Abstract
Heavy metal accumulation in freshwater ecosystem has become one of the major aquatic environmental concerns for freshwater flora and fauna due to their higher stability and bioaccumulation as well as bio-magnification properties. Furthermore, passing through the food web, these heavy metals affect human populations ultimately. This study assessed the heavy metal accumulation in Cirrhinus mrigala in spring, autumn, and winter at different locations (I, II, and III) of Panjnad headwork. Furthermore, the human health risk assessment for the consumption of C. mrigala from the sampling locations was also carried out. Fish were collected from upper (I), middle (II), and lower (III) stream of Panjnad on a monthly basis. The current study evaluated the accumulation of Aluminum (Al), Arsenic (As), Barium (Ba), and Lead (Pb) in various fish organs (liver, kidney, gills, fins, skin, muscles and bones) and assessed their potential hazard to human health through health risk assessment indicators. The results demonstrated a significant difference (p < 0.05) in heavy metal accumulation in different fish organs, seasons, and locations. The accumulation of Al, As, Ba, and Pb were considerably higher in liver and kidney as compared to the other body organs and followed a trend of liver > kidney > gills > fins > skin > bones > muscle and the overall mean concentrations of metals in different body tissues of C. mrigala were in the order of Al > As > Ba > Pb. The results also concluded that C. mrigala caught from the Panjnad headwork is not safe for human consumption due to higher values of TTHQIng (3.76), THQIng for Ba (3.27) and CRIng for As (6.4742).
1. Introduction
Rivers are a crucial global source of fresh water, but in modern times, the presence of heavy metal contamination in river ecosystems has emerged as a significant challenge for humankind. Due to their toxic and non-biodegradable nature, heavy metals pose a serious threat to both plant and animal life, even at very low concentrations. The sources of heavy metals in rivers can be either natural or anthropogenic in nature, with untreated industrial effluent discharges being a significant contributor to heavy metal contamination. This can lead to serious health problems as these toxic metals can enter the bodies of humans and animals through the food chain [1]. The significant presence of trace elements in riverine waters is a critical concern for environmental pollution and poses a risk to the health of animals and humans alike. For example, in the Panjnad River of Pakistan, concentrations of Al, As, and Pb were examined to be significantly higher than the upper limits for safe human consumption set by World Health Organization and US EPA [2,3]. In order to study the impact of human activities on this significant river of Pakistan, it is imperative to implement effective measures for prevention [4]. Metals can generally be categorized as either biologically essential or nonessential. Nonessential metals such as aluminum (Al), cadmium (Cd), mercury (Hg), tin (Sn), and lead (Pb) do not have any known specific biological functions and their toxicity increases as their concentrations rise. On other hand, essential metals including chromium (Cr), zinc (Zn), nickel (Ni), copper (Cu), cobalt (Co), and iron (Fe) have established biological roles, and their toxic effects arise in response to either insufficient or excessive concentrations [5] which could be more severe than non-essential heay metals. Lead is present in the environment through both natural and human-induced sources. Exposure to Pb can occur through various pathways such as ingestion of contaminated drinking water, consumption of contaminated food, inhalation of air and dust, and exposure to soil contaminated with Pb from old paint. As one of the most commonly recycled non-ferrous metals, Pb’s secondary production has steadily increased. However, high levels of Pb exposure can lead to toxic effects [6]. The toxicity of Pb can cause defects in crucial organs of fish, resulting in abnormalities such as irregular and abnormal fins, heads, tails, and various spinal issues [7]. Arsenic (As) is a highly toxic heavy metal that significantly pollutes aquatic environments, making it one of the most prominent toxicants among heavy metals [8,9]. According to the World Health Organization (WHO), As is classified as one of the most hazardous chemicals to public health [9]. Arsenic is a toxic metalloid that is extensively present in various bodies of water, such as rivers, canals, ponds, groundwater, lakes, and seawater. This is primarily due to the unregulated discharge of industrial wastes and pesticides into aquatic environments [10]. As a heavy metal contaminant, As has significant adverse impacts on fish, affecting their morphology, behavior, growth, histopathology, and gene expression levels [11]. The review of literature highlighted a lack of scientific data on the overall impact of As toxicity on commercially important and popular fish species. Arsenic and Pb are the most prevalent pollutants present in various freshwater sources across Pakistan. In addition to As and Pb, industrial and river water samples collected from Punjab, Pakistan, were found to contain toxic levels of Al and barium (Ba). Aluminum is a detrimental metal to aquatic ecosystems, known to cause toxicity events with severe ecological implications [12]. Exposure to Al has been found to result in various physiological changes in different fish species. These alterations can affect the cardiovascular, hematologic, respiratory, ion-regulatory, reproductive, metabolic, endocrine, and gill systems [13]. There is limited research on Al concentrations in the edible tissues of fish in Pakistan. Barium was found abundantly in various samples of bed sediment, water, and plankton from Panjnad. The concentration of Ba in aquatic organisms is based on their age, tissue type, and external environmental conditions [14]. Research on the behavior of Ba in freshwater ecosystems has been limited. A study examining Ba levels in both water and fish, as part of a more comprehensive investigation of Ba in an aquatic-terrestrial ecosystem, suggested that the concentration of Ba increased from the water to the fish [15]. The concentration of Ba in the muscle tissue of juvenile specimens was 2.5 times higher than that of adult individuals, indicating a significant difference [16]. The ability of Ba compounds to easily dissolve in water can potentially result in detrimental health impacts on individuals [17].
Fish have been widely used as biological indicators to determine the presence of high levels of heavy metals in the aquatic environment [18]. Due to their varying sizes, ages, and positions in the food chain, different species of fish have been utilized to study the correlation between metal concentrations in water and fish, both in laboratory and field study [3,4,19]. Furthermore, the acidic nature of the aquatic environment may also lead to the absorption of free divalent ions of various heavy metals by fish gills [20,21].
In countries such as Pakistan, fish, shellfish, and other aquatic organisms constitute a significant portion of the daily diet of the population. The consumption of heavy-metal-contaminated fish over an extended period of time can result in the accumulation of heavy metals in humans. Despite being a subsector of agriculture and contributing only 1% to the country’s GDP, the fisheries sector in Pakistan is rich in marine and freshwater resources. In 2020, the total fish production in Pakistan was estimated at 701,726 metric tons, with 474,025 metric tons being derived from marine fisheries and the rest from inland freshwater sources (Pakistan Economic Survey, 2019–20) [22]. Research showed that fish are a common dietary component, so it is not surprising that contaminated fish could serve as a hazardous source of certain toxic heavy metals in our diet [23]. Heavy metals tend to accumulate in both the soft and hard tissues of fish through the process of bioaccumulation. The build-up of heavy metals in fish serves as a means of detecting the concentration of these metals in aquatic environments. Furthermore, these metals can be passed on from fish to their predators within the food chain [24]. Cirrhinus mrigala is a freshwater fish species that holds significant economic value in numerous countries, including Pakistan [18]. Mrigals are considered detritivore fish that typically inhabit the bottom of aquatic environments and are well-suited for composite aquaculture practices [25]. Cirrhinus mrigala exhibits more capacity to tolerate a broad range of experimental conditions. Additionally, this fish species holds significant commercial value due to its ease of cultivation and is well-regarded for its flavor [26].
