Photocatalytic Degradation of Acetaminophen in Aqueous Environments: A Mini Review
Abstract
1. Introduction
2. ACT Contamination
2.1. ACT Properties and Usage
2.2. Occurrences of ACT in Various Aqueous Environments
2.3. Adverse Effects of ACT
3. Current Technologies for ACT Removal
4. Photocatalytic Degradation of ACT
4.1. Doping
4.2. Heterojunction
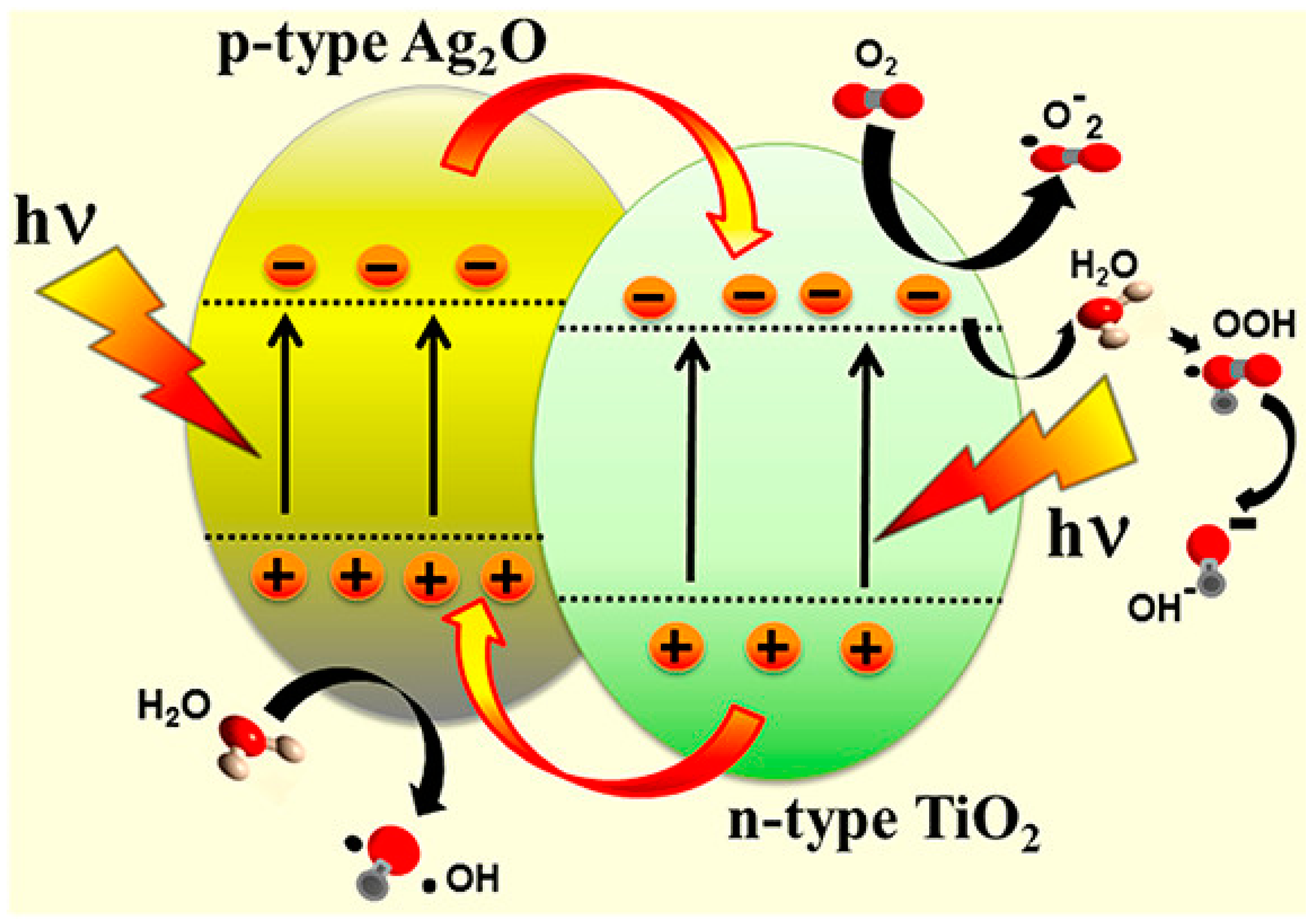
4.2.1. Conventional Type-II Heterojunction
4.2.2. Z-Scheme Heterojunction
4.3. Photocatalytic Degradation Kinetics
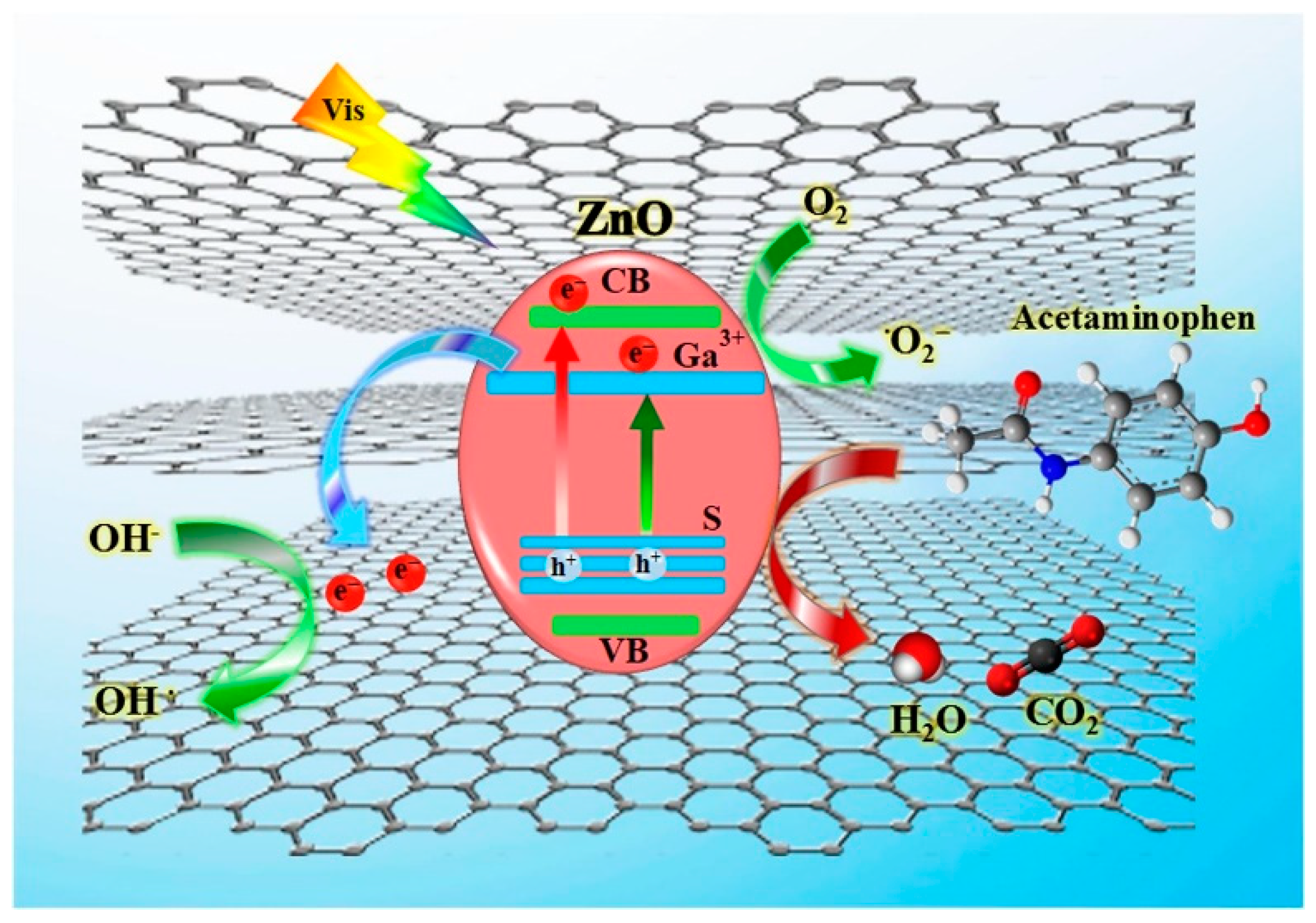
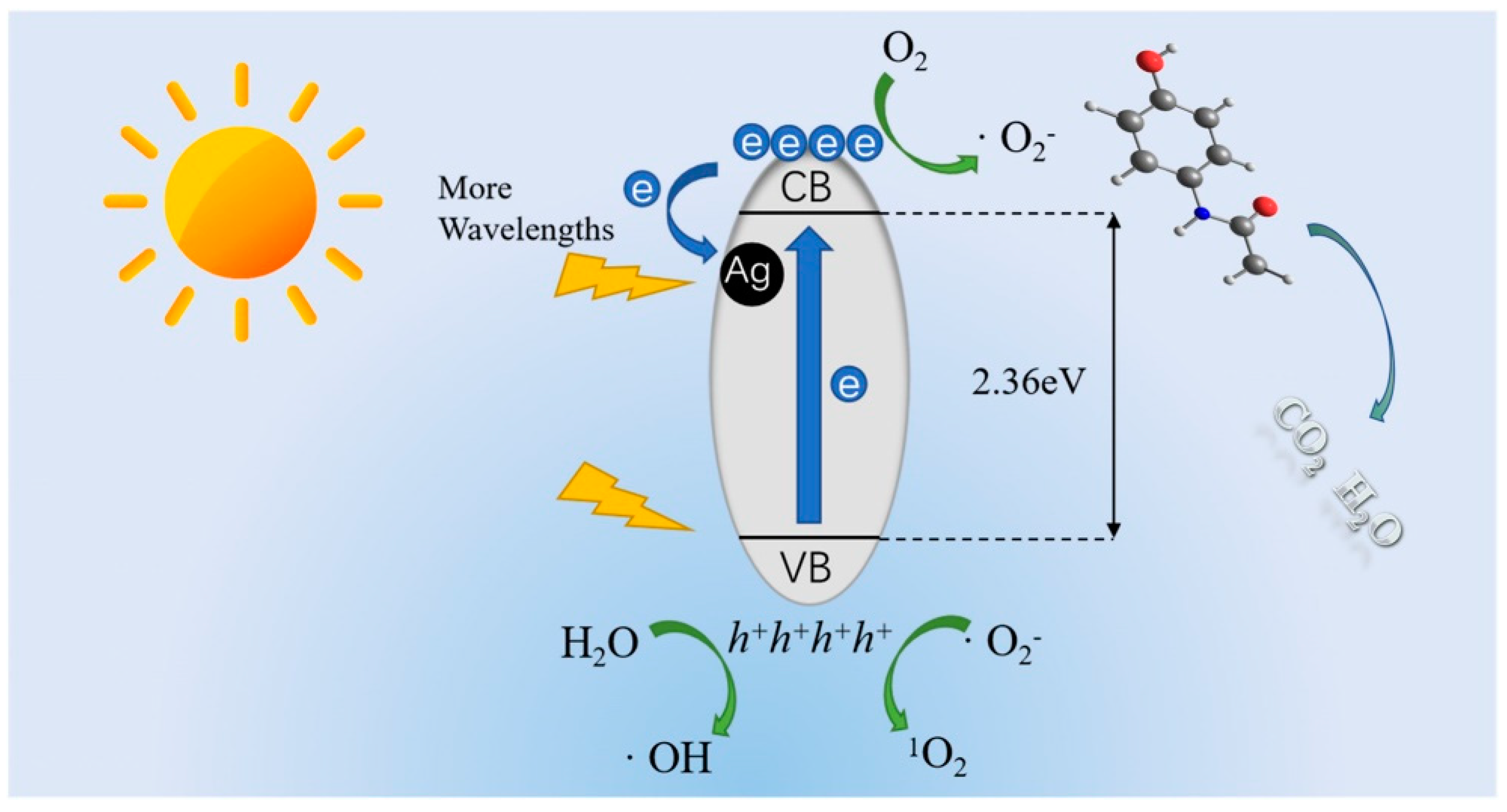
4.4. Degradation Mechanism
5. Challenges and Future Trends of Photocatalytic Degradation of ACT
6. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, M.; Sridharan, S.; Sawarkar, A.D.; Shakeel, A.; Anerao, P.; Mannina, G.; Sharma, P.; Pandey, A. Current research trends on emerging contaminants pharmaceutical and personal care products (PPCPs): A comprehensive review. Sci. Total Environ. 2023, 859, 160031. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Li, Y.; Chen, Y.; Yao, B.; Chen, X.; Yu, Y.; Yang, J.; Zhou, Y. Pharmaceuticals and personal care products (PPCPs) in the aquatic environment: Biotoxicity, determination and electrochemical treatment. J. Clean. Prod. 2023, 388, 135923. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Ferro-García, M.Á.; Prados-Joya, G.; Ocampo-Pérez, R. Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef] [PubMed]
- Cedron, V.P.; Weiner, A.M.J.; Vera, M.; Sanchez, L. Acetaminophen affects the survivor, pigmentation and development of craniofacial structures in zebrafish (Danio rerio) embryos. Biochem. Pharmacol. 2020, 174, 113816. [Google Scholar] [CrossRef]
- Peralta-Hernández, J.M.; Brillas, E. A critical review over the removal of paracetamol (acetaminophen) from synthetic waters and real wastewaters by direct, hybrid catalytic, and sequential ozonation processes. Chemosphere 2023, 313, 137411. [Google Scholar] [CrossRef]
- Pacheco-Álvarez, M.; Picos Benítez, R.; Rodríguez-Narváez, O.M.; Brillas, E.; Peralta-Hernández, J.M. A critical review on paracetamol removal from different aqueous matrices by Fenton and Fenton-based processes, and their combined methods. Chemosphere 2022, 303, 134883. [Google Scholar] [CrossRef]
- Al-Mamun, M.R.; Kader, S.; Islam, M.S.; Khan, M.Z.H. Photocatalytic activity improvement and application of UV-TiO2 photocatalysis in textile wastewater treatment: A review. J. Environ. Chem. Eng. 2019, 7, 103248. [Google Scholar] [CrossRef]
- An, C.; Huang, G.; Yao, Y.; Zhao, S. Emerging usage of electrocoagulation technology for oil removal from wastewater: A review. Sci. Total Environ. 2017, 579, 537–556. [Google Scholar] [CrossRef]
- Cako, E.; Dudziak, S.; Głuchowski, P.; Trykowski, G.; Pisarek, M.; Fiszka Borzyszkowska, A.; Sikora, K.; Zielińska-Jurek, A. Heterojunction of (P, S) co-doped g-C3N4 and 2D TiO2 for improved carbamazepine and acetaminophen photocatalytic degradation. Sep. Purif. Technol. 2023, 311, 123320. [Google Scholar] [CrossRef]
- Dubey, M.; Vellanki, B.P.; Kazmi, A.A. Fate of emerging contaminants in a sequencing batch reactor and potential of biological activated carbon as tertiary treatment for the removal of persisting contaminants. J. Environ. Manag. 2023, 338, 117802. [Google Scholar] [CrossRef]
- Cheng, Y.-X.; Chen, J.; Wu, D.; Liu, Y.-S.; Yang, Y.-Q.; He, L.-X.; Ye, P.; Zhao, J.-L.; Liu, S.-S.; Yang, B.; et al. Highly enhanced biodegradation of pharmaceutical and personal care products in a novel tidal flow constructed wetland with baffle and plants. Water Res. 2021, 193, 116870. [Google Scholar] [CrossRef] [PubMed]
- Maryam, B.; Buscio, V.; Odabasi, S.U.; Buyukgungor, H. A study on behavior, interaction and rejection of Paracetamol, Diclofenac and Ibuprofen (PhACs) from wastewater by nanofiltration membranes. Environ. Technol. Innov. 2020, 18, 100641. [Google Scholar] [CrossRef]
- Poddar, K.; Sarkar, D.; Chakraborty, D.; Patil, P.B.; Maity, S.; Sarkar, A. Paracetamol biodegradation by Pseudomonas strain PrS10 isolated from pharmaceutical effluents. Int. Biodeterior. Biodegrad. 2022, 175, 105490. [Google Scholar] [CrossRef]
- Wang, A.; Jiang, Y.; Yan, Y.; Bu, L.; Wei, Z.; Spinney, R.; Dionysiou, D.D.; Xiao, R. Mechanistic and quantitative profiling of electro-Fenton process for wastewater treatment. Water Res. 2023, 235, 119838. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.R.; Nunes, O.C.; Pereira, M.F.R.; Silva, A.M.T. An overview on the advanced oxidation processes applied for the treatment of water pollutants defined in the recently launched Directive 2013/39/EU. Environ. Int. 2015, 75, 33–51. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, F.; Saravanakumar, K.; Maheskumar, V.; Njaramba, L.K.; Yoon, Y.; Park, C.M. Application of perovskite oxides and their composites for degrading organic pollutants from wastewater using advanced oxidation processes: Review of the recent progress. J. Hazard. Mater. 2022, 436, 129074. [Google Scholar] [CrossRef]
- Wang, Y.L.; Peñas-Garzón, M.; Rodriguez, J.J.; Bedia, J.; Belver, C. Enhanced photodegradation of acetaminophen over Sr@TiO2/UiO-66-NH2 heterostructures under solar light irradiation. Chem. Eng. J. 2022, 446, 137229. [Google Scholar] [CrossRef]
