Stability of Drinking Water Distribution Systems and Control of Disinfection By-Products
Abstract
1. Introduction
2. Stability of Water Quality in the Network
2.1. Chemical Stability of Network Water Quality
2.2. Biological Stability of Pipe Network Water Quality
2.3. Interaction between Chemical Stability and Biological Stability of Pipe Network Water Quality
3. Generation and Removal of Pipe Network Disinfection By-Products
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, X.; Zhang, K.; Zhang, T.; Li, C.; Mao, X. An ignored and potential source of taste and odor (T&O) issues-biofilms in drinking water distribution system (DWDS). Appl. Microbiol. Biotechnol. 2017, 101, 3537–3550. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.; Zheng, W.; Wang, S.; Wang, Y. China’s new national standard for drinking water takes effect. Lancet 2012, 380, e8. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Zhao, P.; Zhang, H.; Tian, Y.; Chen, X.; Zhao, W.; Li, M. Identification and characterization of steady and occluded water in drinking water distribution systems. Chemosphere 2015, 119, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Wen-Bin, Z.; Ri-Hui, Z.; Hai-Yang, J. The Present Hygienic Condition and Countermeasures in Water Factories in Lianyungang Country. Mod. Prev. Med. 2001, 4, 469–470. [Google Scholar]
- Jun, H. Stability of Drinking Water Distribution Systems and Control of Disinfection By-Products; University of Chinese Academy of Sciences: Beijing, China, 2017. [Google Scholar]
- Pasha, M.F.K.; Lansey, K. Effect of parameter uncertainty on water quality predictions in distribution systems-case study. J. Hydroinform. 2010, 12, 1–21. [Google Scholar] [CrossRef]
- Dombay, G. Corrosion in drinking water distribution systems. ResearchGate 1997, 49, 1–8. [Google Scholar]
- Lytle, D.A.; Gerke, T.L.; Maynard, J.B. Effect of bacterial sulfate reduction on iron-corrosion scales. J. Am. Water Work. Assoc. 2005, 97, 109–120. [Google Scholar] [CrossRef]
- Wolska, M.; Molczan, M. Stability Assessment of Water Introduced into the Water Supply Network. Ochr. Srodowiska 2015, 37, 51–56. [Google Scholar]
- Shi, B.; Wan, Y.; Yu, Y.; Gu, J.; Wang, G. Evaluating the chemical stability in drinking water distribution system by corrosivity and precipitation potential. Water Sci. Technol. Water Supply 2018, 18, 383–390. [Google Scholar] [CrossRef]
- Characklis, W.G.; James, D.B.I.B. Bioengineering Report. Fouling biofilm development: A process analysis. Biotechnol. Bioeng. 2009, 102, 309–347. [Google Scholar] [CrossRef]
