Bayesian-Based Probabilistic Risk Assessment of Fipronil in Food: A Case Study in Taiwan
Abstract
1. Introduction
2. Materials and Methods
2.1. Aggregate Dietary Exposure Assessment of Fipronil
2.1.1. Fipronil Residues in Food
2.1.2. Reconstruction of Fipronil Distributions Using Bayesian—Based MCMC Approach
2.1.3. Dietary Exposure Assessment of Fipronil in Taiwan
2.2. Revisit the Health Reference Dose of Fipronil Using Bayesian Benchmark Dose Modeling
2.3. Risk Characterization of Fipronil Exposure in Taiwan
2.4. Statistical Analysis and Data Visualization
3. Results
3.1. Fipronil Residues in Food
3.2. Reconstruction of Fipronil Residues Using MCMC Simulation
3.3. Probabilistic Exposure Assessment of Fipronil in Taiwan
3.4. Probabilistic Dose-Response Assessment of Fipronil
4. Discussion
Supplementary Materials
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Acceptable daily intake | ADI |
| Bayesian Benchmark dose | BBMD |
| Benchmark dose lower confidence limit | BMDL |
| Chemical-specific adjustment factor | CASF |
| Cabbage | CB |
| Chicken egg | CG |
| Citrus fruit | CF |
| Dry bean | DB |
| Fruit vegetable | FV |
| Hazard quotient | HQ |
| Large berry fruit | LF |
| Legume vegetable | LV |
| Lower-bound exposure | LB |
| Markov Chain Monte Carlo simulation | MCMC |
| No-observed adverse effect level | NOAEL |
| Point of departure | POD |
| Probabilistic reference dose | pRfD |
| Root and stem vegetable | RV |
| Small berry fruit | SF |
| Small leafy vegetable | SV |
| Spice | S |
| Upper-bound exposure | UB |
References
- Pino-Otín, M.R.; Ballestero, D.; Navarro, E.; Mainar, A.M.; Val, J. Effects of the insecticide fipronil in freshwater model organisms and microbial and periphyton communities. Sci. Total Environ. 2021, 764, 142820. [Google Scholar] [CrossRef] [PubMed]
- Key, P.; Chung, K.; Siewicki, T.; Fulton, M. Toxicity of three pesticides individually and in mixture to larval grass shrimp (Palaemonetes pugio). Ecotoxicol. Environ. Saf. 2007, 68, 272–277. [Google Scholar] [PubMed]
- Uchida, M.; Mizukawa, H.; Hirano, M.; Tominaga, N.; Arizono, K.; Ishibashi, H. Adverse effects of contamination by fipronil and its derivatives on growth, molting, and gene expression in the mysid crustacean, Americamysis bahia, in Japanese estuaries. Sci. Total Environ. 2023, 892, 164595. [Google Scholar] [CrossRef]
- Singh, N.S.; Sharma, R.; Singh, S.K.; Singh, D.K. A comprehensive review of environmental fate and degradation of fipronil and its toxic metabolites. Environ. Res. 2021, 199, 111316. [Google Scholar] [CrossRef]
- Kartheek, R.M.; David, M. Assessment of fipronil toxicity on wistar rats: A hepatotoxic perspective. Toxicol. Rep. 2018, 5, 448–456. [Google Scholar] [CrossRef]
- Mossa, A.T.H.; Swelam, E.S.; Mohafrash, S.M. Sub-chronic exposure to fipronil induced oxidative stress, biochemical and histopathological changes in the liver and kidney of male albino rats. Toxicol. Rep. 2015, 2, 775–784. [Google Scholar] [CrossRef]
- Wang, X.; Martínez, M.A.; Wu, Q.; Ares, I.; Martínez-Larrañaga, M.R.; Anadón, A.; Yuan, Z. Fipronil insecticide toxicology: Oxidative stress and metabolism. Crit. Rev. Toxicol. 2016, 46, 876–899. [Google Scholar] [CrossRef]
- Kim, Y.A.; Yoon, Y.S.; Kim, H.S.; Jeon, S.J.; Cole, E.; Lee, J.; Kho, Y.; Cho, Y.H. Distribution of fipronil in humans, and adverse health outcomes of in utero fipronil sulfone exposure in newborns. Int. J. Hyg. Environ. Health 2019, 222, 524–532. [Google Scholar] [CrossRef]
- Liang, S.X.; Zhao, Z.; Fan, C.L.; Xu, J.Z.; Li, H.; Chang, Q.Y.; Pang, G.F. Fipronil residues and risk assessment of Chinese marketed fruits and vegetables: A long-term investigation over 6 years. Food Control 2019, 106, 106734. [Google Scholar] [CrossRef]