- Sajid, M.; Bari, S.; Saif Ur Rehman, M.; Ashfaq, M.; Guoliang, Y.; Mustafa, G. Adsorption characteristics of paracetamol removal onto activated carbon prepared from Cannabis sativum Hemp. Alexandria Eng. J. 2022, 61, 7203–7212. [Google Scholar] [CrossRef]
- Wang, K.; Wu, C.; Wang, F.; Jing, N.; Jiang, G. Co/Co3O4 nanoparticles coupled with hollow nanoporous carbon polyhedrons for the enhanced electrochemical sensing of acetaminophen. ACS Sustain. Chem. Eng. 2019, 7, 18582–18592. [Google Scholar] [CrossRef]
- Chokkareddy, R.; Thondavada, N.; Bhajanthri, N.K.; Redhi, G.G. An amino functionalized magnetite nanoparticle and ionic liquid based electrochemical sensor for the detection of acetaminophen. Anal. Methods 2019, 11, 6204–6212. [Google Scholar] [CrossRef]
- Cernat, A.; Tertiş, M.; Săndulescu, R.; Bedioui, F.; Cristea, A.; Cristea, C. Electrochemical sensors based on carbon nanomaterials for acetaminophen detection: A review. Anal. Chim. Acta 2015, 886, 16–28. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, S.; Zhang, Y.; Ding, X.; Jiang, B.; Zhang, Y. An electrochemical sensor based on electro-polymerization of caffeic acid and Zn/Ni-ZIF-8–800 on glassy carbon electrode for the sensitive detection of acetaminophen. Biosens. Bioelectron. 2019, 131, 200–206. [Google Scholar] [CrossRef]
- Ziylan, A.; Ince, N.H. The occurrence and fate of anti-inflammatory and analgesic pharmaceuticals in sewage and fresh water: Treatability by conventional and non-conventional processes. J. Hazard. Mater. 2011, 187, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Warmińska, D.; Nowosielski, B.; Szewczyk, A.; Ruszkowski, J.; Prokopowicz, M. Effect of choline chloride based natural deep eutectic solvents on aqueous solubility and thermodynamic properties of acetaminophen. J. Mol. Liq. 2021, 323, 114834. [Google Scholar] [CrossRef]
- Grzegórska, A.; Gajewicz-Skretna, A.; Trykowski, G.; Sikora, K.; Zielińska-Jurek, A. Design and synthesis of TiO2/Ti3C2 composites for highly efficient photocatalytic removal of acetaminophen: The relationships between synthesis parameters, physicochemical properties, and photocatalytic activity. Catal. Today 2023, 413–415, 113980. [Google Scholar] [CrossRef]
- Jones, O.; Voulvoulis, N.; Lester, J. Aquatic environmental assessment of the top 25 English prescription pharmaceuticals. Water Res. 2002, 36, 5013–5022. [Google Scholar] [CrossRef]
- Huschek, G.; Hansen, P.D.; Maurer, H.H.; Krengel, D.; Kayser, A. Environmental risk assessment of medicinal products for human use according to European Commission recommendations. Environ. Toxicol. 2004, 19, 226–240. [Google Scholar] [CrossRef]
- Feng, L.; van Hullebusch, E.D.; Rodrigo, M.A.; Esposito, G.; Oturan, M.A. Removal of residual anti-inflammatory and analgesic pharmaceuticals from aqueous systems by electrochemical advanced oxidation processes. A review. Chem. Eng. J. 2013, 228, 944–964. [Google Scholar] [CrossRef]
- Huguet, M.; Simon, V.; Gallard, H. Transformation of paracetamol into 1,4-benzoquinone by a manganese oxide bed filter. J. Hazard. Mater. 2014, 271, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Pylypchuk, I.V.; Kessler, V.G.; Seisenbaeva, G.A. Simultaneous removal of acetaminophen, diclofenac, and Cd(II) by trametes versicolor laccase immobilized on Fe3O4/SiO2-DTPA hybrid nanocomposites. ACS Sustain. Chem. Eng. 2018, 6, 9979–9989. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, F.; Wu, X.; Chen, P.; Deng, N. Photodegradation of acetaminophen in TiO2 suspended solution. J. Hazard. Mater. 2008, 157, 300–307. [Google Scholar] [CrossRef] [PubMed]
- De Gusseme, B.; Vanhaecke, L.; Verstraete, W.; Boon, N. Degradation of acetaminophen by Delftia tsuruhatensis and Pseudomonas aeruginosa in a membrane bioreactor. Water Res. 2011, 45, 1829–1837. [Google Scholar] [CrossRef]
- Durán, A.; Monteagudo, J.M.; Carnicer, A.; Ruiz-Murillo, M. Photo-Fenton mineralization of synthetic municipal wastewater effluent containing acetaminophen in a pilot plant. Desalination 2011, 270, 124–129. [Google Scholar] [CrossRef]
- Palas, B.; Ersöz, G.; Atalay, S. Biotemplated copper oxide catalysts over graphene oxide for acetaminophen removal: Reaction kinetics analysis and cost estimation. Chem. Eng. Sci. 2021, 242, 116593. [Google Scholar] [CrossRef]
- Omotola, E.O.; Oluwole, A.O.; Oladoye, P.O.; Olatunji, O.S. Occurrence, detection and ecotoxicity studies of selected pharmaceuticals in aqueous ecosystems—A systematic appraisal. Environ. Toxicol. Pharmacol. 2022, 91, 103831. [Google Scholar] [CrossRef] [PubMed]
- Rathi, B.S.; Kumar, P.S.; Show, P.-L. A review on effective removal of emerging contaminants from aquatic systems: Current trends and scope for further research. J. Hazard. Mater. 2021, 409, 124413. [Google Scholar] [CrossRef]
- Fan, G.; Zheng, X.; Luo, J.; Peng, H.; Lin, H.; Bao, M.; Hong, L.; Zhou, J. Rapid synthesis of Ag/AgCl@ZIF-8 as a highly efficient photocatalyst for degradation of acetaminophen under visible light. Chem. Eng. J. 2018, 351, 782–790. [Google Scholar] [CrossRef]
- Roberts, P.H.; Thomas, K.V. The occurrence of selected pharmaceuticals in wastewater effluent and surface waters of the lower Tyne catchment. Sci. Total Environ. 2006, 356, 143–153. [Google Scholar] [CrossRef]
