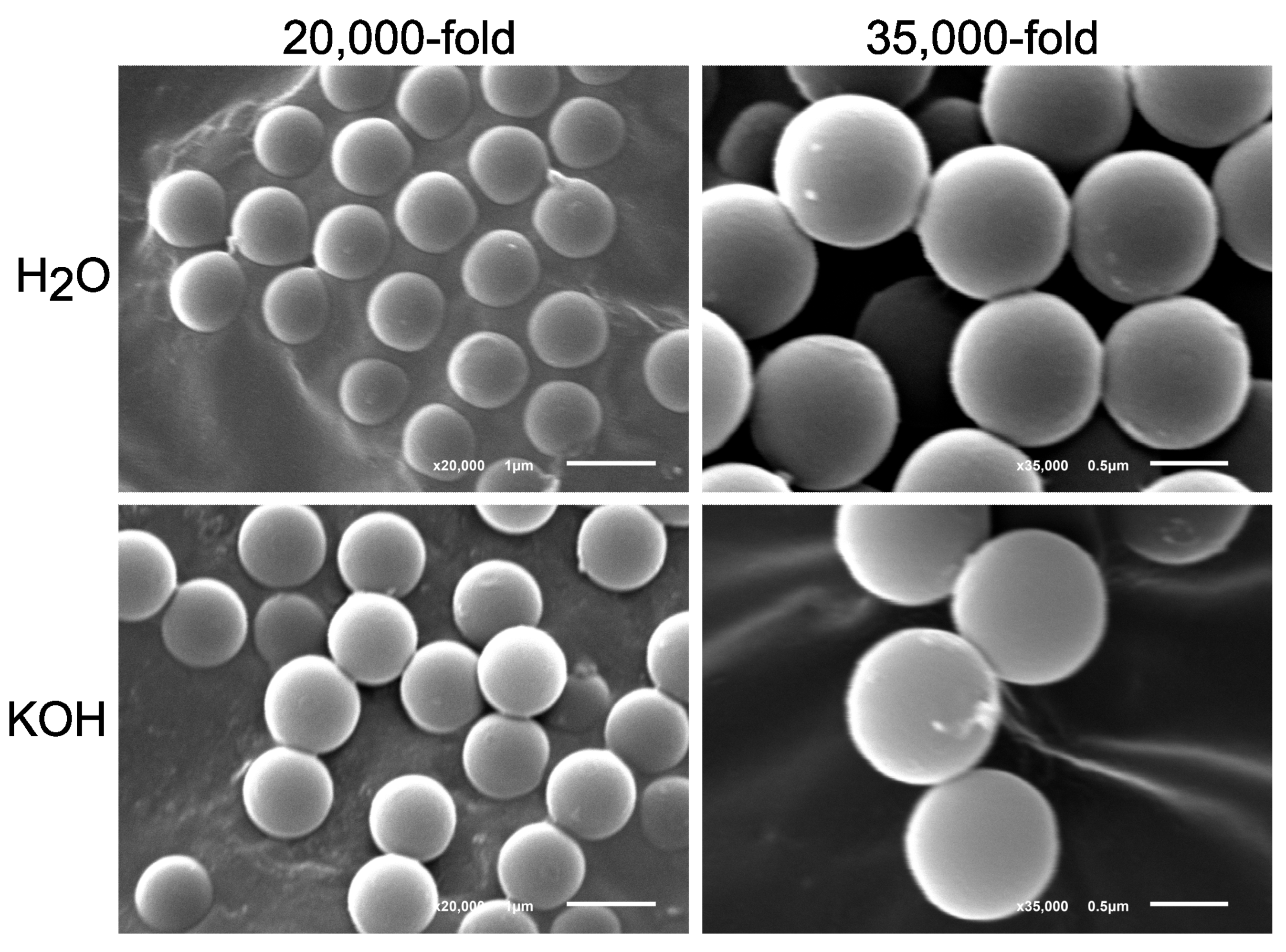
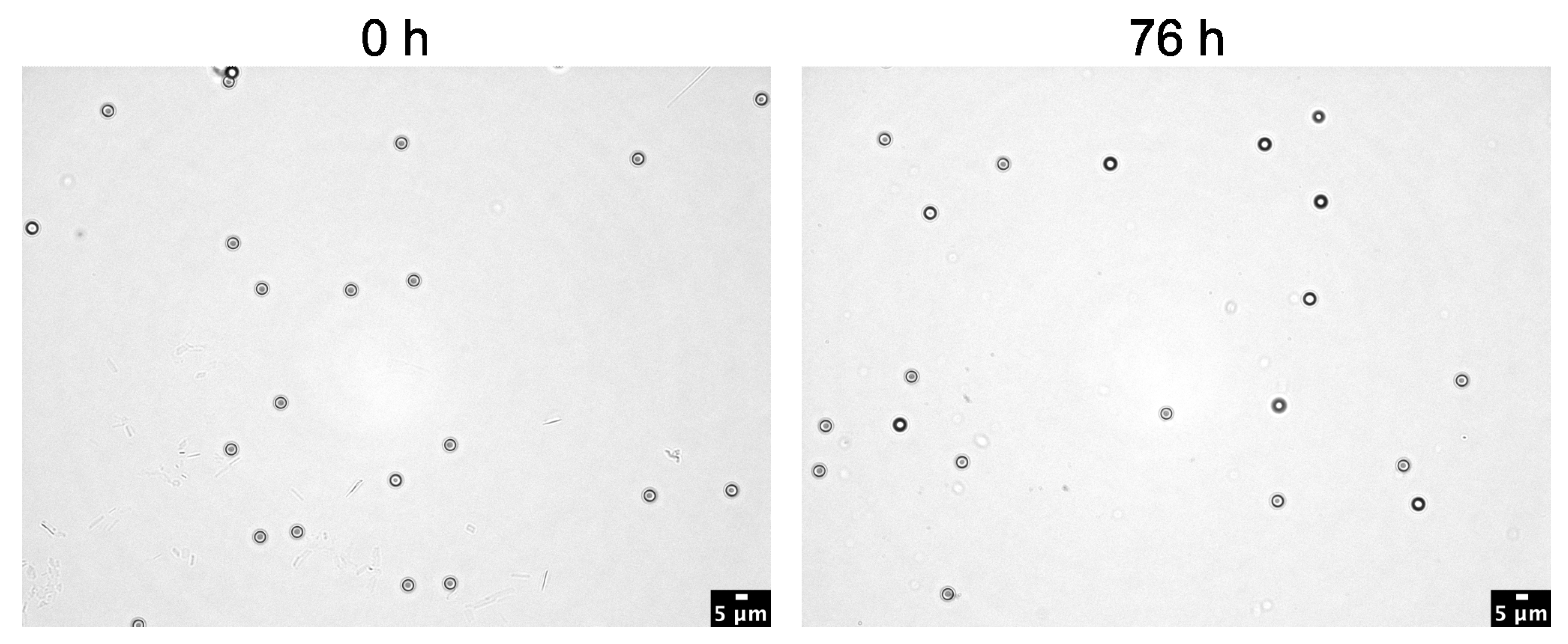
3.4. Effect of 10% KOH Solution on Polystyrene (PS) Microspheres
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koelmans, A.A.; Redondo-Hasselerharm, P.E.; Nor, N.H.M.; De Ruijter, V.N.; Mintenig, S.M.; Kooi, M. Risk Assessment of Microplastic Particles. Nat. Rev. Mater. 2022, 7, 138–152. [Google Scholar] [CrossRef]
- Boucher, J.; Friot, D. Primary Microplastics in the Oceans: A Global Evaluation of Sources; IUCN International Union for Conservation of Nature: Gland, Switzerland, 2017; ISBN 978-2-8317-1827-9. [Google Scholar]
- Wu, X.; Zhao, X.; Chen, R.; Liu, P.; Liang, W.; Wang, J.; Teng, M.; Wang, X.; Gao, S. Wastewater Treatment Plants Act as Essential Sources of Microplastic Formation in Aquatic Environments: A Critical Review. Water Res. 2022, 221, 118825. [Google Scholar] [CrossRef] [PubMed]
- Briassoulis, D. Agricultural Plastics as a Potential Threat to Food Security, Health, and Environment through Soil Pollution by Microplastics: Problem Definition. Sci. Total Environ. 2023, 892, 164533. [Google Scholar] [CrossRef]
- Zuri, G.; Karanasiou, A.; Lacorte, S. Microplastics: Human Exposure Assessment through Air, Water, and Food. Environ. Int. 2023, 179, 108150. [Google Scholar] [CrossRef] [PubMed]
- Santini, S.; De Beni, E.; Martellini, T.; Sarti, C.; Randazzo, D.; Ciraolo, R.; Scopetani, C.; Cincinelli, A. Occurrence of Natural and Synthetic Micro-Fibers in the Mediterranean Sea: A Review. Toxics 2022, 10, 391. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.L.; Kelly, F.J. Plastic and Human Health: A Micro Issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef] [PubMed]
- Schwaferts, C.; Niessner, R.; Elsner, M.; Ivleva, N.P. Methods for the Analysis of Submicrometer- and Nanoplastic Particles in the Environment. TrAC Trends Anal. Chem. 2019, 112, 52–65. [Google Scholar] [CrossRef]
- Frias, J.P.G.L.; Nash, R. Microplastics: Finding a Consensus on the Definition. Mar. Pollut. Bull. 2019, 138, 145–147. [Google Scholar] [CrossRef]
- Ivleva, N.P. Chemical Analysis of Microplastics and Nanoplastics: Challenges, Advanced Methods, and Perspectives. Chem. Rev. 2021, 121, 11886–11936. [Google Scholar] [CrossRef]