- Kuchheuser, P.; Birringer, M. Pesticide residues in food in the European Union: Analysis of notifications in the European Rapid Alert System for Food and Feed from 2002 to 2020. Food Control 2022, 133, 108575. [Google Scholar]
- Gill, J.P.S.; Bedi, J.S.; Singh, R.; Fairoze, M.N.; Hazarika, R.A.; Gaurav, A.; Satpathy, S.K.; Chauhan, A.S.; Lindahl, J.; Grace, D.; et al. Pesticide Residues in Peri-Urban Bovine Milk from India and Risk Assessment: A Multicenter Study. Sci. Rep. 2020, 10, 8054. [Google Scholar] [CrossRef] [PubMed]
- Hatta, S.; Odagawa, Y.; Izumi, J.; Nakamae, S. Study on Fipronil and Other Pesticide Residues in Chicken Eggs Produced and Sold in Japan. J. Food Hyg. Soc. Jpn. 2019, 60, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.S.; Chiu, Z.Y.; Wu, K.Y.; Hsu, C.C. Integrating high-throughput exposure assessment and in vitro screening data to prioritize endocrine-active potential and dietary risks of pesticides and veterinary drug residues in animal products. Food Chem. Toxicol. 2023, 173, 113639. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Agency; Reich, H.; Triacchini, G.A. Occurrence of residues of fipronil and other acaricides in chicken eggs and poultry muscle/fat. EFSA J. 2018, 16, e05164. [Google Scholar]
- Canton, L.; Signorini, M.; Canton, C.; Dominguez, P.; Farias, C.; Alvarez, L.; Lanusse, C.; Moreno, L. Quantitative exposure assessment and risk characterization for fipronil residues in laying hen eggs. J. Food Sci. 2022, 87, 2775–2788. [Google Scholar] [CrossRef]
- Deon van der Merwe, A.J.; van den Berg, M. Case report: Fipronil contamination of chickens in the Netherlands and surrounding countries. In Chemical Hazards in Foods of Animal Origin; ECVPH Food safety assurance; Wageningen Academic Publishers: Wageningen, The Netherlands, 2019; Volume 7, pp. 567–584. [Google Scholar]
- Zhao, H.N.; Huang, D.D.; Zhu, S.H. Multibranch Gold Nanoparticles as Surface-Enhanced Raman Spectroscopy Substrates for Rapid and Sensitive Analysis of Fipronil in Eggs. Sensors 2019, 19, 5354. [Google Scholar] [CrossRef]
- Biswas, S.; Mondal, R.; Mukherjee, A.; Sarkar, M.; Kole, R.K. Simultaneous determination and risk assessment of fipronil and its metabolites in sugarcane, using GC-ECD and confirmation by GC-MS/MS. Food Chem. 2019, 272, 559–567. [Google Scholar] [CrossRef]
- Chawla, S.; Gor, H.N.; Patel, H.K.; Parmar, K.D.; Patel, A.R.; Shukla, V.; Ilyas, M.; Parsai, S.K.; Somashekar Meena, R.S.; Shah, P.G. Validation, residue analysis, and risk assessment of fipronil and flonicamid in cotton (Gossypium sp.) samples and soil. Environ. Sci. Pollut. Res. Int. 2018, 25, 19167–19178. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Liu, Z.; Li, S.; Zhu, F.; Li, L.; Zhao, Y.; Chen, D.; Zhou, Y.; Wu, Y. Occurrence, fate, and probabilistic risk assessment of fipronil residues in Chinese tea. J. Food Compos. Anal. 2023, 115, 105028. [Google Scholar] [CrossRef]
- El-Sheikh, E.A.; Ramadan, M.M.; El-Sobki, A.E.; Shalaby, A.A.; McCoy, M.R.; Hamed, I.A.; Ashour, M.B.; Hammock, B.D. Pesticide Residues in Vegetables and Fruits from Farmer Markets and Associated Dietary Risks. Molecules 2022, 27, 8072. [Google Scholar] [CrossRef]
- El-Sheikh, E.S.A.; Li, D.; Hamed, I.; Ashour, M.B.; Hammock, B.D. Residue Analysis and Risk Exposure Assessment of Multiple Pesticides in Tomato and Strawberry and Their Products from Markets. Foods 2023, 12, 1936. [Google Scholar]
- Chiu, W.A.; Slob, W. A Unified Probabilistic Framework for Dose-Response Assessment of Human Health Effects. Environ. Health Perspect. 2015, 123, 1241–1254. [Google Scholar] [PubMed]
- IPCS. Chemical-Specific Adjustment Factors for Interspecies Differences and Human Variability: Guidance Document for Use of Data in Dose/Concentration–Response Assessment; I.P.o.C. Safety, Ed.; World Health Organization: Geneva, Switzerland, 2005.
