Heated Tobacco Products: Insights into Composition and Toxicity
Abstract
1. Introduction
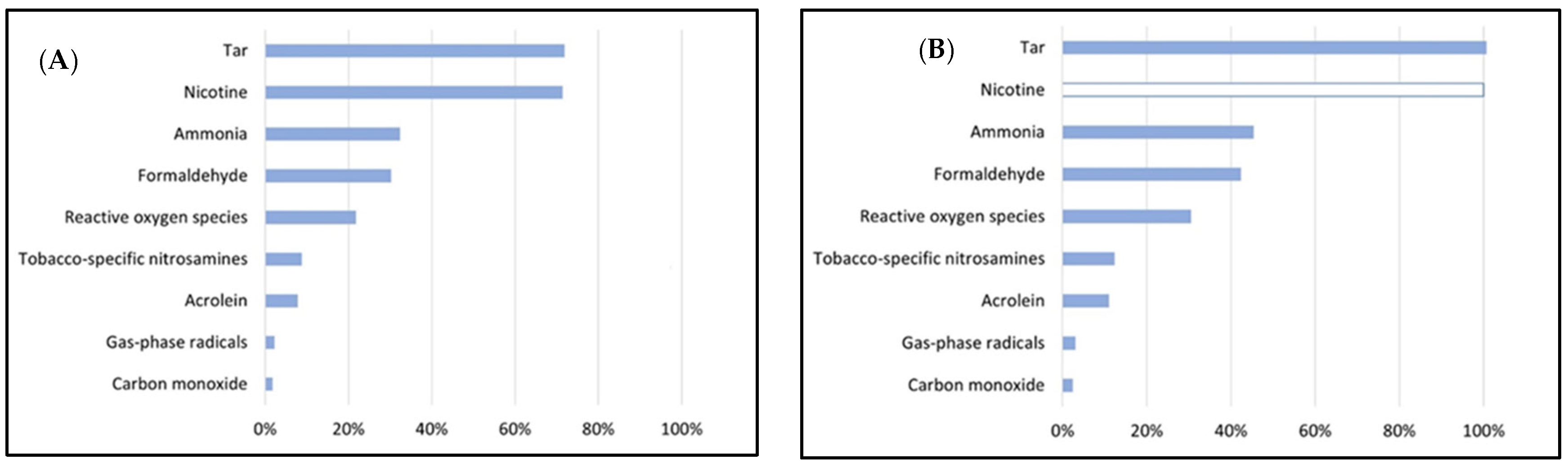
2. Composition of Mainstream HTP Emissions
- ISO: puff volume: 35 mL; duration: 2 s; interval: 60 s; six puffs per HTP stick; six puffs per TCC.
- HCI: puff volume: 55 mL; duration: 2 s; interval: 30 s; 12 puffs per HTP stick; 8–12 puffs per TCC; ECIG: 24–55 puffs.
- CORESTA: puff volume: 75 mL; duration: 2.5 s; interval: 30 s; 7–12 puffs per HTP stick; 11 puffs per TCC; ECIG: 10 puffs.
3. Biomarkers of Exposure
4. Health Effects of Exposure to Mainstream HTP Emissions
4.1. Pulmonary Effects
4.2. Cardiovascular Effects
4.3. Other Effects
4.4. Harm Reduction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heated Tobacco Products—A Brief. 2020. Available online: https://apps.who.int/iris/bitstream/handle/10665/350470/WHO-EURO-2020-4571-44334-64934-eng.pdf?sequence=3&isAllowed=y (accessed on 19 April 2023).
- Heated Tobacco Products: Information Sheet—2nd ed. 2020. Available online: https://www.who.int/publications/i/item/WHO-HEP-HPR-2020.2 (accessed on 18 March 2023).
- Heated Tobacco Products. 2020. Available online: https://www.cdc.gov/tobacco/basic_information/heated-tobacco-products/ (accessed on 18 March 2023).