- Peña-Guzmán, C.; Ulloa-Sánchez, S.; Mora, K.; Helena-Bustos, R.; Lopez-Barrera, E.; Alvarez, J.; Rodriguez-Pinzón, M. Emerging pollutants in the urban water cycle in Latin America: A review of the current literature. J. Environ. Manag. 2019, 237, 408–423. [Google Scholar] [CrossRef]
- Fram, M.S.; Belitz, K. Occurrence and concentrations of pharmaceutical compounds in groundwater used for public drinking-water supply in California. Sci. Total Environ. 2011, 409, 3409–3417. [Google Scholar] [CrossRef]
- Sim, W.-J.; Lee, J.-W.; Oh, J.-E. Occurrence and fate of pharmaceuticals in wastewater treatment plants and rivers in Korea. Environ. Pollut. 2010, 158, 1938–1947. [Google Scholar] [CrossRef]
- Kim, J.; Yoon, S.; Lee, S.; Narumiya, M.; Nakada, N.; Han, I.; Tanaka, H. Occurrence and fate of PPCPs wastewater treatment plants in Korea. Int. Proc. Chem. Biol. Environ. Eng. (IPCBEE) 2012, 35, 57–61. [Google Scholar]
- Wu, J.-L.; Liu, Z.-H.; Ma, Q.-G.; Dai, L.; Dang, Z. Occurrence, removal and risk evaluation of ibuprofen and acetaminophen in municipal wastewater treatment plants: A critical review. Sci. Total Environ. 2023, 891, 164600. [Google Scholar] [CrossRef] [PubMed]
- Gómez, M.J.; Martínez Bueno, M.J.; Lacorte, S.; Fernández-Alba, A.R.; Agüera, A. Pilot survey monitoring pharmaceuticals and related compounds in a sewage treatment plant located on the Mediterranean coast. Chemosphere 2007, 66, 993–1002. [Google Scholar] [CrossRef]
- Phong Vo, H.N.; Le, G.K.; Hong Nguyen, T.M.; Bui, X.-T.; Nguyen, K.H.; Rene, E.R.; Vo, T.D.H.; Thanh Cao, N.-D.; Mohan, R. Acetaminophen micropollutant: Historical and current occurrences, toxicity, removal strategies and transformation pathways in different environments. Chemosphere 2019, 236, 124391. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.K.; Wong, C.S. Distribution and fate of pharmaceuticals and their metabolite conjugates in a municipal wastewater treatment plant. Water Res. 2018, 144, 774–783. [Google Scholar] [CrossRef]
- Botero-Coy, A.M.; Martínez-Pachón, D.; Boix, C.; Rincón, R.J.; Castillo, N.; Arias-Marín, L.P.; Manrique-Losada, L.; Torres-Palma, R.; Moncayo-Lasso, A.; Hernández, F. ‘An investigation into the occurrence and removal of pharmaceuticals in Colombian wastewater’. Sci. Total Environ. 2018, 642, 842–853. [Google Scholar] [CrossRef] [PubMed]
- Subedi, B.; Balakrishna, K.; Joshua, D.I.; Kannan, K. Mass loading and removal of pharmaceuticals and personal care products including psychoactives, antihypertensives, and antibiotics in two sewage treatment plants in southern India. Chemosphere 2017, 167, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Biel-Maeso, M.; Corada-Fernández, C.; Lara-Martín, P.A. Monitoring the occurrence of pharmaceuticals in soils irrigated with reclaimed wastewater. Environ. Pollut. 2018, 235, 312–321. [Google Scholar] [CrossRef]
- Aydin, S.; Aydin, M.E.; Ulvi, A. Monitoring the release of anti-inflammatory and analgesic pharmaceuticals in the receiving environment. Environ. Sci. Pollut. Res. 2019, 26, 36887–36902. [Google Scholar] [CrossRef]
- Najafizadeh, A.; Kaeidi, A.; Rahmani, M.; Hakimizadeh, E.; Hassanshahi, J. The protective effect of carvacrol on acetaminophen-induced renal damage in male rats. Mol. Biol. Rep. 2022, 49, 1763–1771. [Google Scholar] [CrossRef]
- Offor, S.J.; Amadi, C.N.; Chijioke-Nwauche, I.; Manautou, J.E.; Orisakwe, O.E. Potential deleterious effects of paracetamol dose regime used in Nigeria versus that of the United States of America. Toxicol. Rep. 2022, 9, 1035–1044. [Google Scholar] [CrossRef]
- Yang, R.; Tonnesseen, T.I. DAMPs and sterile inflammation in drug hepatotoxicity. Hepatol. Int. 2019, 13, 42–50. [Google Scholar] [CrossRef]
- Bunchorntavakul, C.; Reddy, K.R. Acetaminophen (APAP or N-acetyl-p-aminophenol) and acute liver failure. Clin. Liver Dis. 2018, 22, 325–346. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar Kollarahithlu, S.; Balakrishnan, R.M. Adsorption of pharmaceuticals pollutants, Ibuprofen, Acetaminophen, and Streptomycin from the aqueous phase using amine functionalized superparamagnetic silica nanocomposite. J. Clean. Prod. 2021, 294, 126155. [Google Scholar] [CrossRef]
- Kim, P.; Park, Y.; Ji, K.; Seo, J.; Lee, S.; Choi, K.; Kho, Y.; Park, J.; Choi, K. Effect of chronic exposure to acetaminophen and lincomycin on Japanese medaka (Oryzias latipes) and freshwater cladocerans Daphnia magna and Moina macrocopa, and potential mechanisms of endocrine disruption. Chemosphere 2012, 89, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Żur, J.; Piński, A.; Marchlewicz, A.; Hupert-Kocurek, K.; Wojcieszyńska, D.; Guzik, U. Organic micropollutants paracetamol and ibuprofen—Toxicity, biodegradation, and genetic background of their utilization by bacteria. Environ. Sci. Pollut. Res. 2018, 25, 21498–21524. [Google Scholar] [CrossRef]
- Kim, Y.; Choi, K.; Jung, J.; Park, S.; Kim, P.-G.; Park, J. Aquatic toxicity of acetaminophen, carbamazepine, cimetidine, diltiazem and six major sulfonamides, and their potential ecological risks in Korea. Environ. Int. 2007, 33, 370–375. [Google Scholar] [CrossRef]
- Ding, R.; Liu, S.; He, C.; Nie, X. Paracetamol affects the expression of detoxification- and reproduction-related genes and alters the life traits of Daphnia magna. Ecotoxicology 2020, 29, 398–406. [Google Scholar] [CrossRef]
