Abstract
Nowadays, the problem of inland water pollution is acute. It is caused by vast industrial growth and agricultural intensification. Concentrations of Cd, Pb, Zn, Cu, Mn, Fe, Mg, and Kwere determined in the muscles, liver, and gonads sabrefish from Rybinsk Reservoir areas with different anthropogenic loads. The tissue samples were analyzed by atomic absorption spectrometry. Heavy metals accumulated more intensively in the body of fish from more polluted areas of the reservoir. Among the analyzed elements, the maximum accumulation levels were found for K, Zn, and Fe and the minimum levels were observed for Cd and Pb. The gonads contained the largest concentration of Cd and Mn, the muscles contained the highest concentrations of Mg, and the other elements mainly accumulated in the liver of sabrefish. The THQ and HI values for all elements did not exceed 1, which suggests that there is no potential non-carcinogenic risk to human health. The target values of carcinogenic risk (TR) for cadmium ranged from 8.32 × 10−6 to 1.22 × 10−4 in the muscles. The increased content of cadmium in the gonads of sabrefish not only poses a risk to human health, but also to the reproduction of this species in the Rybinsk Reservoir.
1. Introduction
Nowadays, the problem of inland water pollution is acute. It is caused by vast industrial growth, agricultural intensification, and urban development along the banks of rivers [1,2]. Sources of pollution are often domestic and industrial wastewater, runoff from landfills, agricultural land, and urban areas [3,4,5,6].
Heavy metals are the most ubiquitous environmental pollutants affecting the quality of water resources even in the most remote places on Earth, for example, Tibet, Sundarbans, Amazonia, the Pacific region, the Polar region, and others [7,8,9,10,11,12]. Inland waters such as rivers, lakes, streams, and even groundwater are also subject to heavy metal pollution [13,14,15,16,17,18,19].
The content of pollutants in water reflects only short-term exposure and does not always show the state of biota due to the diffusion of contaminants and their concentrations below the limit of detection, which may increase over time [2,20]. Heavy metals can enter and accumulate in the fish body by chemisorption, mechanical capture of suspended particles, and absorption by gills and through the digestive tract viafood [21]. The latter way is considered the most dangerous because the toxic properties of substances can manifest themselves not only in prey, but also in predators through food webs [22]. Once in the fish body, heavy metals replace important minerals for vital activity and block biological functions, affecting physiological and biochemical parameters [23,24,25]. Therefore, fish are a convenient test object for studying water pollution [26,27,28,29].
Since fish area source of valuable proteins, a number of vitamins, minerals, and fatty acids, especially omega-3, which are essential for human health, they play an important role in the human diet [30,31]. However, after accumulating heavy metals, fish transmit them to humans when eaten [32,33].
Sabrefish Pelecus cultratus is a pelagic species widespread in both fresh and brackish waters of Europe and Russia [34,35,36,37,38,39,40]. The species is a planktophage and ichthyophage, able to consume a very wide range of food organisms. Juveniles feed mainly on plankton, while the food of adults is diverse. Both plankton and benthic larvae of chironomids and terrestrial and aquatic insects are found in its intestines. A significant part of the sabrefish diet is juveniles of other fish species [41,42,43]. Due to its broad dietary spectrum, the species is characterized by a high nutritional value [44,45]. Sabrefish is a target for commercial fisheries, which in a number of countries has led to the threat of its extinction [46,47,48].
There are only a few studies on the elemental composition of this fish species. Attention has mainly been paid to studies of the mercury content in sabrefish meat [49,50,51]. A number of works are devoted to the study of heavy metal concentrations in the muscles of commercial fish species, including sabrefish [52,53,54]. The most comprehensive study, which includes analysis of different tissues of this species, was conducted in the Danube River [55]. We have not found any works on the elemental composition of various tissues of sabrefish from water bodies in Russia or health risk assessments associated with the consumption of this species by humans.
Therefore, studies to determine the elemental composition of sichel tissues are relevant for ecological monitoring of water bodies, as well as for assessing both the physiological state of individuals and the quality of fish products consumed by humans.
The purpose of the work is to study the features of the accumulation of essential and toxic elements in the muscles, liver, and gonads of Pelecus cultratus and to assess the risks to human health when consuming this species.
2. Materials and Methods
2.1. Research Area
Thisresearch was conducted in the Rybinsk Reservoir, the largest artificial water body in the Russian Federation [56,57]. Its area is 4550 km2, its maximum length is 250 km, its width is 70 km, its average depth is 5.6 m, and its catchment area is 150,500 km2 [58,59]. The reservoir is a lake-type eutrophic water body [60]. The profundal macrozoobenthos is represented by an oligochaete–chironomid complex [61]. Intense fishing is carried out in this reservoir. For many people living on its shores, fishing remains the only means of subsistence [62,63]. In Cherepovets, the north-eastern part of the reservoir, chemical and metallurgical industries are widely developed. The largest mining and metallurgical company “Severstal” is located there. This area has been subjected to long-term pollution by industrial wastewater, characterized by high concentrations of persistent organic pollutants and heavy metals [64,65]. Scientists have recorded a significant content of heavy metals in the water and bottom sediments [64,66]. In addition, the area is exposed to household wastewater, as well as diffuse runoff from agricultural land and highways [67]. The adverse impact of industrial pollution has been described in numerous research papers [68,69,70,71,72,73,74,75]. Sampling sites where sabrefish were caught differ in the level of anthropogenic load (Figure 1).
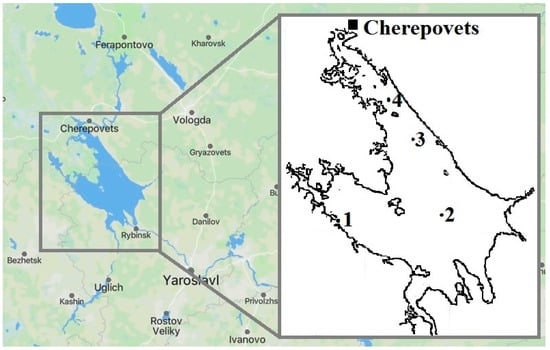
Figure 1.
Schematic map of the Rybinsk Reservoir.
Station 1 (58°23′ N, 37°45′ E.) is considered conditionally clean and at Station 2 (58°25′ N, 38°29′ E), some increased heavy metal concentrations in bottom sediments have been detected. Stations 3 (58°43′ N, 38°16′ E.) and 4 (58°51′ N, 38°06′ E) have the status of heavily polluted. They are located near the metallurgical industrial complex—the main source of reservoir pollution [64,76,77].
2.2. Sampling
All procedures with fish were performed in accordance with the ARRIVE guidelines for the use of animals for research purposes [78].
A total of 39 sabrefish individuals were caught by trawl nets at the end of the feeding period (September–early October) (Table 1). We chose this early fall sampling season to exclude spawning periods, when shifts in biochemical parameters of fish muscles and organs occur.

Table 1.
Dimensional and mass characteristics of sabrefish.
In order to acclimate after capture, the fish were kept in tanks with river water. After this, each individual fish’s length and weight with and without entrails were measured.
The skin was separated from the skeletal muscles on a refrigerant and tissue samples of the muscle along the spine, as well as of the liver and gonads from the internal cavity, were excised. Prior to analysis, all samples were weighed and frozen at a temperature of −18 °C. Since more males were caught than females (35 vs. 4), the elemental content was analyzed only in the testes.
2.3. Heavy Metal Analysis
Concentrations of the following micro- and macro-elements were measured in the fish muscles, liver, and gonads: cadmium (Cd), lead (Pb), zinc, (Zn), copper (Cu), manganese (Mn), iron (Fe), magnesium (Mg), and potassium (K). The tissue samples were analyzed by atomic absorption spectrometry with electrothermal atomization (ETA-AAS) in a KVANT 2-AT spectrophotometer (Kortec Ltd., Moscow, Russia). The ash was dissolved in 5 mL of 20% HCl and filtered through filter paper. The correctness of determining the concentration of elements was checked using the state standard samples for atomic absorption spectrophotometry. The concentration of potassium was determined using the official methods of AOAC International [79]. The results obtained were expressed in mg/kg of wet weight.
2.4. Health Risk Assessment
It is known that heavy metals can enter the human body and affect it in three ways: through the gastrointestinal tract or skin or via inhalation [80]. In this study, the first pathway was considered to assess the risk to human health. To determine the risk over a lifetime of fish consumption, the target hazard quotient (THQ), hazard index (HI), and target cancer risk (TR), determined by generally accepted Formulas (1), (2), and (3), respectively, were used [81,82,83].
where EF—exposure frequency (365 days/year), ED—exposure duration (74 years), Ir—daily consumption of fish (according to FAO data for the Russian Federation in 2020; 0.015 kg/day for pelagic fish), C—metal concentration in fish, mg/kg, RfD—reference peroral dose, mg/kg/day, BW—average human weight (74 kg in the Russian Federation), TA—average exposure time (365 days/year×ED), and CSF—cancer slope factor for carcinogenic metals (mg/kg/day).