Various studies have been conducted to evaluate and analyze heavy metal concentrations in different rivers of Punjab, Pakistan. However, limited information was available on the presence of toxic metals, such as As, Pb, Al, and Ba, in C. mrigala living in the Panjnad River. Located at the far end of the Bahawalpur district in Punjab, the Panjnad River is formed by the successive merging of five rivers. Due to the significant discharge of numerous metallic compounds into the rivers of Pakistan, the freshwater ecosystem has been severely affected. The accumulation and toxicity of heavy metals in aquatic ecosystems pose a significant concern. Consequently, this study aims to contribute to our understanding of the health risks associated with heavy metal (As, Ba, Al, and Pb) contamination in C. mrigala within its environment as little information is available on the toxic profile of these heavy metals at the study sites.
2. Materials and Methods
2.1. Study Area
The Panjnad headwork is situated at an altitude of 99 m above sea level. The area experiences an average annual rainfall of 3.56 mm, an average annual temperature of 29.49 °C, and an average annual relative humidity of 27.64%. As it is the confluence point of various rivers, the Panjnad headwork receives a diverse range of pollutants from these rivers. The primary sources of pollution in the Panjnad headwork are agricultural, domestic, and industrial wastes from various cities, such as Gujarat, Faisalabad, Jhang, and Multan. These wastes come from industries such as textile, dyeing, petrochemical, hosiery, oil refineries, sugar and flour mills, distilleries, tannery, rubber, and plastics. They are discharged into the river ecosystem, which further contributes to the pollution in the region [27]. For this study, samples were collected from three stations (SI, SII, and SIII) between September 2021 and May 2022 (Figure 1). Throughout the study period, the sampling stations were visited every month. The selection of sites was based on different levels of pollutant accumulation. The current study conducted three replications during each season at each site, resulting in a total of nine replications for each experimental factor.
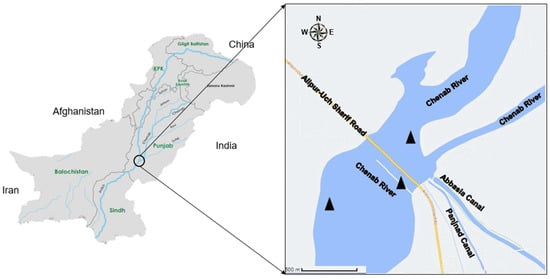
Figure 1.
Map of the study area (Panjnad headwork). The sampling sites are represented by.
2.2. Collection and Digestion of Samples
To conduct the study, we chose C. mrigala being the most common and abundant fish species at the study site. This species was easily caught and is commonly consumed in the surrounding areas. Fish samples were collected randomly from three different locations of Panjnad river in three different seasons (spring, autumn, and winter). The sampling of fish was carried out using a gauze net with the dimension of 100 m × 6 m, having a mesh-size of 60 mm. Samples of C. mrigala were collected randomly from each of the three sampling sites three times a month from September 2021 to May 2022. The samples were then placed in an icebox (Coleman Ice Box 48 Quart) with ice cubes placed at the bottom of the box to preserve the freshness of collected samples. The collected samples were then transported to the zoology laboratory at department of zoology, Government Sadiq College Women University, Bahawalpur and kept in a deep freezer (Haier −25 °C Biomedical Freezer) at a temperature of −20 °C for digestion and further analysis. In the laboratory, the species of the sampled fish were identified using a fish identification manual [28]. The liver, kidney, gills, fins, skin, muscles, and bones of the fish were dissected for analysis of heavy metal content. The acid digestion method was used to digest the dissected organs with analytical-grade HClO4 (60%, Daejung, Siheung-si, Republic of Korea) and HNO3 (65%, Sigma-Aldrich, St. Louis, MO, USA) in a ratio of 1:3 v/v. This method was performed for 5 ± 0.25 h. The digested sample was then heated on a hot plate (ANEX Deluxe Hot Plate AG-2166-EX) at 200 °C for 30 ± 5 min. Once completely digested, the samples were cooled down to 25 ± 2 °C. The cooled samples were then filtered through Whatman No. 42 filter paper. As the atomic absorption spectrophotometer (AAS) requires diluted samples for the analysis, the samples were diluted with double distilled water up to 50 mL for further analysis. After dilution, the digested samples were sealed in analytical-grade 50 mL glass vials and sent to the Central Laboratory, Mian Nawaz Sharif University of Agriculture (MNSUA), Multan, Punjab, Pakistan for heavy metal detection.
2.3. Heavy Metal Detection in Fish Organs
The concentration of heavy metals (Al, As, Ba, and Pb) in different organs of C. mrigala was detected at Central Laboratory, MNSUA, Multan, using an acetylene air flame AAS (Analytik Jena: NovAA 400 P) according to the recommended instrument parameters such as detection limits (Table 1). The precision and accuracy of the AAS analysis was confirmed by comparing the results with reference material (CRM IAEA 407) guided by the International Atomic Agency (IAEA). The heavy metal determination showed an acceptable performance, as the analytical results of the blanks and standards for the studied metals were within the certified values range of 95–101% recovery.

Table 1.
Condition of atomic absorption spectrometer used for the detection of heavy metal concentration.
2.4. Health Risk Assessment
Various health risk assessment indicators were applied to assess heavy metal exposure, carcinogenic effects, and non-carcinogenic effects of studied heavy metals in fish muscle.
2.5. Estimation of Oral Ingestion Exposure
Fish is widely consumed in Pakistan, especially in areas which are close to the rivers and other freshwater resources. Fish is among the cheapest and highest-quality sources of protein, vitamins, and other dietary minerals (Roos et al., 2007).
In order to estimate the heavy metal ingestion exposure to the fish, the following equations were used.
where Ingex is the ingestion exposures of each heavy metal via oral intake of fish. Con. represents the concentration (mg/kg) of respective heavy metals in fish muscles. Various formula constants were also applied in the equation for the estimation of ingestion exposure of heavy metals in fish muscles (Table 2).

Table 2.
Constants used in the formula for the calculation of the health risk assessments.
