Microorganisms and Biochar Improve the Remediation Efficiency of Paspalum vaginatum and Pennisetum alopecuroides on Cadmium-Contaminated Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.1.1. Initial Soil
2.1.2. Plant Material
2.1.3. Microorganism Materials
2.1.4. Biochar
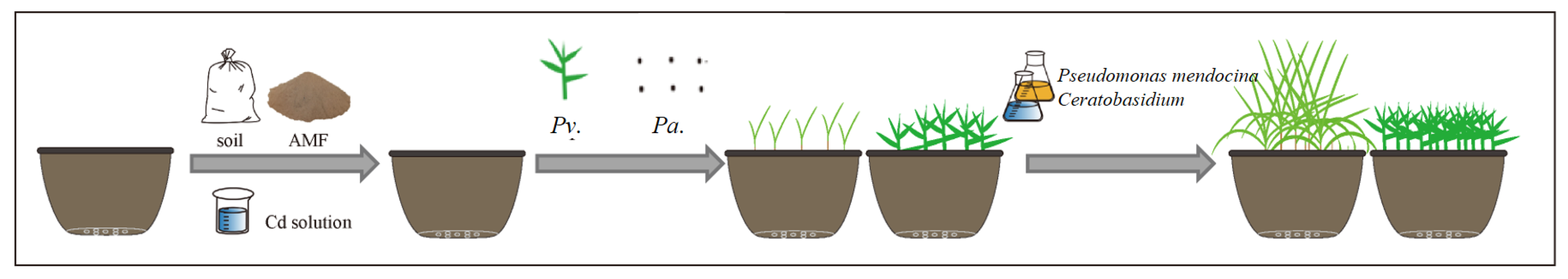
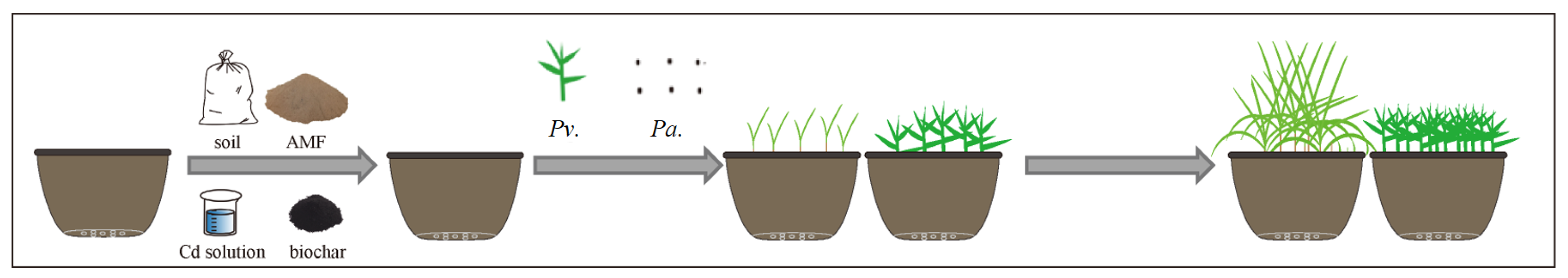
2.2. Pot Experiment Design
2.2.1. The First Stage: Microorganism Evaluation Test
2.2.2. The Second Stage: Microorganism and Biochar Effect Test
2.3. Material Collection and Determination
2.4. Data Processing
3. Results
3.1. The First Stage: Microorganisms Evaluation Test Results
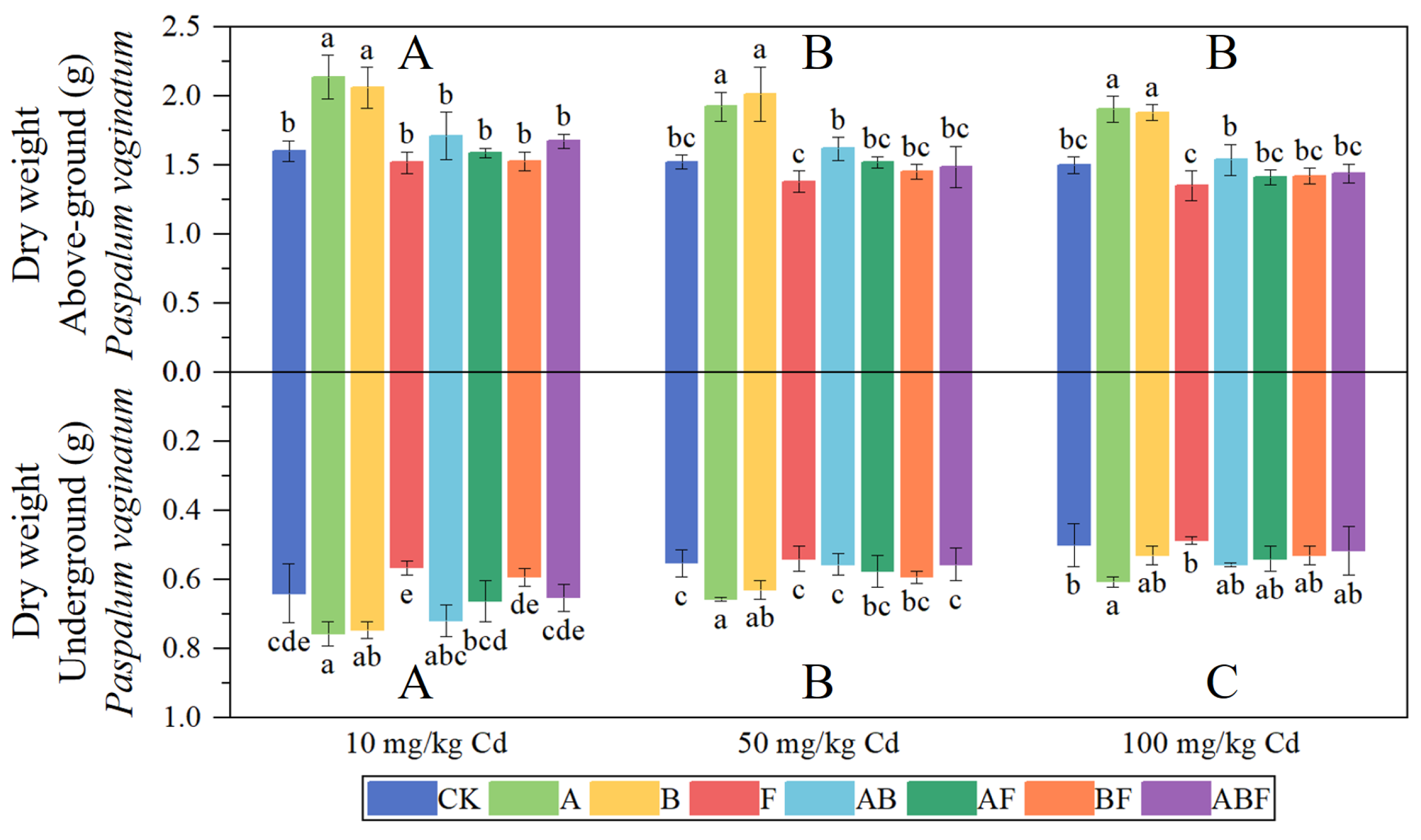
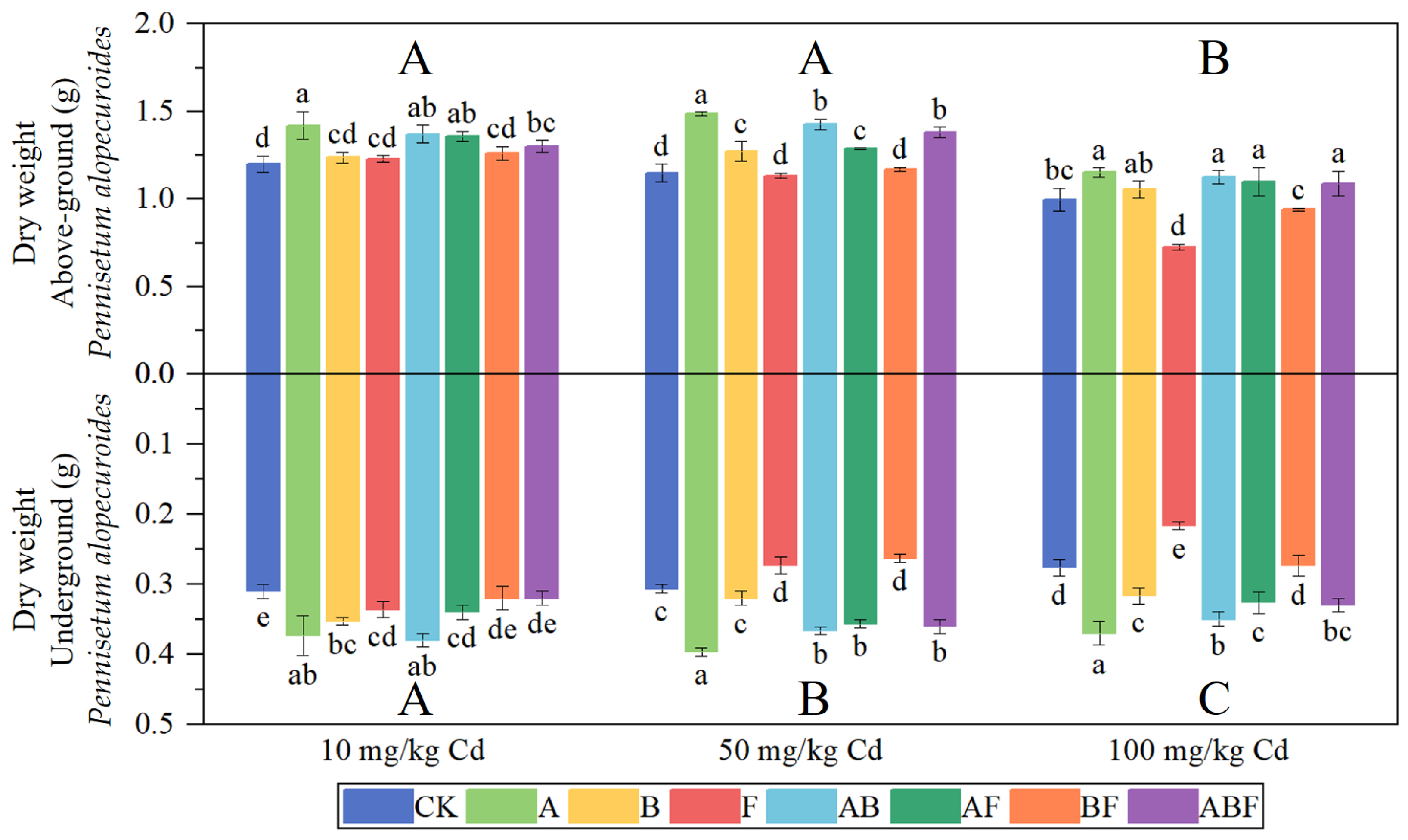
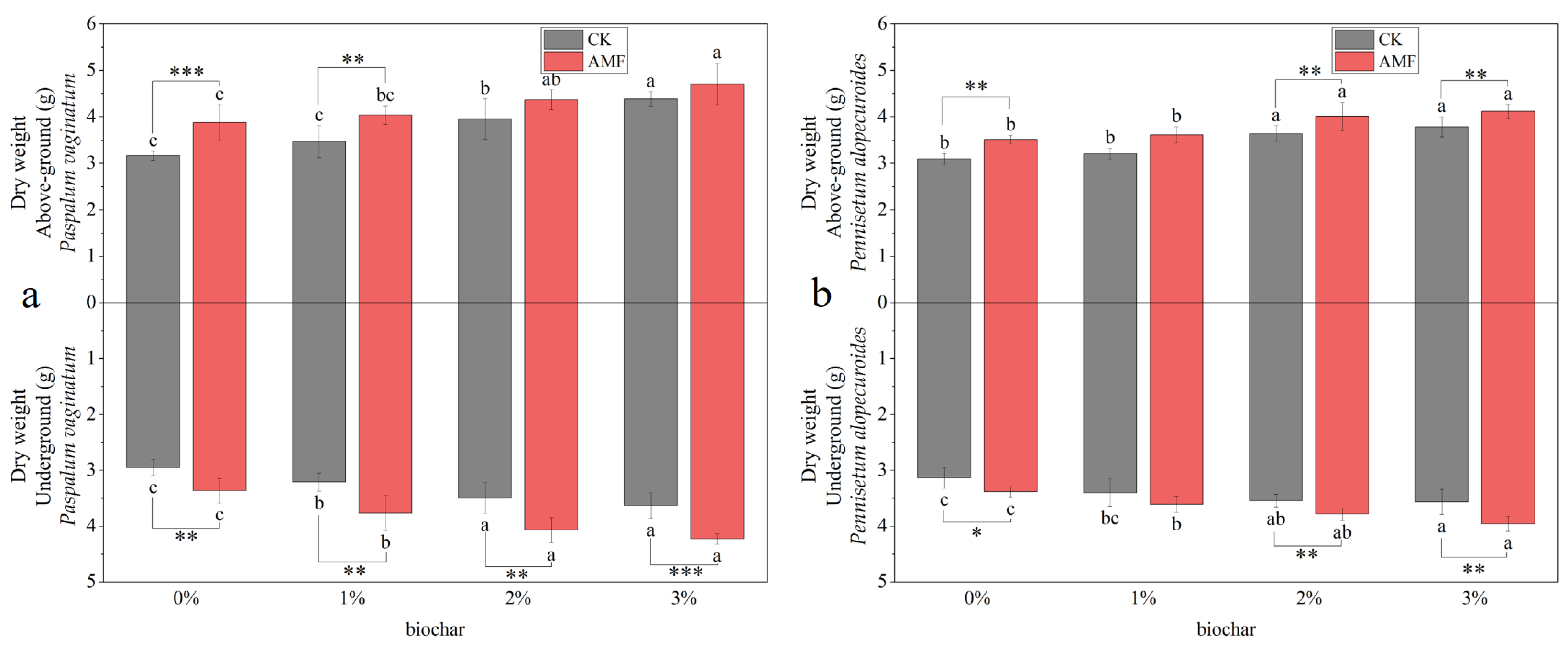
3.1.1. Biomass of Paspalum vaginatum and Pennisetum alopecuroides
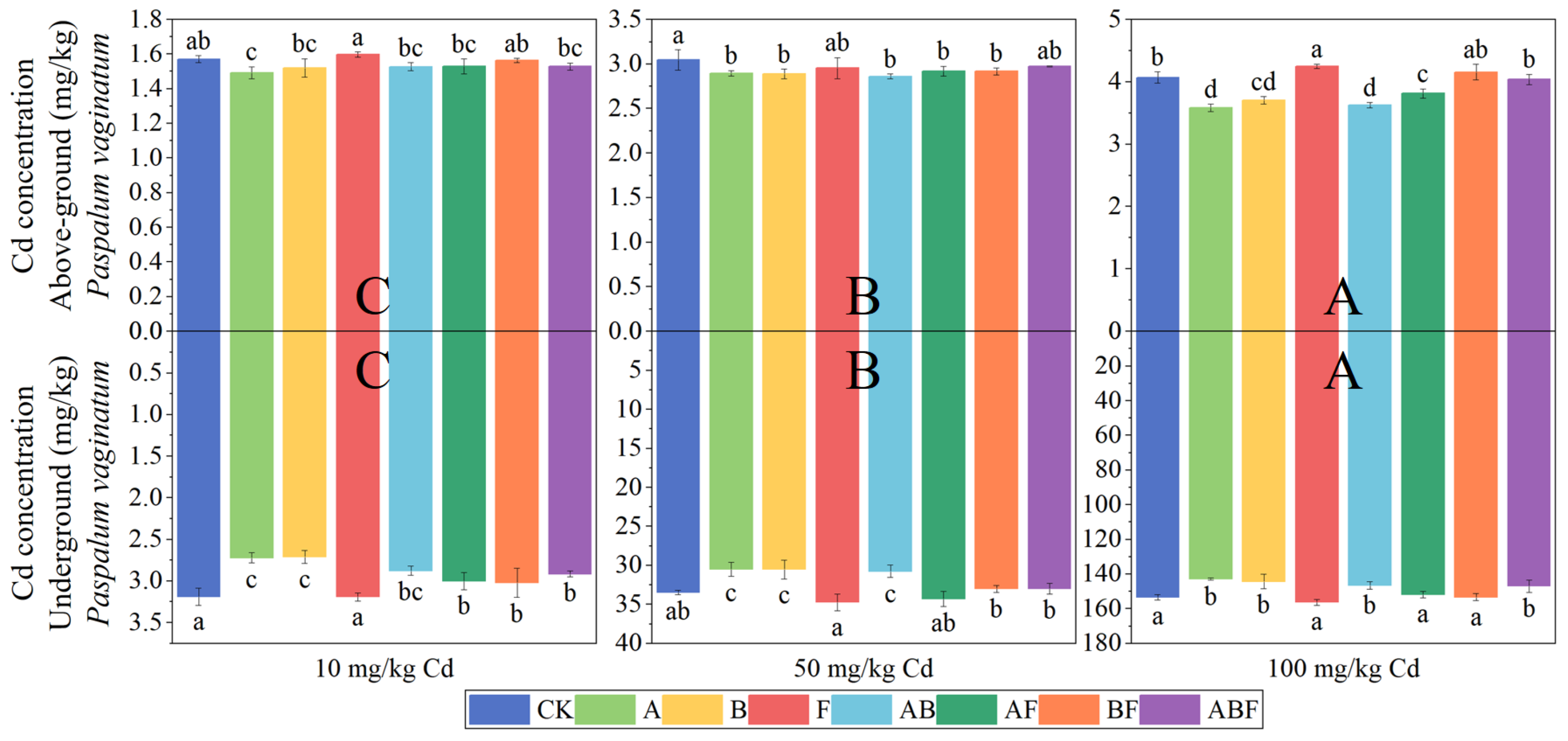
3.1.2. Cadmium Concentration in Plants
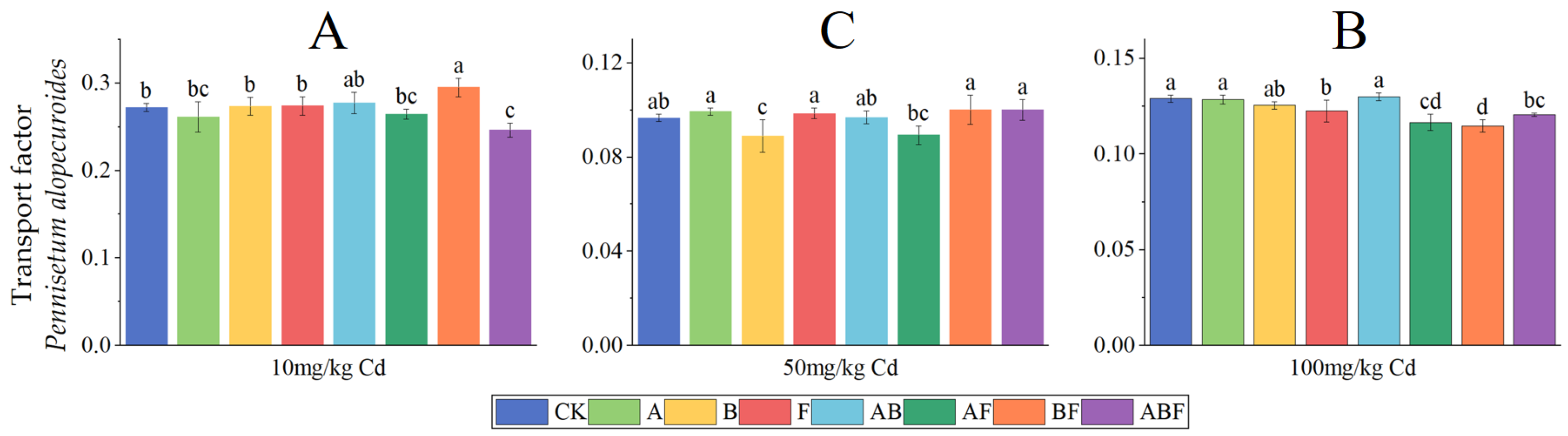
3.1.3. Transport Factors of Cadmium
3.2. The Second Stage: Microorganism and Biochar Effect Test Results
3.2.1. Biomass of Paspalum vaginatum and Pennisetum alopecuroides
3.2.2. Cadmium Concentration in Plants
3.2.3. Transport Factors of Cadmium
4. Discussion
4.1. Growth-Promoting Effect of Microorganisms
4.2. Effect of Biochar on Phytoremediation
4.3. Remediation Ability of Paspalum vaginatum and Pennisetum alopecuroides
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zulkernain, N.H.; Uvarajan, T.; Ng, C.C. Roles and significance of chelating agents for potentially toxic elements (PTEs) phytoremediation in soil: A review. J. Environ. Manag. 2023, 341, 117926. [Google Scholar] [CrossRef]