THQ = EF × ED × Ir × C/RfD × BW × TA,
HI = THQCd + THQPb + … + THQn …,
TR = EF × ED × Ir × C × CSF/BW × TA,
The RfD values of Cd, Pb, Zn, Cu, Mn, and Fe are 0.001, 0.0035, 0.3, 0.04, 0.14, and 0.7 mg/kg/day, respectively [80,81,84,85]. The CSF for Cd is 15 mg/kg/day and for Pb it is 0.0085 mg/kg/day [81,86].
2.5. Statistical Analysis
The data were checked for normality of the distribution using a Shapiro–Wilk test. Since the data did not follow a Gaussian distribution, the Kruskal–Wallis criterion and the Dunn multiple comparison criterion were applied to compare between different sampling areas and between tissues. The results of the study are presented in the form of mean values and their standard deviations (x ± SD). Differences between the compared parameters were considered statistically significant at p < 0.05.
3. Results
3.1. The Concentration of Micro- and Macro-Elements in Sabrefish from Different Areas of the Reservoir, Characterized by Varying Degrees of Anthropogenic Load
The highest Cd and Pb concentrations were found, respectively, in the gonads and liver of sabrefish from Station 4; for Zn, Cu, and Fe, the highest concentrations were found in the liver of individuals from Station 3; for Mn, the highest concentration was found in the gonads of fish from Station 3; and for Mg and K, the highest concentrations were found in the gonads of sabrefish from Station 1 (Table 2).

Table 2.
The content of micro- and macro-elements in the muscles, liver, and gonads of sabrefish.
The lowest concentrations of Cd, Zn, Mn, and K were found in the muscles of sabrefish at Station 1, those of Pb and Cu were found in the gonads of fish from Station 1, and those of Fe and Mg were found in the gonads of individuals at Station 2 (Table 2).
The contents of Cd, Mg, and K in the muscles of sabrefish from Station 1 were significantly lower than at Stations 4, 3, and 2, respectively (Table 2). In the liver of individuals from Station 3, significantly more Zn and Mn had accumulated than in the liver of sabrefish from Station 4, and higher Cu concentrations were recorded than in fish from Station 1. The Cu content in the gonads of sabrefish from Station 1 was significantly lower than in the gonads of fish from Station 3, and the concentration of Fe was higher than in individuals from Station 2.
Regardless of the sampling area, the following pattern of the intensity of element accumulation in the liver of sabrefish was observed: Cd < Pb < Mg < Mn < Cu < Zn < Fe < K. No such dependence was found in the muscles and gonads; however, there was a tendency toward greater accumulation of K, Zn, and Fe in these tissues, the least accumulation of Cd was in the muscles, and the least accumulation of Pb was in the gonads.
3.2. Concentration of Micro- and Macro-Elements in Different Parts of Sabrefish
A comparison of micro- and macro-nutrient concentrations in different tissues of sabrefish from Station 1 shows significant statistical differences between the concentration of Cd and K in the muscles and gonads, as well as between the concentration of Cu in the liver and gonads (Figure 2).
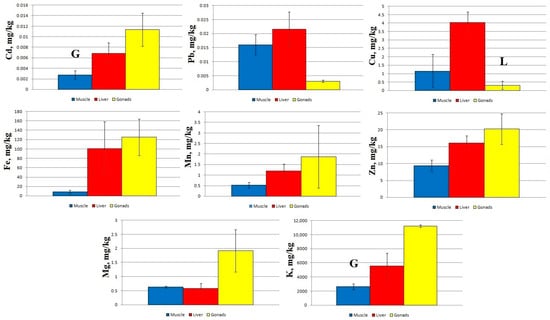
Figure 2.
Concentration of micro- and macro-nutrients in different parts of sabrefish from Station 1 of the Rybinsk Reservoir. G—statistically significant differences from the gonads and muscles, respectively. L—statistically significant differences from the liver and gonads, respectively. Muscles n = 7, liver n = 7, gonads n = 6.
At Station 2, statistically significant differences in Pb, Fe, and K concentrations were shown for the liver and gonads, in Cu and Zn concentrations for the muscles and liver, and in Mn and Mg concentrations for the muscles and gonads (Figure 3).
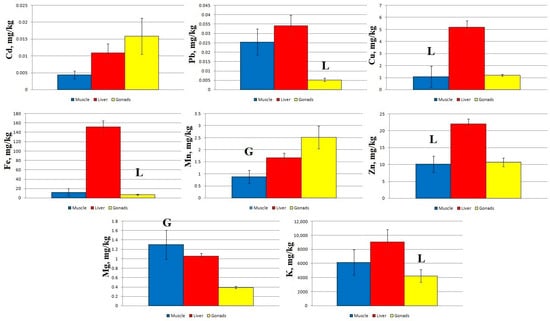
Figure 3.
Concentration of micro- and macro-nutrients in different parts of sabrefish from Station 2 of the Rybinsk Reservoir. G—statistically significant differences from the gonads and muscles or liver, respectively. L—statistically significant differences from the liver and gonads or muscles, respectively. Muscles n = 10, liver n = 10, gonads n = 10.
At Station 3, statistically significant differences are found between the concentrations of Cd and Mn in the muscles and gonads of sabrefish, as well as between the concentrations of Cu, Fe, and Zn in the muscles and liver (Figure 4).
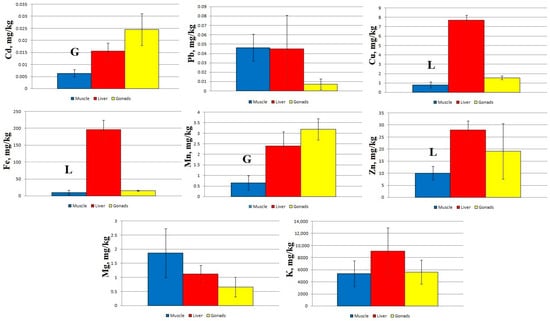
Figure 4.
Concentration of micro- and macro-nutrients in different parts of sabrefish from Station 3 of the Rybinsk Reservoir. G—statistically significant differences from the gonads and muscles, respectively. L—statistically significant differences from the liver and gonads or muscles, respectively. Muscles n = 12, liver n = 12, gonads n = 10.
At Station 4, significant differences were found between the content of Pb in the liver and gonads, between the concentration of Cu and Fe in the muscles and liver, and between Mg concentrations in the muscles and gonads (Figure 5).
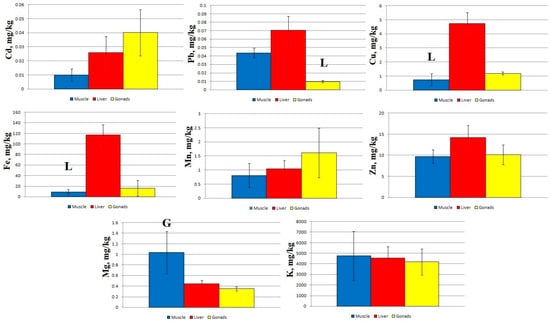
Figure 5.
Concentration of micro- and macro-nutrients in different parts of sabrefish from Station 4 of the Rybinsk Reservoir. G—statistically significant differences from the gonads and muscles, respectively. L—statistically significant differences from the liver and gonads or muscles, respectively. Muscles n = 10, liver n = 10, gonads n = 9.
Regardless of the sampling area, the concentration of Cd and Mn increased in the following order: muscles → liver → gonads. Zn concentration at Stations 2–4 increased in the order muscles → gonads → liver, and at Station 1 it increased in the order muscles → liver → gonads. The other elements accumulated mainly in the liver of sabrefish, with the exception of Mg, which accumulated more intensively in the fish muscles at Stations 2–4.
3.3. Health Risk Assessment
The values of THQ and HI for specific tissues of sabrefish from different areas of the reservoir are presented in Table 3. The results of the study show that the THQ and HI indices for all metals do not exceed the permissible threshold (<1).

Table 3.
Target hazard quotient (THQ) and hazard index (HI) of sabrefish.
The HI value, regardless of the sampling area, decreases in the following order: liver → gonads → muscles.
The carcinogenic risk is calculated only for Cd and Pb because the carcinogenic potency slope factor of carcinogens (CSF) exists only for these metals (Table 4).

Table 4.
Target cancer risk (TR) estimates of sichel sampled.
The calculated Pb values are less than 1 × 10−6 and Cd values range from 8.32 × 10−6 in the muscles at Station 1 to 1.22 × 10−4 in the gonads at Station 4 (Table 4).