- Sarin, P.; Snoeyink, V.L.; Bebee, J.; Kriven, W.M.; Clement, J.A. Physico-chemical characteristics of corrosion scales in old iron pipes. Water Res. 2001, 35, 2961–2969. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Shi, B.; Gu, J.; Wang, D.; Yang, M. Morphological and physicochemical characteristics of iron corrosion scales formed under different water source histories in a drinking water distribution system. Water Res. 2012, 46, 5423–5433. [Google Scholar] [CrossRef] [PubMed]
- Swietlik, J.; Raczyk-Stanislawiak, U.; Piszora, P.; Nawrocki, J. Corrosion in drinking water pipes: The importance of green rusts. Water Res. 2012, 46, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Qu, J.; Liu, H.; Hu, C. Effects of copper(II) and copper oxides on THMs formation in copper pipe. Chemosphere 2007, 68, 2153–2160. [Google Scholar] [CrossRef]
- Feng, Y.; Teo, W.K.; Siow, K.S.; Tan, K.L.; Hsieh, A.K. The corrosion behaviour of copper in neutral tap water. Part I: Corrosion mechanisms. Corros. Sci. 1996, 38, 369–385. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, Y.; Singhal, V.; Giammar, D.E. Effects of pH and carbonate concentration on dissolution rates of the lead corrosion product PbO2. Environ. Sci. Technol. 2010, 44, 1093–1099. [Google Scholar] [CrossRef]
- Lin, Y.P.; Valentine, R.L. Reduction of lead oxide (PbO2) and release of Pb(II) in mixtures of natural organic matter, free chlorine and monochloramine. Environ. Sci. Technol. 2009, 43, 3872–3877. [Google Scholar] [CrossRef]
- Sarin, P.; Snoeyink, V.; Bebee, J.; Jim, K.; Beckett, M.; Kriven, W.; Clement, J. Iron release from corroded iron pipes in drinking water distribution systems: Effect of dissolved oxygen. Water Res. 2004, 38, 1259–1269. [Google Scholar] [CrossRef]
- Wang, H.; Hu, C.; Hu, X.; Yang, M.; Qu, J. Effects of disinfectant and biofilm on the corrosion of cast iron pipes in a reclaimed water distribution system. Water Res. 2012, 46, 1070–1078. [Google Scholar] [CrossRef]
- Ishikawa, T.; Kondo, Y.; Yasukawa, A.; Kandori, K. Formation of Magnetite in the Presence of Ferric Oxyhydroxides. Corros. Sci. 1998, 40, 1239–1251. [Google Scholar] [CrossRef]
- Sontheimer, H.; Kölle, W.; Snoeyink, V.L. The siderite model of the formation of corrosion-resistant scale. J. Am. Water Work. Assoc. 1981, 73, 572–579. [Google Scholar] [CrossRef]
- Gui-Fang, L.I.; Hai-Ying, J.; Jia-Quan, C.; Jian-Jun, W.; Wu-Chang, S.; Rui-Bao, J. Analysis on Characteristics and Causes of Corrosion Scale in a Drinking Water Distribution System in Jinan. China Water Wastewater 2015, 31, 49–51. [Google Scholar]
- Iino, T.; Ito, K.; Wakai, S.; Tsurumaru, H.; Ohkuma, M.; Harayama, S. Iron corrosion induced by nonhydrogenotrophic nitrate-reducing Prolixibacter sp. strain MIC1-1. Appl. Environ. Microbiol. 2015, 81, 1839–1846. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Griffin, A.; Edwards, M. Nitrification in premise plumbing: Role of phosphate, pH and pipe corrosion. Environ. Sci. Technol. 2008, 42, 4280–4284. [Google Scholar] [CrossRef]