- Wang, H.; Xi, H.; Xu, L.; Jin, M.; Zhao, W.; Liu, H. Ecotoxicological effects, environmental fate and risks of pharmaceutical and personal care products in the water environment: A review. Sci. Total Environ. 2021, 788, 147819. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Bae, J.; Park, J.; Suh, J.; Lee, S.; Park, H.; Choi, H. Removal of 12 selected pharmaceuticals by granular mesoporous silica SBA-15 in aqueous phase. Chem. Eng. J. 2014, 256, 475–485. [Google Scholar] [CrossRef]
- Natarajan, R.; Venkataraman, S.; Rajendran, D.S.; Tamilselvam, B.; Zaveri, H.; Jeyachandran, N.; Prashar, H.; Vaidyanathan, V.K. Adsorption performance of magnetic mesoporous silica microsphere support toward the remediation of acetaminophen from aqueous solution. J. Water Process Eng. 2022, 48, 102835. [Google Scholar] [CrossRef]
- Natarajan, R.; Anil Kumar, M.; Vaidyanathan, V.K. Synthesis and characterization of rhamnolipid based chitosan magnetic nanosorbents for the removal of acetaminophen from aqueous solution. Chemosphere 2022, 288, 132532. [Google Scholar] [CrossRef]
- Samal, K.; Mahapatra, S.; Hibzur Ali, M. Pharmaceutical wastewater as Emerging Contaminants (EC): Treatment technologies, impact on environment and human health. Energy Nexus 2022, 6, 100076. [Google Scholar] [CrossRef]
- Zhang, L.L.; Hu, J.; Zhu, R.Y.; Zhou, Q.W.; Chen, J.M. Degradation of paracetamol by pure bacterial cultures and their microbial consortium. Appl. Microbiol. Biotechnol. 2013, 97, 3687–3698. [Google Scholar] [CrossRef]
- Yang, C.-W.; Chen, Y.-E.; Chang, B.-V. Microbial Communities Associated with Acetaminophen Biodegradation from Mangrove Sediment. Sustainability 2020, 12, 5410. [Google Scholar] [CrossRef]
- Shahid, M.K.; Kashif, A.; Fuwad, A.; Choi, Y. Current advances in treatment technologies for removal of emerging contaminants from water—A critical review. Coord. Chem. Rev. 2021, 442, 213993. [Google Scholar] [CrossRef]
- Ling, Y.; Liao, G.; Xu, P.; Li, L. Fast mineralization of acetaminophen by highly dispersed Ag-g-C3N4 hybrid assisted photocatalytic ozonation. Sep. Purif. Technol. 2019, 216, 1–8. [Google Scholar] [CrossRef]
- Mohebali, H.; Moussavi, G.; Karimi, M.; Giannakis, S. Catalytic ozonation of acetaminophen with a magnetic, Cerium-based metal-organic framework as a novel, easily-separable nanocomposite. Chem. Eng. J. 2022, 434, 134614. [Google Scholar] [CrossRef]
- Trellu, C.; Coetsier, C.; Rouch, J.-C.; Esmilaire, R.; Rivallin, M.; Cretin, M.; Causserand, C. Mineralization of organic pollutants by anodic oxidation using reactive electrochemical membrane synthesized from carbothermal reduction of TiO2. Water Res. 2018, 131, 310–319. [Google Scholar] [CrossRef]
- Andreozzi, R.; Caprio, V.; Marotta, R.; Vogna, D. Paracetamol oxidation from aqueous solutions by means of ozonation and H2O2/UV system. Water Res. 2003, 37, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Yin, Y.; Liu, M.; Shi, L.; Zhang, S.; Asif, A.H.; Li, X.; Liu, M.; Duan, X.; Wang, S.; et al. Atomically dispersed Cu-N3 on hollow spherical carbon nitride for acetaminophen degradation: Generation of 1O2 from H2O2. Sep. Purif. Technol. 2023, 318, 124016. [Google Scholar] [CrossRef]
- Wu, J.-C.; Chuang, Y.-H.; Liou, S.Y.H.; Li, Q.; Hou, C.-H. In situ engineering of highly conductive TiO2/carbon heterostructure fibers for enhanced electrocatalytic degradation of water pollutants. J. Hazard. Mater. 2022, 429, 128328. [Google Scholar] [CrossRef]
- Li, M.; Bai, L.; Jiang, S.; Sillanpää, M.; Huang, Y.; Liu, Y. Electrocatalytic transformation of oxygen to hydroxyl radicals via three-electron pathway using nitrogen-doped carbon nanotube-encapsulated nickel nanocatalysts for effective organic decontamination. J. Hazard. Mater. 2023, 452, 131352. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; Wu, T. Electrocatalytic reduction of nitrobenzene using TiO2 nanotube electrodes with different morphologies: Kinetics, mechanism, and degradation pathways. Chem. Eng. J. 2019, 374, 1241–1252. [Google Scholar] [CrossRef]
- Mecha, A.C.; Onyango, M.S.; Ochieng, A.; Momba, M.N.B. Ultraviolet and solar photocatalytic ozonation of municipal wastewater: Catalyst reuse, energy requirements and toxicity assessment. Chemosphere 2017, 186, 669–676. [Google Scholar] [CrossRef]
- Yang, X.; De Buyck, P.-J.; Zhang, R.; Manhaeghe, D.; Wang, H.; Chen, L.; Zhao, Y.; Demeestere, K.; Van Hulle, S.W.H. Enhanced removal of refractory humic- and fulvic-like organics from biotreated landfill leachate by ozonation in packed bubble columns. Sci. Total Environ. 2022, 807, 150762. [Google Scholar] [CrossRef]
- Can-Güven, E.; Daniser, Y.; Yazici Guvenc, S.; Ghanbari, F.; Varank, G. Effective removal of furfural by ultraviolet activated persulfate, peroxide, and percarbonate oxidation: Focus on influencing factors, kinetics, and water matrix effect. J. Photochem. Photobiol. A 2022, 433, 114139. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Jeevanantham, S.; Anubha, M.; Jayashree, S. Degradation of toxic agrochemicals and pharmaceutical pollutants: Effective and alternative approaches toward photocatalysis. Environ. Pollut. 2022, 298, 118844. [Google Scholar] [CrossRef]
