Multiple Assays on Non-Target Organisms to Determine the Risk of Acute Environmental Toxicity in Tebuconazole-Based Fungicides Widely Used in the Black Sea Coastal Area
Abstract
1. Introduction
2. Materials and Methods
2.1. Application of Toxkit Microbiotests
2.1.1. Duckweed Toxkit F
- Preparation of duckweed growth and test dilution medium (Steinberg solution);
- Germination of the S. polyrhiza turions (incubation 72 h at 25 °C, under continuous illumination, at min. 6000 lux);
- Preparation of the toxicant dilutions;
- Filling-in of the test plate with the toxicant dilutions;
- Transfer of the germinated turions in the test cups, photography of the multiwell at the start of the toxicity test (original);
- Incubation of the test plate (72 h at 25 °C, at min. 6000 lux);
- Photography of the multiwell at the end of the toxicity test;
- Measurement of the area of the first fronds using Image J software [27];
- Determination of the test validity: the “mean growth” of the first fronds in the cups of the control column after 3 days incubation at 25 °C and under 6000 lux illumination (=the mean t 72 h–t 0 h area) must be at least 10 mm2.
2.1.2. Daphtoxkit F Test with Daphnia magna Straus, 1820
- Preparation of standard freshwater (S.F.) used as hatching medium for the ephippia and as a dilution medium for preparation of the toxicant dilution series;
- Pre-aeration of S.F. for at least 15 min prior to its use for the hatching of the dormant eggs and for the preparation of the toxicant dilution series;
- Hatching of the ephippia in diluted S.F., 3 days prior to the start of the toxicity test, at 20–22 °C under continuous illumination of min. 6000 lux;
- Preparation of the toxicant dilution series on TEB compound, with two phases: a range-finding test (RFT) with a dilution series of 100 mg/L; 10 mg/L; 1 mg/L; 0.1 mg/L and 0.01 mg/L, and a definitive test with concentrations ranging from 8.33 mg/L (C1) to 0.83 mg/L (C5);
- Filling-in of the test plate with the toxicant (TEB) dilution series and SF for control, in four replicates (A, B, C and D) for each test variant;
- Pre-feeding of the test organisms two hours prior to the start of exposure with a suspension of Spirulina powder into SF;
- Transfer of the neonates to the test wells. There are 5 Daphnia neonates in each well, with a total of 20 neonates for each test variant;
- Incubation of the test plate: the covered multiwell plate is incubated for 24 h and 48 h at 20 °C in darkness;