- Meena, S.L.; Goyal, P.; Sharma, A.; Sharma, I.K.; Verma, P.S. The effect of inhibitors on the corrosion of aluminium in acidic and alkaline media. Bull. Electrochem. 2003, 19, 393–398. [Google Scholar]
- Oguzie, E.E.; Onuoha, G.N.; Ejike, E.N. Effect of Gongronema latifolium extract on aluminium corrosion in acidic and alkaline media. Pigment. Resin Technol. 2007, 36, 44–49. [Google Scholar] [CrossRef]
- Kuch, A. Investigations of the reduction and re-oxidation kinetics of iron(III) oxide scales formed in waters. Corros. Sci. 1988, 28, 221–231. [Google Scholar] [CrossRef]
- Larson, T.E. Chemical Control of Corrosion. J. Am. Water Works Ass. 1966, 58, 354–362. [Google Scholar] [CrossRef]
- Heyer, A.; D’Souza, F.; Bruin, A.; Ferrari, G.; Mol, J.M.C.; de Wit, J.H.W. A new approach to study local corrosion processes on steel surfaces by combining different microscopic techniques. Appl. Surf. Sci. 2012, 258, 8790–8796. [Google Scholar] [CrossRef]
- AlShamaileh, E.; AlRawajfeh, A.; AlMa’abrah, A. Assessment of Quality and Potential of Scale Formation and Corrosivity of Drinking Water Supplied from Disi to Amman, Jordan. Fresenius Environ. Bull. 2017, 26, 634–645. [Google Scholar]
- Luo, B.; Zhao, X.; Liang, B.; Dong, G. (Eds.) Study on Water Quality Chemical Stability of Desalinated Seawater in Municipal Water Supply Systems. In Proceedings of the International Conference on Environmental Science & Information Application Technology, Wuhan, China, 4–5 July 2009. [Google Scholar]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Roddick, F.A.; Fan, L. Biofouling of Water Treatment Membranes: A Review of the Underlying Causes, Monitoring Techniques and Control Measures. Membranes 2012, 2, 804–840. [Google Scholar] [CrossRef] [PubMed]
- Fish, K.E.; Osborn, A.M.; Boxall, J. Characterising and understanding the impact of microbial biofilms and the extracellular polymeric substance (EPS) matrix in drinking water distribution systems. Environ. Sci. Water Res. Technol. 2016, 2, 614–630. [Google Scholar] [CrossRef]
- Hunt, A.P.; Parry, J.D.J.B. The effect of substratum roughness and river flow rate on the development of a freshwater biofilm community. Biofouling 1998, 12, 287–303. [Google Scholar] [CrossRef]
- Revetta, R.P.; Vicente, G.A.; Gerke, T.L.; Claudine, C.; Santo, D.J.W.; Ashbolt, N. Establishment and early succession of bacterial communities in monochloramine-treated drinking water biofilms. Fems Microbiol. Ecol. 2014, 86, 404–414. [Google Scholar] [CrossRef]
- Liu, R.; Yu, Z.; Guo, H.; Liu, M.; Zhang, H.; Yang, M. Pyrosequencing analysis of eukaryotic and bacterial communities in faucet biofilms. Sci. Total Environ. 2012, 435–436, 124–131. [Google Scholar] [CrossRef]