- Bilal, M.; Rasheed, T.; Mehmood, S.; Tang, H.; Ferreira, L.F.R.; Bharagava, R.N.; Iqbal, H.M.N. Mitigation of environmentally-related hazardous pollutants from water matrices using nanostructured materials—A review. Chemosphere 2020, 253, 126770. [Google Scholar] [CrossRef]
- Wong, K.T.; Kim, S.C.; Yun, K.; Choong, C.E.; Nah, I.W.; Jeon, B.-H.; Yoon, Y.; Jang, M. Understanding the potential band position and e–/h+ separation lifetime for Z-scheme and type-II heterojunction mechanisms for effective micropollutant mineralization: Comparative experimental and DFT studies. Appl. Catal. B Environ. 2020, 273, 119034. [Google Scholar] [CrossRef]
- Rahman, M.Z.; Edvinsson, T.; Gascon, J. Hole utilization in solar hydrogen production. Nat. Rev. Chem. 2022, 6, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Lang, Z.; Zhao, Y.; Zhao, X.; Qiu, T.; Hong, Q.; Wei, K.; Tan, H.; Kang, Z.; Li, Y. Copper-bridged tetrakis (4-ethynylphenyl) ethene aggregates with photo-regulated 1O2 and O2.− generation for selective photocatalytic aerobic oxidation. Angew. Chem. Int. Ed. 2022, 61, e202202914. [Google Scholar]
- Kansal, S.K.; Kundu, P.; Sood, S.; Lamba, R.; Umar, A.; Mehta, S.K.; Kansal, S.K. Photocatalytic degradation of the antibiotic levofloxacin using highly crystalline TiO2 nanoparticles. New J. Chem. 2014, 38, 3220–3226. [Google Scholar] [CrossRef]
- Ramalingam, G.; Perumal, N.; Priya, A.K.; Rajendran, S. A review of graphene-based semiconductors for photocatalytic degradation of pollutants in wastewater. Chemosphere 2022, 300, 134391. [Google Scholar] [CrossRef]
- Sun, D.; Zhou, T.; Che, G.; Liu, C. A synergism between Schottky junction and interfacial P-Ni bond for improving the hydrogen evolution of 2D/2D NiS/Phosphorus-doped g-C3N4 photocatalyst. Appl. Surf. Sci. 2022, 578, 152004. [Google Scholar] [CrossRef]
- Xu, P.; Ding, C.; Li, Z.; Yu, R.; Cui, H.; Gao, S. Photocatalytic degradation of air pollutant by modified nano titanium oxide (TiO2)in a fluidized bed photoreactor: Optimizing and kinetic modeling. Chemosphere 2023, 319, 137995. [Google Scholar] [CrossRef]
- Yu, Y.; Huang, H. Coupled adsorption and photocatalysis of g-C3N4 based composites: Material synthesis, mechanism, and environmental applications. Chem. Eng. J. 2023, 453, 139755. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Zhao, X.; Li, C.; Song, X.; Zhang, P.; Huo, P.; Li, X. A review on heterogeneous photocatalysis for environmental remediation: From semiconductors to modification strategies. Chin. J. Catal. 2022, 43, 178–214. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Wang, X.J.; Sun, Q.N.; Yuan, M.; Sun, Z.H.; Xia, S.Q.; Zhao, J.F. Enhanced visible light photo-Fenton-like degradation of tetracyclines by expanded perlite supported FeMo3Ox/g-C3N4 floating Z-scheme catalyst. J. Hazard. Mater. 2022, 424, 127387. [Google Scholar] [CrossRef]
- Jiang, L.B.; Yuan, X.Z.; Pan, Y.; Liang, J.; Zeng, G.M.; Wu, Z.B.; Wang, H. Doping of graphitic carbon nitride for photocatalysis: A reveiw. Appl. Catal. B Environ. 2017, 217, 388–406. [Google Scholar] [CrossRef]
- Wang, L.Y.; Bian, Z.Y. Photocatalytic degradation of paracetamol on Pd–BiVO4 under visible light irradiation. Chemosphere 2020, 239, 124815. [Google Scholar] [CrossRef]
- Ramasamy, B.; Jeyadharmarajan, J.; Chinnaiyan, P. Novel organic assisted Ag-ZnO photocatalyst for atenolol and acetaminophen photocatalytic degradation under visible radiation: Performance and reaction mechanism. Environ. Sci. Pollut. Res. 2021, 28, 39637–39647. [Google Scholar] [CrossRef]
- Gandelman, H.; da Silva, A.L.; Ramos, B.; Gouvêa, D. Interface excess on Sb-doped TiO2 photocatalysts and its influence on photocatalytic activity. Ceram. Int. 2021, 47, 619–625. [Google Scholar] [CrossRef]
- Lee, J.; Kok, S.H.W.; Ng, B.-J.; Kong, X.Y.; Putri, L.K.; Chai, S.-P.; Tan, L.-L. Hole scavenger-free nitrogen photofixation in pure water with non-metal B-doped carbon nitride: Implicative importance of B species for N2 activation. J. Environ. Chem. Eng. 2023, 11, 109511. [Google Scholar] [CrossRef]
- Ng, S.-F.; Chen, X.; Foo, J.J.; Xiong, M.; Ong, W.-J. 2D carbon nitrides: Regulating non-metal boron-doped C3N5 for elucidating the mechanism of wide pH range photocatalytic hydrogen evolution reaction. Chin. J. Catal. 2023, 47, 150–160. [Google Scholar] [CrossRef]
- Chong, W.-K.; Ng, B.-J.; Kong, X.Y.; Tan, L.-L.; Putri, L.K.; Chai, S.-P. Non-metal doping induced dual p-n charge properties in a single ZnIn2S4 crystal structure provoking charge transfer behaviors and boosting photocatalytic hydrogen generation. Appl. Catal. B Environ. 2023, 325, 122372. [Google Scholar] [CrossRef]
- Ranjith Kumar, D.; Ranjith, K.S.; Haldorai, Y.; Kandasami, A.; Rajendra Kumar, R.T. Nitrogen-implanted ZnO nanorod arrays for visible light photocatalytic degradation of a pharmaceutical drug acetaminophen. ACS Omega 2019, 4, 11973–11979. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Liu, Y.Q.; Ren, M.; Yang, G.; Qin, L.; Guo, Y.H.; Meng, J.Q. Precise carbon doping regulation of porous graphitic carbon nitride nanosheets enables elevated photocatalytic oxidation performance towards emerging organic pollutants. Chem. Eng. J. 2022, 433, 134551. [Google Scholar] [CrossRef]