- Bitzer, Z.T.; Goel, R.; Trushin, N.; Muscat, J.; Richie, J.P., Jr. Free Radical Production and Characterization of Heat-Not-Burn Cigarettes in Comparison to Conventional and Electronic Cigarettes. Chem. Res. Toxicol. 2020, 33, 1882–1887. [Google Scholar] [CrossRef] [PubMed]
- Simonavicius, E.; McNeill, A.; Shahab, L.; Brose, L.S. Heat-not-burn tobacco products: A systematic literature review. Tob. Control 2019, 28, 582–594. [Google Scholar] [CrossRef] [PubMed]
- Heat-Not-Burn Market Size, Share & Trends Analysis Report by Component (Capsules, Heating Module, Sticks, Vaporizers), by Distribution Channel, by Region, and Segment Forecasts, 2020–2027. 2020. Available online: https://www.grandviewresearch.com/industry-analysis/heat-not-burn-market (accessed on 18 March 2023).
- Laverty, A.A.; Vardavas, C.I.; Filippidis, F.T. Prevalence and reasons for use of Heated Tobacco Products (HTP) in Europe: An analysis of Eurobarometer data in 28 countries. Lancet Reg. Health Eur. 2021, 8, 100159. [Google Scholar] [CrossRef] [PubMed]
- Gallus, S.; Lugo, A.; Liu, X.; Borroni, E.; Clancy, L.; Gorini, G.; Lopez, M.J.; Odone, A.; Przewozniak, K.; Tigova, O.; et al. Use and Awareness of Heated Tobacco Products in Europe. J. Epidemiol. 2022, 32, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Czoli, C.D.; White, C.M.; Reid, J.L.; OConnor, R.J.; Hammond, D. Awareness and interest in IQOS heated tobacco products among youth in Canada, England and the USA. Tob. Control 2020, 29, 89–95. [Google Scholar] [CrossRef]
- Bekki, K.; Inaba, Y.; Uchiyama, S.; Kunugita, N. Comparison of Chemicals in Mainstream Smoke in Heat-not-burn Tobacco and Combustion Cigarettes. J. UOEH 2017, 39, 201–207. [Google Scholar] [CrossRef]
- Farsalinos, K.E.; Yannovits, N.; Sarri, T.; Voudris, V.; Poulas, K. Nicotine Delivery to the Aerosol of a Heat-Not-Burn Tobacco Product: Comparison with a Tobacco Cigarette and E-Cigarettes. Nicotine Tob. Res. 2018, 20, 1004–1009. [Google Scholar] [CrossRef]
- Farsalinos, K.E.; Yannovits, N.; Sarri, T.; Voudris, V.; Poulas, K.; Leischow, S.J. Carbonyl emissions from a novel heated tobacco product (IQOS): Comparison with an e-cigarette and a tobacco cigarette. Addiction 2018, 113, 2099–2106. [Google Scholar] [CrossRef]
- Leigh, N.J.; Palumbo, M.N.; Marino, A.M.; O’Connor, R.J.; Goniewicz, M.L. Tobacco-specific nitrosamines (TSNA) in heated tobacco product IQOS. Tob. Control 2018, 27 (Suppl. S1), s37–s38. [Google Scholar] [CrossRef]
- Li, X.; Luo, Y.; Jiang, X.; Zhang, H.; Zhu, F.; Hu, S.; Hou, H.; Hu, Q.; Pang, Y. Chemical Analysis and Simulated Pyrolysis of Tobacco Heating System 2.2 Compared to Conventional Cigarettes. Nicotine Tob. Res. 2019, 21, 111–118. [Google Scholar] [CrossRef]
- Salman, R.; Talih, S.; El-Hage, R.; Haddad, C.; Karaoghlanian, N.; El-Hellani, A.; Saliba, N.A.; Shihadeh, A. Free-Base and Total Nicotine, Reactive Oxygen Species, and Carbonyl Emissions From IQOS, a Heated Tobacco Product. Nicotine Tob. Res. 2019, 21, 1285–1288. [Google Scholar] [CrossRef]
- Davis, B.; Williams, M.; Talbot, P. iQOS: Evidence of pyrolysis and release of a toxicant from plastic. Tob. Control 2019, 28, 34–41. [Google Scholar] [CrossRef]
- Auer, R.; Concha-Lozano, N.; Jacot-Sadowski, I.; Cornuz, J.; Berthet, A. Heat-Not-Burn Tobacco Cigarettes: Smoke by Any Other Name. JAMA Intern. Med. 2017, 177, 1050–1052. [Google Scholar] [CrossRef]
- Azzopardi, D.; Haswell, L.E.; Foss-Smith, G.; Hewitt, K.; Asquith, N.; Corke, S.; Phillips, G. Evaluation of an air-liquid interface cell culture model for studies on the inflammatory and cytotoxic responses to tobacco smoke aerosols. Toxicol. Vitr. 2015, 29, 1720–1728. [Google Scholar] [CrossRef]
- Glantz, S.A. Heated tobacco products: The example of IQOS. Tob. Control 2018, 27 (Suppl. S1), s1–s6. [Google Scholar] [CrossRef]
- St Helen, G.; Jacob Iii, P.; Nardone, N.; Benowitz, N.L. IQOS: Examination of Philip Morris International’s claim of reduced exposure. Tob. Control 2018, 27 (Suppl. 1), s30–s36. [Google Scholar] [CrossRef]
- IQOS. Available online: https://se.iqos.com/en/discover-iqos/what-is-iqos (accessed on 18 March 2023).