- Scoring of the results: after 24 h and 48 h of incubation, the plate is placed under a dissection microscope, and the dead and immobilized neonates are scored (they are considered dead if they do not show any movement during 15 s of observation);
- Estimation of the EC50 at 24 h and 48 h: a data treatment program based on Macro “REGTOX” (available on request from MicroBioTests Inc.) was applied using a sigmoid function with the EC50 calculation application [26].
2.1.3. Thamnotoxkit F
- Preparation of standard freshwater (S.F.), according to the US EPA formula, used as a hatching medium for the cysts and for the toxicant dilution series’ preparation;
- Pre-aeration of S.F. and storage of 1 L S.F. (six bioassays of each Toxkit);
- Hatching of the cysts (in diluted S.F. for 20–22 h at 25°C, under continuous illumination at min. 3000–4000 lux);
- The preparation of the toxicant dilution series on chemical compounds has two phases:
- ▪
- a range-finding test (RFT) with a dilution series of 100 mg/L; 10 mg/L; 1 mg/L; 0.1 mg/L and 0.01 mg/L, and
- ▪
- the definitive test, in which concentrations ranged from 1 mg/L (C1) to 0.03 mg/L (C5).
- Filling of the test plate with the toxicant (TEB) dilution series and SF for control, in three replicates (A, B and C) for each test variant.
- Transfer of the larvae to the test wells (10 larvae in each well, with a total of 30 larvae for each test variant).
- Incubation of the test plate: the covered multiwell plate is incubated for 24 h at 25 °C in darkness.
- Scoring of the result: under a dissection microscope, the mortality of the larvae was scored (they were considered dead if they do not show any movement during 10 s of observation).
2.2. Acute Toxicity Testing on Marine Fish (Chelon auratus)
2.2.1. Species Selection
2.2.2. Collection, Adaptation and Conditioning
2.2.3. Preparation of Dilution Water
2.2.4. Fish Randomization
2.2.5. Experimental Design of the Acute Toxicity Test
2.2.6. Mortality Observation and Euthanasia
2.2.7. Statistical Analysis and Interpretation
2.3. Experiments on Bacteria and Yeasts
2.3.1. Diffusimetric Test Assay
2.3.2. Minimal Inhibitory Concentration (MIC) Assay
2.3.3. Time-Kill Assay
2.4. Tebuconazole Detection in the Environment
3. Results and Discussion
3.1. Results of Toxkit Microbiotests
3.1.1. Spirodela polyrhiza
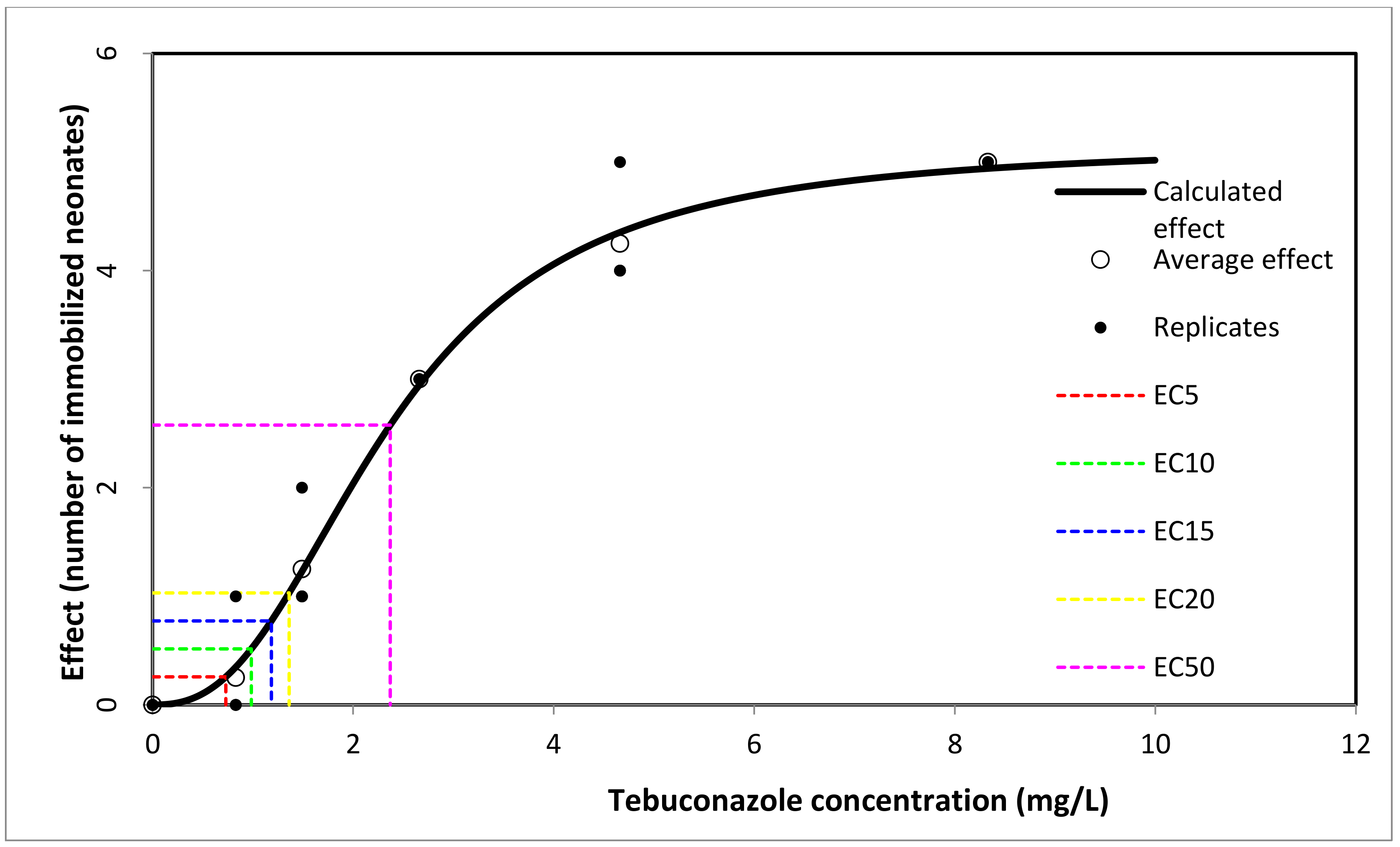
3.1.2. Daphnia magna
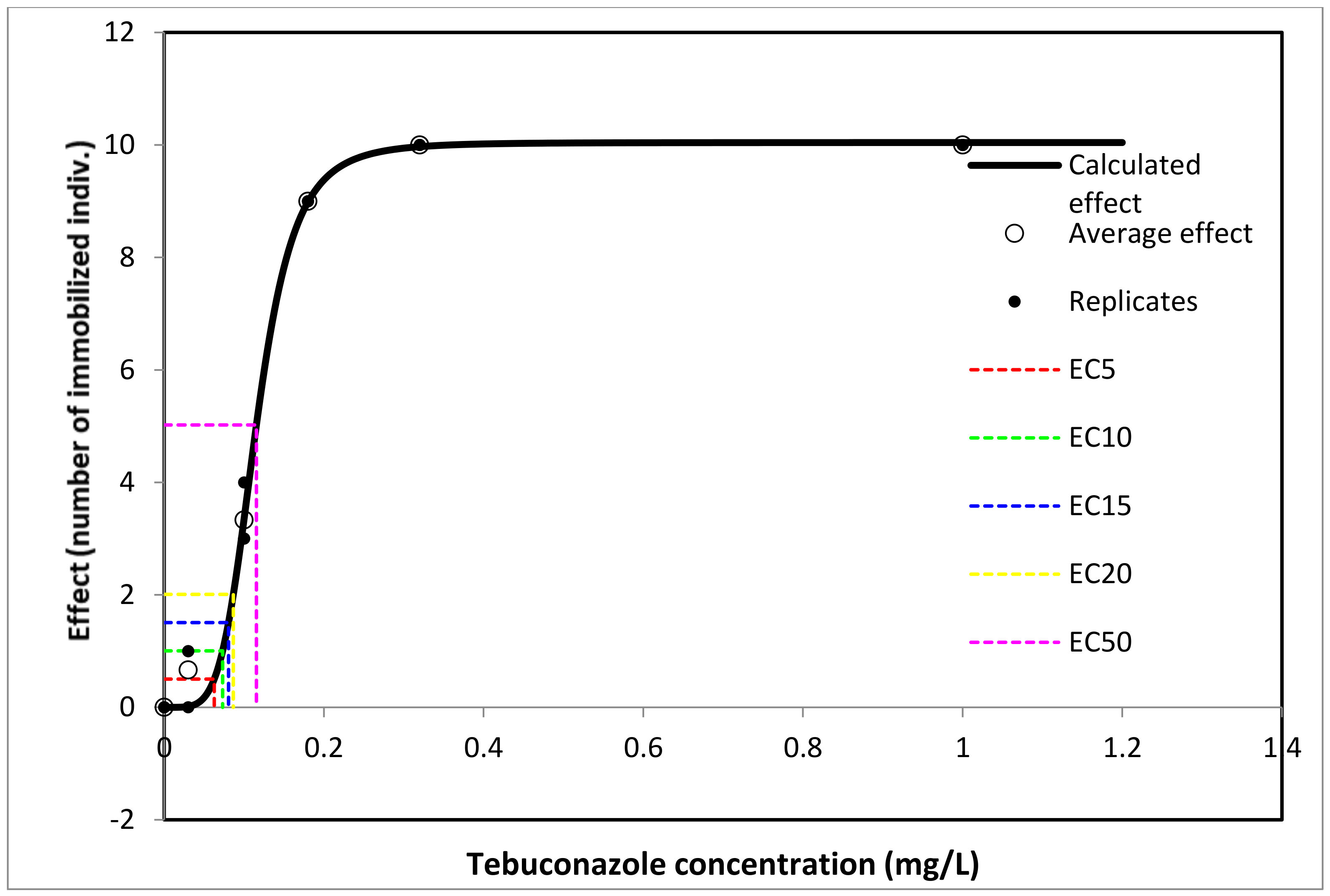
3.1.3. Thamnocephalus platyurus
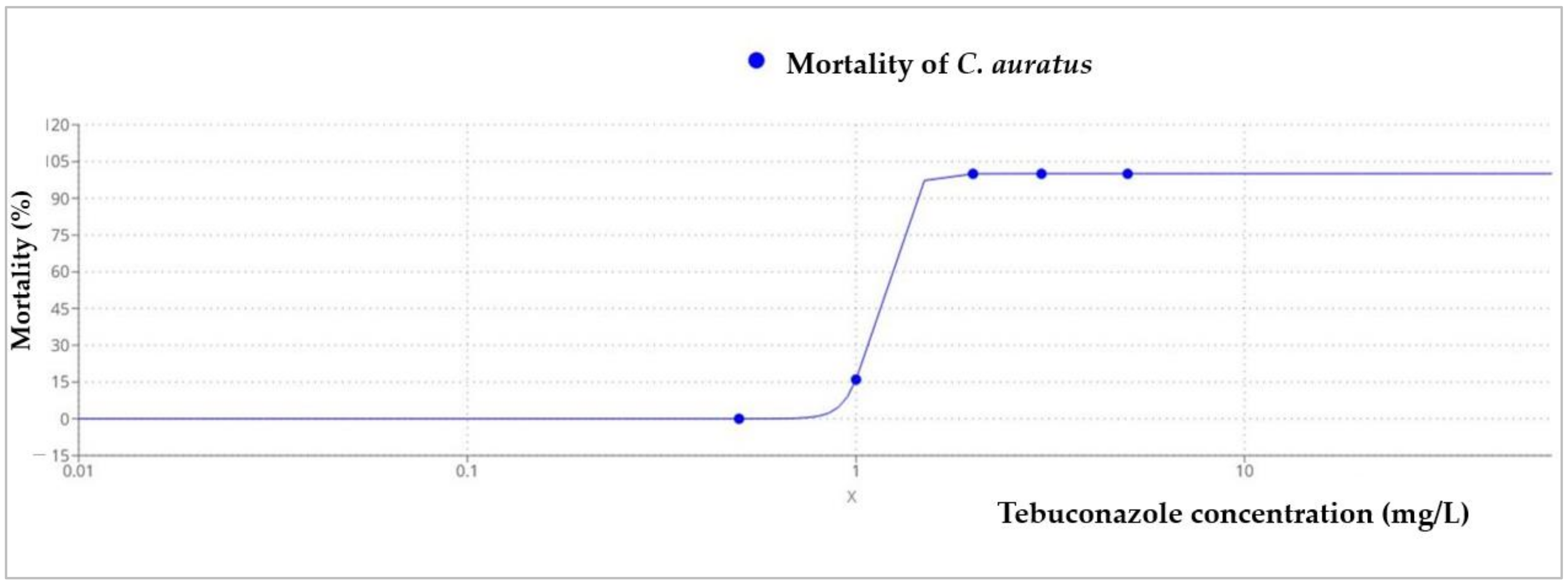
3.2. Acute Toxicity Results on C. auratus
3.3. Results Obtained from Bacteria and Yeast Tests
3.3.1. Diffusimetric Test