2.6. Target Hazardous Quotients via Ingestion (THQIng)
The THQIng for the oral ingestion exposure of the heavy metals was estimated using Equation (2). The equation is based on USEPA Region III risk-based concentration criteria assuming the duration of heavy metal exposure of fish consumers is 30 years.
where Ingex is the estimated ingestion exposures through oral intake and is derived from Equation (1). Reference dose (RfoD) is the permissible limit (mg) of each metal per kg per day of fish. For the metals in the present study, RfoD (mg/kg/day) are; Al = 1.0, As = 0.0003, Ba = 0.2, and Pb = 0.0035 [29]. A THQIng < 1 shows non-lethal health impact for human consumption, while a THQing > 1 represents a significant impact of contaminated fish on the exposed consumers [29].
2.7. Total Target Hazardous Quotients (TTHQIng)
The TTHQIng is estimated as a summation (∑) of THQIng of studied heavy fish muscles. The TTHQIng were estimated based on the following equations.
where “k” represents the total number of heavy metals in this study, and it is the sum of each metal THQIng for each sampling location. A TTHQIng < 1 shows non-lethal health impact for human consumption, while a TTHQIng > 1 represents a significant impact of contaminated fish on the exposed consumers [29].
2.8. Carcinogenic Risk (CR)
The CR represents the potential risk posed by heavy metal intake to cause cancer. The CR was calculated based on Equation (4) for fish consumers [3,30].
where SFC is the cancer risk slope factor (0.0085 and 1.5 (mg/kg per day) for Pb and As, respectively) [31,32,33]. For Al and Ba, SFC is not known as these heavy metals are dietary requirements, and the cancer risk slope is not defined for these heavy metals.
2.9. Statistical Analysis
The assessment of heavy metal contamination in different organs of C. mrigala at different locations (SI, SII, and SIII) and different seasons (spring, autumn, and winter) was based on the analysis of individual heavy metal concentration in the fish organs. The heavy metals concentration in the liver, kidney, gills, fins, skin, muscles, and bones of C. mrigala were compared using a one-way analysis of variance (ANOVA) and the Duncan’s new multiple range test with α = 0.05 was performed to compare heavy metal accumulation in different fish organs in all sampling sites and seasons using IBM SPSS (Version 25.0). Furthermore, correlation analysis was also conducted to observe the relation between heavy metal concentration in different organs of C. mrigala in various sampling months (October 2021–May 2022) using Minitab (version 19.11).
3. Results
A study was conducted to investigate the aquatic pollution at Panjnad headwork, Punjab, Pakistan, caused by four (Al, Ba, As, and Pb) heavy metals in different organs (liver, kidney, gills, fins, skin, muscles, and bones) of C. mrigala during autumn, winter, and spring seasons. The investigation of Al contamination in different organs of C. mrigala at three different locations (SI, SII, and SIII) showed that highest concentration of Al (µg·g−1) at SI was found in fish kidney with the overall mean (151.90 ± 6.10), followed by liver, gills, skin, fins, and bones, and the lowest concentration was found in fish muscles (72.38 ± 6.11). Similar pattern of Al concentration was also recorded in SII and SIII, with a slight change in Al concentration in SII where the lowest Al concentration was recorded in fish bones (72.25 ± 6.21). During comparison of Al concentration at different locations in different seasons, the highest Al concentration in fish kidney at SI was found in spring season (156.51 ± 6.02) followed by winter, and the lowest concentration was found in fish muscles in autumn (66.84 ± 4.94). Similar results were also recorded for other two locations (SII and SIII) with a slight difference in the lowest Al concentration at SII, which was recorded in fish bones (66.62 ± 6.30) instead of muscles. Among the comparison of different locations (SI, SII, and SIII), the highest Al concentration was found in kidney at SIII (152.31 ± 4.89) while the lowest concentration was recorded in fish bones (72.25 ± 6.21) at the SII location (Table 3).

Table 3.
Al concentration (mg kg−1) in tissue samples (Means ± SD) of C. mrigala caught from different sites at Panjnad headwork, Pakistan.
The investigation of As contamination in different organs of C. mrigala at three different locations (SI, SII, and SIII) showed that the highest concentration of As (µgg−1) at SI was found in fish kidney with the overall mean (26.86 ± 5.36), followed by liver, gills, fins, skin, and bones, and the lowest concentration was found in fish muscles (13.77 ± 3.53). Similar patterns of As concentration were also recorded in SII and SIII, with the highest concentrations in kidney (26.70 ± 4.60 and 26.73 ± 4.87) and the lowest in fish muscles (13.25 ± 2.83 and 13.63 ± 3.77) at SII and SIII, respectively. During comparison of As concentration at different locations in different seasons, the highest As concentration in fish kidney at SI was found in spring season (32.71 ± 2.43) followed by winter, and the lowest concentration was found in fish bones in autumn (10.04 ± 1.70). Similar results were also recorded in other two locations (SII and SIII) with the highest concentrations in kidney (31.87 ± 1.81 and 32.08 ± 1.42) and the lowest in fish bones (9.59 ± 2.40 and 9.24 ± 2.52) at SII and SIII, respectively. The comparison of different locations (SI, SII, and SIII) revealed that the highest As concentration was found in kidney at SI (26.86 ± 5.36) while the lowest concentration was recorded in fish bones (9.24 ± 2.52) at the SII location (Table 4).

Table 4.
As concentration (mg kg−1) in tissue samples (Means ± SD) of C. mrigala caught from different sites at Panjnad headwork, Pakistan.
The investigation of Ba contamination in different organs of C. mrigala at three different locations (SI, SII, and SIII) showed that highest concentration of Al (µgg−1) at SI was found in fish liver with the overall mean (5.12 ± 1.88), followed by kidney, gills, fins, skin, and muscles, and the lowest concentration was found in fish bones (3.32 ± 2.01). A similar pattern of Ba concentration was also recorded in SII and SIII, with a slight change in Ba concentration in SII where the lowest Al concentration was recorded in fish muscles (2.70 ± 1.10). During comparison of Ba concentration at different locations in different seasons, the highest Ba concentration at SI was found in the spring season (7.43 ± 0.70) in fish liver followed by winter, and the lowest concentration was found in autumn (1.38 ± 0.83) in fish bones. Similar results were also recorded in other two locations (SII and SIII) with a slight difference in the lowest Ba concentration at SIII, which was recorded in fish muscles (1.19 ± 0.31) instead of muscles. The comparison of different locations (SI, SII, and SIII) revealed that the highest Ba concentration was found in liver at SI (5.12 ± 1.88) while the lowest concentration was recorded in fish muscles (2.70 ± 1.10) at the SII location (Table 5).