- Bartrand, T.A.; Causey, J.J.; Clancy, J.L. Naegleria fowleri: An emerging drinking water pathogen. J. Am. Water Work. Assoc. 2014, 106, E32–E418. [Google Scholar] [CrossRef]
- Zhang, P.; Lapara, T.M.; Goslan, E.H.; Xie, Y.; Parsons, S.A.; Hozalski, R.M. Biodegradation of haloacetic acids by bacterial isolates and enrichment cultures from drinking water systems. Environ. Sci. Technol. 2009, 43, 3169–3175. [Google Scholar] [CrossRef]
- Learbuch, K.L.G.; Smidt, H.; van der Wielen, P.W.J.J. Influence of pipe materials on the microbial community in unchlorinated drinking water and biofilm. Water Res. 2021, 194, 116922. [Google Scholar] [CrossRef]
- Simes, L.C.; Simes, M. Biofilms in drinking water: Problems and solutions. Rsc Adv. 2013, 3, 2520–2533. [Google Scholar] [CrossRef]
- Ndiongue, S.; Huck, P.M.; Slawson, R.M. Effects of temperature and biodegradable organic matter on control of biofilms by free chlorine in a model drinking water distribution system. Water Res. 2005, 39, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Marabini, L.; Frigerio, S.; Chiesara, E.; Radice, S. Toxicity evaluation of surface water treated with different disinfectants in HepG2 cells. Water Res. 2006, 40, 267–272. [Google Scholar] [CrossRef]
- Lee, W.; Westerhoff, P.; Yang, X.; Shang, C. Comparison of colorimetric and membrane introduction mass spectrometry techniques for chloramine analysis. Water Res. 2007, 41, 3097–3102. [Google Scholar] [CrossRef]
- Liu, S.; Gunawan, C.; Barraud, N.; Rice, S.A.; Harry, E.J.; Amal, R. Understanding, Monitoring, and Controlling Biofilm Growth in Drinking Water Distribution Systems. Environ. Sci. Technol. 2016, 50, 8954–8976. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Gunten, U.V.; Croue, J.P. Chlorination of bromide-containing waters: Enhanced bromate formation in the presence ofsynthetic metal oxides and deposits formed indrinking water distribution systems. Water Res. 2013, 47, 5307–5315. [Google Scholar] [CrossRef]
- Zhang, H.; Andrews, S.A. Catalysis of copper corrosion products on chlorine decay and HAA formation in simulated distribution systems. Water Res. 2012, 46, 2665–2673. [Google Scholar] [CrossRef]
- Zhang, H.; Andrews, S.A. Factors affecting catalysis of copper corrosion products in NDMA formation from DMA in simulated premise plumbing. Chemosphere 2013, 93, 2683–2689. [Google Scholar] [CrossRef]
- Chen, G.; Palmer, R.J.; White, D.C. Instrumental analysis of microbiologically influenced corrosion. Biodegradation 1997, 8, 189–200. [Google Scholar] [CrossRef]
- Sharma, S.K.; Petrusevski, B.; Schippers, J.C. Biological iron removal from groundwater: A review. J. Water Supply Res. Technol. Aqua 2005, 54, 239–247. [Google Scholar] [CrossRef]
- Starosvetsky, D.; Armon, R.; Yahalom, J.; Starosvetsky, J. Pitting corrosion of carbon steel caused by iron bacteria. Int. Biodeterior. 2001, 47, 79–87. [Google Scholar] [CrossRef]