3.3.2. Results of MIC Estimation
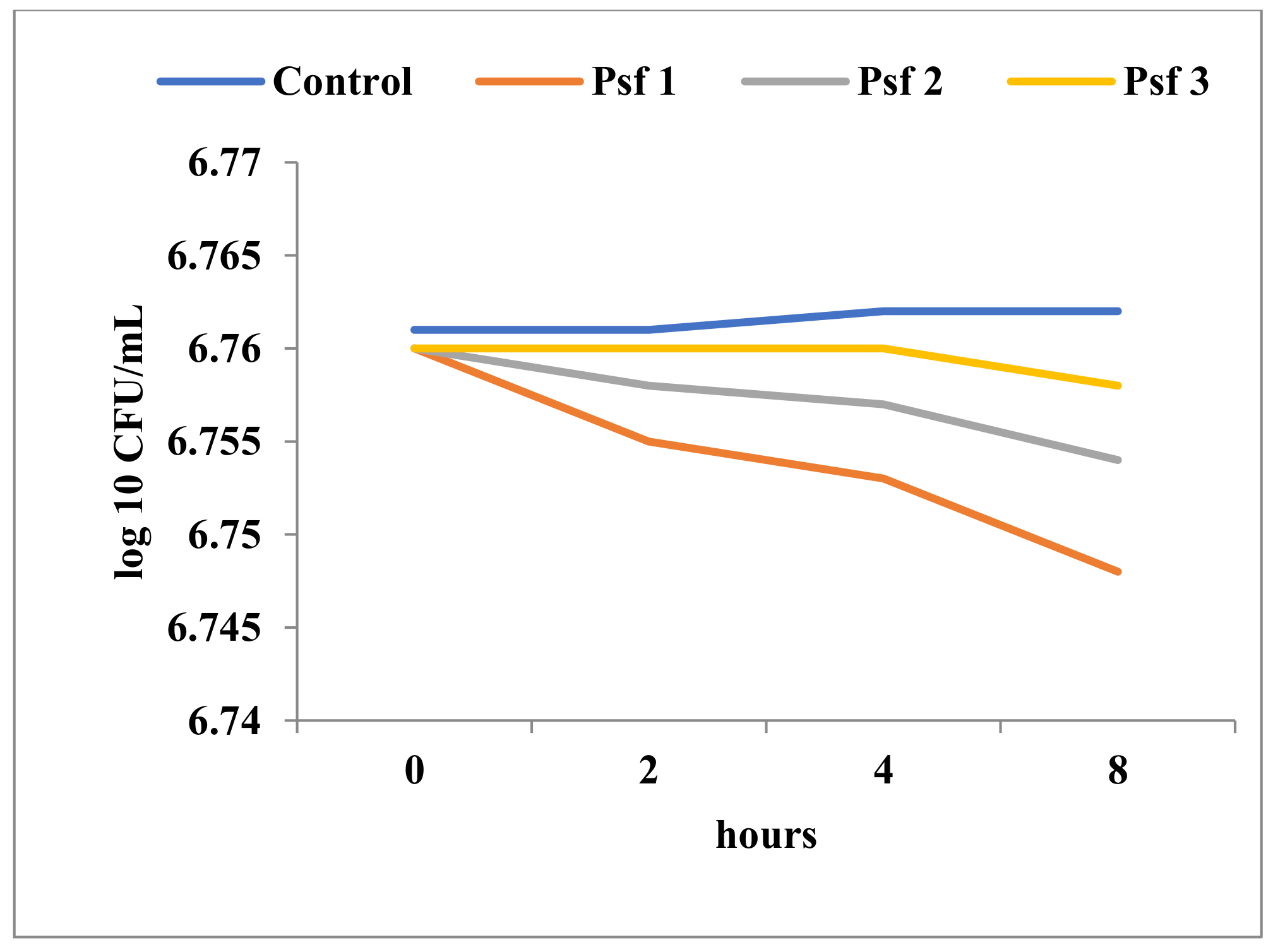
3.3.3. Results of Time-Kill Assay
3.4. Tebuconazole Detection in the Environment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ANSES (Agence Nationale de Sécurité Sanitaire de L’alimentation, de L’environnement et du Travail). Report ANSES/France (4 June 2023): Emerging Pollutants in Drinking Water: Update on the Main Results of the Last National Campaign. Available online: https://www.anses.fr/fr/content/polluants-emergents-dans-leau-potable-le-point-sur-les-principaux-resultats-de-la-derniere-2 (accessed on 10 March 2023).
- Wu, W.Z.; Guo, M.; Kong, D.Y.; Xu, J.; Shan, Z.J. Degradation of 1,2,4-Triazole Fungicides in the Environment. J. Ecol. Rural Environ. 2016, 32, 837–841. [Google Scholar] [CrossRef]
- Tebustar EW—Solarex. 2023. Available online: https://solarex.ro/catalog/tebustar-ew/ (accessed on 24 February 2023).
- EFSA (European Food Safety Authority). Conclusion Regarding the Peer Review of the Pesticide Risk Assessment of the Active Substance Tebuconazole. EFSA J. 2008, 176, 1–109. [Google Scholar] [CrossRef]
- Wei, Y.; Guangyu, L.; Qiqi, L.; Jianjun, H.; Meiqi, P.; Maomin, P.; Xitian, P.; Hui, W.; Xixia, L.; Qin, W. Molecular Mechanisms of Tebuconazole Affecting the Social Behavior and Reproduction of Zebrafish. Int. J. Environ. Res. Public Health 2023, 20, 3928. [Google Scholar] [CrossRef]
- EPA. Memorandum: Applicability of RCRA Section 3020 to In-Situ Treatment of Ground Water, 27 December 2000. Available online: https://www.epa.gov/sites/default/files/documents/3020insitugwtr-mem.pdf (accessed on 17 April 2023).
- Diaz-Biancas, V.; Medina, D.; Padilla-Ortega, E.; Bortolini-Zavala, R.; Olvera-Romero, M.; Luna-Barcenas, G. Nanoemulsion Formulations of Fungicide Tebuconazole for Agricultural Applications. Molecules 2016, 21, 1271. [Google Scholar] [CrossRef] [PubMed]
- Casado-Martinez, C.; Lefranc, M.; Kroll, A. SQC (EQSsed)—Proposal by the Ecotox Centre for: Tebuconazole; Swiss Centre for Applied Ecotoxicology: Lausanne, Switzerland, 2020. [Google Scholar]
- EFSA (European Food Safety Authority). Conclusion on the Peer Review of the Pesticide Risk Assessment of the Active Substance Tebuconazole. EFSA J. 2014, 12, 98. [Google Scholar] [CrossRef]
- EU Pesticide Database. Active Substances, Safeners and Synergists. EU Pesticides Database (v.2.2) Search Active Substances, Safeners and Synergists. Available online: https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/start/screen/active-substances (accessed on 23 March 2023).