4. Discussion
This study has revealed a tendency for more intensive accumulation of heavy metals (Cd, Pb, Zn, Cu, Mn, and Fe) in sabrefish from an area with a high anthropogenic load. A number of studies have also shown that stations located near the industrial complex (Stations 3 and 4) are considered the most unfavorable for aquatic organisms, in terms of heavy metal concentrations in both the water and bottom sediments and according to the results of biotesting in different areas of the reservoir [66,77,87,88].
In order to assess the degree of water pollution at the studied stations, the concentrations of heavy metals recorded in sabrefish during this study were compared with those from other studies. As mentioned above, only limited information is available on heavy metal concentrations in this fish species. Acomparison of our results with the data published by Subotić [55] suggests that in our study Cd, Cu, Fe, and Mn concentrations in the muscles and gonads of sabrefish are higher since they were obtained for wet weight and are comparable or exceed those in sabrefish from the Danube River, expressed on a dry weight basis. The heavy metal contents of Cd, Cu, Pb, and Zn in the muscles of sabrefish from the Rybinsk Reservoir are higher than from Lake Ladoga and lower than in this fish species from the Caspian Sea and water bodies of Moldova [52,53,54]. The literature data and the results of our study show that the metal concentrations in fish tissues vary widely depending on the sampling area. It is known that heavy metal contents in aquatic organisms are influenced not only by the anthropogenic load on the water body, the concentration of elements, and the duration of exposure, but also by the hydrochemical factors of the aquatic environment, because the solubility of a number of trace elements located in hard-to-reach compounds in silts depends on the oxygen level, pH, and other environmental parameters [89,90,91,92].
In our study, no single pattern of distribution of metals in the sabrefish body was found, with the exception of the liver—the organ responsible for the redistribution and detoxification of heavy metals [93,94,95]. In addition, the concentrations of metals in the liver are proportional to those present in the aquatic environment [96,97]. Probably due to this reason, in this organ, a general pattern of element accumulation was revealed for sabrefish from different stations of the reservoir, which may reflect the level of pollution of the entire reservoir.
In all the tissues of sabrefish, the content of elements such as K, Fe, and Zn significantly exceeded those of the other substances studied. At the same time, the concentration of K was several times higher than other elements. These metals are vital for living organisms and are called essential [98,99]. Normally, they should accumulate in large quantities because of their important role in the work of biological systems (enzymatic, metabolic, regulatory, and other roles) [100,101,102,103]. Deficiency of essential elements can lead to improper enzyme-mediated metabolic functions, congenital anomalies, immunological disorders, and chronic diseases [102,104,105,106,107,108]. High levels of K, Fe, and Zn in fish, including pelagic species, have also been reported by other researchers both in freshwater and marine ecosystems [101,104,109,110,111]. The highest K values, in comparison with other elements, were observed in the Black Sea kalkan Psetta Maxima Maeotica [108]. Potassium is one of the most important minerals in the body. It is involved in acid–base balance, glycogenesis reactions, regulation of osmotic pressure, conduction of nerve impulses, and muscle contractions [105,112].
In our study, the contents of Cd and Pb in the analyzed tissues of sabrefish were the lowest. Similar results, where the concentrations of essential elements exceeded those of Cd and Pb, were shown for both marine and freshwater pelagic fish species inhabiting rivers as well as lakes [96,113,114,115,116,117,118]. The reason for the low accumulation of nonessential metals (Cd and Pb) is the lack of need for them in physiological processes in living organisms [119]. Nevertheless, there are studies showing that the content of toxic elements in fish organs may exceed the concentration of essential ones due to the high level of anthropogenic load [120].
The pattern of macro- and micro-element distribution in the organs of sabrefish had some special features: an increased content of Cd and Mn was recorded in the gonads, an increased content of Mg was found in the muscle tissue, and other elements accumulated mainly in the liver of this fish species (Figure 2, Figure 3, Figure 4 and Figure 5).
Gonads are important reproductive organs responsible for producing gametes needed for fertilization [121]. In general, lower concentrations of heavy metals in the sex glands of fish may indicate a certain physiological mechanism of protection in these organs from the effects of heavy metals in order to avoid disruption of their work [122]. However, elevated Cd levels in the testes of sabrefish pose a risk for the reproduction of this species in the reservoir. Our results are consistent with findings of a number of studies on freshwater fish species, which have also reported higher Cd concentrations in the gonads in comparison with those in the liver and muscles [122,123,124]. In the gonads of sabrefish from the Danube River, Cd concentrations were below the detection limit [55].
Manganese is an essential microelement that acts as a cofactor in many enzymatic processes, and its deficiency can lead to reproductive abnormalities [102,125,126,127]. A higher Mn content in the gonads than in the muscles and liver of marine and freshwater fish species has been reported in a number of studies [33,123,128]. However, in the gonads of sabrefish from the Danube River, the content of Mn was lower compared to the muscles and liver [55].
It is known that magnesium ions are concentrated in the intercellular space of soft tissues and are in a bound form in fish muscles [129]. In the marine pelagic species Caesio varilineata and Caesio lunaris, the magnesium content is higher in the muscles than in the gonads and liver [130]. However, in Van fish (Alburnus tarichi), a cyprinid fish species which lives in the alkaline Lake Van, the Mg content in the muscles is lower than in the liver and gonads [131].
The liver is considered the main organ of metabolism and the most metabolically active tissue which accumulates and neutralizes toxic substances, including heavy metals [33,122,132]. It is known that the liver is a target organ for most metals, regardless of their route of entry, and is the optimum tissue for water monitoring, since higher concentrations of metals remain in this organ for a long time [96,97,115]. The results of our study regarding the greater accumulation of Fe and Cu in the liver of sabrefish are comparable to the work by Subotić [55] on a similar fish species. The literature data confirm that Pb accumulates more intensively in the liver of freshwater fish than in muscle tissue [92,133]. Despite the fact that muscles are not an active participant in the process of accumulation of the elements under study, they need to be analyzed, since they are considered the main edible part of fish and are important for human health [33,134].
It is interesting to note that in our study, the concentration of Zn at conditionally clean Station 1 was higher in the gonads, while at the other stations it was higher in the liver. It is known that this element plays an important role in the development of reproductive organs and fish reproduction, and its concentration in the gonads can be several times higher than in the muscles [108,132]. The tissues of sabrefish from Stations 2–4, subjected to anthropogenic load, contained higher concentrations of cadmium than the fish collected at Station 1. Zinc can be replaced by cadmium, reducing the harmful effects of the latter [135,136]. Perhaps for this reason, the zinc content in the gonads of fish from Station 1 was higher than in fish caught at the other stations.
Fish consumption is one of the main sources of heavy metal exposure to humans [84]. A human health risk assessment, associated with the duration of exposure to heavy metals, was performed using the recorded concentrations of Cd, Pb, Zn, Cu, Mn, and Fe in the tissues of sabrefish. The THQ data obtained in this study show that there is no potential risk for people related to the consumption of sabrefish from the Rybinsk Reservoir. The HI corresponded to the THQ model and did not exceed the permissible limit (<1). Thus, people will not experience non-carcinogenic health effects when consuming sabrefish from the Rybinsk Reservoir.
It is known that a number of toxic elements, such as lead, cadmium, methylmercury, and arsenic, have carcinogenic, mutagenic, and teratogenic effects when they enter the human body due to their insufficient excretion [86,137]. Carcinogenic risk (TR) indicates an increased likelihood of developing cancer in a person over the course of their lifetime due to the exposure to potential carcinogens [138]. The risk of developing cancer is considered insignificant, at TR < 1 × 10−6. When TR > 1 × 10−4, the consumers are in the unacceptable risk zone and some correction is needed [86,139,140].
In our study, the carcinogenic risks of Pb were less than 1 × 10−6, which indicates the absence of carcinogenic effects from sabrefish associated with this metal. As for Cd, theresults showed a slightly different picture. The consumption of muscle tissue of fish caught at Station 1 can be considered conditionally safe. For the other tissues, the risk of cancer from Cd is 1 per 100,000, and with regular lifetime consumption of fish gonads from Station 4 it is 1 per 10,000, because the TR values for these tissues exceeded 1 × 10−4. Long-term exposure to Cd can cause kidney failure, disrupt the gastrointestinal tract, reduce bone mineral density, and cause osteoporosis [102,104,117]. It is worth noting that in the areas adjacent to the reservoir, there is a steady increase in oncological diseases among all age groups of the population, and the highest number of cancer cases in the country [141,142].
Thus, due to potential human health risks associated with consumption of sabrefish, it is recommended to constantly monitor the levels of metals in their tissues, especially in fish caught from areas with an increased anthropogenic load. Given the target values of the carcinogenic risk for cadmium, we do not recommend using the internal organs of sabrefish from the Rybinsk Reservoir for food.