Table 5.
Ba concentration (mg kg−1) in tissue samples (Means ± SD) of C. mrigala caught from different sites at Panjnad headwork, Pakistan.
The investigation of Pb contamination in different organs of C. mrigala at three different locations (SI, SII, and SIII) showed that highest concentration of Pb (µg·g−1) at SI was found in fish liver with the overall mean (4.73 ± 1.96), followed by kidney, gills, fins, skin, and muscles, and the lowest concentration was found in fish bones (2.53 ± 1.33). A similar pattern of Pb concentration was also recorded in SII and SIII, with the highest concentration in fish liver (4.34 ± 1.69 and 4.67 ± 1.84) and the lowest concentration in fish bones (2.43 ± 1.42 and 2.63 ± 1.62) for SII and SIII, respectively. During comparison of Pb concentration at different locations in different seasons, the highest Pb concentration at SI was found in fish kidney during spring (7.57 ± 0.42) followed by winter, and the lowest concentration was found in fish muscles in autumn (1.22 ± 0.58). However, SII and SIII locations showed different varying results. During sampling at SII, the highest Pb concentration was found in fish kidney during spring (6.45 ± 0.95) followed by winter, and the lowest concentration was found in fish bones in autumn (0.77 ± 0.32), while at SIII, the highest Pb concentration was recorded in fish liver during spring (6.67 ± 1.84) followed by winter, and the lowest concentration was found in fish bones in autumn (0.80 ± 0.59). Among the comparison of different locations (SI, SII, and SIII), the highest Al concentration was found in liver at SI (4.73 ± 1.96) while the lowest concentration was recorded in fish bones (0.77 ± 0.32) at the SII location (Table 6). Overall, these findings showed that fish liver and kidney are most affected organs for heavy metal contamination in any season or location while fish bones and fish muscles are the least affected fish organs. The results also showed that heavy metal accumulation is significantly higher (p < 0.05) in spring while being significantly lower (p < 0.05) during autumn.

Table 6.
Pb concentration (mg kg−1) in tissue samples (Means ± SD) of C. mrigala caught from different sites at Panjnad headwork, Pakistan.
The relation between sampling months (September–May) and heavy metal (Al, As, Ba, and Pb) accumulation in different organs (liver, kidney, gills, finds, skin, muscles and bones) of C. mrigala was analyzed with Pearson correlation. The obtained results showed a combination of significant and non-significant positive as well as negative correlation between sampling months and accumulation of different heavy metals. Overall, most of the significant correlation between sampling month and heavy metal accumulation was observed in spring months as compared to autumn and winter.
The correlation between sampling months and Al accumulation in fish showed significant negative as well as positive correlation among various months. The highest significant correlation was observed between January and October (r = 0.819, p = 0.007) in fish liver while the least significant correlation was found between January and April (r = 0.674, p = 0.047) in fish muscles. It was also observed that most of the significant correlations for Al concentration in various fish organs occurred from January to May (Table 7).

Table 7.
Correlation among different sampling months (October 2021–May 2022) for Al accumulation in different organs of C. mrigala at Panjnad headwork. Bold values represent statistically significant values.
The correlation between sampling months and As accumulation in fish showed significant negative as well as positive correlation among various months. It was found that January and November had the highest significant correlation for As accumulation (r = 0.907, p < 0.000) in fish muscles while the least significant correlation was observed between October and May (r = 0.672, p = 0.047) in fish liver. Like Al, most of the significant correlations for As concentration in various fish organs occurred in later months. (Table 8).

Table 8.
Correlation among different sampling months (October 2021–May 2022) for As accumulation in different organs of C. mrigala at Panjnad headwork. Bold values represent statistically significant values.
The correlation between sampling months and Ba accumulation in fish showed a number of significant negative as well as positive correlation among various months. For Ba, February and March showed the highest significant correlation for Ba concentration (r = 0.984, p < 0.000) in skin, while the lowest significant correlation was observed between February and October (r = 0.670, p = 0.048) in fish liver. Like Al and Ba, most of the significant correlations for Ba accumulation in various fish organs were observed in late winter or in spring. (Table 9).

Table 9.
Correlation among different sampling months (October 2021–May 2022) for Ba accumulation in different organs of C. mrigala at Panjnad headwork. Bold values represent statistically significant values.
The correlation between sampling months and Pb concentration in fish organs showed a number of significant negative as well as positive correlations among various months. For Pb, the highest significant correlation was observed in fish kidney between February and March (r = 0.956, p < 0.000), while the least significant correlation was found between January and March (r = 0.672, p = 0.047) in fish muscles. Like other heavy metals, it was also observed that most of the significant correlations for Pb concentration in various fish organs occurred from January to March. (Table 10).

Table 10.
Correlation among different sampling months (October 2021–May 2022) for Pb accumulation in different organs of C. mrigala at Panjnad headwork. Bold values represent statistically significant values.
The correlation analysis also showed that most of the significant correlations between sampling months for various heavy metals were found in Ba followed by Pb and As, and the least significant correlation was observed for Al accumulation.
Fish are among the most consumed and important diets worldwide. The accumulation of heavy metals in fish muscles could pose a serious health risk to the consumers if the heavy metal contamination in fish muscles is above permissible limits. The health risk assessment of studied fish was carried out to evaluate the potential health hazards due to oral exposure of studied heavy metals. The observed oral exposure (Ingex) was calculated in milligrams per kilogram per day (mg kg−1/day) for ingestion of C. mrigala. The results showed that the highest oral exposure through fish consumption was recorded for Al (23.05) followed by As, Ba, and Pb with Ingex values of (4.3, 0.98, and 0.89), respectively. However, the highest THQIng was found in Ba followed by Pb, Al, and As with the values of (0.44, 0.02, and 0.02), respectively. Furthermore, the TTHQIng of the studied heavy metals was recorded to be 3.76. The CRIng values for Ba and Al were not calculated as these metals and dietary requirement do not have a cancer slope factor. However, the CRIng of As and Pb was found to be 6.4742 and 0.0076, respectively (Table 11).

Table 11.
Human Health Risk Assessment of heavy metals in C. mrigala.
The obtained results showed that the Ingex values of studied heavy metal were within the permissible limits according to the guidelines of ATSDR (2007) and EFSA (2008) except for Al and As which were higher than the permissible limits of 1 mg·kg−1 bw/week and 2.14 μg·kg−1 bw /day for Al and As, respectively. THQIng values of Al, As, and Pb showed that these heavy metals are not hazardous. However, Ba was found to be hazardous as its THQIng (3.27 mg·kg−1/day) was greater than 1. Similarly, based on higher THQIng value of Ba in the fish muscle samples, the TTHQIng also showed potential hazard for the consumption of the C. mrigala from the sampling sites. CRIng for As was found to be potentially carcinogenic in C. mirgala for human consumption. Overall, the results indicated that the studied sites possess potentially elevated accumulation of heavy metals and that the C. mrigala species captured from these sites are not safe for long term human consumption.