- Commission Implementing Decision (EU) 2022/1307 of 22 July 2022 Establishing a Watch List of Substances for Union-Wide Monitoring in the Field of Water Policy Pursuant to Directive 2008/105/EC of the European Parliament and of the Council. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32022D1307 (accessed on 16 April 2023).
- Chițescu, C.L.; Ene, A.; Geană, E.I.; Vasile, A.M.; Ciucure, C.T. Emerging and Persistent Pollutants in the Aquatic Ecosystems of the Lower Danube Basin and North-West Black Sea Region—A Review. Appl. Sci. 2021, 11, 9721. [Google Scholar] [CrossRef]
- Golshani, R.; Mashinchian, M.; Mosavi Nodoshan, R.; Fatemi, S.M.; Ghavam Mostafavi, P. Organophosphorus Pesticides (Diazinon, Malathion and Azinfos Methyl) Accumulation in Three Fish Species, in South coasts of the Caspian Sea, Iran. Iran. J. Fish. Sci. 2020, 19, 3050–3062. [Google Scholar] [CrossRef]
- Tresnakova, N.; Famulari, S.; Zicarelli, G.; Impellitteri, F.; Pagano, M.; Presti, G.; Filice, M.; Caferro, F.; Gulotta, E.; Salvatore, G.; et al. Multi-characteristic Toxicity of Enantioselective Chiral Fungicide Tebuconazole to a Model Organism Mediterranean Mussel Mytilus galloprovincialis Lamarck, 1819 (Bivalve: Mytilidae). Sci. Total Environ. 2023, 862, 160874. [Google Scholar] [CrossRef] [PubMed]
- Sancho, E.; Villarroel, M.J.; Fernandez, C.; Andreu, E.; Ferrando, M.D. Short-term Exposure to Sublethal Tebuconazole Induces Physiological Impairment in Male Zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2010, 73, 370–376. [Google Scholar] [CrossRef]
- Duarte, S.; Pascoal, C.; Alves, A.; Correia, A.; Cassio, F. Assessing the dynamic of microbial communities during leaf decomposition in a low-order stream by microscopic and molecular techniques. Microbiol. Res. 2010, 165, 351–362. [Google Scholar] [CrossRef]
- Veraart, A.J.; Audet, J.; Dimitrov, M.R.; Hoffmann, C.C.; Gillissen, F.; De Klein, J.J.M. Denitrification in restored and unrestored Danish streams. Ecol. Eng. 2014, 66, 129–140. [Google Scholar] [CrossRef]
- Dimitrov, M.; Kosol, S.; Smidt, H.; Buijse, L.; Van Den Brink, P.; Van Wijngaarden, R.; Brock, T.; Maltyby, L. Assessing Effects of the Fungicide Tebuconazole to Heterotrophic Microbes in Aquatic Microcosms. Sci. Total Environ. 2014, 490, 1002–1011. [Google Scholar] [CrossRef]
- Persoone, G.; Marsalek, B.; Blinova, I.; Törökne, A.; Zarina, D.; Manusadzianas, L.; Nalecz-Jawecki, G.; Tofan, L.; Stepanova, N.; Tothova, L.; et al. A Practical and User-Friendly Toxicity Classification System with Microbiotests for Natural waters and Wastewaters. Environ. Toxicol. 2003, 18, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Tofan, L.; O’Grady, E.; Ehlinger, J.T. Setting Up a Lab on Aquatic Ecotoxicology for Students. Int. J. Cross-Cult. Stud. Environ. Commun. 2014, 2, 72–78. [Google Scholar]
- Persoone, G.; Baudo, R.; Cotman, M.; Blaise, C.; Thompson, K.C.; Moreira-Santos, M.; Vollat, B.; Törökne, A.; Han, T. Review on the Acute Daphnia magna Toxicity Test—Evaluation of the Sensitivity and the Precision of Assays Performed with Organisms from Laboratory Cultures or Hatched from Dormant Eggs. Knowl. Manag. Aquat. Ecosyst. 2009, 393, 01. [Google Scholar] [CrossRef]
- Ruck, J.G.; Martin, M.; Mabon, M. Evaluation of Toxkits as Methods for Monitoring Water Quality in New Zealand. In New Microbiotests for Routine Toxicity Screening and Biomonitoring; Persoone, G., Janssen, C., De Coen, W., Eds.; Kluwer Academic Plenum Publishers: Dordrecht, The Netherlands, 2000; Chapter 9; pp. 103–119. [Google Scholar]
- Fochtman, P. Acute Toxicity of Nine Pesticides as Determined with Conventional Assays and Alternative Microbiotests. In New Microbiotests for Routine Toxicity Screening and Biomonitoring; Persoone, G., Janssen, C., De Coen, W., Eds.; Kluwer Academic Plenum Publishers: Dordrecht, The Netherlands, 2000; Chapter 25; pp. 233–241. [Google Scholar]
- MicroBioTests Inc.; Duckweed Toxkit F. Growth Inhibition Microbiotest with Spirodela polyrhiza. Standard Operating Procedure. Available online: https://www.microbiotests.com/wp-content/uploads/2019/07/duckweed-toxicity-test_duckweed-toxkit-f_standard-operating-procedure.pdf (accessed on 11 April 2023).
- ISO 20227:2017; ISO International Standard, Water Quality–Determination of the Growth Inhibition Effects of Waste Waters, Natural Waters and Chemicals on the Duckweed Spirodela polyrhiza—Method Using a Stock Culture Independent Microbiotest. ISO: Geneva, Switzerland, 2017; pp. 1–11.
- Spreadsheet Instructions for Toxkits Microbiotests LC50 Calculation. Available online: http://www.normalesup.org/~vindimian/en_index.html (accessed on 27 March 2023).
- Ferreira, T.; Rasband, W. Image J User Guide. Available online: https://imagej.nih.gov/ij/docs/guide/user-guide.pdf (accessed on 11 April 2023).
- ISO 6341: 2012; Water Quality–Determination of the Inhibition of the Mobility of Daphnia magna Straus (Cladocera, Crustacea)—Acute Toxicity Test. ISO: Geneva, Switzerland, 2012. Available online: https://www.iso.org/standard/54614.html (accessed on 14 March 2023).
- Test No. 202; Daphnia sp. Acute Immobilisation Test, Test No. 202: Daphnia sp. Acute Immobilisation Test|OECD Guidelines for the Testing of Chemicals, Section 2: Effects on Biotic Systems. OECD iLibrary. 2004. Available online: Oecd-ilibrary.org (accessed on 25 March 2023).
- ISO 14380:2011; Water Quality—Determination of the Acute Toxicity to Thamnocephalus platyurus (Crustacea, Anostraca). ISO: Geneva, Switzerland, 2011. Available online: https://www.iso.org/standard/54613.html (accessed on 15 March 2023).