- Pan, L.-B.; Ma, J.; Wang, X.-L.; Hou, H. Heavy metals in soils from a typical county in Shanxi Province, China: Levels, sources and spatial distribution. Chemosphere 2016, 148, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Dai, M.; Yang, J.; Sun, L.; Tan, X.; Peng, C.; Ali, I.; Naz, I. A critical review on the phytoremediation of heavy metals from environment: Performance and challenges. Chemosphere 2022, 291, 132979. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Zeng, G.; Huang, D.; Chen, M.; Zhang, C.; Huang, C.; Wan, J. Remediation of contaminated soils by enhanced nanoscale zero valent iron. Environ. Res. 2018, 163, 217–227. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- Gough, H.L.; Dahl, A.L.; Nolan, M.A.; Gaillard, J.-F.; Stahl, D.A. Metal impacts on microbial biomass in the anoxic sediments of a contaminated lake. J. Geophys. Res. Biogeosci. 2008, 113, G02017. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Tsadilas, C.D.; Rinklebe, J. A review of the distribution coefficients of trace elements in soils: Influence of sorption system, element characteristics, and soil colloidal properties. Adv. Colloid Interface Sci. 2013, 201–202, 43–56. [Google Scholar] [CrossRef]
- Taha, M.M.; Mahdy-Abdallah, H.; Shahy, E.M.; Ibrahim, K.S.; Elserougy, S. Impact of occupational cadmium exposure on bone in sewage workers. Int. J. Occup. Environ. Health 2018, 24, 101–108. [Google Scholar] [CrossRef]
- Cristaldi, A.; Conti, G.O.; Jho, E.H.; Zuccarello, P.; Grasso, A.; Copat, C.; Ferrante, M. Phytoremediation of contaminated soils by heavy metals and PAHs. A brief review. Environ. Technol. Innov. 2017, 8, 309–326. [Google Scholar] [CrossRef]
- Hou, D.; Abir, A.T. Sustainability: A new imperative in contaminated land remediation. Environ. Sci. Policy 2014, 39, 25–34. [Google Scholar] [CrossRef]
- Wan, X.; Lei, M.; Chen, T. Cost–benefit calculation of phytoremediation technology for heavy-metal-contaminated soil. Sci. Total Environ. 2016, 563–564, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Liu, C.; Li, B.; Dong, Y. Trifolium repens L. regulated phytoremediation of heavy metal contaminated soil by promoting soil enzyme activities and beneficial rhizosphere associated microorganisms. J. Hazard. Mater. 