- Drovandi, A.; Salem, S.; Barker, D.; Booth, D.; Kairuz, T. Human Biomarker Exposure from Cigarettes Versus Novel Heat-Not-Burn Devices: A Systematic Review and Meta-Analysis. Nicotine Tob. Res. 2020, 22, 1077–1085. [Google Scholar] [CrossRef]
- Kopa, P.N.; Pawliczak, R. IQOS—A heat-not-burn (HnB) tobacco product—Chemical composition and possible impact on oxidative stress and inflammatory response. A systematic review. Toxicol. Mech. Methods 2020, 30, 81–87. [Google Scholar] [CrossRef]
- Moazed, F.; Chun, L.; Matthay, M.A.; Calfee, C.S.; Gotts, J. Assessment of industry data on pulmonary and immunosuppressive effects of IQOS. Tob. Control 2018, 27 (Suppl. S1), s20–s25. [Google Scholar] [CrossRef]
- Polosa, R.; Morjaria, J.B.; Prosperini, U.; Busà, B.; Pennisi, A.; Gussoni, G.; Rust, S.; Maglia, M.; Caponnetto, P. Health outcomes in COPD smokers using heated tobacco products: A 3-year follow-up. Intern. Emerg. Med. 2021, 16, 687–696, Erratum in Intern. Emerg. Med. 2022, 17, 1849. [Google Scholar] [CrossRef]
- Pataka, A.; Kotoulas, S.; Chatzopoulos, E.; Grigoriou, I.; Sapalidis, K.; Kosmidis, C.; Vagionas, A.; Perdikouri, Ε.I.; Drevelegas, K.; Zarogoulidis, P.; et al. Acute Effects of a Heat-Not-Burn Tobacco Product on Pulmonary Function. Medicina 2020, 56, 292. [Google Scholar] [CrossRef] [PubMed]
- Aokage, T.; Tsukahara, K.; Fukuda, Y.; Tokioka, F.; Taniguchi, A.; Naito, H.; Nakao, A. Heat-not-burn cigarettes induce fulminant acute eosinophilic pneumonia requiring extracorporeal membrane oxygenation. Respir. Med. Case Rep. 2018, 26, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Chaaban, T. Acute eosinophilic pneumonia associated with non-cigarette smoking products: A systematic review. Adv. Respir. Med. 2020, 88, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Kamada, T.; Yamashita, Y.; Tomioka, H. Acute eosinophilic pneumonia following heat-not-burn cigarette smoking. Respirol. Case Rep. 2016, 4, e00190. [Google Scholar] [CrossRef] [PubMed]
- Bhat, T.A.; Kalathil, S.G.; Leigh, N.; Muthumalage, T.; Rahman, I.; Goniewicz, M.L.; Thanavala, Y.M. Acute Effects of Heated Tobacco Product (IQOS) Aerosol Inhalation on Lung Tissue Damage and Inflammatory Changes in the Lungs. Nicotine Tob. Res. 2021, 23, 1160–1167. [Google Scholar] [CrossRef]
- Kaur, G.; Muthumalage, T.; Rahman, I. Mechanisms of toxicity and biomarkers of flavoring and flavor enhancing chemicals in emerging tobacco and non-tobacco products. Toxicol. Lett. 2018, 288, 143–155. [Google Scholar] [CrossRef]
- Leigh, N.J.; Tran, P.L.; O’Connor, R.J.; Goniewicz, M.L. Cytotoxic effects of heated tobacco products (HTP) on human bronchial epithelial cells. Tob. Control 2018, 27 (Suppl. 1), s26–s29. [Google Scholar] [CrossRef]