- Xiao, C.F.; Chen, X.Q.; Tao, X.M.; Liu, X.; Wang, X.; Zhu, L. In situ generation of hydroxyl radicals by B-doped TiO2 for efficient photocatalytic degradation of acetaminophen in wastewater. Environ. Sci. Pollut. Res. 2023, 30, 46997–47011. [Google Scholar] [CrossRef]
- Wang, Q.; He, R.; Yang, F.; Tian, X.; Sui, H.; Feng, L. An overview of heteroatom doped cobalt phosphide for efficient electrochemical water splitting. Chem. Eng. J. 2023, 456, 141056. [Google Scholar] [CrossRef]
- Jafarisani, M.; Cheshme Khavar, A.H.; Mahjoub, A.R.; Luque, R.; Rodríguez-Padrón, D.; Satari, M.; Gharravi, A.M.; Khastar, H.; Kazemi, S.S.; Masoumikarimi, M. Enhanced visible-light-driven photocatalytic degradation of emerging water contaminants by a modified zinc oxide-based photocatalyst; In-vivo and in-vitro toxicity evaluation of wastewater and PCO-treated water. Sep. Purif. Technol. 2020, 243, 116430. [Google Scholar] [CrossRef]
- Paragas, L.K.B.; Dien Dang, V.; Sahu, R.S.; Garcia-Segura, S.; de Luna, M.D.G.; Pimentel, J.A.I.; Doong, R.-A. Enhanced visible-light-driven photocatalytic degradation of acetaminophen over CeO2/I, K-codoped C3N4 heterojunction with tunable properties in simulated water matrix. Sep. Purif. Technol. 2021, 272, 117567. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Chinthala, M.; Polagani, R.K.; Vo, D.-V.N. Removal of tetracycline from wastewater using g-C3N4 based photocatalysts: A review. Environ. Res. 2023, 216, 114660. [Google Scholar] [CrossRef] [PubMed]
- Muelas-Ramos, V.; Belver, C.; Rodriguez, J.J.; Bedia, J. Synthesis of noble metal-decorated NH2-MIL-125 titanium MOF for the photocatalytic degradation of acetaminophen under solar irradiation. Sep. Purif. Technol. 2021, 272, 118896. [Google Scholar] [CrossRef]
- Montalvo, C.; Gines, R.S.; Cantu, D.; Ruiz, A.; Aguilar, C.A.; Perez, I.; Ceron, R.M. Fluidized bed photoreactor for the removal of acetaminophen and pyridine using Al-doped TiO2 supported on alumina. Iran. J. Catal. 2022, 12, 295–313. [Google Scholar]
- Alzamly, A.; Hamed, F.; Ramachandran, T.; Bakiro, M.; Ahmed, S.H.; Mansour, S.; Salem, S.; Abdul al, K.; Al Kaabi, N.S.; Meetani, M. Tunable band gap of Bi3+-doped anatase TiO2 for enhanced photocatalytic removal of acetaminophen under UV-visible light irradiation. J. Water Reuse Desalin. 2019, 9, 31–46. [Google Scholar] [CrossRef]
- Sheikh Asadi, A.M.; Malakootian, M. Preparation and characterization of Fe/TiO2 in the presence of ionic liquid to optimize the photocatalytic degradation of acetaminophen using the response surface methodology. J. Mater. Sci. Mater. Electron. 2019, 30, 14878–14889. [Google Scholar] [CrossRef]
- Sayegh, S.; Abid, M.; Tanos, F.; Cretin, M.; Lesage, G.; Zaviska, F.; Petit, E.; Navarra, B.; Iatsunskyi, I.; Coy, E.; et al. N-doped TiO2 nanotubes synthesized by atomic layer deposition for acetaminophen degradation. Colloids Surf. A 2022, 655, 130213. [Google Scholar] [CrossRef]
- Abid, M.; Makhoul, E.; Tanos, F.; Iatsunskyi, I.; Coy, E.; Lesage, G.; Cretin, M.; Cornu, D.; Ben Haj Amara, A.; Bechelany, M. N-doped HNT/TiO2 nanocomposite by electrospinning for acetaminophen degradation. Membranes 2023, 13, 204. [Google Scholar] [CrossRef]
- Shaban, Y.A.; Fallata, H.M. Sunlight-induced photocatalytic degradation of acetaminophen over efficient carbon doped TiO2 (CTiO2) nanoparticles. Res. Chem. Intermed. 2019, 45, 2529–2547. [Google Scholar] [CrossRef]
- Wang, X.Y.; Sang, L.B.; Zhang, L.; Yang, G.; Guo, Y.H.; Yang, Y.X. Controllable synthesis for carbon self-doping and structural defect co-modified g-C3N4: Enhanced photocatalytic oxidation performance and the mechanism insight. J. Alloys Compd. 2023, 941, 168921. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Chinthala, M. Comprehensive review on advanced reusability of g-C3N4 based photocatalysts for the removal of organic pollutants. Chemosphere 2022, 297, 134190. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Zeng, D.; Ong, W.-J. Interfacial engineering of graphitic carbon nitride (g-C3N4)-based metal sulfide heterojunction photocatalysts for energy conversion: A review. Chin. J. Catal. 2019, 40, 289–319. [Google Scholar] [CrossRef]
- Brillas, E.; Manuel Peralta-Hernández, J. Removal of paracetamol (acetaminophen) by photocatalysis and photoelectrocatalysis. A critical review. Sep. Purif. Technol. 2023, 309, 122982. [Google Scholar] [CrossRef]
- Ali, A.Z.; Wu, Y.; Bennani, Y.-D.; Spanjers, H.; van der Hoek, J.P. Photo-electrocatalytic based removal of acetaminophen: Application of visible light driven heterojunction based BiVO4/BiOI photoanode. Chemosphere 2023, 324, 138322. [Google Scholar] [CrossRef]
- Sarkar, D.; Ghosh, C.K.; Mukherjee, S.; Chattopadhyay, K.K. Three dimensional Ag2O/TiO2 type-ii (p–n) nanoheterojunctions for superior photocatalytic activity. ACS Appl. Mater. Interfaces 2013, 5, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Cheshme Khavar, A.H.; Moussavi, G.; Mahjoub, A.R.; Luque, R.; Rodríguez-Padrón, D.; Sattari, M. Enhanced visible light photocatalytic degradation of acetaminophen with Ag2S-ZnO@rGO core-shell microsphere as a novel catalyst: Catalyst preparation and characterization and mechanistic catalytic experiments. Sep. Purif. Technol. 2019, 229, 115803. [Google Scholar] [CrossRef]