- Ramsperger, A.F.R.M.; Bergamaschi, E.; Panizzolo, M.; Fenoglio, I.; Barbero, F.; Peters, R.; Undas, A.; Purker, S.; Giese, B.; Lalyer, C.R.; et al. Nano-and Microplastics: A Comprehensive Review on Their Exposure Routes, Translocation, and Fate in Humans. NanoImpact 2023, 29, 100441. [Google Scholar] [CrossRef]
- Primpke, S.; Christiansen, S.H.; Cowger, W.; De Frond, H.; Deshpande, A.; Fischer, M.; Holland, E.B.; Meyns, M.; O’Donnell, B.A.; Ossmann, B.E.; et al. Critical Assessment of Analytical Methods for the Harmonized and Cost-Efficient Analysis of Microplastics. Appl. Spectrosc. 2020, 74, 1012–1047. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Webb, H.; Lindeque, P.K.; Fileman, E.S.; Halsband, C.; Galloway, T.S. Isolation of Microplastics in Biota-Rich Seawater Samples and Marine Organisms. Sci. Rep. 2014, 4, 4528. [Google Scholar] [CrossRef]
- Li, X.; Chen, L.; Ji, Y.; Li, M.; Dong, B.; Qian, G.; Zhou, J.; Dai, X. Effects of Chemical Pretreatments on Microplastic Extraction in Sewage Sludge and Their Physicochemical Characteristics. Water Res. 2020, 171, 115379. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, F.; Fischer, E.K. Various Digestion Protocols Within Microplastic Sample Processing—Evaluating the Resistance of Different Synthetic Polymers and the Efficiency of Biogenic Organic Matter Destruction. Front. Environ. Sci. 2020, 8, 572424. [Google Scholar] [CrossRef]
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and Quantification of Plastic Particle Pollution in Human Blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Amato-Lourenço, L.F.; Carvalho-Oliveira, R.; Júnior, G.R.; dos Santos Galvão, L.; Ando, R.A.; Mauad, T. Presence of Airborne Microplastics in Human Lung Tissue. J. Hazard. Mater. 2021, 416, 126124. [Google Scholar] [CrossRef]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First Evidence of Microplastics in Human Placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; Dias-Pereira, P. Microplastics in Terrestrial Domestic Animals and Human Health: Implications for Food Security and Food Safety and Their Role as Sentinels. Animals 2023, 13, 661. [Google Scholar] [CrossRef]
- Lee, S.; Kang, K.-K.; Sung, S.-E.; Choi, J.-H.; Sung, M.; Seong, K.-Y.; Lee, S.; Yang, S.Y.; Seo, M.-S.; Kim, K. Toxicity Study and Quantitative Evaluation of Polyethylene Microplastics in ICR Mice. Polymers 2022, 14, 402. [Google Scholar] [CrossRef]