- Niță, V.; Nenciu, M.; Coatu, V. Suitability and Sensitivity of Golden Grey Mullet Chelon auratus (Risso, 1810) as a Reference Fish Species for Ecotoxicity Tests in the Black Sea. Toxics 2022, 10, 222. [Google Scholar] [CrossRef] [PubMed]
- Niță, V.; Nenciu, M.; Galațchi, M. Fish Species of the Romanian Coast. Updated Atlas; NIMRD: Constanța, Romania, 2022; 152p, ISBN 978-973-0-36642-6. [Google Scholar]
- Basar, E.; Sivri, N.; Ugurlu, O.; Zulal Sonmez, V. Potential Impact of Oil Spill Damage around the Planned Oil Rigs at the Black Sea. Indian J. Mar. Sci. 2018, 47, 2198–2206. [Google Scholar]
- Bu-Olayan, A.H.; Thomas, B.V. Assessment on Biocides Bioaccumulation in Mullet Liza klunzingeri in Kuwaiti Waters, off the Arabian Gulf. Am. J. Environ. Sci. 2006, 2, 109–113. [Google Scholar] [CrossRef]
- Hariharan, G.; Purvaja, R.; Ramesh, R. Environmental Safety Level of Lead (Pb) Pertaining to Toxic Effects on Grey Mullet (Mugil cephalus) and Tiger Perch (Terapon jarbua). Environ. Toxicol. 2016, 31, 24–43. [Google Scholar] [CrossRef]
- Inaz, E.; Elahe, E.; Kasalkhe, N. Acute Toxicity and the Effects of Copper Sulphate [CuSO4.5H2O] on the Behavior of the Gray Mullet (Mugil cephalus). Int. J. Sci. Res. Environ. Sci. Toxicol. 2018, 3, 771–778. [Google Scholar]
- Krajnović-Ozretić, M.; Ozretić, B. The ALA-D Activity Test in Lead-Exposed Grey Mullet Mugil auratus. Mar. Ecol. Prog. Ser. 1980, 3, 187–191. [Google Scholar] [CrossRef]
- Parrino, V.; De Marco, G.; Minutoli, R.; Lo Paro, G.; Giannetto, A.; Cappello, T.; De Plano, L.M.; Cecchini, S.; Fazio, F. Effects of Pesticides on Chelon labrosus (Risso, 1827) Evaluated by Enzymatic Activities along the North-Eastern Sicilian Coastlines (Italy). Eur. Zool. J. 2021, 88, 540–548. [Google Scholar] [CrossRef]
- Grasshoff, K.; Kremling, K.; Ehrhardt, M. Methods of Seawater Analysis; Wiley-VCH: Hoboken, NJ, USA, 1999; pp. 1–599. [Google Scholar]
- Strickland, J.D.H.; Parsons, T.R. A Practical Handbook of Seawater Analysis; Fisheries Research Board of Canada: Andrews, NB, Canada, 1972; pp. 1–3. [Google Scholar]
- Random Lists. Available online: https://www.randomlists.com/random-numbers?min=1&max=120&qty=120&dup=false (accessed on 10 March 2023).
- US-EPA-OCSPP850; Freshwater and Saltwater Fish Acute Toxicity Test. EPA 712-C-16-007. U.S. Environmental Protection Agency—Office of Chemical Safety and Pollution Prevention: Washington, DC, USA, 2016; pp. 1–19.
- Mullin, J.B.; Riley, J.P. The Spectrophotometric Determination of Nitrate in Natural Waters, with Particular Reference to Seawater. Anal. Chim. Acta 1955, 12, 464–480. [Google Scholar] [CrossRef]
- AVMA. Guidelines for the Euthanasia of Animals; American Veterinary Medical Association: Schaumburg, IL, USA, 2013; pp. 1–102. ISBN 978-1-882691-21-0. [Google Scholar]
- AAT Bioquest. Quest Graph™ LC50 Calculator. Available online: https://www.aatbio.com/tools/lc50-calculator (accessed on 18 April 2023).
- Magaldi, S.; Mata-Essayag, S.; Hartung de Capriles, C.; Perez, C.; Colella, M.T.; Olaizola, C.; Ontiveros, Y. Well Diffusion for Antifungal Susceptibility Testing. Int. J. Infect. Dis. 2004, 8, 39–45. [Google Scholar] [CrossRef]
- M100-S21; Performance Standards for Antimicrobial Susceptibility Testing: Twenty-First Informational Supplement. Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA; Volume 31, pp. 1–169.
- Koeth, L.M. 5.14 Tests to Assess Bactericidal Activity. In Clinical Microbiology Procedures Handbook, 4th ed.; ASM Press: Washington, DC, USA, 2022. [Google Scholar]
- Marsalek, B.; Rojickova-Padrtova, R. Selection of a Battery of Microbiotests for Various Purposes—The Czech Experience. In New Microbiotests for Routine Toxicity Screening and Biomonitoring; Persoone, G., Janssen, C., De Coen, W., Eds.; Kluwer Academic Plenum Publishers: Dordrecht, The Netherlands, 2000; Chapter 8; pp. 95–101. [Google Scholar]
- Montague, B.; Al-Mudallal, A. Ecological Risk Assessment for Section 3 Registration of Tebuconazole on Wheat, Cucurbits, Bananas, Turnips, Tree nuts, Hops, and Sunflowers. US EPA. Available online: https://www3.epa.gov/pesticides/chem_search/cleared_reviews/csr_PC-128997_25-Jul-00_a.pdf (accessed on 11 April 2023).
- Valimaña-Traverso, J.; Amariei, G.; Boltes, K.; García, M.A.; Marina, M.L. Stability and Toxicity Studies for Duloxetine and Econazole on Spirodela polyrhiza Using Chiral Capillary Electrophoresis. J. Hazard. Mater. 2019, 374, 203–210. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.; Tang, Q.; Mei, L.; Cao, J.; Hunag, H.; Zhang, Z. Aquatic Ecological Risk Evaluation of Chiral Triazole Fungicide Prothioconazole and Its Metabolite Prothioconazole-desthio on Lemna minor. Sustainability 2022, 14, 16292. [Google Scholar] [CrossRef]
- Coors, A.; Vollmar, P.; Sacher, F.; Thoma, A. Joint Effects of Pharmaceuticals and Chemicals Regulated under REACH in Wastewater Treatment Plant Effluents—Evaluating Concepts for a Risk Assessment by Means of Experimental Scenarios. Environmental Research of the Federal Ministry for the Environment, Nature Conservation, Building and Nuclear Safety, Bonn, Germany. 2016, pp. 1–126. Available online: https://www.bmuv.de/fileadmin/Daten_BMU/Pools/Forschungsdatenbank/fkz_3712_64_419_klaeranlagenablaeufe_bf.pdf (accessed on 19 April 2023).