5. Conclusions
In this study, the concentrations of macro- and micro-elements in the muscles, liver, and gonads of sabrefish from Rybinsk Reservoir areas with different levels of anthropogenic load were determined. Heavy metals accumulated more intensively in the body of fish from more polluted areas of the reservoir. Among the analyzed elements, the maximum accumulation levels were found for K, Zn, and Fe and the minimum for Cd and Pb. The gonads contained the highest concentration of Cd and Mn, the muscles contained the highest concentration Mg, and the other elements accumulated mainly in the liver of sabrefish. In regard to human health, the THQ and HI values for all the elements did not exceed 1, which suggests that there is no potential non-carcinogenic risk to human health from heavy metals. The target values of carcinogenic risk (TR) for lead in all the tissues of fish from all the stations were below the threshold of 10−6, and for cadmium they ranged from 8.32 × 10−6 in the muscles of fish from Station 1 to 1.22 × 10−4 in the gonads of fish from Station 4. The increased content of cadmium in the gonads of sabrefish not only poses a risk to human health, but also to the reproduction of this species in the Rybinsk Reservoir. The data obtained in this study on the elemental content, including concentrations of toxic elements in the tissues of sabrefish, complement and expand our knowledge on the chemical and environmental situation in the Rybinsk Reservoir.
Author Contributions
Conceptualization, E.A.F. and A.A.P.; methodology, Y.V.Z.; formal analysis Y.V.Z.; investigation, E.A.F., A.A.P. and Y.V.Z.; resources, E.A.F. and A.A.P.; data curation E.A.F.; writing—original draft preparation, A.A.P.; writing—review and editing, E.A.F. and A.A.P.; visualization, E.A.F.; supervision, E.A.F.; project administration, E.A.F.; funding acquisition, E.A.F. and Y.V.Z. All authors have read and agreed to the published version of the manuscript.
Funding
The study was performed with the support of the Russian Ministry of Science and Higher Education, Project No. FENZ-2023-0004.
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee of P.G. Demidov Yaroslavl State University (protocol number: 01, approved on 8 June 2023).
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.-Q. Heavy metals and pesticides toxicity in agricultural soil and plants: Ecological risks and human health implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef]
- Lee, S.J.; Mamun, M.; Atique, U.; An, K.G. Fish Tissue Contamination with Organic Pollutants and Heavy Metals: Link between Land Use and Ecological Health. Water 2023, 15, 1845. [Google Scholar] [CrossRef]
- Hasan, M.K.; Shahriar, A.; Jim, K.U. Water pollution in Bangladesh and its impact on public health. Heliyon 2019, 5, e02145. [Google Scholar] [CrossRef]
- Mokarram, M.; Saber, A.; Sheykhi, V. Effects of heavy metal contamination on river water quality due to release of industrial effluents. J. Clean. Prod. 2020, 277, 123380. [Google Scholar] [CrossRef]
- Mushtaq, N.; Singh, D.V.; Bhat, R.A.; Dervash, M.A.; Hameed, O.B. Freshwater Contamination: Sources and Hazards to Aquatic Biota. In Fresh Water Pollution Dynamics and Remediation; Springer: Singapore, 2020; pp. 27–50. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, J.H.; Kim, K.; Mun, H.; Park, N.; Jeon, J. Identification, quantification, and prioritization of new emerging pollutants in domestic and industrial effluents, Korea: Application of LC-HRMS based suspect and non-target screening. J. Hazard. Mater. 2021, 402, 123706. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Gui, D.; Liu, W.; Wu, Y.P. Risk for Indo-Pacific humpback dolphins (Sousa chinensis) and human health related to the heavy metal levels in fish from the Pearl River Estuary, China. Chemosphere 2020, 240, 124844. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lu, Z.; Xiao, K.; Zeng, L.; Wang, J.; Gabrielsen, G.W. Antarctic Adélie penguin feathers as bio-indicators of geographic and temporal variations in heavy metal concentrations in their habitats. Ecotoxicol. Environ. Saf. 2020, 206, 111135. [Google Scholar] [CrossRef] [PubMed]
- Diarra, I.; Prasad, S. The current state of heavy metal pollution in Pacific Island Countries: A review. Appl. Spectrosc. Rev. 2021, 56, 27–51. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Q.; Su, W.; Cao, G.; Fu, G.; Du, W. Potential Sources, Pollution, and Ecological Risk Assessment of Potentially Toxic Elements in Surface Soils on the North–Eastern Margin of the Tibetan Plateau. Toxics 2022, 10, 368. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.; Naz, A.; Maiti, S.K. Distribution, speciation, and bioaccumulation of potentially toxic elements in the grey mangroves at Indian Sundarbans, in relation to vessel movements. Mar. Environ. Res. 2023, 189, 106042. [Google Scholar] [CrossRef]
- Silva, M.H.L.; Castro, A.C.L.; Silva, I.S.D.; Cabral, P.F.P.; Azevedo, J.W.J.; Soares, L.S.; Bandeira, A.M.; Basso, M.J.; Nunes, J.L.S. Determination of metals in estuarine fishes in a metropolitan region of the coastal zone of the Brazilian Amazon. Mar. Pollut. Bull. 2023, 186, 114477. [Google Scholar] [CrossRef]
- Kumari, P.; Chowdhury, A.; Maiti, S.K. Assessment of Heavy Metal in the Water, Sediment, and Two Edible Fish Species of Jamshedpur Urban Agglomeration, India with Special Emphasis on Human Health Risk. Hum. Ecol. Risk Assess. Int. J. 2018, 24, 1477–1500. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Q.; Ren, B.; Luo, J.; Yuan, J.; Ding, X.; Bian, H.; Yao, X. Trends and Health Risks of Dissolved Heavy Metal Pollution in Global River and Lake Water from 1970 to 2017. In Reviews of Environmental Contamination and Toxicology; Springer: Cham, The Netherland, 2020; Volume 251, pp. 1–24. [Google Scholar] [CrossRef]
- Algül, F.; Beyhan, M. Concentrations and sources of heavy metals in shallow sediments in Lake Bafa, Turkey. Sci. Rep. 2020, 10, 11782. [Google Scholar] [CrossRef] [PubMed]
- Akindele, E.O.; Omisakin, D.A.; Oni, O.A.; Aliu, O.O.; Omoniyi, G.E.; Akinpelu, O.T. Heavy metal toxicity in the water column and benthic sediments of a degraded tropical stream. Ecotoxicol. Environ. Saf. 2020, 190, 110153. [Google Scholar] [CrossRef]
- Egbueri, J.C. Heavy metals pollution source identification and probabilistic health Risk Assessment of Shallow Groundwater in Onitsha, Nigeria. Anal. Lett. 2020, 53, 1620–1638. [Google Scholar] [CrossRef]
- Sivakumar, K.; Shanmugasundaram, A.; Jayaprakash, M.; Prabakaran, K.; Muthusamy, S.; Ramachandran, A.; Venkatramanan, S.; Selvam, S. Causes of heavy metal contamination in groundwater of Tuticorin industrial block, Tamil Nadu, India. Environ. Sci. Pollut. Res. 2021, 28, 18651–18666. [Google Scholar] [CrossRef]
- Krivokapić, M. Study on the Evaluation of (Heavy) Metals in Water and Sediment of Skadar Lake (Montenegro), with BCF Assessment and Translocation Ability (TA) by Trapa natans and a Review of SDGs. Water 2021, 13, 876. [Google Scholar] [CrossRef]