- Tsou, T.-Y.; Lee, S.-H.; Kuo, T.-H.; Chien, C.-C.; Chen, H.-C.; Cheng, T.-J. Distribution and Toxicity of Submicron Plastic Particles in Mice. Environ. Toxicol. Pharmacol. 2023, 97, 104038. [Google Scholar] [CrossRef]
- Wang, Y.; Tibbetts, S.M.; Berrue, F.; McGinn, P.J.; MacQuarrie, S.P.; Puttaswamy, A.; Patelakis, S.; Schmidt, D.; Melanson, R.; MacKenzie, S.E. A Rat Study to Evaluate the Protein Quality of Three Green Microalgal Species and the Impact of Mechanical Cell Wall Disruption. Foods 2020, 9, 1531. [Google Scholar] [CrossRef] [PubMed]
- Kęska, P.; Wójciak, K.M.; Stasiak, D.M. Influence of Sonication and Taraxacum Officinale Addition on the Antioxidant and Anti-ACE Activity of Protein Extracts from Sous Vide Beef Marinated with Sour Milk and after In Vitro Digestion. Molecules 2020, 25, 4692. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liu, H.; Li, Q.; Zhao, D.; Shan, K.; Ke, W.; Zhang, M.; Li, C. The Degree of Doneness Affected Molecular Changes and Protein Digestibility of Pork. Front. Nutr. 2023, 9, 1084779. [Google Scholar] [CrossRef] [PubMed]
- Hurley, R.R.; Lusher, A.L.; Olsen, M.; Nizzetto, L. Validation of a Method for Extracting Microplastics from Complex, Organic-Rich, Environmental Matrices. Environ. Sci. Technol. 2018, 52, 7409–7417. [Google Scholar] [CrossRef]
- Karami, A.; Golieskardi, A.; Choo, C.K.; Romano, N.; Ho, Y.B.; Salamatinia, B. A High-Performance Protocol for Extraction of Microplastics in Fish. Sci. Total Environ. 2017, 578, 485–494. [Google Scholar] [CrossRef]
- Kühn, S.; van Werven, B.; van Oyen, A.; Meijboom, A.; Bravo Rebolledo, E.L.; van Franeker, J.A. The Use of Potassium Hydroxide (KOH) Solution as a Suitable Approach to Isolate Plastics Ingested by Marine Organisms. Mar. Pollut. Bull. 2017, 115, 86–90. [Google Scholar] [CrossRef]
- Alfonso, M.B.; Takashima, K.; Yamaguchi, S.; Tanaka, M.; Isobe, A. Microplastics on Plankton Samples: Multiple Digestion Techniques Assessment Based on Weight, Size, and FTIR Spectroscopy Analyses. Mar. Pollut. Bull. 2021, 173, 113027. [Google Scholar] [CrossRef]
- Liang, B.; Zhong, Y.; Huang, Y.; Lin, X.; Liu, J.; Lin, L.; Hu, M.; Jiang, J.; Dai, M.; Wang, B.; et al. Underestimated Health Risks: Polystyrene Micro- and Nanoplastics Jointly Induce Intestinal Barrier Dysfunction by ROS-Mediated Epithelial Cell Apoptosis. Part. Fibre Toxicol. 2021, 18, 20. [Google Scholar] [CrossRef]