- King, R.A.; Miller, J.D. Corrosion by the sulphate-reducing bacteria. Nature 1971, 233, 491–492. [Google Scholar] [CrossRef] [PubMed]
- Pacheco Aguilar, J.R.; Pena Cabriales, J.J.; Maldonado Vega, M. Identification and characterization of sulfur-oxidizing bacteria in an artificial wetland that treats wastewater from a tannery. Int. J. Phytoremediation. 2008, 10, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, D.Y.; Tourova, T.P.; Antipov, A.N.; Muyzer, G.; Kuenen, J.G. Anaerobic growth of the haloalkaliphilic denitrifying sulfur-oxidizing bacterium Thialkalivibrio thiocyanodenitrificans sp. nov. with thiocyanate. Microbiology 2004, 150, 2435–2442. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R.; Stolz, J.F.; Nord, G.L., Jr.; Phillips, E.J. Anaerobic production of magnetite by a dissimilatory iron-reducing microorganism. Nature 1987, 330, 252–254. [Google Scholar] [CrossRef]
- Bell, P.E.; Mills, A.L.; Herman, J.S. Biogeochemical Conditions Favoring Magnetite Formation during Anaerobic Iron Reduction. Appl. Environ. Microbiol. 1987, 53, 2610–2616. [Google Scholar] [CrossRef]
- Sorokin, D.Y.; Tourova, T.P.; Sjollema, K.A.; Kuenen, J.G. Thialkalivibrio nitratireducens sp. nov., a nitrate-reducing member of an autotrophic denitrifying consortium from a soda lake. Int. J. Syst. Evol. Microbiol. 2003, 53, 1779–1783. [Google Scholar] [CrossRef]
- Straub, K.L.; Benz, M.; Schink, B.; Widdel, F. Anaerobic, nitrate-dependent microbial oxidation of ferrous iron. Appl. Environ. Microbiol. 1996, 62, 1458–1460. [Google Scholar] [CrossRef]
- Peng, X.; Chen, S.; Zhou, H.; Zhang, L.; Xu, H. Diversity of biogenic minerals in low-temperature Si-rich deposits from a newly discovered hydrothermal field on the ultraslow spreading Southwest Indian Ridge. J. Geophys. Res. Biogeosciences 2015, 116, 162. [Google Scholar] [CrossRef]
- Beech, I.B.; Sunner, J. Biocorrosion: Towards understanding interactions between biofilms and metals. Curr. Opin. Biotechnol. 2004, 15, 181–186. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, H.; Ang, H.M.; Tade, M.O.; Wang, S. 3D-hierarchically structured MnO2 for catalytic oxidation of phenol solutions by activation of peroxymonosulfate: Structure dependence and mechanism. Appl. Catal. B-Environ. 2015, 164, 159–167. [Google Scholar] [CrossRef]
- Jeffrey, R.; Melchers, R.E. Bacteriological influence in the development of iron sulphide species in marine immersion environments. Corros. Sci. 2003, 45, 693–714. [Google Scholar] [CrossRef]
- Iii, E.R.B.; Margetas, D.; Duggirala, R. Copper catalysis in chloroform formation during water chlorination. Water Res. 2003, 37, 4385–4394. [Google Scholar]