- Anishchenko, O.V.; Gladyshev, M.I.; Kravchuk, E.S.; Kalacheva, G.S.; Gribovskaya, I.V. Assessment of the Yenisei River Anthropogenic Pollution by Metals Concentrations in the Main Ecosystem Compartments Upstream and Downstream Krasnoyarsk City (Russia). J. SibFU. Biol. 2010, 3, 82–98. (In Russian) [Google Scholar]
- Karim, S.; Aouniti, A.; Taleb, M.; El hajjaji, F.; Belbachir, C.; Rahhou, I.; Achmit, M.; Hammouti, B. Evaluation of heavy metal concentrations in seven commercial marine fishes caught in the Mediterranean coast of Morocco and their associated health risks to consumers. J. Environ. Biotechnol. Res. 2019, 8, 1–13. [Google Scholar]
- Liu, J.; Cao, L.; Dou, S. Trophic transfer, biomagnification and risk assessments of four common heavy metals in the food web of Laizhou Bay, the Bohai Sea. Sci. Total Environ. 2019, 670, 508–522. [Google Scholar] [CrossRef]
- Abah, J.; Mashebe, P.; Sylvanus, O.A. Preliminary Assessment of Some Heavy Metals Pollution Status of Lisikili River Water in Zambezi Region, Namibia. Int. J. Environ. Pollut. Res. 2016, 4, 13–30. [Google Scholar]
- Bhat, S.A.; Hassan, T.; Majid, S. Heavy metal toxicity and their harmful effects on living organisms—A review. Int. J. Med. Sci. Diagn. Res. 2019, 3, 106–122. [Google Scholar] [CrossRef]
- Vajargah, M.F. A review on the effects of heavy metals on aquatic animals. Environ. Sci. 2021, 2, 865–869. [Google Scholar] [CrossRef]
- Authman, M.M.; Zaki, M.S.; Khallaf, E.A.; Abbas, H.H. Use of fish as bio-indicator of the effects of heavy metals pollution. J. Aquac. Res. Dev. 2015, 6, 4. [Google Scholar] [CrossRef]
- Andriani, I.; Litaay, M.; Tahir, D. Medaka fish Oryziasjavanicus Bleeker as bio-indicator of lead content in waters. J. Phys. Conf. Ser. 2019, 1341, 022028. [Google Scholar] [CrossRef]
- Moiseenko, T.I.; Gashkina, N.A. Distribution and bioaccumulation of heavy metals (Hg, Cd and Pb) in fish: Influence of the aquatic environment and climate. Environ. Res. Lett. 2020, 15, 115013. [Google Scholar] [CrossRef]
- Kaymak, G.; Kayhan, F.E.; Ertuğ, N.D.Y. A biomonitoring study: Using the biomarkers in Cyprinus carpio for the evaluation of water pollution in Sapanca lake (Sakarya, Turkey). Int. J. Agric. Environ. Food Sci. 2021, 5, 107–121. [Google Scholar] [CrossRef]
- Balami, S.; Sharma, A.; Karn, R. Significance of Nutritional Value of Fish for Human Health. Malays. J. Halal Res. 2019, 2, 32–34. [Google Scholar] [CrossRef]
- Pradeepkiran, J.A. Aquaculture role in global food security with nutritional value: A review. Transl. Anim. Sci. 2019, 3, 903–910. [Google Scholar] [CrossRef]
- Yang, G.; Sun, X.; Song, Z. Trophic level and heavy metal pollution of Sardinella albella in Liusha Bay, Beibu Gulf of the South China Sea. Mar. Pollut. Bull. 2020, 156, 111204. [Google Scholar] [CrossRef]
- El-Shenawy, N.S.; EL-Hak, H.N.G.; Ghobashy, M.A.; Mansour, F.A.; Soliman, M.F. Using antioxidant changes in liver and gonads of Oreochromis niloticus as biomarkers for the assessment of heavy metals pollution at Sharkia province, Egypt. Reg. Stud. Mar. Sci. 2021, 46, 101863. [Google Scholar] [CrossRef]
- Kujawa, R.; Furgała-Selezniow, G.; Mamcarz, A.; Lach, M.; Kucharczyk, D. Influence of temperature on the growth and survivability of sichel larvae Pelecuscultratus reared under controlled conditions. Ichthyol. Res. 2015, 62, 163–170. [Google Scholar] [CrossRef]
- Kujawa, R.; Lach, M.; Pol, P.; Ptaszkowski, M.; Mamcarz, A.; Nowosad, J.; Furgała-Selezniow, G.; Kucharczyk, D. Influence of water salinity on the survival of embryos and growth of the sichel larvae Pelecuscultratus (L.) under controlled conditions. Aquac. Res. 2017, 48, 1302–1314. [Google Scholar] [CrossRef]
- Kolosyuk, G.G.; Izherskaya, V.A. Sabrefish distribution in Northern Caspian and factors influencing its concentrations during the summer season. Vestn. ASTUSer. Fish. Ind. 2017, 1, 26–34. (In Russian) [Google Scholar] [CrossRef]
- Karabanov, D.P.; Pavlov, D.D.; Bazarov, M.I.; Borovikova, E.A.; Gerasimov, Y.V.; Kodukhova, Y.V.; Smirnov, A.K.; Stolbunov, I.A. Alien species of fish in the littoral of Volga and Kama reservoirs (Results of complex expeditions of IBIW RAS in 2005–2017). Trans. IBIW RAS 2018, 82, 67–80. [Google Scholar] [CrossRef]
- Solomatin, Y.I.; Bazarov, M.I. Density of the Fish Population in River Channel Parts of the Ivankovo Reservoir in 2012–2015. Inland Water Biol. 2018, 11, 359–362. [Google Scholar] [CrossRef]
- Flerova, E.A.; Malin, M.I.; Klyuchnikov, A.S.; Payuta, A.A.; Bogdanova, A.A.; Andreeva, M.I. Species composition and biological characteristics of small river fishes of the state natural order “Yaroslavsky”. Trans. IBIW RAS 2019, 86, 80–89. (In Russian) [Google Scholar] [CrossRef]
- Năstase, A.; Honț, S.; Iani, M.; Paraschiv, M.; Cernișencu, I.; Năvodaru, I. Ecological status of fish fauna from Razim Lake and the adjacent area, the Danube Delta Biosphere Reserve, Romania. Acta Ichthyol. Piscat. 2022, 52, 43–52. [Google Scholar] [CrossRef]
- Tatrai, I.; Herzig, A. Effect of habitat structure on the feeding efficiency of young stages of razor fish (Pelecuscultratus (L.)): An experimental approach. Hydrobiologia 1995, 299, 75–81. [Google Scholar] [CrossRef]
- Liu, Z.; Herzig, A. Food and feeding behaviour of a planktivorous cyprinid, Pelecuscultratus (L.), in a shallow eutrophic lake, Neusiedler See (Austria). Hydrobiologia 1996, 333, 71–77. [Google Scholar] [CrossRef]
- Naumenko, E.N.; Khlopnikov, M.M.; Rudynskaya, L.V. Energy flows in the Vistula Lagoon of the Baltic Sea. J. SibFUBiol. 2012, 5, 184–202. (In Russian) [Google Scholar]
- Payuta, A.A.; Flerova, E.A. Some indicators of metabolism in the muscles, liver, and gonads of pike-perch Sander lucioperca and sichel Pelecuscultratus from the Gorky Reservoir. J. Ichthyol. 2019, 59, 255–262. [Google Scholar] [CrossRef]
- Payuta, A.A.; Bogdanova, A.A.; Flerova, E.A.; Miroshnichenko, D.A.; Malin, M.I.; Andreeva, M.I. Chemical composition of fish muscle of small rivers of the Yaroslavl region. Vestn. ASTU Ser. Fish. Ind. 2019, 1, 112–121. (In Russian) [Google Scholar] [CrossRef]
- Curtean-Bănăduc, A.; Cismaș, I.-C.; Bănăduc, D. Pelecuscultratus (Linnaeus, 1758) on site management decisions support system—A Carpathian Natura 2000 site study case. Rom. J. Biol. Zool. 2015, 60, 27–37. [Google Scholar]
- Kujawa, R.; Kucharczyk, D.; Furgała–Selezniow, G.; Mamcarz, A.; Ptaszkowski, M.; Mateuszj, M. Substitution of natural food with artificial feed during rearing larvae of Sichel Pelecuscultratus (L.) under controlled conditions. Turk. J. Fish. Aquat. Sci. 2016, 16, 643–650. [Google Scholar] [CrossRef]
- Traczuk, P.; Kalinowska, K.; Ulikowski, D.; Kapusta, A. Protected and alien fish species in Polish lakes in 2014–2021. Ecohydrology. Hydrobiol. 2023, in press. [Google Scholar] [CrossRef]
- Falandysz, J.; Chwir, A.; Wyrzykowska, B. Total mercury contamination of some fish species in the firth of Vistula and the lower Vistula river, Poland. Pol. J. Environ. Stud. 2000, 9, 335–339. [Google Scholar]
- Nowosad, J.; Sieszputowska, J.; Kucharczyk, D.; Łuczyńska, J.; Sikora, M.; Kujawa, R. Dynamics of mercury content in adult sichel (Pelecuscultratus L.) tissues from the Baltic Sea before and during spawning. Mar. Environ. Res. 2019, 14, 75–80. [Google Scholar] [CrossRef]