4. Discussion
Heavy metal accumulation in freshwater ecosystems has become one of the major aquatic environmental concerns for freshwater organisms such as fish due to their higher stability and bioaccumulation as well as bio-magnification properties [34,35]. The unplanned expansion of urban areas and the rapid growth of industries near the Panjnad headwork on the Chenab River in Bahawalpur have led to significant pollution in the surrounding environment. This pollution is caused by the release of large amounts of untreated hazardous waste and domestic sewage, resulting in detrimental effects on the local ecosystems [36,37]. Current study evaluated the bioaccumulation of heavy metals in different organs of C. mrigala in various seasons and sampling locations. Furthermore, the health risk assessment of heavy metal accumulation in fish muscles was also explored to assess the impact of fish consumption from the selected locations.
The present study concluded that kidney and liver of C. mrigala accumulated significantly more heavy metals as compared to other organs. It is now widely known that the kidney and the liver are more actively involved in metabolic process of fish as compared to other organs such as muscles, bones, and skin [38]. A number of studies on various fish species have concluded that the kidney and the liver usually accumulate higher concentrations of heavy metals as compared to other fish organs [39]. Kidney and liver are the primary site of metal detoxification in fish body, due to which these organs tend to accumulate higher levels of various pollutants including heavy metals [40].
Our primary concern is the accumulation of toxic compounds in fish in their flesh (muscle), which is the most consumed organ. However, in comparison to other tissues, muscles generally contain lower levels of metals [41]. It is possible for muscle levels to be undetectable even when the concentration in the liver is high [42]. In contrast, to other fish organs, muscles are not considered a primary site for metal intake in fish, as they typically contain lower levels of trace elements and metals compared to other organs but in a human health perspective the accumulation of heavy metals in fish muscles becomes more concerned.
The current study showed that the muscles of C. mrigala accumulated the lowest quantity of heavy metal pollutants. Similar results have been concluded previously in various fish species, suggesting that fish muscles are among the least affected body organ by heavy metal pollution and have no or fewer active mechanisms for releasing them [43,44]. Li et al. [45] concluded similar results while studying Cd, Pb, As, and Hg accumulation in Carassius auratus, Pelteobagrus fulvidraco, and Hippelates nobilis sampled in the Nansi Lake in China.
The current study recorded higher concentration of heavy metals (Al, As, Ba, and Pb) during spring as compared to the autumn and winter. Higher levels of physical and physiological activities performed by the fish during the hot weathers as compared to the winter and autumn could a plausible explanation for this higher heavy metal accumulation in spring and lower accumulation in autumn season [18,46,47]. These findings are coherent with a number of other studies which have concluded that accumulation of heavy metals is significantly higher in spring as compared to autumn and winter in the Mediterranean fish [48,49]. Similarly, another study has shown that higher growth rate in spring and summer in fish may cause significant more heavy metal accumulation in these seasons as compared to cold seasons [46].
The bio-accumulation of metals in fish bodies is a complex process, and its variation depends on the mode of action of the metals [50]. The toxicity of metals found in the fish species studied showed a direct correlation with the eco-toxicity of the Panjnad, as well as the metabolism, feeding patterns, and ecological requirements of the fish species under investigation. The current study revealed significant differences in metal accumulation among various organs, likely attributable to variations in the physiological functions of these organs within the fish body. Specifically, the liver emerged as the primary target organ for metal toxicity, followed by the kidney, gills, scales, fins, bones, and muscles, in descending order of metal accumulation. Fish muscles and bones exhibited notably lower concentrations of the studied metals. It is important to note that bio-accumulation refers to the uptake of metals by an organism, but within the organism, metals are distributed among various tissues, each with a specific affinity for accumulation [51].
The mean accumulation of various metals in different body organs of C. mrigala were in the order of Al > As > Ba > Pb. Naz et al. [3] concluded similar trends for the contamination of these heavy metals in water and sediments at Panjnad. Hence, it can be inferred that the ability of fish to bio-accumulate heavy metals is directly influenced by the concentration of these metals in the water and sediment of the aquatic environment.
The current study is coherent with Mahboob et al. [52] and Chatha et al. [18] who concluded that heavy metal concentration in fish organs was significantly greater in the summer as compared to other seasons. The correlation analysis conducted in this study showed that there is a higher statistically significant correlation between sampling months of late winter (January and February) and spring season (March–May) for accumulation of different heavy metals in various fish organs among sampling months. Fishes such as C. mrigala being larger in size tend to accumulate more heavy metals contents due to their widespread column-feeding nature [53,54], which is also observed in the current study, and higher heavy metals accumulation was found in C. mrigala as compared to the permissible limits.
In order to assess the impact of heavy metal accumulation in fish to the human health, Ingex, THQIng, TTHQIng, and CRIng parameters were calculated for the consumption of muscles of C. mrigala from Panjnad headwork, Punjab, Pakistan. THQIng readings for Al metal analyzed in C. mrigala were the highest for fish consumers, following the arrangement Al > As > Ba > Pb. Similarly, Harmanescu et al. [55] concluded a similar pattern of health assessment of heavy metals such as Pb and Cd. The THQIng analysis showed that Ba values for THQIng were >1, suggesting a potential hazard for the Ba intake. Apart from Ba, the THQIng values for other heavy metals were <1, which suggests that the accumulation of these heavy metals in fish muscles do not pose any potential health hazards to its consumers. The TTHQIng was also >1 due to the additive effect of Ba in the mixture of other heavy metals. It suggests that C. mrigala collected from the sampling sites were not safe for human consumption. Our results are coherent with [56], who also found lower concentrations of As in fish samples. On the other hand, studies have concluded that As, even at very low concentrations, disrupts the hormonal activity of endocrine glands [57]. Therefore, a number of health issues in organs such as the gastrointestinal tract, respiratory tract, skin, liver, cardiovascular system, hematopoietic system, and nervous system may arise by the chronic exposure to inorganic As even at lower concentrations [58].
It is observed that human populations may encounter trace metals by consuming water or beverages that are rich in metals, inhaling air contaminated with trace metals, or ingesting other food substances [59]. Additionally, there are various pathways through which trace metals can enter fish ponds, extending beyond the water within the pond and the fish feed [60]. Naz et al. [3] found similar health risk pattern for studied heavy metals in water, plankton, and sediments from Panjnad river.