- Fu, J.; Qu, J.; Liu, R.; Qiang, Z.; Liu, H.; Zhao, X. Cu(II)-catalyzed THM formation during water chlorination and monochloramination: A comparison study. J. Hazard. Mater. 2009, 170, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Sun, P.; Zhou, X.; Zhang, Y.; Huang, C.H. Cu(II)–Catalyzed Transformation of Benzylpenicillin Revisited: The Overlooked Oxidation. Environ. Sci. Technol. 2015, 49, 4218. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.; Xue, D.; Pan, H.; Feng, J.; Li, Y. Degradation of ibuprofen by a synergistic UV/Fe(III)/Oxone process. Chem. Eng. J. 2016, 283, 65–75. [Google Scholar] [CrossRef]
- Liu, C.; von Gunten, U.; Croue, J.P. Enhanced bromate formation during chlorination of bromide-containing waters in the presence of CuO: Catalytic disproportionation of hypobromous acid. Environ. Sci. Technol. 2012, 46, 11054–11061. [Google Scholar] [CrossRef]
- Pham, A.N.; Rose, A.L.; Waite, T.D. Kinetics of Cu(II) Reduction by Natural Organic Matter. J. Phys. Chem. A 2012, 116, 6590–6599. [Google Scholar] [CrossRef]
- Brown, D.; Bridgeman, J.; West, J.R. Predicting chlorine decay and THM formation in water supply systems. Rev. Environ. Sci. Biotechnol. 2011, 10, 79–99. [Google Scholar] [CrossRef]
- Tsagkari, E.; Sloan, W.T. Impact of Methylobacterium in the drinking water microbiome on removal of trihalomethanes. Int. Biodeter. Biodegr. 2019, 141, 10–16. [Google Scholar] [CrossRef]
- Lin, Y.-P.; Valentine, R.L. Release of Pb(II) from Monochloramine-Mediated Reduction of Lead Oxide (PhO2). Environ. Sci. Technol. 2008, 42, 9137–9143. [Google Scholar] [CrossRef]
- Berbey, C.; Allard, B. Divalent Cation Current And Influx Investigated By The Mn2+Quenching Method In Resting And Active Voltage-Controlled Mouse Skeletal Muscle Fibres. Biophys. J. 2009, 96, 5A–234A. [Google Scholar] [CrossRef] [PubMed]
- Sathasivan, A.; Fisher, I.; Tam, T. Onset of severe nitrification in mildly nitrifying chloraminated bulk waters and its relation to biostability. Water Res. 2008, 42, 3623–3632. [Google Scholar] [CrossRef]
- Bal Krishna, K.C. Does an Unknown Mechanism Accelerate Chemical Chloramine Decay in Nitrifying Waters? J. Am. Water Work. Assoc. 2010, 102, 96. [Google Scholar] [CrossRef]
- Krishna, K.C.B.; Sathasivan, A.; Sarker, D.C. Evidence of soluble microbial products accelerating chloramine decay in nitrifying bulk water samples. Water Res. 2012, 46, 3977–3988. [Google Scholar] [CrossRef] [PubMed]
- Sathasivan, A.; Krishna, K.C.B. Major mechanism(s) of chloramine decay in rechloraminated laboratory scale system waters. Desalination Water Treat. 2012, 47, 112–119. [Google Scholar] [CrossRef]
- Sadiq, R.; Rodriguez, M.J. Disinfection by-products (DBPs) in drinking water and predictive models for their occurrence: A review. Sci. Total Environ. 2004, 321, 21–46. [Google Scholar] [CrossRef]