- Tyutin, A.V.; Medyantseva, E.N.; Gremyachikh, V.A.; Komov, V.T. Host—Parasite relationships in the plerocercoids of Ligula intestinalis (L.) (Cestoda: Pseudophyllidea)—Carp fish system and the mercury accumulation in the muscles of hosts. Parazitologiya 2019, 53, 241–250. (In Russian) [Google Scholar] [CrossRef]
- Zarei, M.; Asadi, A.; Zarei, S.M. Levels of some heavy metal concentration in fishes tissue of southern Caspian Sea. Int. J. Phys. Sci. 2011, 6, 6220–6225. [Google Scholar] [CrossRef]
- Zubcova, E.; Zubcova, N.; Bileţchi, L.; Bulat, D.; Bulat, D. Monitoring the accumulation of heavy metals in fish products. In Fiziologiaşisănătatea; Furdui, T., Lacusta, V., Vovc, V., Ciochină, V., Eds.; Materialelecongresului VII al fi ziologilor din Republica Moldova: Chișinău, Republic of Moldova, 2012; pp. 395–400. (In Russian) [Google Scholar]
- Arshanitsa, N.M.; Grebtsov, M.R.; Stekolnikov, A.A. The content of metals in muscle tissue of fish from some waterbodies of the North-West of Russia. Int. Bull. Vet. Med. 2016, 3, 57–63. (In Russian) [Google Scholar]
- Subotić, S.; Višnjić-Jeftić, Ž.; Spasić, S.; Hegediš, A.; Krpo-Ćetković, J.; Lenhardt, M. Concentrations of 18 elements in muscle, liver, gills, and gonads of Sichel (Pelecuscultratus), ruffe (Gymnocephaluscernua), and European perch (Percafluviatilis) in the Danube River near Belgrade (Serbia). Water Air Soil. Pollut. 2015, 226, 287. [Google Scholar] [CrossRef]
- Gerasimov, Y.V.; Brazhnik, S.Y.; Strelnikov, A.S. Dynamics of structural parameters of populations of the bream Abramis brama (Cyprinidae) in Rybinsk Reservoir in 1954–2007. J. Ichthyol. 2010, 50, 465–474. [Google Scholar] [CrossRef]
- Lazareva, V.I.; Sokolova, E.A. Metazooplankton of the plain reservoir during climate warming: Biomass and production. Inland Water Biol. 2015, 8, 250–258. [Google Scholar] [CrossRef]
- Avakyan, A.B.; Litvinov, A.S.; Riv’er, I.K. Sixty Year’s Experience in Operating the Rybinsk Reservoir. Water Resour. 2002, 29, 1–11. [Google Scholar] [CrossRef]
- Sakharova, E.G.; Korneva, L.G. Influence of Temperature and Water Level on the Phytoplankton in the Estuarine Zone of the Rybinsk Reservoir Tributary. Inland Water Biol. 2019, 12, 25–32. [Google Scholar] [CrossRef]
- Mineeva, N.M.; Makarova, O.S. Chlorophyll content as an indicator of the modern (2015–2016) trophic state of Volga River reservoirs. Inland Water Biol. 2018, 11, 367–370. [Google Scholar] [CrossRef]
- Perova, S.N. Taxonomic composition and abundance of macrozoobenthos in the Rybinsk Reservoir at the beginning of the 21st century. Inland Water Biol. 2012, 5, 199–207. [Google Scholar] [CrossRef]
- Gerasimov, Y.V.; Strelnikov, A.S.; Brazhnik, S.Y. Dynamics and the state of fishery resources in the Rybinsk Reservoir from 1950–2010. J. Ichthyol. 2013, 53, 486–498. [Google Scholar] [CrossRef]
- Gerasimov, Y.V. Population dynamics of the Rybinsk reservoir Fishes throughout the whole period of its existence: Role of natural and anthropogenic factors. VNIRO Proc. 2015, 156, 67–90. (In Russian) [Google Scholar]
- Chuiko, G.M.; Tomilina, I.I.; Brodsky, E.S.; Shelepchikov, A.A.; Mir-Kadyrova, E.Y.; Pavlov, D.F.; Tillitt, D.E. Accumulation of polychlorinated biphenyls (PCB) associated with bottom sediments in larvae of Chironomus riparius Meigen. Limnologica 2021, 90, 125912. [Google Scholar] [CrossRef]
- Konyukhov, Y.V. Heavy-metal extraction from wastewater by means of iron nanopowder. Steel Transl. 2018, 48, 135–141. [Google Scholar] [CrossRef]
- Tomilina, I.I.; Lozhkina, R.A.; Gapeeva, M.V. Toxicity of Bottom Sediments of the Rybinsk Reservoir According to Long-Term Biotesting Data: Report 1. Toxicological Studies. Inland Water Biol. 2021, 14, 777–787. [Google Scholar] [CrossRef]
- Lozhkina, R.A.; Tomilina, I.I.; Gapeeva, M.V. Long-term dynamics of the water quality in the Rybinsk reservoir according to biotesting. Ecosyst. Transform. 2020, 3, 48–61. [Google Scholar] [CrossRef]
- Shapovalov, D.A.; Gruzdev, V.S.; Vedeshin, L.A. Ecological monitoring of plant “Severstal” (North Steel) technological wastes on ecosystems of water protection zone of Rybin Reservoir within the Sheksna stretch of open water. Ecol. Syst. Devices 2007, 8, 18–25. (In Russian) [Google Scholar]
- Lapirova, T.B.; Zabotkina, E.A. Comparative analysis of the indices of immunophysiological state in bream (Abramis brama (L.)) from parts of the Rybinsk Reservoir with different extents of pollution. Inland Water Biol. 2010, 3, 181–186. [Google Scholar] [CrossRef]
- Tomilina, I.I.; Grebenyuk, L.P.; Chuiko, G.M. Toxicological and teratogenic assessment of bottom sediments from the Rybinsk Reservoir. Inland Water Biol. 2011, 4, 373–382. [Google Scholar] [CrossRef]
- Silkina, N.I.; Mikryakov, D.V.; Mikryakov, V.R. Effect of anthropogenic pollution on oxidative processes in the liver of fish from the Rybinsk Reservoir. Russ. J. Ecol. 2012, 43, 386–389. [Google Scholar] [CrossRef]
- Golovanova, I.L.; Filippov, A.A.; Chuiko, G.M. Effects of heavy metals (Cu and Zn) on digestive glycosidases in benthivorous fish of areas of Rybinsk Reservoir differing in anthropogenic loads. Inland Water Biol. 2014, 7, 286–293. [Google Scholar] [CrossRef]
- Klimova, Y.S.; Chuiko, G.M.; Gapeeva, M.V.; Pesnya, D.S. The use of biomarkers of oxidative stress in zebra mussel Dreissena polymorpha (Pallas, 1771) for chronic anthropogenic pollution assessment of the Rybinsk Reservoir. Contemp. Probl. Ecol. 2017, 10, 178–183. [Google Scholar] [CrossRef]
- Klimova, Y.S.; Chuiko, G.M.; Gapeeva, M.V.; Pesnya, D.S.; Ivanova, E.I. The Use of Oxidative Stress Parameters of Bivalve Mollusks Dreissena Polymorpha (Pallas, 1771) as Biomarkers for Ecotoxicological Assessment of Environment. Inland Water Biol. 2019, 12, 88–95. [Google Scholar] [CrossRef]
- Shcherbina, G.K. Comparative Analysis of the Feeding Spectrum of Bream Abramis brama L. (Cyprinidae, Pisces) in Different Areas of the Rybinsk Reservoir. Inland Water Biol. 2021, 14, 590–596. [Google Scholar] [CrossRef]
- Kozlovskaya, V.I.; German, A.V. Polychlorinated biphenyls and polyaromatic hydrocarbons in the ecosystem of the Rybinskoe Reservoir. Water Resour. 1997, 24, 520–526. [Google Scholar]
- Gapeeva, M.V. Heavy metals in water and sediments of the Rybinsk reservoir. Water Chem. Ecol. 2013, 5, 3–7. (In Russian) [Google Scholar]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]
- Cundiff, P. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists (AOAC): Arlington, VA, USA, 1995. [Google Scholar]
- Elumalai, V.; Brindha, K.; Lakshmanan, E. Human Exposure Risk Assessment Due to Heavy Metals in Groundwater by Pollution Index and Multivariate Statistical Methods: A Case Study from South Africa. Water 2017, 9, 234. [Google Scholar] [CrossRef]
- Vu, C.T.; Lin, C.; Yeh, G.; Villanueva, M.C. Bioaccumulation and potential sources of heavy metal contamination in fish species in Taiwan: Assessment and possible human health implications. Environ. Sci. Pollut. Res. 2017, 24, 19422–19434. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.S.S.; Rahman, M.; Sultana, S.; Babu, S.M.O.F.; Sarker, M.S.I. Bioaccumulation and heavy metal concentration in tissues of some commercial fishes from the Meghna River Estuary in Bangladesh and human health implications. Mar. Pollut. Bull. 2019, 145, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Traina, A.; Bono, G.; Bonsignore, M.; Falco, F.; Giuga, M.; Quinci, E.M.; Vitale, S.; Sprovieri, M. Heavy Metals Concentrations in Some Commercially Key Species from Sicilian Coasts (Mediterranean Sea): Potential Human Health Risk Estimation. Ecotoxicol. Environ. Saf. 2019, 168, 466–478. [Google Scholar] [CrossRef]