- Başaran, R.; Güven, N.M.; Eke, B.C. An Overview of iQOS® as a New Heat-Not-Burn Tobacco Product and Its Potential Effects on Human Health and the Environment. Turk. J. Pharm. Sci. 2019, 16, 371–374. [Google Scholar] [CrossRef]
- Sohal, S.S.; Eapen, M.S.; Naidu, V.G.M.; Sharma, P. IQOS exposure impairs human airway cell homeostasis: Direct comparison with traditional cigarette and e-cigarette. ERJ Open Res. 2019, 5, 00159–02018. [Google Scholar] [CrossRef]
- Rahman, M.; Irmler, M.; Introna, M.; Beckers, J.; Palmberg, L.; Johanson, G.; Upadhyay, S.; Ganguly, K. Insight into the pulmonary molecular toxicity of heated tobacco products using human bronchial and alveolar mucosa models at air-liquid interface. Sci. Rep. 2022, 12, 16396. [Google Scholar] [CrossRef]
- Fried, N.D.; Gardner, J.D. Heat-not-burn tobacco products: An emerging threat to cardiovascular health. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H1234–H1239. [Google Scholar] [CrossRef]
- Ioakeimidis, N.; Emmanouil, E.; Terentes-Printzios, D.; Dima, I.; Aznaouridis, K.; Tousoulis, D.; Vlachopoulos, C. Acute effect of heat-not-burn versus standard cigarette smoking on arterial stiffness and wave reflections in young smokers. Eur. J. Prev. Cardiol. 2021, 28, e9–e11. [Google Scholar] [CrossRef]
- Franzen, K.F.; Belkin, S.; Goldmann, T.; Reppel, M.; Watz, H.; Mortensen, K.; Droemann, D. The impact of heated tobacco products on arterial stiffness. Vasc. Med. 2020, 25, 572–574. [Google Scholar] [CrossRef]
- Nabavizadeh, P.; Liu, J.; Havel, C.M.; Ibrahim, S.; Derakhshandeh, R.; Jacob Iii, P.; Springer, M.L. Vascular endothelial function is impaired by aerosol from a single IQOS HeatStick to the same extent as by cigarette smoke. Tob. Control 2018, 27 (Suppl. S1), s13–s19. [Google Scholar] [CrossRef]
- Chun, L.; Moazed, F.; Matthay, M.; Calfee, C.; Gotts, J. Possible hepatotoxicity of IQOS. Tob. Control 2018, 27 (Suppl. S1), s39–s40. [Google Scholar] [CrossRef]
- Jankowski, M.; Brożek, G.M.; Lawson, J.; Skoczyński, S.; Majek, P.; Zejda, J.E. New ideas, old problems? Heated tobacco products—A systematic review. Int. J. Occup. Med. Environ. Health 2019, 32, 595–634. [Google Scholar] [CrossRef]
- Zanetti, F.; Zhao, X.; Pan, J.; Peitsch, M.C.; Hoeng, J.; Ren, Y. Effects of cigarette smoke and tobacco heating aerosol on color stability of dental enamel, dentin, and composite resin restorations. Quintessence Int. 2019, 50, 156–166. [Google Scholar] [CrossRef]
- Zhao, X.; Zanetti, F.; Majeed, S.; Pan, J.; Malmstrom, H.; Peitsch, M.C.; Hoeng, J.; Ren, Y. Effects of cigarette smoking on color stability of dental resin composites. Am. J. Dent. 2017, 30, 316–322. [Google Scholar]
- Pisinger, C.; on behalf of the ERS Tobacco Control Committee. Position Paper on Heated Tobacco Products. Available online: https://www.ersnet.org/news-and-features/news/ers-position-paper-on-heated-tobacco-products/ (accessed on 18 March 2023).