- Azeem, S.M.A.; Burham, N.; Borik, M.G.; El Shahat, M.F. Trihalomethanes formation in water treatment plants and distribution lines: A monitoring and modeling scheme. Toxicol. Environ. Chem. 2014, 96, 12–26. [Google Scholar] [CrossRef]
- Liu, C.; Croue, J.P. Formation of Bromate and Halogenated Disinfection Byproducts during Chlorination of Bromide-Containing Waters in the Presence of Dissolved Organic Matter and CuO. Environ. Sci. Technol. 2016, 50, 135–144. [Google Scholar] [CrossRef]
- Gallard, H.; Allard, S.; Nicolau, R.; Gunten, U.V.; Croue, J.P. Formation of lodinated Organic Compounds by Oxidation of Iodide-Containing Waters with Manganese Dioxide. Environ. Sci. Technol. 2009, 43, 7003–7009. [Google Scholar] [CrossRef]
- Chun, C.L.; Hozalski, R.M.; Arnold, W.A. Degradation of disinfection byproducts by carbonate green rust. Environ. Sci. Technol. 2007, 41, 1615–1621. [Google Scholar] [CrossRef]
- Heeb, M.B.; Criquet, J.; Zimmermann-Steffens, S.G.; Von Gunten, U. Oxidative treatment of bromide-containing waters: Formation of bromine and its reactions with inorganic and organic compounds—A critical review. Water Res. 2014, 48, 15–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Liu, X.; Ng, T.W.; Xiao, J.W.; Chow, A.T.; Wong, P.K. Disinfection byproduct formation from chlorination of pure bacterial cells and pipeline biofilms. Water Res. 2013, 47, 2701–2709. [Google Scholar] [CrossRef] [PubMed]
- Borgmann-Strahsen, R. Comparative assessment of different biocides in swimming pool water. Int. Biodeterior. 2003, 51, 291–297. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D. Transition metal/UV-based advanced oxidation technologies for water decontamination. Appl. Catal. B-Environ. 2004, 54, 155–163. [Google Scholar] [CrossRef]
- Luo, C.; Jiang, J.; Ma, J.; Pang, S.; Liu, Y.; Song, Y.; Guan, C.; Li, J.; Jin, Y.; Wu, D. Oxidation of the odorous compound 2,4,6-trichloroanisole by UV activated persulfate: Kinetics, products, and pathways. Water Res. 2016, 96, 12–21. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Tufano, T.P.; Dionysiou, D.D. Chemical and microbial decontamination of pool water using activated potassium peroxymonosulfate. Water Res. 2008, 42, 2899–2910. [Google Scholar] [CrossRef]
- Guerra-Rodriguez, S.; Rodriguez, E.; Singh, D.N.; Rodriguez-Chueca, J. Assessment of Sulfate Radical-Based Advanced Oxidation Processes for Water and Wastewater Treatment: A Review. Water 2018, 10, 1828. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Reactive species in advanced oxidation processes: Formation, identification and reaction mechanism. Chem. Eng. J. 2020, 401, 126158. [Google Scholar] [CrossRef]