- Botwe, B.O. Heavy metal concentrations in five fish species from the Gulf of Guinea and their human health implications. Reg. Stud. Mar. Sci. 2021, 44, 101763. [Google Scholar] [CrossRef]
- Ab Manan, W.N.A.; Zulkifli, N.N. Evaluation of heavy metals content in different local brands of bottled drinking water. ESTEEM Acad. J. 2021, 17, 47–55. [Google Scholar]
- Barone, G.; Storelli, A.; Garofalo, R.; Mallamaci, R.; Storelli, M.M. Residual Levels of Mercury, Cadmium, Lead and Arsenic in Some Commercially Key Species from Italian Coasts (Adriatic Sea): Focus on Human Health. Toxics 2022, 10, 223. [Google Scholar] [CrossRef] [PubMed]
- Tomilina, I.I.; Grebenyuk, L.P.; Lozhkina, R.A. Toxicity of Bottom Sediments of the Rybinsk Reservoir According to Long-Term Biotesting Data. Part 2. Teratological Studies. Inland Water Biol. 2022, 15, 68–79. [Google Scholar] [CrossRef]
- Payuta, A.A.; Pryanichnikova, E.G.; Shcherbina, G.K.; Perova, S.N.; Flerova, E.A. Physiological parameters of bream (Abramis brama L.) in parts of the Rybinsk Reservoir of different types. Inland Water Biol. 2019, 12, 217–224. [Google Scholar] [CrossRef]
- Golovanova, I.L. Effects of heavy metals on the physiological and biochemical status of fishes and aquatic invertebrates. Inland Water Biol. 2008, 1, 93–101. [Google Scholar] [CrossRef]
- Moiseenko, T.I.; Kudryavtseva, L.P.; Gashkina, N.A. Assessment of the geochemical background and anthropogenic load by bioaccumulation of microelements in fish. Water Resour. 2005, 32, 640–652. [Google Scholar] [CrossRef]
- Sheikhzadeh, H.; Hamidian, A.H. Bioaccumulation of Heavy Metals in Fish Species of Iran: A Review. Environ. Geochem. Health 2021, 43, 3749–3869. [Google Scholar] [CrossRef]
- Özparlak, H.; Arslan, G.; Arslan, E. Determination of some metal levels in muscle tissue of nine fish species from the Beyşehir Lake, Turkey. Turk. J. Fish. Aquat. Sci. 2012, 12, 761–770. [Google Scholar] [CrossRef]
- Al-Najjar, T.; Al-Momani, R.; Khalaf, M.; Wahsha, M.; Sbaihat, M.; Khalaf, N.; Abu Khadra, K.; Magames, H. Levels of heavy metals in fishes (Cheilinustrilobatus) from the Gulf of Aqaba, Jordan. Nat. Sci. 2016, 8, 256. [Google Scholar] [CrossRef][Green Version]
- Bawuro, A.; Voegborlo, R.; Adimado, A. Bioaccumulation of heavy metals in some tissues of fish in Lake Geriyo, Adamawa State, Nigeria. J. Environ. Public Health 2018, 2018, 1854892. [Google Scholar] [CrossRef]
- El-Hak, H.N.G.; Ghobashy, M.A.; Mansour, F.A.; El-Shenawy, N.S.; El-Din, M.I.S. Heavy metals and parasitological infection associated with oxidative stress and histopathological alteration in the Clariasgariepinus. Ecotoxicology 2022, 31, 1096–1110. [Google Scholar] [CrossRef] [PubMed]
- Dural, M.; Göksu, M.L.; Özak, A.A.; Derici, B. Bioaccumulation of some heavy metals in different tissues of Dicentrarchuslabrax L., 1758, Sparus aurata L., 1758 and Mugil cephalus L., 1758 from the Camlik lagoon of the eastern cost of mediterranean (Turkey). Environ. Monit. Assess. 2006, 118, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Fazio, F.; D’Iglio, C.; Capillo, G.; Saoca, C.; Peycheva, K.; Piccione, G.; Makedonski, L. Environmental Investigations and Tissue Bioaccumulation of Heavy Metals in Grey Mullet from the Black Sea (Bulgaria) and the Ionian Sea (Italy). Animals 2020, 10, 1739. [Google Scholar] [CrossRef]
- Aparna, C.A.; Singh, P.K. Estimation of toxic, trace and essential metals (Pb, cd, Fe, Zn, Mn, Cu, Mg, K) in fruit and vegetable product (jam, ketchup, pickles) by atomic absorption spectrophotometer. Pharma Innov. J. 2018, 7, 313–317. [Google Scholar]
- Zoroddu, M.A.; Aaseth, J.; Crisponi, G.; Medici, S.; Peana, M.; Nurchi, V.M. The essential metals for humans: A brief overview. J. Inorg. Biochem. 2019, 195, 120–129. [Google Scholar] [CrossRef]
- Abadi, D.R.V.; Dobaradaran, S.; Nabipour, I.; Lamani, X.; Ravanipour, M.; Tahmasebi, R.; Nazmara, S. Comparative Investigation of Heavy Metal, Trace, and Macro Element Contents in Commercially Valuable Fish Species Harvested off from the Persian Gulf. Environ. Sci. Pollut. Res. Int. 2015, 22, 6670–6678. [Google Scholar] [CrossRef]
- Rakocevic, J.; Sukovic, D.; Maric, D. Distribution and relationships of eleven trace elements in muscle of six fish species from Skadar Lake (Montenegro). Turk. J. Fish. Aquat. Sci. 2018, 18, 647–657. [Google Scholar] [CrossRef]
- Baki, M.A.; Hossain, M.M.; Akter, J.; Quraishi, S.B.; Shojib, M.F.H.; Ullah, A.A.; Khan, M.F. Concentration of heavy metals in seafood (fishes, shrimp, lobster and crabs) and human health assessment in Saint Martin Island, Bangladesh. Ecotoxicol. Environ. Saf. 2018, 159, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Garai, P.; Banerjee, P.; Mondal, P.; Chandra Saha, N. Effect of heavy metals on fishes: Toxicity and bioaccumulation. J. Toxicol. Clin. Toxicol. 2021, 11, S18. [Google Scholar]
- Ersoy, B.; Çelik, M. Essential elements and contaminants in tissues of commercial pelagic fish from the Eastern Mediterranean Sea. J. Sci. Food Agric. 2009, 89, 1615–1621. [Google Scholar] [CrossRef]
- Soetan, K.O.; Olaiya, C.O.; Oyewole, O.E. The importance of mineral elements for humans, domestic animals and plants: A review. Afr. J. Food Sci. 2010, 4, 200–222. [Google Scholar]
- Adewumi, A.A.; Adewole, H.A.; Olaleye, V.F. Proximate and elemental composition of the fillets of some fish species in Osinmo Reservoir, Nigeria. ABJNA 2014, 5, 109–117. [Google Scholar] [CrossRef]
- Stancheva, M.; Makedonski, L.; Peycheva, K. Determination of heavy metal concentrations of most consumed fish species from Bulgarian Black Sea coast. Bulg. Chem. Commun. 2014, 46, 195–203. [Google Scholar]
- Simionov, I.A.; Cristea, V.; Petrea, S.M.; Mogodan, A.; Nicoara, M.; Baltag, E.S.; Strungaru, S.A.; Faggio, C. Bioconcentration of essential and nonessential elements in Black Sea turbot (Psetta Maxima Maeotica Linnaeus, 1758) in relation to fish gender. J. Mar. Sci. Eng. 2019, 7, 466. [Google Scholar] [CrossRef]
- El-Faer, M.Z.; Rawdah, T.N.; Attar, K.M.; Arab, M. Mineral and proximate composition of some commercially important fish of the Arabian Gulf. Food Chem. 1992, 45, 95–98. [Google Scholar] [CrossRef]
- Gu, Y.-G.; Lin, Q.; Wang, X.-H.; Du, F.-Y.; Yu, Z.-L.; Huang, H.-H. Heavy metal concentrations in wild fishes captured from the South China Sea and associated health risks. Mar. Pollut. Bull. 2015, 96, 508–512. [Google Scholar] [CrossRef]
- Hassan, A.H.; Al-Zanbagi, N.A.; Al-Nabati, E.A. Impact of nematode helminthes on metal concentrations in the muscles of Koshar fish, Epinephelussummana, in Jeddah, Saudi Arabia. J. Basic Appl. Zool. 2016, 74, 56–61. [Google Scholar] [CrossRef][Green Version]