2020, 402, 123829. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.H.D. Phytoremediation: Where do we go from here? Biocatal. Agric. Biotechnol. 2023, 50, 102721. [Google Scholar] [CrossRef]
- Lee, E.-H.; Eo, J.-K.; Ka, K.-H. Diversity of Arbuscular Mycorrhizal Fungi and Their Roles in Ecosystems. Mycobiology 2013, 41, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wu, S.; Wu, F.; Leung, H.M.; Lin, X.; Wong, M.H. Arbuscular mycorrhizal fungi enhance both absorption and stabilization of Cd by Alfred stonecrop (Sedum alfredii Hance) and perennial ryegrass (Lolium perenne L.) in a Cd-contaminated acidic soil. Chemosphere 2013, 93, 1359–1365. [Google Scholar] [CrossRef]
- Audet, P.; Charest, C. Dynamics of arbuscular mycorrhizal symbiosis in heavy metal phytoremediation: Meta-analytical and conceptual perspectives. Environ. Pollut. 2007, 147, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Waigi, M.G.; Sun, K.; Gao, Y. Sphingomonads in Microbe-Assisted Phytoremediation: Tackling Soil Pollution. Trends Biotechnol. 2017, 35, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.-L.; Zhu, Q.-Y.; Xu, P.-X.; Jing, Y.-X. The intercropping and arbuscular mycorrhizal fungus decrease Cd accumulation in upland rice and improve phytoremediation of Cd-contaminated soil by Sphagneticola calendulacea (L.) Pruski. J. Environ. Manag. 2021, 298, 113516. [Google Scholar] [CrossRef]
- Sharma, P.; Tripathi, S.; Chaturvedi, P.; Chaurasia, D.; Chandra, R. Newly isolated Bacillus sp. PS-6 assisted phytoremediation of heavy metals using Phragmites communis: Potential application in wastewater treatment. Bioresour. Technol. 2021, 320, 124353. [Google Scholar] [CrossRef]