- Poonsuk, K.; Arunorat, J.; Talummuk, S.; Kunalintip, R.; Anuvongnukroh, W.; Thanawongnuwech, R. Field Efficacy of Potassium Peroxymonosulfate (PMS) Oxidizing Disinfectant (VirusnipTM) against Porcine Circovirus Type 2 (PCV2) in Gilt Acclimatization Unit. Thai. J. Vet. Med. 2013, 43, 601–604. [Google Scholar]
- Cancho, B.; Ventura, F.; Galceran, M.T.; Diaz, A.; Ricart, S. Determination, synthesis and survey of iodinated trihalomethanes in water treatment processes. Water Res. 2000, 34, 3380–3390. [Google Scholar] [CrossRef]
- Xiao, Y.; Fan, R.; Zhang, L.; Yue, J.; Webster, R.D.; Lim, T.T. Photodegradation of iodinated trihalomethanes in aqueous solution by UV 254 irradiation. Water Res. 2014, 49, 275–285. [Google Scholar] [CrossRef]
- Sharma, V.K.; Yang, X.; Cizmas, L.; McDonald, T.J.; Luque, R.; Sayes, C.M.; Yuan, B.; Dionysiou, D.D. Impact of metal ions, metal oxides, and nanoparticles on the formation of disinfection byproducts during chlorination. Chem. Eng. J. 2017, 317, 777–792. [Google Scholar] [CrossRef]
- Wen, G.; Xu, X.; Zhu, H.; Huang, T.; Ma, J. Inactivation of four genera of dominant fungal spores in groundwater using UV and UV/PMS: Efficiency and mechanisms. Chem. Eng. J. 2017, 328, 619–628. [Google Scholar] [CrossRef]
- Rodríguez-Chueca, J.; Giannakis, S.; Marjanovic, M.; Kohantorabi, M.; Gholami, M.R.; Grandjean, D.; de Alencastro, L.F.; Pulgarín, C. Solar-assisted bacterial disinfection and removal of contaminants of emerging concern by Fe2+-activated HSO5− vs. S2O82− in drinking water. Appl. Catal. B-Environ. 2019, 248, 62–72. [Google Scholar] [CrossRef]
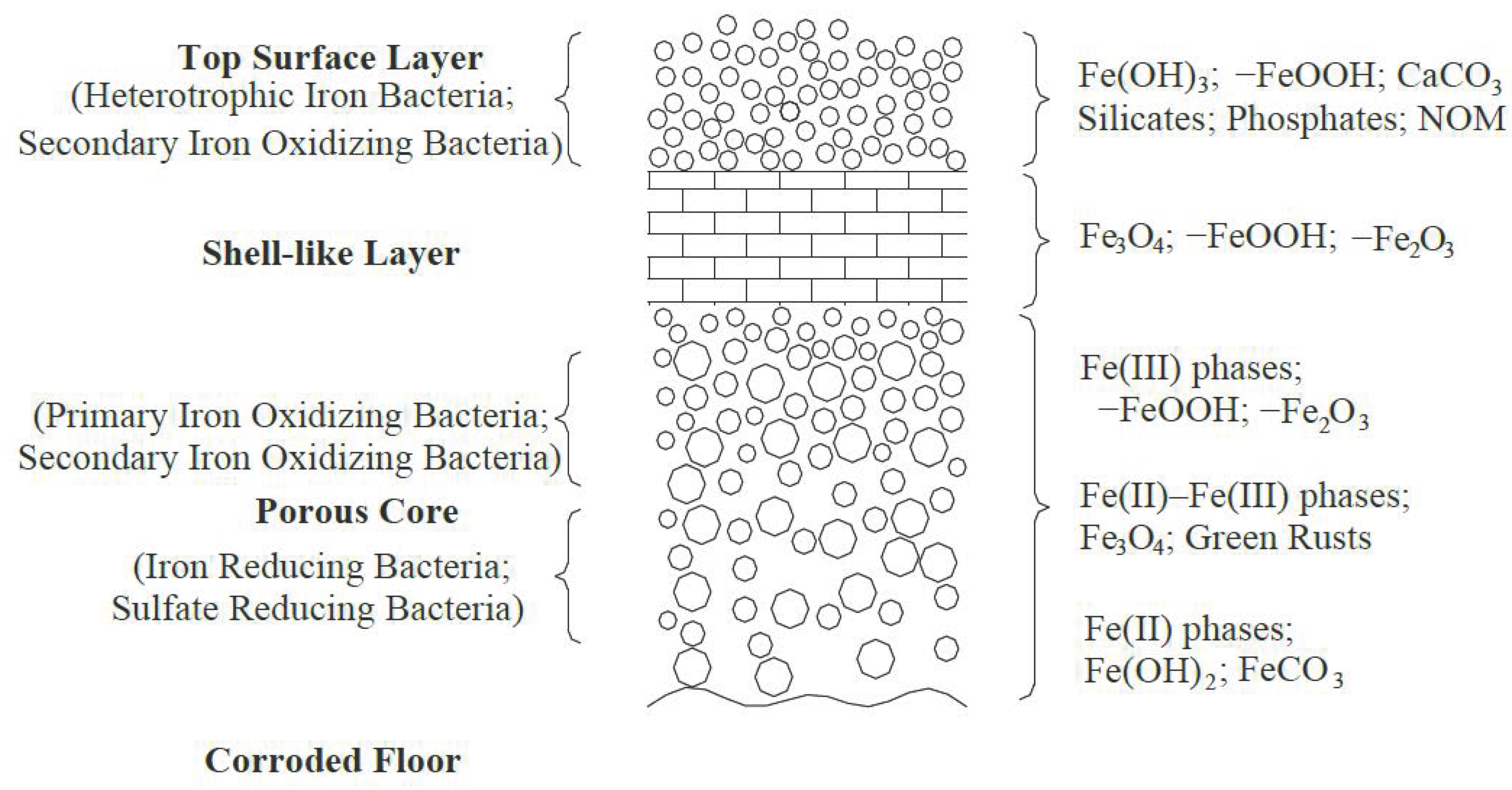
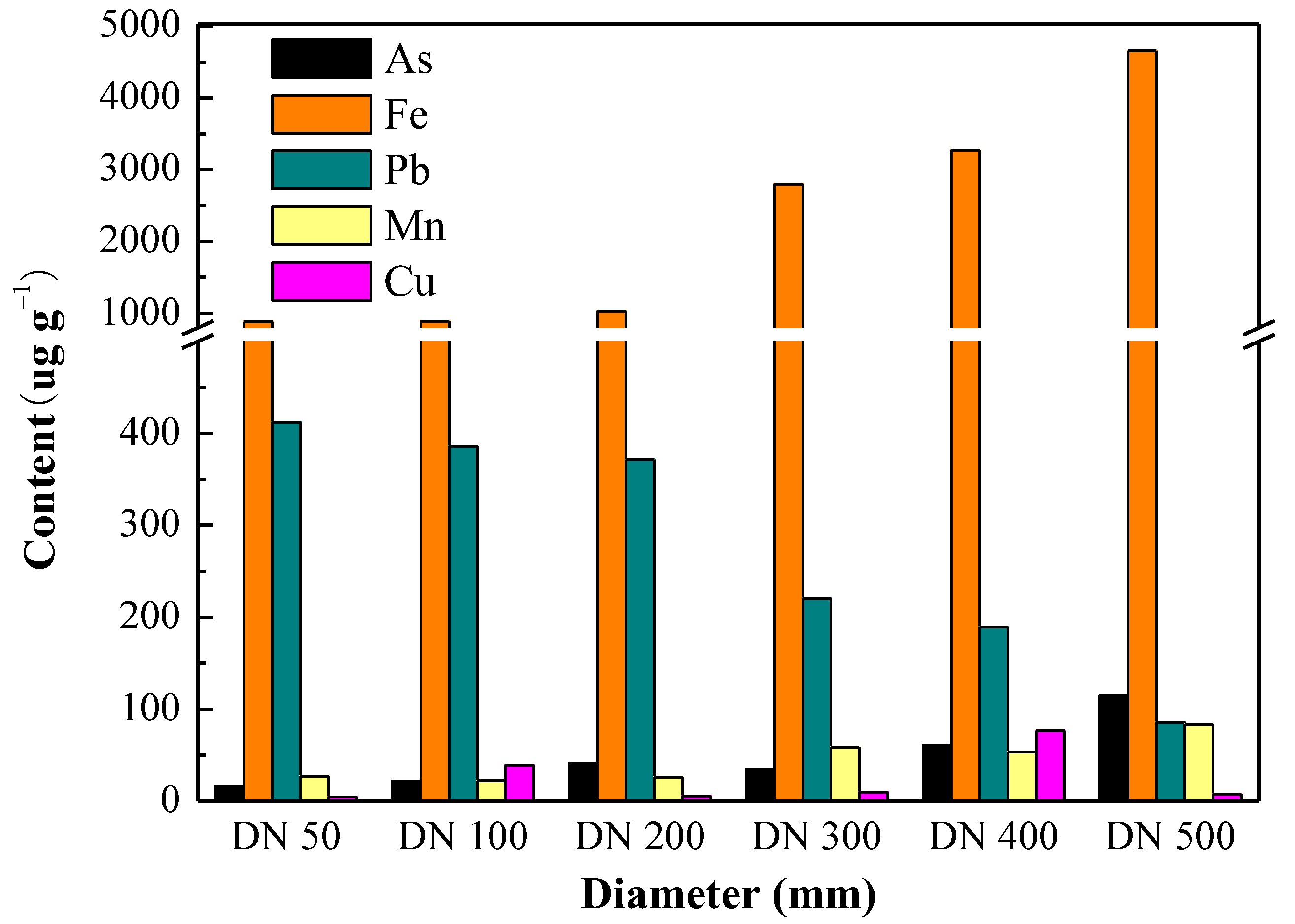
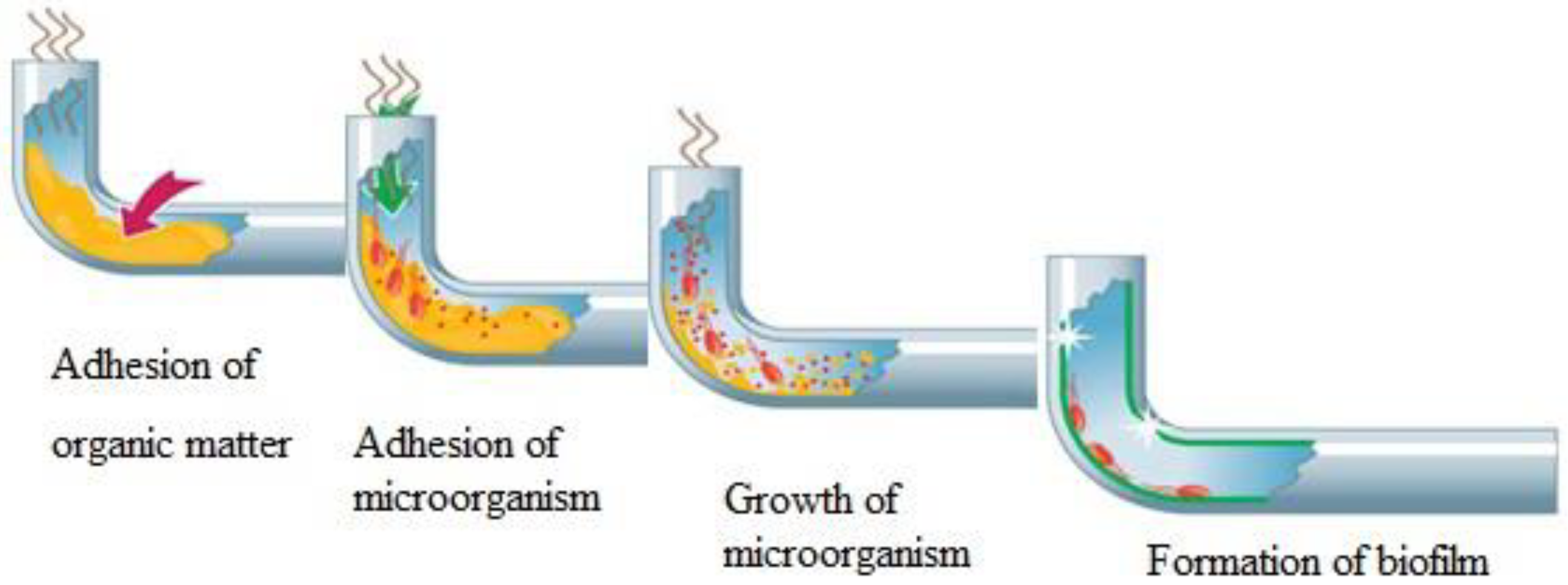
| Name of Disinfectant | Contact Time with Water (min) | Treated Water Limit (mg/L) | Ex-Treated Water Allowance (mg/L) | Water Allowance at the End of Pipe Network (mg/L) |
|---|---|---|---|---|
| Free chlorine | ≥30 | 4 | ≥0.3 | ≥0.05 |
| Monochloramine | ≥120 | 3 | ≥0.5 | ≥0.05 |
| Chlorine dioxide | ≥30 | 0.8 | ≥0.1 | ≥0.02 |
| ozone | ≥12 | 0.3 | ≥0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Q.; Bian, Z.; Yang, D.; Fu, L. Stability of Drinking Water Distribution Systems and Control of Disinfection By-Products. Toxics 2023, 11, 606. https://doi.org/10.3390/toxics11070606
Zhou Q, Bian Z, Yang D, Fu L. Stability of Drinking Water Distribution Systems and Control of Disinfection By-Products. Toxics. 2023; 11(7):606. https://doi.org/10.3390/toxics11070606
Chicago/Turabian StyleZhou, Qingwei, Zhengfu Bian, Dejun Yang, and Li Fu. 2023. "Stability of Drinking Water Distribution Systems and Control of Disinfection By-Products" Toxics 11, no. 7: 606. https://doi.org/10.3390/toxics11070606
APA StyleZhou, Q., Bian, Z., Yang, D., & Fu, L. (2023). Stability of Drinking Water Distribution Systems and Control of Disinfection By-Products. Toxics, 11(7), 606. https://doi.org/10.3390/toxics11070606