The carcinogenic risk (CRIng) of heavy metals (Al, As, Ba, and Pb) was determined using the consumption levels of C. mrigala. The study indicated that heavy metals such as Al, Ba, and Pb did not show carcinogenic risks for human health. However, As levels were above the borderline for safe human consumption suggested by USEPA [61] for oral intake. It could be due to the higher toxic properties of As when taken orally. It has been shown that even very little accumulation of As in water could cause carcinogenic effects in humans when taken orally [62].
5. Conclusions
It is widely known that various heavy metals such as Al, As, Ba, and Pb accumulate in aquatic organisms when present in fresh water and may cause serious damage to fish organs. The consumption of these polluted fish causes potential health hazards to its consumers. This study concluded that various heavy metals were accumulated at various concentrations in different fish organs. However, health risk assessment suggests that C. mrigala was not safe for human consumption due to its associated carcinogenic and non-carcinogenic potential health risks. An understanding of the adverse effects of heavy metals in freshwater organisms and their permissible concentrations in the aquatic environment would be extremely essential for fish conservation, fisheries development, and safe human consumption.
Author Contributions
S.N., wrote original draft, A.M.M.C., data curation, D.D., wrote methodology, M.F.K., performed formal analysis, Y.X., visualized data, P.Z., funding acquisition and L.S., Project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
All experimental study were approved and reviewed by the Departmental Animal welfare & ethical Committee of Department of Zoology, Government Sadiq College Women University, Bahawalpur 36100, Pakistan (NO. 602b/Zool and Dated. 19 June 2021).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
All authors declare that they have no conflict of interest.
References
- Rani, L.; Srivastav, A.L.; Kaushal, J.; Grewal, A.S.; Madhav, S. Chapter 3—Heavy Metal Contamination in the River Ecosystem. In Ecological Significance of River Ecosystems; Madhav, S., Kanhaiya, S., Srivastav, A., Singh, V., Singh, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 37–50. [Google Scholar]
- Bawuro, A.A.; Voegborlo, R.B.; Adimado, A.A. Bioaccumulation of Heavy Metals in Some Tissues of Fish in Lake Geriyo, Adamawa State, Nigeria. J. Environ. Public Health 2018, 2018, 1854892. [Google Scholar] [CrossRef] [PubMed]
- Naz, S.; Mansouri, B.; Chatha, A.M.M.; Ullah, Q.; Abadeen, Z.U.; Khan, M.Z.; Khan, A.; Saeed, S.; Bhat, R.A. Water quality and health risk assessment of trace elements in surface water at Punjnad Headworks, Punjab, Pakistan. Environ. Sci. Pollut. Res. 2022, 29, 61457–61469. [Google Scholar] [CrossRef] [PubMed]
- Naz, S.; Chatha, A.M.M. Metals Mixture Effects on Growth Performance and Their Bioaccumulation in Fish. Iran. J. Fish. Sci. 2022, 21, 605–618. [Google Scholar]
- Taslima, K.; Al-Emran, M.; Rahman, M.S.; Hasan, J.; Ferdous, Z.; Rohani, M.F.; Shahjahan, M. Impacts of Heavy Metals on Early Development, Growth and Reproduction of Fish—A Review. Toxicol. Rep. 2022, 9, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Huseen, H.M.; Mohammed, A.J. Heavy Metals Causing Toxicity in Fishes. J. Phys. Conf. Ser. 2019, 1294, 062028. [Google Scholar] [CrossRef]
- Osman, A.G.M.; Wuertz, S.; Mekkawy, I.A.; Exner, H.-J.; Kirschbaum, F. Lead induced malformations in embryos of the African catfish Clarias gariepinus (Burchell, 1822). Environ. Toxicol. 2007, 22, 375–389. [Google Scholar] [CrossRef]
- Oremland, R.S.; Stolz, J.F. The Ecology of Arsenic. Science 2003, 300, 939–944. [Google Scholar] [CrossRef]
- Babich, R.; Van Beneden, R.J. Effect of arsenic exposure on early eye development in zebrafish (Danio rerio). J. Appl. Toxicol. 2019, 39, 824–831. [Google Scholar] [CrossRef]
- Kumari, B.; Kumar, V.; Sinha, A.K.; Ahsan, J.; Ghosh, A.; Wang, H.; De Boeck, G. Toxicology of arsenic in fish and aquatic systems. Environ. Chem. Lett. 2017, 15, 43–64. [Google Scholar] [CrossRef]
- Rabbane, M.G.; Kabir, M.A.; Habibullah-Al-Mamun, M.; Mustafa, M.G. Toxic Effects of Arsenic in Commercially Important Fish Rohu Carp, Labeo Rohita of Bangladesh. Fishes 2022, 7, 217. [Google Scholar] [CrossRef]
- Correia, T.; Narcizo, A.; Bianchini, A.; Moreira, R. Aluminum as an endocrine disruptor in female Nile tilapia (Oreochromis niloticus). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2010, 151, 461–466. [Google Scholar] [CrossRef]
- Vuorinen, P.J.; Keinänen, M.; Peuranen, S.; Tigerstedt, C. Reproduction, blood and plasma parameters and gill histology of vendace (Coregonus albula L.) in long-term exposure to acidity and aluminum. Ecotoxicol. Environ. Saf. 2003, 54, 255–276. [Google Scholar] [CrossRef]
- Donald, D.B. Trophic decline and distribution of barium in a freshwater ecosystem. Hydrobiologia 2017, 784, 237–247. [Google Scholar] [CrossRef]
- Hope, B.; Loy, C.; Miller, P. Uptake and Trophic Transfer of Barium in a Terrestrial Ecosystem. Bull. Environ. Contam. Toxicol. 1996, 56, 683–689. [Google Scholar] [CrossRef]
- Donald, D.B.; Sardella, G.D. Mercury and other metals in muscle and ovaries of goldeye (Hiodon alosoides). Environ. Toxicol. Chem. 2010, 29, 373–379. [Google Scholar] [CrossRef]