- El Shehawy, S.M.; Gab-Alla, A.A.; Mutwally, H.M. Proximate and elemental composition of important fish species in Makkah central fish market, Saudi Arabia. Food Nutr. Sci. 2016, 7, 429–439. [Google Scholar] [CrossRef]
- Yilmaz, A.B. Levels of heavy metals (Fe, Cu, Ni, Cr, Pb, and Zn) in tissue of Mugil cephalus and Trachurus mediterraneus from Iskenderun Bay, Turkey. Environ. Res. 2003, 92, 277–281. [Google Scholar] [CrossRef]
- Okoro, D.; Olaleye, V.F.; Djeresa, J.O. Bioaccumulation of nickel, lead, copper, mercury and cadmium in tissues and organs of Ethmalosa fimbriata from the Forcados River, Niger-Delta, Nigeria. Glob. J. Pure Appl. Sci. 2007, 13, 339–346. [Google Scholar] [CrossRef]
- Chi, Q.; Zhu, G.; Langdon, A. Bioaccumulation of Heavy Metals in Fishes from Taihu Lake, China. J. Environ. Sci. 2007, 19, 1500–1504. [Google Scholar] [CrossRef] [PubMed]
- Alkan, N.; Alkan, A.; Gedik, K.; Fisher, A. Assessment of metal concentrations in commercially important fish species in Black Sea. Toxicol. Ind. Health 2016, 32, 447–456. [Google Scholar] [CrossRef]
- Sarker, M.; Islam, M.; Rahman, F.; Anisuzzaman, M. Heavy Metals in the Fish Tenualosailisha Hamilton, 1822 in the Padma–Meghna River Confluence: Potential Risks to Public Health. Toxics 2021, 9, 341. [Google Scholar] [CrossRef]
- Payuta, A.A.; Flerova, E.A.; Zaitseva, Y.V. Heavy metal content in muscle tissue of pikeperch Sander lucioperca in different reaches of Rybinsk reservoir. Vestn. ASTUSer. Fish. Ind. 2022, 4, 135–142. [Google Scholar] [CrossRef]
- Wu, D.; Feng, H.; Zou, Y.; Xiao, J.; Zhang, P.; Ji, Y.; Lek, S.; Guo, Z.; Fu, Q. Feeding Habit-Specific Heavy Metal Bioaccumulation and Health Risk Assessment of Fish in a Tropical Reservoir in Southern China. Fishes 2023, 8, 211. [Google Scholar] [CrossRef]
- Mensoor, M.; Said, A.M. Determination of Heavy Metals in Freshwater Fishes of the Tigris River in Baghdad. Fishes 2018, 3, 23. [Google Scholar] [CrossRef]
- Bhat, I.A.; Dar, J.Y.; Ahmad, I.; Mir, I.N.; Bhat, H.; Bhat, R.A.H.; Ganie, P.A.; Sharma, R. Testicular development and spermatogenesis in fish: Insights into molecular aspects and regulation of gene expression by different exogenous factors. Rev. Aquac. 2021, 13, 2142–2168. [Google Scholar] [CrossRef]
- Shovon, M.N.H.; Majumdar, B.C.; Rahman, Z. Heavy metals (Lead, Cadmium and Nickel) concentration in different organs of three commonly consumed fishes in Bangladesh. Fish. Aquac. J. 2017, 8, 1000207. [Google Scholar] [CrossRef]
- Gomaa, M.; Abou-Arab, A.A.K.; Badawy, A.; Khayria, N. Distribution pattern of some heavy metals in Egyptian fish organs. Food Chem. 1995, 53, 385–389. [Google Scholar] [CrossRef]
- Yilmaz, A.B.; Dogan, M. Heavy metals in water and in tissues of Himri (Carasobarbus luteus) from Orontes (Asi) River, Turkey. Environ. Monit. Assess. 2008, 144, 437–444. [Google Scholar] [CrossRef]
- Asare, M.L.; Cobbina, S.J.; Akpabey, F.J.; Duwiejuah, A.B.; Abuntori, Z.N. Heavy metal concentration in water, sediment and fish species in the bontanga reservoir, Ghana. Toxicol. Environ. Health Sci. 2018, 10, 49–58. [Google Scholar] [CrossRef]
- Tahity, T.; Islam, M.R.U.; Bhuiyan, N.Z.; Choudhury, T.R.; Yu, J.; Noman, M.A.; Hosen, M.M.; Quraishi, S.B.; Paray, B.A.; Arai, T.; et al. Heavy Metals Accumulation in Tissues of Wild and Farmed Barramundi from the Northern Bay of Bengal Coast, and Its Estimated Human Health Risks. Toxics 2022, 10, 410. [Google Scholar] [CrossRef] [PubMed]
- Tanhan, P.; Lansubsakul, N.; Phaochoosak, N.; Sirinupong, P.; Yeesin, P.; Imsilp, K. Human Health Risk Assessment of Heavy Metal Concentration in Seafood Collected from Pattani Bay, Thailand. Toxics 2023, 11, 18. [Google Scholar] [CrossRef]
- Hilal, A.A.; Ismail, N.S. Heavy Metals in Eleven Common Species of Fish from the Gulf of Aqaba, Red Sea. Jordan J. Biol. Sci. 2008, 1, 13–18. [Google Scholar]
- Kadiyani, Y.; Teraiya, S. Studies on changes of magnesium, phosphorus and iron content in white and red muscles of fishes. Life Sci. Leafl. 2013, 9, 1–11. [Google Scholar]
- Al-Najjar, T.; Khalaf, N.; Allawi, M.; Disi, A. Levels of Trace Metals in Two Fish Species (Caesiovarilineata and Caesiolunaris) of the Family Caesionidae from the Gulf of Aqaba, Red Sea. Fresen. Environ. Bull. 2012, 21, 1152–1157. [Google Scholar]
- Yeltekin, A.Ç.; Oğuz, A.R. Some macro and trace elements in various tissues of Van fish variations according to gender and weight. Arq. Bras. Med. Vet. Zootec. 2018, 70, 231–237. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chen, M.H. Heavy metal concentrations in nine species of fishes caught in coastal waters off Ann-Ping, SW Taiwan. J. Food Drug Anal. 2001, 9, 107–114. [Google Scholar] [CrossRef]
- Mahjoub, M.; Fadlaoui, S.; El Maadoudi, M.; Smiri, Y. Mercury, Lead, and Cadmium in the Muscles of Five Fish Species from the Mechraa-Hammadi Dam in Morocco and Health Risks for Their Consumers. J. Toxicol. 2021, 2021, 8865869. [Google Scholar] [CrossRef]
- Begum, A.; Amin, M.N.; Kaneco, S.; Ohta, K. Selected elemental composition of the muscle tissue of three species of fish, Tilapia nilotica, Cirrhinamrigala and Clariusbatrachus, from the fresh water Dhanmondi Lake in Bangladesh. Food Chem. 2005, 93, 439–443. [Google Scholar] [CrossRef]
- Madhusudan, S.; Liyaquat, F.; Nadim, C. Bioaccumulation of zinc and cadmium in freshwater fishes. IndianJ. Fish. 2003, 50, 53–65. [Google Scholar]
- Chouchene, L.; Pellegrini, E.; Gueguen, M.-M.; Hinfray, N.; Brion, F.; Piccini, B.; Kah, O.; Saïd, K.; Messaoudi, I.; Pakdel, F. Inhibitory effect of cadmium on estrogen signaling in zebrafish brain and protection by zinc. J. Appl. Toxicol. 2016, 36, 863–871. [Google Scholar] [CrossRef]
- Rezaei, H.; Zarei, A.; Kamarehie, B.; Jafari, A.; Fakhri, Y.; Bidarpoor, F.; Karami, M.A.; Farhang, M.; Ghaderpoori, M.; Sadeghi, H. Levels, Distributions and Health Risk Assessment of Lead, Cadmium and Arsenic Found in Drinking Groundwater of Dehgolan’s Villages, Iran. Toxicol. Environ. Health Sci. 2019, 11, 54–62. [Google Scholar] [CrossRef]
- Saha, N.; Mollah, M.; Alam, M.; Rahman, M.S. Seasonal investigation of heavy metals in marine fishes captured from the Bay of Bengal and the implications for human health risk assessment. Food Control 2016, 70, 110–118. [Google Scholar] [CrossRef]
- Bamuwamye, M.; Ogwok, P.; Tumuhairwe, V. Cancer and Non-Cancer Risks Associated with Heavy Metal Exposures from Street Foods, Evaluation of Roasted Meats in an Urban Setting. J. Environ. Pollut. Hum. Health 2015, 3, 24–30. [Google Scholar] [CrossRef]
- Kakar, A.; Hayat, M.T.; Abbasi, A.M.; Pervez, A.; Mahmood, Q.; Farooq, U.; Akbar, T.A.; Ali, S.; Rizwan, M.; El-Serehy, H.A.; et al. Risk Assessment of Heavy Metals in Selected Marine Fish Species of Gadani Shipbreaking Area and Pakistan. Animals 2020, 10, 1738. [Google Scholar] [CrossRef] [PubMed]
- Sinitsyn, I.S. Spatial and Age Peculiarities of Ecology-Caused Diseases of the Yaroslavl Region Population. Yarosl. Pedagog. Bull. 2011, 3, 160–164. (In Russian) [Google Scholar]
- Modestov, A.A.; Safontsev, I.P.; Zukov, R.A.; Slepov, E.V.; Klimenok, M.P.; Gaas, E.N. Cancer incidence in the Krasnoyarsk Krai. Russ. J. Oncol. 2016, 21, 76–80. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).