- Haeba, M.; Bláha, L. Comparison of Different Endpoints Responses in Aquatic Plant Lemna minor Exposed to Ketoconazole. Egypt. J. Nat. Toxins 2011, 8, 49–57. [Google Scholar]
- Herrera, J.M.; Armijos, C.I.; Alcarria, D.R.; Ramírez, S.A. Toxicity of Difenoconazole and Atrazine and Their Photodegradation Products on Aquatic Biota: Environmental Implications in Countries Lacking Good Agricultural Practices. Toxics 2023, 11, 213. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fang, Y.; Huang, M.; Jin, Y.; Sun, J.; Tao, X.; Zhang, G.; He, K.; Zhao, Y.; Zhao, H. Uniconazole-induced Starch Accumulation in the Bioenergy Crop duckweed (Landoltia punctata) II: Transcriptome Alterations of Pathways Involved in Carbohydrate Metabolism and Endogenous Hormone Crosstalk. Biotechnol. Biofuels 2015, 8, 64. [Google Scholar] [CrossRef]
- Roman, D.L.; Voiculescu, D.I.; Ostafe, V.; Ciorsac, A.; Isvoran, A. A review of the Toxicity of Triazole Fungicides Approved to be Used in European Union to the Soil and Aqueous Environment. Ovidius Univ. Ann. Chem. 2022, 33, 113–120. [Google Scholar] [CrossRef]
- Păunescu, A.; Ponepal, C.M.; Tofan, L.; Brînzea, G.; Tantu, M.M.; Mihăescu, C.F.; Drăghiceanu, O.A.; Popoviciu, D.R.; Făgăraș, M.M.; Vasile, D.; et al. Ecotoxicological Risk Assessment of Actellic 50 EC Insecticide on Non-target Organisms in Parallel with the Application of Standardized Tests. Toxics 2022, 10, 745. [Google Scholar] [CrossRef]
- Qi, Z.; Chen, X.; Jinag, J.; Wang, C. Comparative Toxicity of Rac- and S-Tebuconazole to Daphnia magna. J. Environ. Sci. Health B 2015, 50, 456–462. [Google Scholar] [CrossRef] [PubMed]
- CHL Report. Proposal for Harmonised Classification and Labelling Based on Regulation (EC) No. 1272/2008 (CLP Regulation), Annex VI, Part 2 Substance Name: Tebuconazole. 2012. Available online: https://echa.europa.eu/documents/10162/2f902639–3c60–38a5-d61f-76757d87aee4 (accessed on 20 March 2023).
- Bien, C.M.; Espenshade, P.J. Sterol Regulatory Element Binding Proteins in Fungi: Hypoxic Transcription Factors Linked to Pathogenesis. Eukaryot. Cell 2010, 9, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Strickland, D.A.; Villani, S.M.; Cox, K.D. Optimizing use of DMI fungicides for management of apple powdery mildew caused by Podosphaera leucotricha in New York State. Plant Dis. 2022, 106, 1226–1237. [Google Scholar] [CrossRef]
- Widenfalk, A.; Bertilsson, S.; Sundh, I.; Goedkoop, W. Effects of pesticides on community composition and activity of sediment microbes—Responses at various levels of microbial community organization. Environ. Pollut. 2008, 152, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Bundschuh, M.; Zubrod, J.P.; Kosol, S.; Maltby, L.; Stang, C.; Duester, L.; Schulz, R. Fungal composition on leaves explains pollutant-mediated indirect effects on amphipod feeding. Aquat. Toxicol. 2011, 104, 32–37. [Google Scholar] [CrossRef]
- Zubrod, J.P.; Bundschuh, M.; Feckler, A.; Englert, D.; Schulz, R. Ecotoxicological impact of the fungicide Tebuconazole on an aquatic decomposer-detritivore system. Environ. Toxicol. Chem. 2011, 30, 2718–2724. [Google Scholar] [CrossRef]
- Bacmaga, M.; Wyszkowska, J.; Borowik, A.; Kucharski, J. Effects of Tebuconazole Application on Soil Microbiota and Enzymes. Molecules 2022, 27, 7501. [Google Scholar] [CrossRef]
- Muñoz-Leoz, B.; Ruiz-Romera, E.; Antigüedad, I.; Garbisu, C. Tebuconazole application decreases soil microbial biomass and activity. Soil Biol. Biochem. 2011, 43, 2176–2183. [Google Scholar] [CrossRef]
- Wang, C.; Wang, F.; Zhang, Q.; Liang, W. Individual and combined effects of Tebuconazole and carbendazim on soil microbial activity. Eur. J. Soil Biol. 2016, 72, 6–13. [Google Scholar] [CrossRef]
- Milenkovski, S.; Baath, E.; Lindgren, P.E.; Berglund, O. Toxicity of fungicides to natural bacterial communities in wetland water and sediment measured using leucine incorporation and potential denitrification. Ecotoxicology 2010, 19, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Dijksterhuis, J.; Van Doorn, T.; Samson, R.; Postma, J. Effects of seven fungicides on non-target aquatic fungi. Water Air Soil Pollut. 2011, 222, 421–425. [Google Scholar] [CrossRef]
- Artigas, J.; Majerholc, J.; Foulquier, A.; Margoum, C.; Volat, B.; Neyra, M.; Pesce, S. Effects of the fungicide Tebuconazole on microbial capacities for litter breakdown in streams. Aquat. Toxicol. 2012, 122–123, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Artigas, J.; Pascault, N.; Bouchez, A.; Chastaine, J.; Debroas, D.; Humbertg, J.F.; Leloup, J.; Tadonleke, R.D.; Ter Halle, A.; Pesce, S. Comparative sensitivity to the fungicide Tebuconazole of biofilm and plankton microbial communities in freshwater ecosystems. Sci. Total Environ. 2014, 468–469, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Youness, M.; Sancelme, M.; Combourieu, B.; Besse-Hoggan, P. Identification of new metabolic pathways in the enantioselective fungicide Tebuconazole biodegradation by Bacillus sp. 3B6. J. Hazard. Mater. 2018, 351, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Podbielska, M.; Kus-Liśkiewicz, M.; Jagusztyn, B.; Szpyrka, E. Effect of microorganisms on degradation of fluopyram and Tebuconazole in laboratory and field studies. Environ. Sci. Pollut. Res. Int. 2023, 30, 47727–47741. [Google Scholar] [CrossRef]
- Blattner, F.R.; Plunkett, G.; Bloch, C.A.; Perna, N.T.; Burland, V.; Riley, M.; Collado-Vides, J.; Glasner, J.; Rode, C.; Mayhew, G.; et al. The complete genome sequence of Escherichia coli K-12. Science 1997, 277, 1453–1462. [Google Scholar] [CrossRef]
- Kunst, F.; Ogasawara, N.; Moszer, I.; Albertini, A.M.; Alloni, G.; Azevedo, V.; Bertero, M.G.; Bessières, P.; Bolotin, A.; Borchert, S.; et al. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature 1997, 390, 249–256. [Google Scholar] [CrossRef]
- Lentz, O.; Urlacher, V.; Schmid, R.D. Substrate specificity of native and mutated cytochrome P450 (CYP102A3) from Bacillus subtilis. J. Biotechnol. 2004, 108, 41–44. [Google Scholar] [CrossRef]
- Padayachee, T.; Nomfundo Nzuza, N.; Chen, W.; Nelson, D.R.; Syed, K. Impact of lifestyle on cytochrome P450 monooxygenase repertoire is clearly evident in the bacterial phylum Firmicutes. Sci. Rep. 2020, 10, 13982. [Google Scholar] [CrossRef]
- Nelson, D.R.; Koymans, L.; Kamataki, T.; Stegeman, J.J.; Feyereisen, R.; Waxman, D.J.; Waterman, M.R.; Gotoh, O.; Coon, M.J.; Estabrook, R.W.; et al. P450 superfamily: Update on new sequences, gene mapping, accession numbers and nomenclature. Pharmacogenetics 1996, 6, 1–42. [Google Scholar] [CrossRef] [PubMed]
- McLean, K.J.; Marshall, K.R.; Richmond, A.; Hunter, I.S.; Fowler, K.; Kieser, T.; Gurcha, S.S.; Besra, G.S.; Munro, A.W. Azole antifungals are potent inhibitors of cytochrome P450 mono-oxygenases and bacterial growth in mycobacteria and streptomycetes. Microbiology 2002, 148, 2937–2949. [Google Scholar] [CrossRef] [PubMed]
- Toxkit Microbiotests Procedures. Available online: http://www.microbiotests.com (accessed on 23 March 2023).