- Ullah, A.; Heng, S.; Munis, M.F.H.; Fahad, S.; Yang, X. Phytoremediation of heavy metals assisted by plant growth promoting (PGP) bacteria: A review. Environ. Exp. Bot. 2015, 117, 28–40. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, K.; Zhan, W.; Huang, L.; Liu, Y.; Li, T.; Yang, Z.; Liao, Q.; Chen, R.; Zhang, C.; et al. Highly effective stabilization of Cd and Cu in two different soils and improvement of soil properties by multiple-modified biochar. Ecotoxicol. Environ. Saf. 2021, 207, 111294. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, L.H.; Peng, L.; Luo, S.; Zeng, Q.R. Phytoremediation of heavy metals under an oil crop rotation and treatment of biochar from contaminated biomass for safe use. Chemosphere 2020, 247, 125856. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, S.; Zhou, W. Enhanced phytoremediation of petroleum-contaminated soil by biochar and urea. J. Hazard. Mater. 2023, 453, 131404. [Google Scholar] [CrossRef]
- Simiele, M.; Lebrun, M.; Miard, F.; Trupiano, D.; Poupart, P.; Forestier, O.; Scippa, G.S.; Bourgerie, S.; Morabito, D. Assisted phytoremediation of a former mine soil using biochar and iron sulphate: Effects on as soil immobilization and accumulation in three Salicaceae species. Sci. Total Environ. 2019, 710, 136203. [Google Scholar] [CrossRef]
- He, L.; Zhong, H.; Liu, G.; Dai, Z.; Brookes, P.C.; Xu, J. Remediation of heavy metal contaminated soils by biochar: Mechanisms, potential risks and applications in China. Environ. Pollut. 2019, 252, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Singh, G.; Santal, A.R.; Singh, N.P. Omics approaches in effective selection and generation of potential plants for phytoremediation of heavy metal from contaminated resources. J. Environ. Manag. 2023, 336, 117730. [Google Scholar] [CrossRef]
- Lee, G.J.; Carrow, R.N.; Duncan, R.R. Growth and water relation responses to salinity stress in halophytic Paspalum vaginatum ecotypes. Sci. Hortic. 2005, 104, 221–236. [Google Scholar] [CrossRef]