- Outbreak of Lung Injury Associated with the Use of E-Cigarette, or Vaping, Products. Available online: https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html (accessed on 10 July 2023).
- Bals, R.; Boyd, J.; Esposito, S.; Foronjy, R.; Hiemstra, P.S.; Jiménez-Ruiz, C.A.; Katsaounou, P.; Lindberg, A.; Metz, C.; Schober, W.; et al. Electronic cigarettes: A task force report from the European Respiratory Society. Eur. Respir. J. 2019, 53, 1801151. [Google Scholar] [CrossRef]
- Hedman, L.; Backman, H.; Stridsman, C.; Bosson, J.A.; Lundbäck, M.; Lindberg, A.; Rönmark, E.; Ekerljung, L. Association of Electronic Cigarette Use with Smoking Habits, Demographic Factors, and Respiratory Symptoms. JAMA Netw. Open 2018, 1, e180789. [Google Scholar] [CrossRef]
- Hedman, L.; Backman, H.; Stridsman, C.; Lundbäck, M.; Andersson, M.; Rönmark, E. Predictors of electronic cigarette use among Swedish teenagers: A population-based cohort study. BMJ Open 2020, 10, e040683. [Google Scholar] [CrossRef] [PubMed]
- Kasza, K.A.; Ambrose, B.K.; Conway, K.P.; Borek, N.; Taylor, K.; Goniewicz, M.L.; Cummings, K.M.; Sharma, E.; Pearson, J.L.; Green, V.R.; et al. Tobacco-Product Use by Adults and Youths in the United States in 2013 and 2014. N. Engl. J. Med. 2017, 376, 342–353, Erratum in N. Engl. J. Med. 2018, 378, 492. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.; Case, K.R.; Loukas, A.; Creamer, M.R.; Perry, C.L. E-cigarette Dual Users, Exclusive Users and Perceptions of Tobacco Products. Am. J. Health Behav. 2016, 40, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Goniewicz, M.L.; Leigh, N.J.; Gawron, M.; Nadolska, J.; Balwicki, L.; McGuire, C.; Sobczak, A. Dual use of electronic and tobacco cigarettes among adolescents: A cross-sectional study in Poland. Int. J. Public Health 2016, 61, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Rüther, T.; Wissen, F.; Linhardt, A.; Aichert, D.S.; Pogarell, O.; de Vries, H. Electronic Cigarettes-Attitudes and Use in Germany. Nicotine Tob. Res. 2016, 18, 660–669. [Google Scholar] [CrossRef]
- Cotinine Factsheet. Available online: https://www.cdc.gov/biomonitoring/Cotinine_FactSheet.html (accessed on 10 July 2023).
- Kim, J.; Lee, S. Daily Cigarette Consumption and Urine Cotinine Level between Dual Users of Electronic and Conventional Cigarettes, and Cigarette-Only Users. J. Psychoact. Drugs 2020, 52, 20–26. [Google Scholar] [CrossRef]
- Park, M.B.; Choi, J.K. Differences between the effects of conventional cigarettes, e-cigarettes and dual product use on urine cotinine levels. Tob. Induc. Dis. 2019, 17, 12. [Google Scholar] [CrossRef]
- Osei, A.D.; Mirbolouk, M.; Orimoloye, O.A.; Dzaye, O.; Uddin, S.M.I.; Benjamin, E.J.; Hall, M.E.; DeFilippis, A.P.; Stokes, A.; Bhatnagar, A.; et al. Association Between E-Cigarette Use and Cardiovascular Disease among Never and Current Combustible-Cigarette Smokers. Am. J. Med. 2019, 132, 949–954.e2. [Google Scholar] [CrossRef]