- Xu, Q.; Deng, T.; LeBlanc, G.A.; An, L. An Effective Method for Evaluation of Microplastic Contaminant in Gastropod from Taihu Lake, China. Environ. Sci. Pollut. Res. 2020, 27, 22878–22887. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Dong, P.; An, R.; Xue, C.; Ge, Y.; Wei, L.; Liang, X. Digestion of Nucleic Acids Starts in the Stomach. Sci. Rep. 2015, 5, 11936. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A Standardised Static in Vitro Digestion Method Suitable for Food–an International Consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Al-Azzawi, M.S.M.; Kefer, S.; Weißer, J.; Reichel, J.; Schwaller, C.; Glas, K.; Knoop, O.; Drewes, J.E. Validation of Sample Preparation Methods for Microplastic Analysis in Wastewater Matrices—Reproducibility and Standardization. Water 2020, 12, 2445. [Google Scholar] [CrossRef]
- Habib, R.Z.; Kindi, R.A.; Salem, F.A.; Kittaneh, W.F.; Poulose, V.; Iftikhar, S.H.; Mourad, A.-H.I.; Thiemann, T. Microplastic Contamination of Chicken Meat and Fish through Plastic Cutting Boards. Int. J. Environ. Res. Public. Health 2022, 19, 13442. [Google Scholar] [CrossRef] [PubMed]
- Habib, R.Z.; Poulose, V.; Alsaidi, R.; Al Kendi, R.; Iftikhar, S.H.; Mourad, A.-H.I.; Kittaneh, W.F.; Thiemann, T. Plastic Cutting Boards as a Source of Microplastics in Meat. Food Addit. Contam. Part A 2022, 39, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-Y.; Ke, D.-S.; Chen, J.-Y. Essential Fatty Acids and Human Brain. Acta Neurol. Taiwanica 2009, 18, 231–241. [Google Scholar]
- Söderberg, M.; Edlund, C.; Kristensson, K.; Dallner, G. Lipid Compositions of Different Regions of the Human Brain during Aging. J. Neurochem. 1990, 54, 415–423. [Google Scholar] [CrossRef]
- Alvarez-Román, R.; Naik, A.; Kalia, Y.N.; Guy, R.H.; Fessi, H. Skin Penetration and Distribution of Polymeric Nanoparticles. J. Control. Release 2004, 99, 53–62. [Google Scholar] [CrossRef]
- Schwarzfischer, M.; Niechcial, A.; Lee, S.S.; Sinnet, B.; Wawrzyniak, M.; Laimbacher, A.; Atrott, K.; Manzini, R.; Morsy, Y.; Häfliger, J.; et al. Ingested Nano-and Microsized Polystyrene Particles Surpass the Intestinal Barrier and Accumulate in the Body. NanoImpact 2022, 25, 100374. [Google Scholar] [CrossRef]