- Baudo, R.; Foudoulakis, M.; Arapis, G.; Perdaen, K.; Lanneau, W.; Paxinou, A.C.M.; Kouvdou, S.; Persoone, G. History and sensitivity comparison of the Spirodela polyrhiza microbiotest and Lemna toxicity tests. Knowl. Manag. Aquat. Ecosyst. 2015, 416, 23. [Google Scholar] [CrossRef]
- Wang, W.; Kerstetter, R.A.; Michael, T.P. Evolution of Genome Size in Duckweeds (Lemnaceae). J. Bot. 2011, 2011, 570319. [Google Scholar] [CrossRef]
- Image of Spirodela polyrhiza (Spirodela polyrrhiza marais poitevin—Wikipedia). Available online: https://zh.wikipedia.org/wiki/File:Spirodela_polyrrhiza_marais_poitevin.jpg (accessed on 14 April 2023).
- Image of Daphnia magna a. Available online: https://www.google.ro/url?sa=i&url=https%3A%2F%2Fen.wikipedia.org%2Fwiki%2FDaphnia_magna&psig=AOvVaw2bpxOLMn59Qbpmzy7uo4RU&ust=1681554345020000&source=images&cd=vfe&ved=0CBEQjRxqFwoTCMiJrZ6Uqf4CFQAAAAAdAAAAABAQ (accessed on 14 April 2023).
- Image of Daphnia magna b. Available online: https://www.google.ro/url?sa=i&url=https%3A%2F%2Fen.wikipedia.org%2Fwiki%2FDaphnia_magna&psig=AOvVaw2bpxOLMn59Qbpmzy7uo4RU&ust=1681554345020000&source=images&cd=vfe&ved=0CBEQjRxqFwoTCMiJrZ6Uqf4CFQAAAAAdAAAAABAI (accessed on 14 April 2023).
- Image of Thamnocephalus platyurus. Available online: https://www.biotoxicity.com (accessed on 14 April 2023).
- Romanian Parliament. Law No. 43/2014 on the Protection of Animals Used for Scientific Purposes. Off. Gaz. Rom. 2014, 326. (In Romanian). Available online: http://www.dreptonline.ro/legislatie/legea_43_2014_protectia_animalelor_utilizate_scopuri_stiintifice.php (accessed on 23 March 2023).


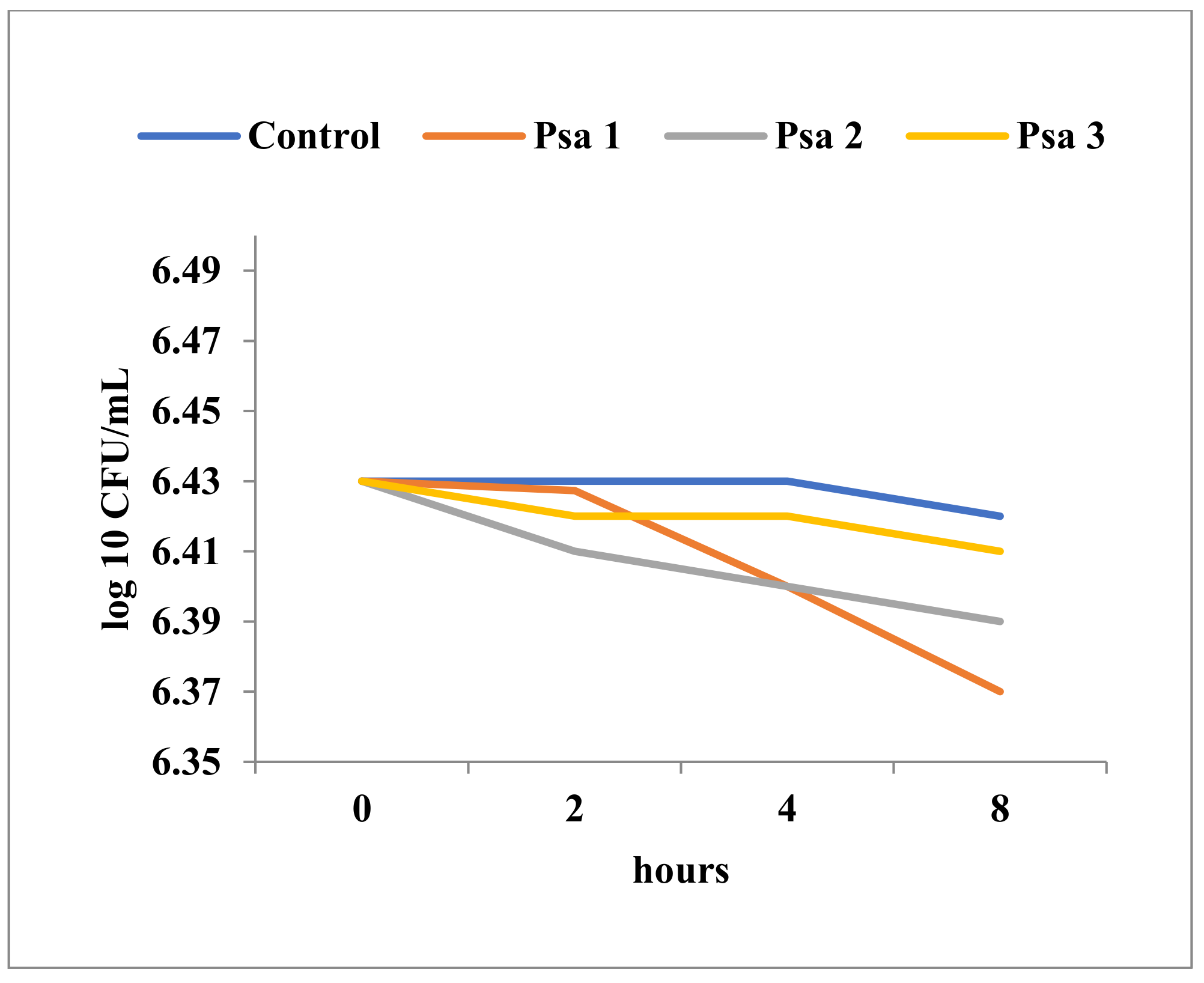
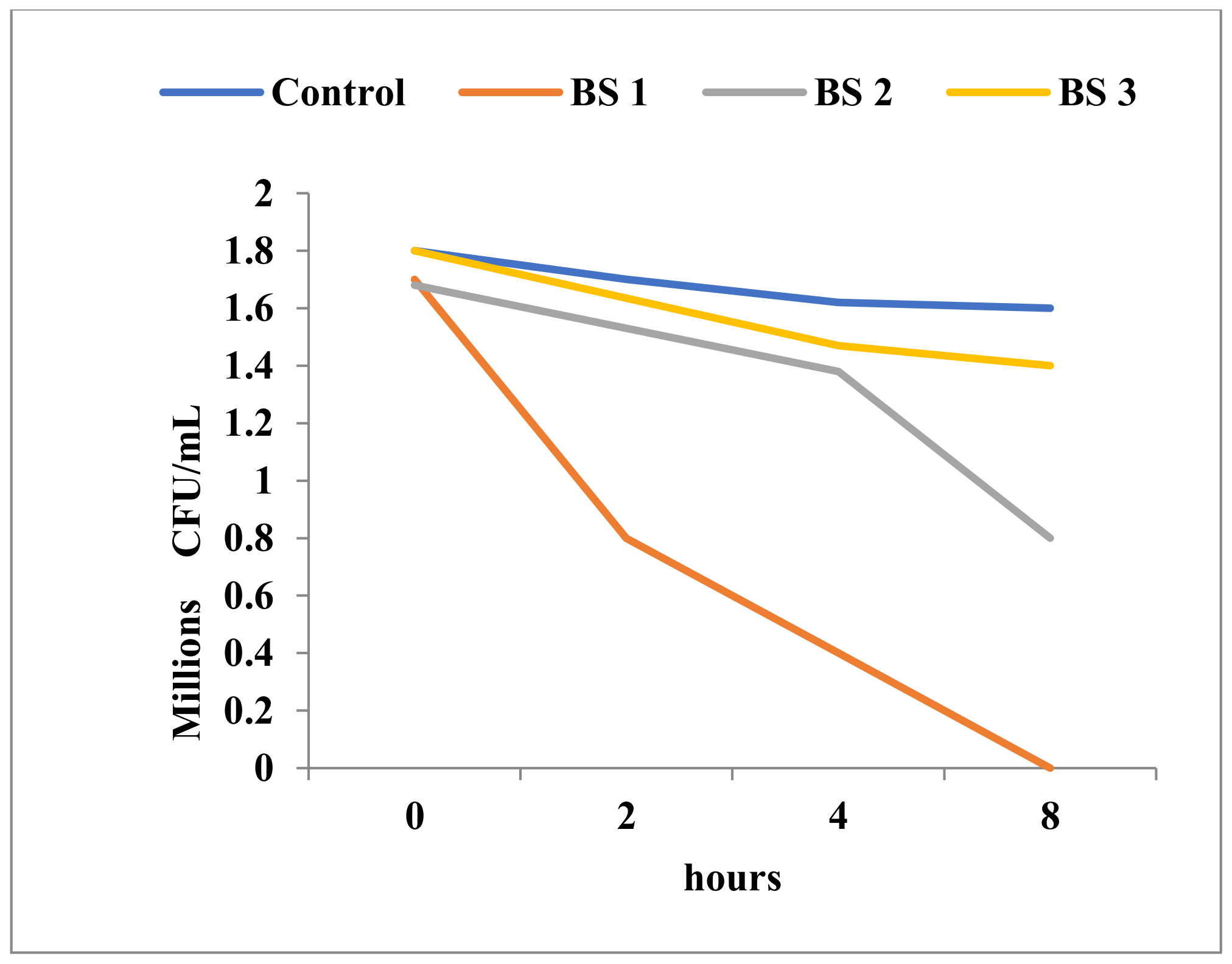

| No. | Strain | Observation |
|---|---|---|
| 1 | Escherichia coli ATCC 25922 | Reference strain |
| 2 | Pseudomonas aeruginosa ATCC 27853 | Reference strain |
| 3 | Pseudomonas fluorescens | Clinical strain |
| 4 | Bacillus sp. | Gram-positive, spore-forming soil isolate |
| 5 | Candida albicans ATCC 10231 | Reference strain |
| Indicative | Concentration (mg/L) | Average Inhibition (%) | 72 h EC50 (mg/L) | 95% Confidence Interval |
|---|---|---|---|---|
| C1 | 6.25 | 91.65 | 2.204 | 1.031–2.946 |
| C2 | 3.12 | 80.78 | ||
| C3 | 1.56 | 28.84 | ||
| C4 | 0.78 | 22.09 | ||
| C5 | 0.39 | 15.67 | ||
| M | 0 (control) | - |
| Parameter | Value |
|---|---|
| LC50 | 1.1372 |
| Equation | |
| Equation Form |
| Two-Fold Dilutions | I | II | III | IV | V | VI | VII | VIII | IX | X | XI | XII | XIII |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tebuconazole concentration mg/mL | 125 | 62.5 | 31.25 | 15.625 | 7.81 | 3.90 | 1.95 | 0.97 | 0.48 | 0.24 | 0.12 | 0.061 | 0.030 |