- Wang, G.; Wang, L.; Ma, F.; You, Y.; Wang, Y.; Yang, D. Integration of earthworms and arbuscular mycorrhizal fungi into phytoremediation of cadmium-contaminated soil by Solanum nigrum L. J. Hazard. Mater. 2019, 389, 121873. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Gao, B.; Wu, P.; Chen, M.; He, C.; Zhang, X. Effects of microplastics on the phytoremediation of Cd, Pb, and Zn contaminated soils by Solanum photeinocarpum and Lantana camara. Environ. Res. 2023, 231, 116312. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Liu, Y.; Shi, B.; Yuan, H. Exogenous IAA improves the seedling growth of Syringa villosa via regulating the endogenous hormones and enhancing the photosynthesis. Sci. Hortic. 2023, 308, 111585. [Google Scholar] [CrossRef]
- Mnafgui, W.; Hajlaoui, H.; Rizzo, V.; Muratore, G.; Elleuch, A. Priming with EDTA, IAA and Fe Alleviates Pb Toxicity in Trigonella Foneum graecum L. growth: Phytochemicals and secondary metabolites. J. Biotechnol. 2022, 356, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, M.; Karuppusamy, I.; Alshiekheid, M.; Sabour, A.; Chi, N.T.L.; Pugazhendhi, A. Phytoremediation potential of Gossypium hirsutum on abandoned polluted chromium sludge soil with the amalgamation of Streptomyces tritici D5. Chemosphere 2022, 306, 135526. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Zhao, Y.; Zhou, R.; Lin, J.; Su, T.; Tong, H.; Wang, Z. Biodegradation of polybutylene adipate-co-terephthalate by Priestia megaterium, Pseudomonas mendocina, and Pseudomonas pseudoalcaligenes following incubation in the soil. Chemosphere 2022, 307, 135700. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Shen, J.; Zhang, H.; Zheng, C.; Wei, R.; Gao, Y.; Yang, L. Efficient nitrate removal by Pseudomonas mendocina GL6 immobilized on biochar. Bioresour. Technol. 2021, 320, 124324. [Google Scholar] [CrossRef] [PubMed]