- Peñas-Garzón, M.; Gómez-Avilés, A.; Álvarez-Conde, J.; Bedia, J.; García-Frutos, E.M.; Belver, C. Azaindole grafted titanium dioxide for the photodegradation of pharmaceuticals under solar irradiation. J. Colloid Interface Sci. 2023, 629, 593–603. [Google Scholar] [CrossRef]
- Solís, R.R.; Quintana, M.A.; Martín-Lara, M.Á.; Pérez, A.; Calero, M.; Muñoz-Batista, M.J. Boosted activity of g-C3N4/UiO-66-NH2 heterostructures for the photocatalytic degradation of contaminants in water. Int. J. Mol. Sci. 2022, 23, 12871. [Google Scholar] [CrossRef] [PubMed]
- Chau, J.H.F.; Lai, C.W.; Leo, B.F.; Juan, J.C.; Johan, M.R. Advanced photocatalytic degradation of acetaminophen using Cu2O/WO3/TiO2 ternary composite under solar irradiation. Catal. Commun. 2022, 163, 106396. [Google Scholar] [CrossRef]
- Li, K.Y.; Chen, J.; Ao, Y.H.; Wang, P.F. Preparation of a ternary g-C3N4-CdS/Bi4O5I2 composite photocatalysts with two charge transfer pathways for efficient degradation of acetaminophen under visible light irradiation. Sep. Purif. Technol. 2021, 259, 118177. [Google Scholar] [CrossRef]
- Bi, X.; Du, G.H.; Kalam, A.; Sun, D.F.; Zhao, W.Q.; Yu, Y.; Su, Q.M.; Xu, B.S.; Al-Sehemi, A.G. Constructing anatase TiO2/Amorphous Nb2O5 heterostructures to enhance photocatalytic degradation of acetaminophen and nitrogen oxide. J. Colloid Interface Sci. 2021, 601, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Hojamberdiev, M.; Czech, B.; Göktaş, A.C.; Yubuta, K.; Kadirova, Z.C. SnO2@ZnS photocatalyst with enhanced photocatalytic activity for the degradation of selected pharmaceuticals and personal care products in model wastewater. J. Alloys Compd. 2020, 827, 154339. [Google Scholar] [CrossRef]
- Hassan, N.S.; Jalil, A.A.; Khusnun, N.F.; Bahari, M.B.; Hussain, I.; Firmansyah, M.L.; Nugraha, R.E.; Saravanan, R. Extra-modification of zirconium dioxide for potential photocatalytic applications towards environmental remediation: A critical review. J. Environ. Manag. 2023, 327, 116869. [Google Scholar] [CrossRef]
- Kumar, A.; Khan, M.; He, J.; Lo, I.M.C. Recent developments and challenges in practical application of visible–light–driven TiO2–based heterojunctions for PPCP degradation: A critical review. Water Res. 2020, 170, 115356. [Google Scholar] [CrossRef]
- Xia, T.; Hou, Q.; Qian, H.; Lai, R.; Bai, X.; Yu, G.; Zhang, W.; Laiq Ur Rehman, M.; Ju, M. Fabricating metal-free Z-scheme heterostructures with nitrogen-deficient carbon nitride for fast photocatalytic removal of acetaminophen. Sep. Purif. Technol. 2023, 308, 122964. [Google Scholar] [CrossRef]
- He, X.H.; Kai, T.H.; Ding, P. Heterojunction photocatalysts for degradation of the tetracycline antibiotic: A review. Environ. Chem. Lett. 2021, 19, 4563–4601. [Google Scholar] [CrossRef]
- Moradi, S.; Isari, A.A.; Hayati, F.; Rezaei Kalantary, R.; Kakavandi, B. Co-implanting of TiO2 and liquid-phase-delaminated g-C3N4 on multi-functional graphene nanobridges for enhancing photocatalytic degradation of acetaminophen. Chem. Eng. J. 2021, 414, 128618. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Ren, M.; Zhang, X.Y.; Yang, G.; Qin, L.; Pan, Y.; Meng, J.Q.; Guo, Y.H. Ti–N coordination bonds boost Z-scheme interfacial charge transfer in TiO2/C-deficient g-C3N4 heterojunctions for enhanced photocatalytic phenolic pollutant degradation. Appl. Surf. Sci. 2023, 614, 156118. [Google Scholar] [CrossRef]
- Meng, J.Q.; Wang, X.Y.; Liu, Y.Q.; Ren, M.; Zhang, X.Y.; Ding, X.H.; Guo, Y.H.; Yang, Y.X. Acid-induced molecule self-assembly synthesis of Z-scheme WO3/g-C3N4 heterojunctions for robust photocatalysis against phenolic pollutants. Chem. Eng. J. 2021, 403, 126354. [Google Scholar] [CrossRef]
- Liu, F.Y.; Nie, C.Y.; Dong, Q.Q.; Ma, Z.Y.; Liu, W.; Tong, M.P. AgI modified covalent organic frameworks for effective bacterial disinfection and organic pollutant degradation under visible light irradiation. J. Hazard. Mater. 2020, 398, 122865. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-E.; Moru, S.; Jo, W.-K.; Tonda, S. Porous g-C3N4-encapsulated TiO2 hollow sphere as a high-performance Z-scheme hybrid for solar-induced photocatalytic abatement of environmentally toxic pharmaceuticals. J. Mater. Sci. Technol. 2021, 82, 21–32. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Ren, M.; Zhang, X.Y.; Yang, G.; Qin, L.; Meng, J.Q.; Guo, Y.H. Supramolecule self-assembly approach to direct Z-scheme TiO2/g-C3N4 heterojunctions for efficient photocatalytic degradation of emerging phenolic pollutants. Appl. Surf. Sci. 2022, 593, 153401. [Google Scholar] [CrossRef]
- Rubio-Govea, R.; Orona-Návar, C.; Lugo-Bueno, S.F.; Hernández, N.; Mahlknecht, J.; García-García, A.; Ornelas-Soto, N. Bi2O3/rGO/MonO3n-1 all-solid-state ternary Z-scheme for visible-light driven photocatalytic degradation of bisphenol A and acetaminophen in groundwater. J. Environ. Chem. Eng. 2020, 8, 104170. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, W.-L.; Wang, Z.-W.; Wang, J.; Wu, Q.-Y. Single-atom Ag-loaded carbon nitride photocatalysts for efficient degradation of acetaminophen: The role of Ag-atom and O2. J. Environ. Sci. 2024, 139, 12–22. [Google Scholar] [CrossRef]
- Yang, L.; Yu, L.E.; Ray, M.B. Degradation of paracetamol in aqueous solutions by TiO2 photocatalysis. Water Res. 2008, 42, 3480–3488. [Google Scholar] [CrossRef]