- Frančišković-Bilinski, S.; Bilinski, H.; Grbac, R.; Žunić, J.; Nečemer, M.; Hanžel, D. Multidisciplinary work on barium contamination of the karstic upper Kupa River drainage basin (Croatia and Slovenia); calling for watershed management. Environ. Geochem. Health 2007, 29, 69–79. [Google Scholar] [CrossRef]
- Chatha, A.M.; Naz, S.; Mansouri, B.; Nawaz, A. Accumulation and Human Health Risk Assessment of Trace Elements in Two Fish Species, Cirrhinus mrigala and Oreochromis niloticus, at Tarukri Drain, District Rahimyar Khan, Punjab, Pakistan. Environ. Sci. Pollut. Res. 2023, 30, 56522–56533. [Google Scholar] [CrossRef]
- Al-Saeed, F.A.; Naz, S.; Saeed, M.H.; Hussain, R.; Iqbal, S.; Chatha, A.M.M.; Ghaffar, A.; Akram, R. Oxidative Stress, Antioxidant Enzymes, Genotoxicity and Histopathological Profile in Oreochromis niloticus Exposed to Lufenuron. Pak. Veter J. 2023, 43, 160–166. [Google Scholar] [CrossRef]
- Naz, S.; Javed, M. Growth Responses of Fish During Chronic Exposure of Metal Mixture under Laboratory Conditions. Pak. Vet. J. 2013, 33. [Google Scholar]
- Pärt, P.; Svanberg, O.; Kiessling, A. The availability of cadmium to perfused rainbow trout gills in different water qualities. Water Res. 1985, 19, 427–434. [Google Scholar] [CrossRef]
- Haseeb, A.; Fozia, U.; Ahmad, I.; Ullah, H.; Iqbal, A.; Ullah, R.; Moharram, B.A.; Kowalczyk, A. Ecotoxicological Assessment of Heavy Metal and Its Biochemical Effect in Fishes. BioMed Res. Int. 2022, 2022, 3787838. [Google Scholar] [CrossRef]
- Çelik, M.; Diler, A.; Küçükgülmez, A. A Comparison of the Proximate Compositions and Fatty Acid Profiles of Zander (Sander Lucioperca) from Two Different Regions and Climatic Conditions. Food Chem. 2005, 92, 637–641. [Google Scholar] [CrossRef]
- Valkova, E.; Atanasov, V.; Tzanova, M.; Atanassova, S.; Sirakov, I.; Velichkova, K.; Marinova, M.H.; Yakimov, K. Content of Pb and Zn in Sediments and Hydrobionts as Ecological Markers for Pollution Assessment of Freshwater Objects in Bulgaria—A Review. Int. J. Environ. Res. Public Health 2022, 19, 9600. [Google Scholar] [CrossRef]
- Khangembam, B.K.; Chakrabarti, R. Trypsin from the Digestive System of Carp Cirrhinus mrigala: Purification, Characterization and Its Potential Application. Food Chem. 2015, 175, 386–394. [Google Scholar] [CrossRef]
- Ramesh, M.; Sujitha, M.; Anila, P.A.; Ren, Z.; Poopal, R.K. Responses of Cirrhinus mrigala to Second-Generation Fluoroquinolone (Ciprofloxacin) Toxicity: Assessment of Antioxidants, Tissue Morphology, and Inorganic Ions. Environ. Toxicol. 2021, 36, 887–902. [Google Scholar] [CrossRef]
- Ali, Z.; Malik, R.N.; Qadir, A. Heavy metals distribution and risk assessment in soils affected by tannery effluents. Chem. Ecol. 2013, 29, 676–692. [Google Scholar] [CrossRef]
- Rafique, M.; Khan, N.U. Distribution and Status of Significant Freshwater Fishes of Pakistan. Rec. Zool. Surv. Pak. 2012, 21, 90–95. [Google Scholar]
- USEPA. Integrated Risk Information System, Us Environmental Protection Agency Region I, Washington, DC 20460. Available online: http://www.epa.gov/iris/ (accessed on 6 August 2022).
- Jiang, Y.; Chao, S.; Liu, J.; Yang, Y.; Chen, Y.; Zhang, A.; Cao, H. Source apportionment and health risk assessment of heavy metals in soil for a township in Jiangsu Province, China. Chemosphere 2017, 168, 1658–1668. [Google Scholar] [CrossRef]
- ATSDR. Toxicological Profile Information Sheet; Agency for Toxic Substances and Disease Registry, Public Health Service, U.S. Department of Health and Human Services: Atlanta, GA, USA, 2010. [Google Scholar]
- USEPA. Supplemental Guidance for Assessing Cancer Susceptibility from Early-Life Exposure to Carcinogens; Risk Assessment Forum: Washington, DC, USA, 2005. [Google Scholar]
- Onuoha, S.C.; Anelo, P.C.; Nkpaa, K.W. Human Health Risk Assessment of Heavy Metals in Snail (Archachatina marginata) from Four Contaminated Regions in Rivers State, Nigeria. Am. Chem. Sci. J. 2016, 11, 1–8. [Google Scholar] [CrossRef]
- Yi, Y.; Tang, C.; Yi, T.; Yang, Z.; Zhang, S. Health risk assessment of heavy metals in fish and accumulation patterns in food web in the upper Yangtze River, China. Ecotoxicol. Environ. Saf. 2017, 145, 295–302. [Google Scholar] [CrossRef]
- Hanif, N.; Eqani, S.A.M.A.S.; Ali, S.M.; Cincinelli, A.; Ali, N.; Katsoyiannis, I.A.; Tanveer, Z.I.; Bokhari, H. Geo-accumulation and enrichment of trace metals in sediments and their associated risks in the Chenab River, Pakistan. J. Geochem. Explor. 2016, 165, 62–70. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E. Assessment of Potentially Toxic Heavy Metals and Health Risk in Water, Sediments, and Different Fish Species of River Kabul, Pakistan. Hum. Ecol. Risk Assess. 2018, 24, 2101–2118. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Liu, Y.; Zhang, X.; Ravikumar, B.; Bai, G.; Li, X. Studies on seasonal pollution of heavy metals in water, sediment, fish and oyster from the Meiliang Bay of Taihu Lake in China. Chemosphere 2018, 191, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Maleki, A.; Azadi, N.; Mansouri, B.; Majnoni, F.; Rezaei, Z.; Gharibi, F. Health risk assessment of trace elements in two fish species of Sanandaj Gheshlagh Reservoir, Iran. Toxicol. Environ. Health Sci. 2015, 7, 43–49. [Google Scholar] [CrossRef]
- Moiseenko, T.I.; Gashkina, N.A. Distribution and bioaccumulation of heavy metals (Hg, Cd and Pb) in fish: Influence of the aquatic environment and climate. Environ. Res. Lett. 2020, 15, 115013. [Google Scholar] [CrossRef]