- Oh, S.S.; Jang, J.E.; Lee, D.W.; Park, E.C.; Jang, S.I. Cigarette type or smoking history: Which has a greater impact on the metabolic syndrome and its components? Sci. Rep. 2020, 10, 10467. [Google Scholar] [CrossRef]
- Xie, Z.; Ossip, D.J.; Rahman, I.; Li, D. Use of Electronic Cigarettes and Self-Reported Chronic Obstructive Pulmonary Disease Diagnosis in Adults. Nicotine Tob. Res. 2020, 22, 1155–1161. [Google Scholar] [CrossRef]

| Product Name | Company | Type |
|---|---|---|
| Accord | Philip Morris International | HTP |
| Eclipse | RJ Reynolds Tobacco Company | HTP |
| iFuse | British American Tobacco | Hybrid |
| IQOS | Philip Morris International | HTP |
| Kent Glo | British American Tobacco | HTP |
| lil hybrid, lil vapor | The Korea Tobacco and Ginseng Corporation | Hybrid |
| lil plus, lil mini | The Korea Tobacco and Ginseng Corporation | HTP |
| PloomTech | Japan Tobacco International | Hybrid |
| Premier | RJ Reynolds Tobacco Company | HTP |
| (Per Cigarette) | HTP Stick | TCC Cigarette |
|---|---|---|
| Nicotine (mg) | 4.7–5.1 | 8.7–15.0 |
| Tobacco-specific nitrosamines (TSNAs) (ng) | ||
| N-Nitrosonornicotine (NNN) | 94.4–101.0 | 1899.0–1691.0 |
| N’-Nitrosoanatabine (NAT) | 94.5–99.8 | 913.0–1341.0 |
| N-Nitrosoanabasine (NAB) | 2.6–5.6 | 46.0–65.0 |
| Nicotine-derived nitrosoamine ketone (NNK) | 51.1–58.2 | 412.0–532.0 |
| HTP Aerosol | TCC Smoke | |
| Total particulate matter (mg) | 12.9–55.8 | 9.8–37.7 |
| % Free base | 5.7–13.6 | 5.8–14.5 |
| Tar (mg) | 7.5–16.6 | 8.0–25.50 |
| Propylene glycol (mg) | 0.2–0.6 | - |
| Glycerin (mg) | 1.6–3.8 | 0.80–2.3 |
| Nicotine (mg) | 0.5–1.5 | 0.7–2.1 |
| Carbon monoxide (mg) | 0.3–0.5 | 11.2–33.0 |
| TSNAs (ng) | ||
| NNN | 5.00–24.9 | 92.1–311.1 |
| NAT | 6.1–37.2 | 92.9–246.4 |
| NAB | 2.6–5.5 | 9.60–30.4 |
| NNK | 3.5–13.8 | 85.50–250.4 |
| Aromatic amines (ng) | ||
| 1-Aminonaphthalene | - | 10.6–21.6 |
| 2-Aminonaphthalene | - | 5.7–10.1 |
| 3-Aminobiphenyl | - | 2.0–4.2 |
| 4-Aminobiphenyl | - | 1.0–2.2 |
| Hydrogen cyanide (µg) | - | 70.9–319.0 |
| Ammonia (µg) | 2.4–10.5 | 11.1–28.7 |
| Phenol (µg) | 1.2 | 7.0–14.8 |
| Polycyclic aromatic hydrocarbon (PAH) Benzo(a)pyrene (ng) | - | 6.7–16.2 |
| Reactive oxygen species (nmol H2O2) | 6.3 | 10.7–46.8 |
| Gas phase | 1.9 | 2.3–2210 |
| Particle phase | 4.3 | 7.8–24.7 |
| Particulate-phase radicals (pmol) | - | 79.4 |
| Volatile organic compounds (µg) | ||
| 1,3-Butadiene | 0.5 | 38.5–76.5 |
| Isoprene | 0.6–3.0 | 395.0–863.0 |
| Acrylonitrile | 0.2 | 26.4–67.0 |
| Benzene | 0.1 –0.6 | 47.7–104.0 |
| Toluene | 0.8–2.5 | 73.6–208.0 |
| HPHC | HTP Aerosol | TCC Smoke | ECIG Aerosol |
|---|---|---|---|
| Nicotine (mg) | 0.5–1.5 | 0.7–2.1 | 0.07–1.73 |