- Peñas-Garzón, M.; Gómez-Avilés, A.; Belver, C.; Rodriguez, J.J.; Bedia, J. Degradation pathways of emerging contaminants using TiO2-activated carbon heterostructures in aqueous solution under simulated solar light. Chem. Eng. J. 2020, 392, 124867. [Google Scholar] [CrossRef]
- Rashidashmagh, F.; Doekhi-Bennani, Y.; Tizghadam-Ghazani, M.; van der Hoek, J.P.; Mashayekh-Salehi, A.; Heijman, B.S.G.J.; Yaghmaeian, K. Synthesis and characterization of SnO2 crystalline nanoparticles: A new approach for enhancing the catalytic ozonation of acetaminophen. J. Hazard. Mater. 2021, 404, 124154. [Google Scholar] [CrossRef]
- Zhao, B.; Yu, H.; Liu, Y.; Lu, Y.; Fan, W.; Qin, W.; Huo, M. Enhanced photoelectrocatalytic degradation of acetaminophen using a bifacial electrode of praseodymium-polyethylene glycol-PbO2/Ti/TiO2-nanotubes. Chem. Eng. J. 2021, 410, 128337. [Google Scholar] [CrossRef]
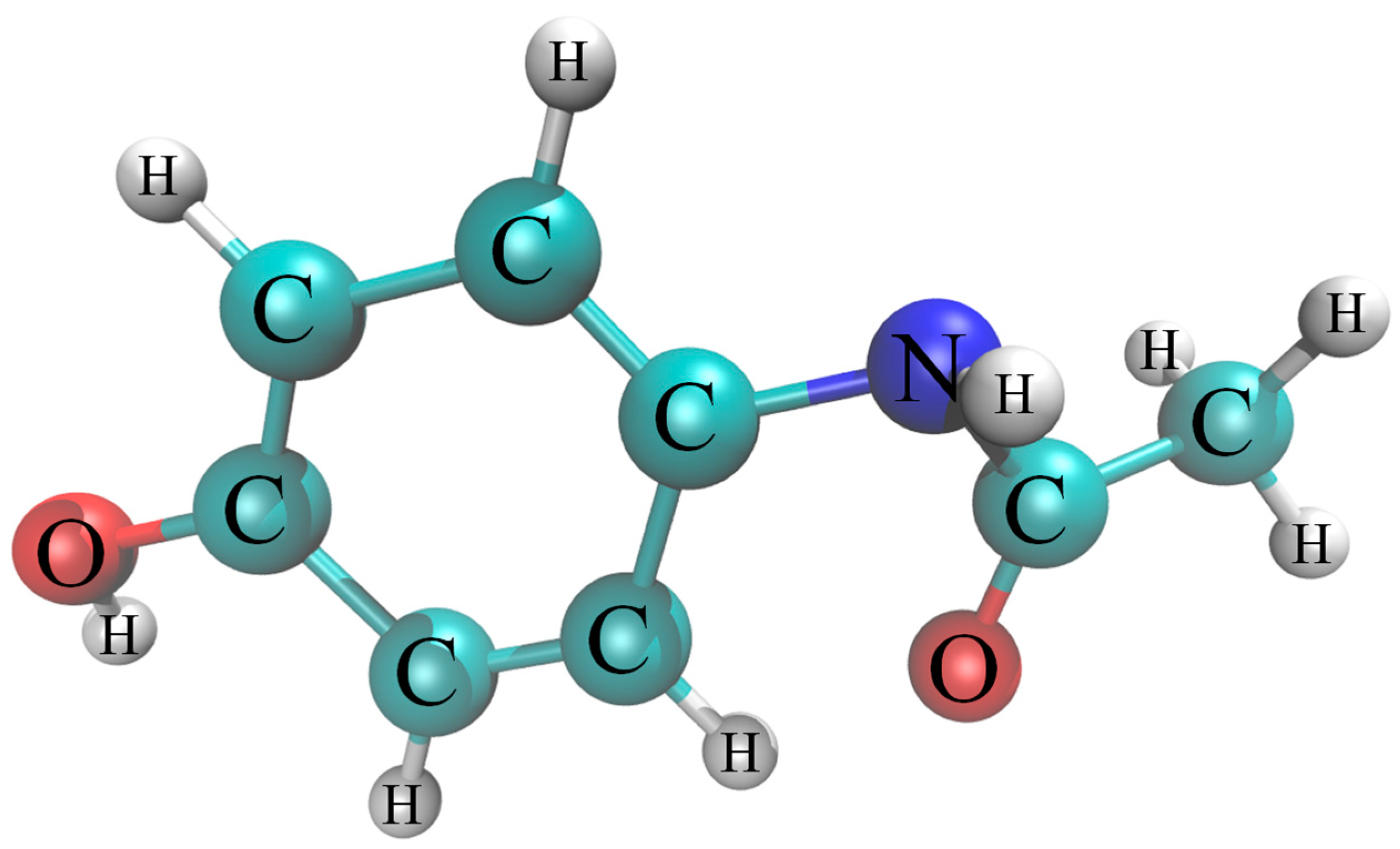

| Properties | Values |
|---|---|
| Chemical formula | C8H9NO2 |
| CAS number | 103-90-2 |
| Molecular weight (g/mol) | 151.2 |
| Melting point (°C) | 168–172 |
| Vapor pressure (mmHg) | 5.2 × 10−6 |
| Log Kow | 2.0 |
| Henry’s constant (at m3/m) | 6.4 × 10−13 |
| Water solubility (mg/L) | 1400–2400 |
| Country | Concentration (ng/L) | Year | Ref. |
|---|---|---|---|
| Canada | 320 | 2018 | [46] |
| Colombia | 25–410 | 2018 | [47] |
| India | 330–1200 | 2017 | [48] |
| Spain | 17–441 | 2018 | [49] |
| Turkey | 436 | 2019 | [50] |
| AOPs | Pollutant | Energy Requirements (kWh/m3/Order) | Ref. |
|---|---|---|---|
| Electrocatalysis | Crystal violet | 0.7 | [73] |
| Electrocatalysis | Bisphenol A | 0.07 | [74] |
| Electrocatalysis | Nitrobenzene | 2.07 | [75] |
| Photocatalysis | Phenol | 38.9–47.1 | [76] |
| Ozonation | Phenol | 26.2 | [76] |
| Ozonation | Humic- and fulvic-like organics | 18.0 | [77] |
| UV-activated persulfate | Furfural | 20.9 | [78] |
| UV-activated peroxide | Furfural | 34.5 | [78] |
| UV-activated percarbonate | Furfural | 26.6 | [78] |
| Catalyst | Type | Light Source | CACT (mg/L) | Time (min) | Efficiency (%) | Ref. |
|---|---|---|---|---|---|---|
| Pt/NH2-MIL-125 | Pt-doped | Xe lamp(λ ≤ 290 nm filter) | 5 | 180 | 100.0 | [105] |
| Pd-BiVO4 | Pd-doped | Xe lamp | 10 | 60 | 100.0 | [92] |
| Al-TiO2/Al2O3 | Al-doped | UV lamp | 40 | 300 | 85.0 | [106] |
| Sb-TiO2 | Sb-doped | UVA LED | 4.6 | 120 | 70.0 | [94] |
| Bi3+-TiO2 | Bi3+-doped | UVA lamp | 15 | 240 | 98.0 | [107] |
| Fe/TiO2 | Fe-doped | UVC lamp | 10 | 90 | 91.4 | [108] |
| Ag-ZnO | Ag-doped | Halogen lamp | 5 | 120 | 90.8 | [93] |
| B-TiO2 | B-doped | Halogen lamp | 10 | 30 | 98.8 | [100] |
| N-TiO2 NTs | N-doped | Halogen lamp | 5 | 90 | 98.3 | [109] |
| HNT/TiO2 | N-doped | Halogen lamp | 10 | 270 | 95.0 | [110] |
| N-ZnO | N-doped | Sunlight | 20 | 120 | 98.5 | [98] |
| C-TiO2 | C-doped | UV lamp | 3 | 90 | 100.0 | [111] |
| HCN-Cx | C-doped | Xe lamp | 10 | 10 | 98.4 | [99] |
| C-DCN | C-doped | Xe lamp | 10 | 60 | 99.4 | [112] |
| GaS@ZG | Ga, S co-doped | Xe lamp | 50 | 60 | 100.0 | [102] |
| CeO2/IK-C3N4 | CeO/I, K-co-doped | Visible light | 10 | 90 | 99.0 | [103] |
| Catalyst | Light Source | CACT (mg/L) | Time (min) | Efficiency (%) | Ref. |
|---|---|---|---|---|---|
| Cu2O/WO3/TiO2 | Xe lamp | 1 | 60 | 92.5 | [121] |
| g-C3N4-CdS/Bi4O5I2 | Xe lamp | 3 | 25 | 80.0 | [122] |
| TiO2-AZA4 | Xe lamp (λ ≤ 320 nm filter) | 5 | 120 | 100.0 | [119] |
| Sr@TiO2/UiO-66-NH2-2 | Xe lamp (λ ≤ 320 nm filter) | 5 | 240 | 93.5 | [17] |
| Ag2S-ZnO@rGO | Xe lamp | 20 | 60 | 100.0 | [118] |
| g-C3N4/UiO-66-NH2 | UVA | 5 | 240 | 100.0 | [120] |
| TiO2/0.25Nb2O5 | 40 W LED | 30 | 20 | 90.6 | [123] |
| SnO2@ZnS | Mercury lamp | 10 | 120 | 70.0 | [124] |
| Catalyst | Light Source | CACT (mg/L) | Time (min) | Efficiency (%) | Ref. |
|---|---|---|---|---|---|
| COF-PD/AgI | Visible light | 5 | 160 | 100.0 | [132] |
| BNCN350/BNCN400 | Xe lamp | 10 | 30 | 100.0 | [127] |
| TiO2/VC-CN | Xe lamp | 10 | 90 | 100.0 | [130] |
| g-C3N4/TiO2 | Xe lamp | 10 | 60 | 99.0 | [133] |
| WO3/g-C3N4 | Xe lamp | 10 | 60 | 98.2 | [131] |
| TiO2/g-C3N4 | Xe lamp | 10 | 45 | 96.7 | [134] |
| Bi2O3/rGO/MonO3n-1 | Xe lamp | 10 | 360 | 76.5 | [135] |
| TiO2/graphene/g-C3N4 | Xe-lamp (λ ≤ 420 nm filter) | 50 | 120 | 100.0 | [129] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Chen, H.; Rong, C.; Li, A.; Hua, X.; Dong, D.; Liang, D.; Liu, H. Photocatalytic Degradation of Acetaminophen in Aqueous Environments: A Mini Review. Toxics 2023, 11, 604. https://doi.org/10.3390/toxics11070604
Wang Z, Chen H, Rong C, Li A, Hua X, Dong D, Liang D, Liu H. Photocatalytic Degradation of Acetaminophen in Aqueous Environments: A Mini Review. Toxics. 2023; 11(7):604. https://doi.org/10.3390/toxics11070604
Chicago/Turabian StyleWang, Zhuowen, Haijun Chen, Chang Rong, Anfeng Li, Xiuyi Hua, Deming Dong, Dapeng Liang, and Haiyang Liu. 2023. "Photocatalytic Degradation of Acetaminophen in Aqueous Environments: A Mini Review" Toxics 11, no. 7: 604. https://doi.org/10.3390/toxics11070604
APA StyleWang, Z., Chen, H., Rong, C., Li, A., Hua, X., Dong, D., Liang, D., & Liu, H. (2023). Photocatalytic Degradation of Acetaminophen in Aqueous Environments: A Mini Review. Toxics, 11(7), 604. https://doi.org/10.3390/toxics11070604