- Baramaki Yazdi, R.; Ebrahimpour, M.; Mansouri, B.; Rezaei, M.R.; Babaei, H. Contamination of Metals in Tissues of Ctenopharyngodon Idella and Perca Fluviatilis, from Anzali Wetland, Iran. Bull. Environ. Contam. Toxicol. 2012, 89, 831–835. [Google Scholar] [CrossRef]
- Sivaperumal, P.; Sankar, T.; Viswanathannair, P. Heavy metal concentrations in fish, shellfish and fish products from internal markets of India vis-a-vis international standards. Food Chem. 2007, 102, 612–620. [Google Scholar] [CrossRef]
- Madu, J.; Odo, G.; Asogwa, C.; Nwani, C. Heavy metal concentrations in, and human health risk assessment of, three commercially valuable fish species in the lower Niger River, Nigeria. Afr. J. Aquat. Sci. 2017, 42, 341–349. [Google Scholar] [CrossRef]
- Shah, B.R.; Mraz, J. Advances in nanotechnology for sustainable aquaculture and fisheries. Rev. Aquac. 2020, 12, 925–942. [Google Scholar] [CrossRef]
- Majnoni, F.; Rezaei, M.; Mansouri, B.; Hamidian, A. Metal concentrations in tissues of common carp, Cyprinus carpio, and silver carp, Hypophthalmichthys molitrix from the Zarivar Wetland in Western Iran. Arch. Pol. Fish. 2013, 2, 11–18. [Google Scholar] [CrossRef]
- Li, P.; Zhang, J.; Xie, H.; Liu, C.; Liang, S.; Ren, Y.; Wang, W. Heavy Metal Bioaccumulation and Health Hazard Assessment for Three Fish Species from Nansi Lake, China. Bull. Environ. Contam. Toxicol. 2015, 94, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Zubcov, E.; Zubcov, N.; Ene, A.; Biletchi, L. Assessment of copper and zinc levels in fish from freshwater ecosystems of Moldova. Environ. Sci. Pollut. Res. 2012, 19, 2238–2247. [Google Scholar] [CrossRef] [PubMed]
- Campolim, M.B.; Henriques, M.B.; Petesse, M.L.; Rezende, K.F.O.; Barbieri, E. Metal Trace Elements in Mussels in Urubuqueçaba Island, Santos Bay, Brazil. Pesqui. Agropecuária Bras. 2017, 52, 1131–1139. [Google Scholar] [CrossRef]
- Çoğun, H.; Yüzereroğlu, T.A.; Kargin, F.; Firat, Ö. Seasonal Variation and Tissue Distribution of Heavy Metals in Shrimp and Fish Species from the Yumurtalik Coast of Iskenderun Gulf, Mediterranean. Bull. Environ. Contam. Toxicol. 2005, 75, 707–715. [Google Scholar] [CrossRef]
- Kargın, F.; Dönmez, A.; Çoğun, H.Y. Distribution of Heavy Metals in Different Tissues of the Shrimp Penaeus Semiculatus and Metapenaeus Monocerus from the Iskenderun Gulf, Turkey: Seasonal Variations. Bull. Environ. Contam. Toxicol. 2001, 66, 102–109. [Google Scholar] [CrossRef]
- Luoma, S.N. Bioavailability of trace metals to aquatic organisms—A review. Sci. Total. Environ. 1983, 28, 1–22. [Google Scholar] [CrossRef]
- Luo, L.; Ke, C.; Guo, X.; Shi, B.; Huang, M. Metal accumulation and differentially expressed proteins in gill of oyster (Crassostrea hongkongensis) exposed to long-term heavy metal-contaminated estuary. Fish Shellfish. Immunol. 2014, 38, 318–329. [Google Scholar] [CrossRef]
- Mahboob, S.; Al-Ghanim, K.A.; Al-Balawi, H.; Al-Misned, F.; Ahmed, Z. Toxicological effects of heavy metals on histological alterations in various organs in Nile tilapia (Oreochromis niloticus) from freshwater reservoir. J. King Saud Univ. Sci. 2020, 32, 970–973. [Google Scholar] [CrossRef]
- Maurya, P.K.; Malik, D.S.; Yadav, K.K.; Gupta, N.; Kumar, S. Haematological and histological changes in fish Heteropneustes fossilis exposed to pesticides from industrial waste water. Hum. Ecol. Risk Assess. Int. J. 2019, 25, 1251–1278. [Google Scholar] [CrossRef]
- Farkas, A.; Salánki, J.; Specziár, A. Age- and Size-Specific Patterns of Heavy Metals in the Organs of Freshwater Fish Abramis Brama L. Populating a Low-Contaminated Site. Water Res. 2003, 37, 959–964. [Google Scholar] [CrossRef]
- Harmanescu, M.; Alda, L.M.; Bordean, D.M.; Gogoasa, I.; Gergen, I. Heavy metals health risk assessment for population via consumption of vegetables grown in old mining area; a case study: Banat County, Romania. Chem. Central J. 2011, 5, 64. [Google Scholar] [CrossRef]
- Ahmed, M.K.; Baki, M.A.; Islam, M.S.; Kundu, G.K.; Habibullah-Al-Mamun, M.; Sarkar, S.K.; Hossain, M.M. Human health risk assessment of heavy metals in tropical fish and shellfish collected from the river Buriganga, Bangladesh. Environ. Sci. Pollut. Res. 2015, 22, 15880–15890. [Google Scholar] [CrossRef] [PubMed]
- Stoica, A.; Pentecost, E.; Martin, M.B. Effects of Arsenite on Estrogen Receptor-Alpha Expression and Activity in Mcf-7 Breast Cancer Cells. Endocrinology 2000, 141, 3595–3602. [Google Scholar] [CrossRef] [PubMed]
- Mandal, B.K.; Suzuki, K.T. Arsenic round the world: A review. Talanta 2002, 58, 201–235. [Google Scholar] [CrossRef] [PubMed]
- Al-Khatib, I.A.; Arafeh, G.A.; Al-Qutob, M.; Jodeh, S.; Hasan, A.R.; Jodeh, D.; van der Valk, M. Health Risk Associated with Some Trace and Some Heavy Metals Content of Harvested Rainwater in Yatta Area, Palestine. Water 2019, 11, 238. [Google Scholar] [CrossRef]
- Renieri, E.A.; Goumenou, M.; Kardonsky, D.A.; Veselov, V.; Alegakis, A.; Buha, A.; Tzatzarakis, M.N.; Nosyrev, A.E.; Rakitskii, V.N.; Kentouri, M.; et al. Indicator PCBs in farmed and wild fish in Greece–Risk assessment for the Greek population. Food Chem. Toxicol. 2019, 127, 260–269. [Google Scholar] [CrossRef]
- USEPA. 2018 Edition of the Drinking Water Standards and Health Advisories Tables; USEPA: Washington, DC, USA, 2018. [Google Scholar]
- Alidadi, H.; Sany, S.B.T.; Oftadeh, B.Z.G.; Mohamad, T.; Shamszade, H.; Fakhari, M. Health risk assessments of arsenic and toxic heavy metal exposure in drinking water in northeast Iran. Environ. Health Prev. Med. 2019, 24, 59. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).