| Total gas-phase radicals (pmol) | 12.5–12.6 | 567.6 | 5.3–47.8 |
| Non-polar | 13.9–14.3 | 449.9 | 2.4–19.2 |
| Polar | 6.8–8.2 | 9.6 | 5.9–43.3 |
| Carbonyls (µg) | |||
| Formaldehyde | 0.9–22.6 | 3.2–74.4 | 0.5–3.7 |
| Acetaldehyde | 128.5–301. 5 | 567.0–1534 | 0.8–2.9 |
| Acetone | 18.8–48.37 | 210–775.6 | - |
| Acrolein | 4.0–13.1 | 56.7–160.9 | 0.3–1.1 |
| Propionaldehyde | 9.6–22.3 | 48.4–124.0 | - |
| Crotonaldehyde | 1.4–6.4 | 10.10–65.7 | - |
| Methacrolein | 6.5 | 85.5 | - |
| Butyraldehyde | 14.9–30.7 | 22.2–65.0 | - |
| Valeraldehyde | 20.1 | - | - |
| Glyoxal | 3.1 | - | - |
| Methyl glyoxal | 33.5 | - | - |
| 2-Butanone | 4.2–6.5 | 11.0–220.5 | - |
| HPHC | Fold Increase in HTP Aerosol Compared to TCC Smoke | |
|---|---|---|
| 1 | 1,4-Dioxane, 2-ethyl-5-methyl- | 137 |
| 2 | Hexadecanoic acid, ethyl ester | 60 |
| 3 | Trans-4-hydroxymethyl-2-methyl-1,3-dioxolane | 47 |
| 4 | Stearate, ethyl- | 24 |
| 5 | 12,14-Labdadiene-7,8-diol, (8a,12E) | 21 |
| 6 | Butylated hydroxytoluene | 18 |
| 7 | Ethyl linoleate | 16 |
| 8 | Labdane-8,15-diol, (13S) | 9 |
| 9 | Propylene glycol | 6 |
| 10 | 2-Furanmethanol | 4 |
| 11 | Butyrolactone | 5 |
| 12 | Methyl furoate | 4 |
| 13 | 2-Cyclopentene-1,4-dione | 4 |
| 14 | 2-Furanmethanol, 5-methyl- | 3 |
| 15 | Ethyl linolenate | 3 |
| 16 | 2-Methylcyclobutane-1,3-dione | 3 |
| 17 | Lanost-8-en-3-ol, 24-methylene-, (3beta) | 3 |
| 18 | 2-Furancarboxaldehyde, 5-methyl- | 3 |
| 19 | Eicosane, 2-methyl- | 3 |
| 20 | 1,2,3-Propanetriol, diacetate (diacetin) | 2 |
| 21 | Glycidol | 2 |
| 22 | Heneicosane, 2-methyl- | 2 |
| Biomarkers of Exposure | Effect Size | p-Value |
|---|---|---|
| 3-Hydroxypropylmercaptauric acid (3-HPMA) | 0.21 (0.02, 0.40) | 0.027 |
| 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) | 0.11 (0.03, 0.18) | 0.005 |
| N-Nitrosonornicotine (NNN) | 0.22 (0.01, 0.43) | 0.041 |
| Total nicotine equivalents (TNeq) | 1.91 (1.40, 2.41) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Upadhyay, S.; Rahman, M.; Johanson, G.; Palmberg, L.; Ganguly, K. Heated Tobacco Products: Insights into Composition and Toxicity. Toxics 2023, 11, 667. https://doi.org/10.3390/toxics11080667
Upadhyay S, Rahman M, Johanson G, Palmberg L, Ganguly K. Heated Tobacco Products: Insights into Composition and Toxicity. Toxics. 2023; 11(8):667. https://doi.org/10.3390/toxics11080667
Chicago/Turabian StyleUpadhyay, Swapna, Mizanur Rahman, Gunnar Johanson, Lena Palmberg, and Koustav Ganguly. 2023. "Heated Tobacco Products: Insights into Composition and Toxicity" Toxics 11, no. 8: 667. https://doi.org/10.3390/toxics11080667
APA StyleUpadhyay, S., Rahman, M., Johanson, G., Palmberg, L., & Ganguly, K. (2023). Heated Tobacco Products: Insights into Composition and Toxicity. Toxics, 11(8), 667. https://doi.org/10.3390/toxics11080667