- Durán-López, M.; Caroca-Cáceres, R.; Jahreis, K.; Narváez-Vera, M.; Ansaloni, R.; Cazar, M. The micorryzal fungi Ceratobasidium sp. and Sebacina vermifera promote seed germination and seedling development of the terrestrial orchid Epidendrum secundum Jacq. S. Afr. J. Bot. 2019, 125, 54–61. [Google Scholar] [CrossRef]
- Hietala, A.M.; Vahala, J.; Hantula, J. Molecular evidence suggests that Ceratobasidium bicorne has an anamorph known as a conifer pathogen. Mycol. Res. 2001, 105, 555–562. [Google Scholar] [CrossRef]
- Laurence, B.; Marc, S.A.; Michel, L. Phytoextraction of heavy metals by two Salicaceae clones in symbiosis with arbuscular mycorrhizal fungi during the second year of a field trial. Plant Soil. 2010, 332, 55–67. [Google Scholar]
- Hidri, R.; Mahmoud, O.M.-B.; Debez, A.; Abdelly, C.; Barea, J.-M.; Azcon, R. Modulation of C:N:P stoichiometry is involved in the effectiveness of a PGPR and AM fungus in increasing salt stress tolerance of Sulla carnosa Tunisian provenances. Appl. Soil Ecol. 2019, 143, 161–172. [Google Scholar] [CrossRef]
- Khan, A.Z.; Khan, S.; Khan, M.A.; Alam, M.; Ayaz, T. Biochar reduced the uptake of toxic heavy metals and their associated health risk via rice (Oryza sativa L). grown in Cr-Mn mine contaminated soils. Environ. Technol. Innov. 2020, 17, 100590. [Google Scholar] [CrossRef]
- Ibrahim, M.; Li, G.; Tang, Y.-T. Biochar effects acidic soil remediation and Brassica oleracea L. toxicity—A case study in subtropical area of China. Environ. Technol. Innov. 2021, 23, 101588. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Mosa, A.; Natasha; Jeyasundar, P.G.S.A.; Hassan, N.E.E.; Yang, X.; Antoniadis, V.; Li, R.; Wang, J.; Zhang, T.; et al. Pros and Cons of Biochar to Soil Potentially Toxic Element Mobilization and Phytoavailability: Environmental Implications. Earth Syst. Environ. 2023, 7, 321–345. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Bio/Technol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Wang, S.; Gao, B.; Zimmerman, A.R.; Li, Y.; Ma, L.; Harris, W.G.; Migliaccio, K.W. Physicochemical and sorptive properties of biochars derived from woody and herbaceous biomass. Chemosphere 2015, 134, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Yin, H.; Zhu, M.; Yu, X.; Shao, P.; Dang, Z. MgO-loaded nitrogen and phosphorus self-doped biochar: High-efficient adsorption of aquatic Cu2+, Cd2+, and Pb2+ and its remediation efficiency on heavy metal contaminated soil. Chemosphere 2022, 294, 133733. [Google Scholar] [CrossRef]
- Visconti, D.; Álvarez-Robles, M.J.; Fiorentino, N.; Fagnano, M.; Clemente, R. Use of Brassica juncea and Dactylis glomerata for the phytostabilization of mine soils amended with compost or biochar. Chemosphere 2020, 260, 127661. [Google Scholar] [CrossRef] [PubMed]
- Wen, E.; Yang, X.; Chen, H.; Shaheen, S.M.; Sarkar, B.; Xu, S.; Song, H.; Liang, Y.; Rinklebe, J.; Hou, D.; et al. Iron-modified biochar and water management regime-induced changes in plant growth, enzyme activities, and phytoavailability of arsenic, cadmium and lead in a paddy soil. J. Hazard. Mater. 2021, 407, 124344. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Peng, C.; Tariq, M.; Lin, S.; Wan, J.; Liang, W.; Zhang, W.; Zhang, L. Mechanistic insight and bifunctional study of a sulfide Fe3O4 coated biochar composite for efficient As(III) and Pb(II) immobilization in soils. Environ. Pollut. 2022, 293, 118587. [Google Scholar] [CrossRef] [PubMed]
- Dexter, F.; Edward, B.; Jeff, B.; Kathyrn, F. Evaluating Bermudagrass (Cynodon dactylon), Seashore paspalum (Paspalum vaginatum), and Weeping Lovegrass (Eragrostis curvula), as a Vegetative Cap for Industrial Brine Landform Stabilization and Phytoremediation. J. Plant Nutr. 2015, 38, 237–245. [Google Scholar]