- Chiu, W.A.; Axelrad, D.A.; Dalaijamts, C.; Dockins, C.; Shao, K.; Shapiro, A.J.; Paoli, G. Beyond the RfD: Broad Application of a Probabilistic Approach to Improve Chemical Dose-Response Assessments for Noncancer Effects. Environ. Health Perspect. 2018, 126, 067009. [Google Scholar] [CrossRef] [PubMed]
- NAS. Science and Decisions: Advancing Risk Assessment; National Academy of Sciences: Washington, DC, USA, 2009. [Google Scholar]
- Bokkers, B.G.H.; Mengelers, M.J.; Bakker, M.I.; Chiu, W.A.; Slob, W. APROBA-Plus: A probabilistic tool to evaluate and express uncertainty in hazard characterization and exposure assessment of substances. Food Chem. Toxicol. 2017, 110, 408–417. [Google Scholar]
- Shao, K.; Shapiro, A.J. A Web-Based System for Bayesian Benchmark Dose Estimation. Environ. Health Perspect. 2018, 126, 017002. [Google Scholar] [CrossRef]
- Blessinger, T.; Davis, A.; Chiu, W.A.; Stanek, J.; Woodall, G.M.; Gift, J.; Thayer, K.A.; Bussard, D. Application of a unified probabilistic framework to the dose-response assessment of acrolein. Environ. Int. 2020, 143, 105953. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Tanaka, N.; Akiyama, H. Attempt of Bayesian Estimation from Left-censored Data Using the Markov Chain Monte Carlo Method: Exploring Cr(VI) Concentrations in Mineral Water Products. Food Saf. 2020, 8, 67–89. [Google Scholar]
- NHRI. National Food Consumption Database; National Health Research Institute: Taipei City, Taiwan, 2023. [Google Scholar]
- FAO. Pesticide residues in food 2021. In Proceedings of the Joint FAO/WHO Meeting on Pesticide Residues, Online, 6–17 September and 4–7 October 2021; Evaluation Part II—Toxicological; World Health Organization and Food and Agriculture Organization of the United Nations: Geneva, Switzerland, 2022. [Google Scholar]
- IPCS. Uncertainty and Data Quality in Exposure Assessment; World Health Organization: Geneva, Switzerland, 2008.
- WHO/IPCS. Guidance Document on Evaluating and Expressing Uncertainty in Hazard Characterization; World Health Organization International Program on Chemical Safety: Geneva, Switzerland, 2014.
- WHO/IPCS. Guidance Document on Evaluating and Expressing Uncertainty in Hazard Characterization, 2nd ed.; World Health Organization: Geneva, Switzerland, 2018.
- Lu, E.H.; Huang, S.Z.; Yu, T.H.; Chiang, S.Y.; Wu, K.Y. Systematic probabilistic risk assessment of pesticide residues in tea leaves. Chemosphere 2020, 247, 125692. [Google Scholar] [CrossRef]
- Khoshnam, F.; Ziaee, M.; Daei, M.; Mahdavi, V.; Mousavi Khaneghah, A. Investigation and probabilistic health risk assessment of pesticide residues in cucumber, tomato, and okra fruits from Khuzestan, Iran. Environ. Sci. Pollut. Res. Int. 2022, 29, 25953–25964. [Google Scholar]
- Helsel, D.R. Fabricating data: How substituting values for nondetects can ruin results, and what can be done about it. Chemosphere 2006, 65, 2434–2439. [Google Scholar]
- EFSA. Management of Left-Censored Data in Dietary Exposure Assessment of Chemical Substances; European Food Safety Authority: Parma, Italy, 2010.