- Shan, S.; Zhang, Y.; Zhao, H.; Zeng, T.; Zhao, X. Polystyrene Nanoplastics Penetrate across the Blood-Brain Barrier and Induce Activation of Microglia in the Brain of Mice. Chemosphere 2022, 298, 134261. [Google Scholar] [CrossRef]
- Vlacil, A.-K.; Bänfer, S.; Jacob, R.; Trippel, N.; Kuzu, I.; Schieffer, B.; Grote, K. Polystyrene Microplastic Particles Induce Endothelial Activation. PLoS ONE 2021, 16, e0260181. [Google Scholar] [CrossRef]
- Gulizia, A.M.; Brodie, E.; Daumuller, R.; Bloom, S.B.; Corbett, T.; Santana, M.M.F.; Motti, C.A.; Vamvounis, G. Evaluating the Effect of Chemical Digestion Treatments on Polystyrene Microplastics: Recommended Updates to Chemical Digestion Protocols. Macromol. Chem. Phys. 2022, 223, 2100485. [Google Scholar] [CrossRef]
- Thiele, C.J.; Hudson, M.D.; Russell, A.E. Evaluation of Existing Methods to Extract Microplastics from Bivalve Tissue: Adapted KOH Digestion Protocol Improves Filtration at Single-Digit Pore Size. Mar. Pollut. Bull. 2019, 142, 384–393. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geppner, L.; Karaca, J.; Wegner, W.; Rados, M.; Gutwald, T.; Werth, P.; Henjakovic, M. Testing of Different Digestion Solutions on Tissue Samples and the Effects of Used Potassium Hydroxide Solution on Polystyrene Microspheres. Toxics 2023, 11, 790. https://doi.org/10.3390/toxics11090790
Geppner L, Karaca J, Wegner W, Rados M, Gutwald T, Werth P, Henjakovic M. Testing of Different Digestion Solutions on Tissue Samples and the Effects of Used Potassium Hydroxide Solution on Polystyrene Microspheres. Toxics. 2023; 11(9):790. https://doi.org/10.3390/toxics11090790
Chicago/Turabian StyleGeppner, Liesa, Jakob Karaca, Wencke Wegner, Moritz Rados, Tobias Gutwald, Philemon Werth, and Maja Henjakovic. 2023. "Testing of Different Digestion Solutions on Tissue Samples and the Effects of Used Potassium Hydroxide Solution on Polystyrene Microspheres" Toxics 11, no. 9: 790. https://doi.org/10.3390/toxics11090790
APA StyleGeppner, L., Karaca, J., Wegner, W., Rados, M., Gutwald, T., Werth, P., & Henjakovic, M. (2023). Testing of Different Digestion Solutions on Tissue Samples and the Effects of Used Potassium Hydroxide Solution on Polystyrene Microspheres. Toxics, 11(9), 790. https://doi.org/10.3390/toxics11090790