| E coli ATCC 25922 | 4 | 3 | 2 | 2 | 0 | 0 | 0 | ||||||
| P. aeruginosa ATCC 27853 | 3 | 2 | 1 | 0 | 0 | 0 | 0 | ||||||
| P. fluorescens | 3 | 3 | 1 | 0 | 0 | 0 | 0 | ||||||
| Candida albicans ATCC 10231 | 20 | 20 | 16 | 12 | 12 | 11 | 11.5 | 9.5 | 9 | 7.5 | 6 | 3 | 0 |
| Bacillus sp. | 12 | 10 | 9 | 8 | 8 | 7 | 6.5 | 7.5 | 6 | 4 | 4 | 3.5 | 0 |
| Strain | mg/mL Tebuconazole |
|---|---|
| E. coli ATCC 25922 | 15.62 |
| P. aeruginosa ATCC 27853 | 31.25 |
| P. fluorescens | 31.25 |
| Candida albicans ATCC 10231 | 0.0012 |
| Bacillus sp. | 0.0024 |
| Sampling Station | TEB (mg/L) | SD (±) |
|---|---|---|
| Ramada | 2.02 | 0.04 |
| Large bridge | 1.47 | 0.11 |
| On Plonge Jr. | 0.5 | 0.06 |
| Tăbăcărie Microreserve | 0.96 | 0.15 |
| Church | 0.41 | 0.08 |
| Sports Camp | 0.38 | 0.05 |
| Expo | 0.27 | 0.036 |
| Station 2 trees | 0.084 | 0.009 |
| Station 1 stairs | 0.098 | 0.021 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tofan, L.; Niță, V.; Nenciu, M.; Coatu, V.; Lazăr, L.; Damir, N.; Vasile, D.; Popoviciu, D.R.; Brotea, A.-G.; Curtean-Bănăduc, A.M.; et al. Multiple Assays on Non-Target Organisms to Determine the Risk of Acute Environmental Toxicity in Tebuconazole-Based Fungicides Widely Used in the Black Sea Coastal Area. Toxics 2023, 11, 597. https://doi.org/10.3390/toxics11070597
Tofan L, Niță V, Nenciu M, Coatu V, Lazăr L, Damir N, Vasile D, Popoviciu DR, Brotea A-G, Curtean-Bănăduc AM, et al. Multiple Assays on Non-Target Organisms to Determine the Risk of Acute Environmental Toxicity in Tebuconazole-Based Fungicides Widely Used in the Black Sea Coastal Area. Toxics. 2023; 11(7):597. https://doi.org/10.3390/toxics11070597
Chicago/Turabian StyleTofan, Lucica, Victor Niță, Magda Nenciu, Valentina Coatu, Luminița Lazăr, Nicoleta Damir, Daniela Vasile, Dan Răzvan Popoviciu, Alina-Giorgiana Brotea, Angela Maria Curtean-Bănăduc, and et al. 2023. "Multiple Assays on Non-Target Organisms to Determine the Risk of Acute Environmental Toxicity in Tebuconazole-Based Fungicides Widely Used in the Black Sea Coastal Area" Toxics 11, no. 7: 597. https://doi.org/10.3390/toxics11070597
APA StyleTofan, L., Niță, V., Nenciu, M., Coatu, V., Lazăr, L., Damir, N., Vasile, D., Popoviciu, D. R., Brotea, A.-G., Curtean-Bănăduc, A. M., Avramescu, S., & Aonofriesei, F. (2023). Multiple Assays on Non-Target Organisms to Determine the Risk of Acute Environmental Toxicity in Tebuconazole-Based Fungicides Widely Used in the Black Sea Coastal Area. Toxics, 11(7), 597. https://doi.org/10.3390/toxics11070597