- Shoari, N.; Dube, J.S. Toward improved analysis of concentration data: Embracing nondetects. Environ. Toxicol. Chem. 2018, 37, 643–656. [Google Scholar] [CrossRef] [PubMed]
- Chu, Q.; Li, Y.; Wang, X. Bayesian inference of heavy metals exposure in crayfish for assessing human non-carcinogenic health risk. Food Chem. Toxicol. 2023, 173, 113595. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.C.; Lin, Z. Probabilistic human health risk assessment of perfluorooctane sulfonate (PFOS) by integrating in vitro, in vivo toxicity, and human epidemiological studies using a Bayesian-based dose-response assessment coupled with physiologically based pharmacokinetic (PBPK) modeling approach. Environ. Int. 2020, 137, 105581. [Google Scholar] [PubMed]
- Aurisano, N.; Jolliet, O.; Chiu, W.A.; Judson, R.; Jang, S.; Unnikrishnan, A.; Kosnik, M.B.; Fantke, P. Probabilistic Points of Departure and Reference Doses for Characterizing Human Noncancer and Developmental/Reproductive Effects for 10,145 Chemicals. Environ. Health Perspect. 2023, 131, 37016. [Google Scholar] [CrossRef]
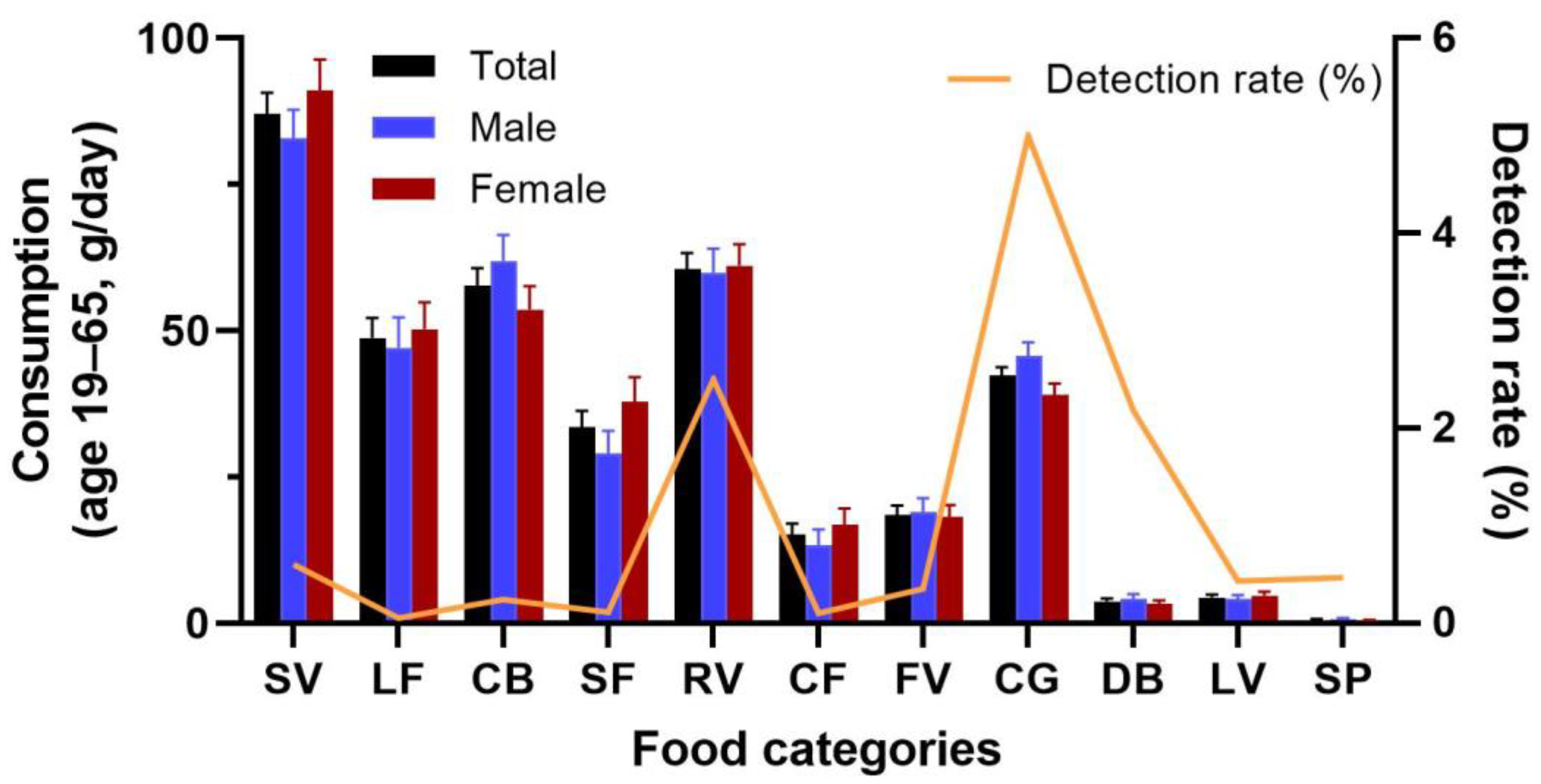

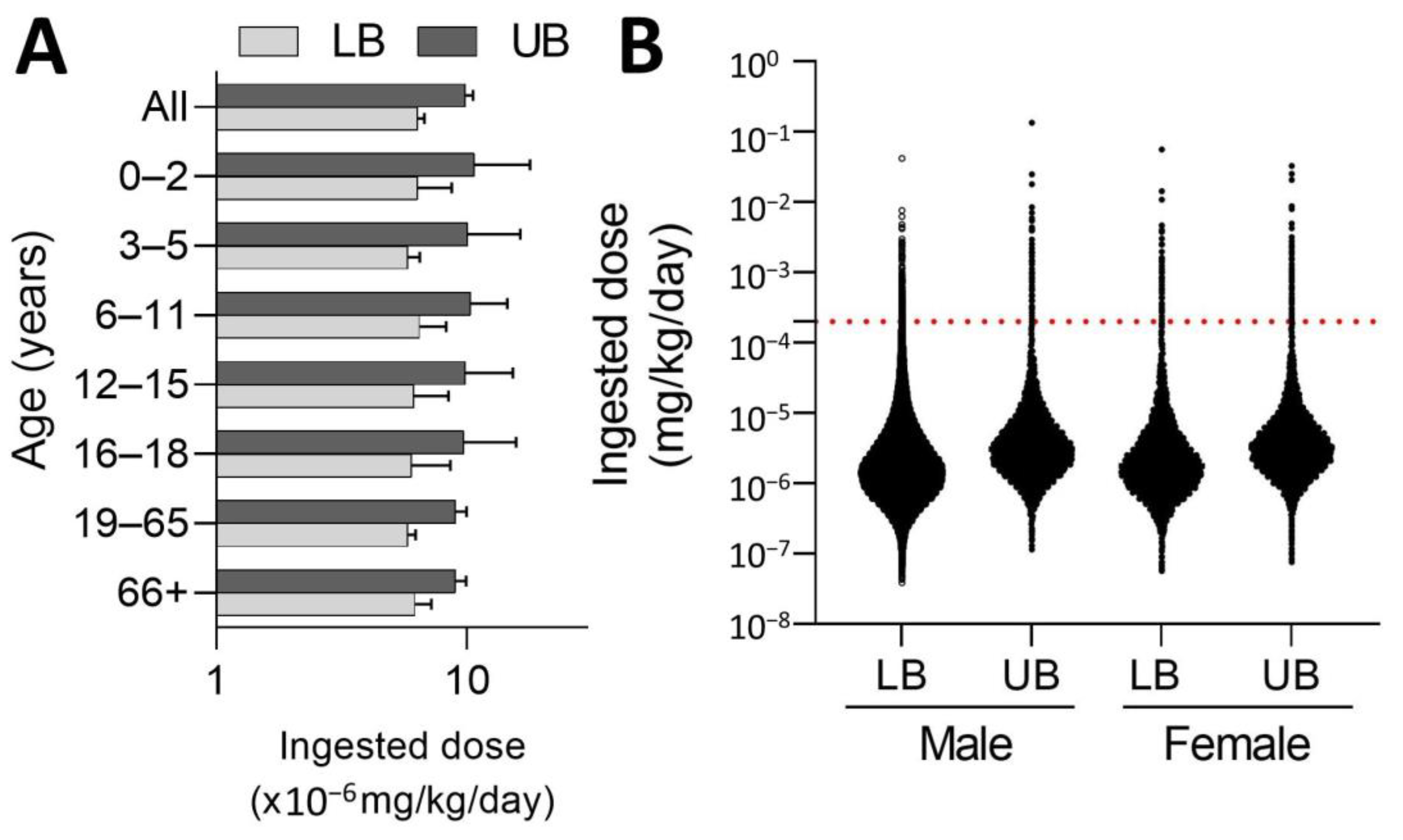

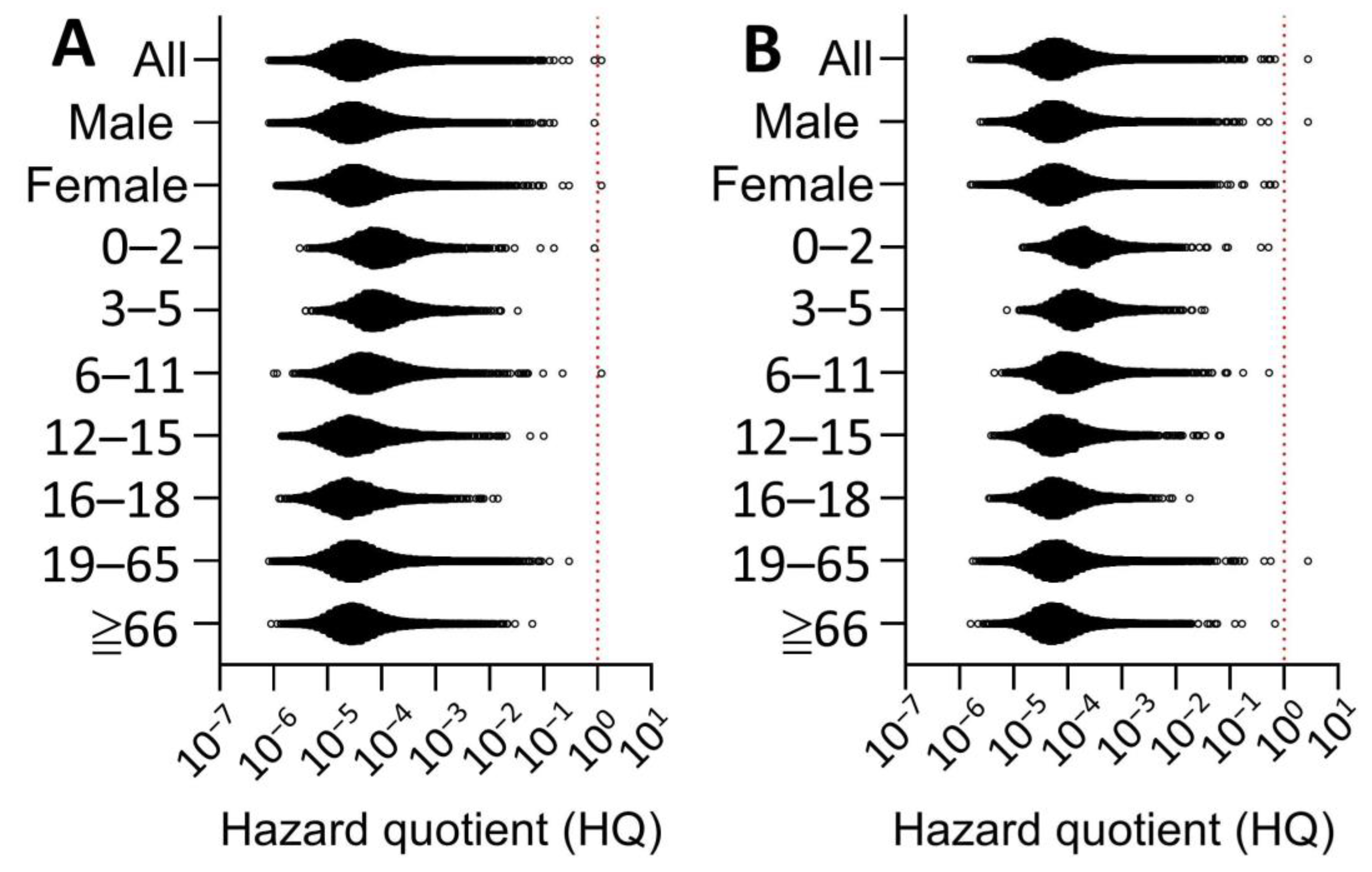
| Food Category | Abbreviation | Food Items | Total | Detects | Conc. | Std. |
|---|---|---|---|---|---|---|
| Large berry fruit | LF | dragon fruit, pineapple, banana, sugar apple, papaya, passion fruit, avocado, caimito, aiyu, rambutan, kiwi fruit, star apple, jack fruit, and canistel fruit | 2042 | 1 | 0.003 | - |
| Small berry fruit | SF | guava, grape, wax apple, strawberry, fig, carambola, jabuticaba, and mulberry | 923 | 1 | 0.002 | - |
| Citrus fruit | CF | pomelo, orange, madarin, tangerine, lemon, lime, grapefruit, and kumquat | 1046 | 1 | 0.014 | - |
| Cabbage | CB | broccoli, cabbage, garden lettuce, and kohlrabi | 1265 | 3 | 0.229 | 0.138 |
| Small leafy vegetable | SV | bok choy, water spinach, rape, pak choy, lettuce, basil, spinach, celery, leaf of sweat potato, chives, kale, mustard, chayote vine, bird-nest fern, scallion, Ganges amaranth, Japanese mustard spinach, garlic, Okinawa spinach, chrysanthemum greens, Malabar spinach, garland chrysanthemum, white water, vegetable fern, white mugwort, Rangoon ceeper, shallot, purslane, jute, beefsteak plant, aloe, and Chinese violet | 6974 | 42 | 0.115 | 0.328 |
| Fruit vegetables | FV | tomato, eggplant, bell pepper, baby corn, chili, okra, and roselle | 2556 | 9 | 0.018 | 0.025 |
| Legume vegetables | LV | snap bean, kidney bean, french bean, navy bean, string bean, garden pea, sugar pea, snow pea, and lima bean | 1867 | 8 | 0.010 | 0.005 |
| Root and stem vegetables | RV | carrot, bamboo, potato, sweet potato, onion, taro, turnip, water bamboo, common yam, ginger, asparagus, Arctium, garlic, lotus root, water chestnut, beetroot, and cassava | 1760 | 44 | 0.013 | 0.013 |
| Dry beans | DB | soybean, peanut, mung bean, adzuki bean, jackfruit seed, yardlong bean, sunflower seed, rapeseed, sunflower seed, cottonseed, hyacinth bean, lotus seed, sesame seed | 229 | 5 | 0.006 | 0.004 |
| Chicken egg | CG | chicken egg | 40 | 2 | 0.014 | 0.011 |
| Spice | SP | butterfly pea flower, goji berry root, Chinese celery, mesona, star anise, chamomile, rose, mugwort, chia seeds, Sichuan peppercorn, and mint | 858 | 4 | 0.051 | 0.027 |
| Unit: mg/kg/day | BMDL10 | HD0.5 | ADI | |
|---|---|---|---|---|
| Median | 13.48 | 2.80 | 0.46 | 0.0002 |
| 90th percentile | 9.96 | 1.06 | 0.088 | |
| 95th percentile | 8.67 | 0.80 | 0.048 † | |
| 99th percentile | 6.49 | 0.48 | 0.012 | |
| Mean | 22.21 | 4.96 | 1.03 | |
| Standard deviation | 107.37 | 107.37 | 39.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.-S. Bayesian-Based Probabilistic Risk Assessment of Fipronil in Food: A Case Study in Taiwan. Toxics 2023, 11, 677. https://doi.org/10.3390/toxics11080677
Luo Y-S. Bayesian-Based Probabilistic Risk Assessment of Fipronil in Food: A Case Study in Taiwan. Toxics. 2023; 11(8):677. https://doi.org/10.3390/toxics11080677
Chicago/Turabian StyleLuo, Yu-Syuan. 2023. "Bayesian-Based Probabilistic Risk Assessment of Fipronil in Food: A Case Study in Taiwan" Toxics 11, no. 8: 677. https://doi.org/10.3390/toxics11080677
APA StyleLuo, Y.-S. (2023). Bayesian-Based Probabilistic Risk Assessment of Fipronil in Food: A Case Study in Taiwan. Toxics, 11(8), 677. https://doi.org/10.3390/toxics11080677