- Chen, Y.; Chen, C.; Tan, Z.; Liu, J.; Zhuang, L.; Yang, Z.; Huang, B. Functional Identification and Characterization of Genes Cloned from Halophyte Paspalum vaginatum Conferring Salinity and Cadmium Tolerance. Front. Plant Sci. 2016, 7, 102. [Google Scholar] [PubMed]




| Cd Concentration | pH | Electric Conductivity | Ammoniacal Nitrogen | Nitrate Nitrogen | Available Phosphorous |
|---|---|---|---|---|---|
| 0.31 ± 0.06 (mg/kg) | 6.51 ± 0.17 | 65.26 ± 9.14 (μS/cm) | 14.37 ± 1.36 (mg/kg) | 11.33 ± 1.23 (mg/kg) | 17.58 ± 2.36 (mg/kg) |
| Index | Plant | Microorganism | Cadmium | Plant × Microorganism | Plant × Cadmium | Cadmium × Microorganism | Plant × Microorganism × Cadmium |
|---|---|---|---|---|---|---|---|
| Dry weight (aboveground) | 1105.098 <0.001 *** | 66.946 <0.001 *** | 104.935 <0.001 *** | 26.749 <0.001 *** | 21.024 <0.001 *** | 0.947 0.513 | 1.635 0.083 |
| Dry weight (underground) | 2533.946 <0.001 *** | 27.317 <0.001 *** | 83.807 <0.001 *** | 2.618 <0.05 * | 31.521 <0.001 *** | 1.221 0.273 | 3.205 <0.001 *** |
| Cd Plant (aboveground) | 29,275.762 <0.001 *** | 31.308 <0.001 *** | 18,345.585 <0.001 *** | 7.547 <0.001 *** | 6320.867 <0.001 *** | 5.658 <0.001 *** | 2.564 <0.01 ** |
| Cd Plant (underground) | 1509.490 <0.001 *** | 46.931 <0.001 *** | 71,657.740 <0.001 *** | 3.132 <0.01 ** | 9897.477 <0.001 *** | 116.628 <0.001 *** | 3.184 <0.001 *** |
| Transport factor | 1478.382 <0.001 *** | 7.169 <0.001 *** | 25,960.472 <0.001 *** | 7.820 <0.001 *** | 6539.869 <0.001 *** | 5.611 <0.001 *** | 7.179 <0.001 *** |
| Index | Plant | Biochar | AMF | Plant × Biochar | Plant × AMF | AMF × Biochar | Plant × Biochar × AMF |
|---|---|---|---|---|---|---|---|
| Dry weight (aboveground) | 52.303 <0.001 *** | 54.383 <0.001 *** | 73.934 <0.001 *** | 2.371 <0.077 | 21.024 <0.001 *** | 1.018 0.389 | 0.440 0.725 |
| Dry weight (underground) | 1.157 0.285 | 50.525 <0.001 *** | 104.858 <0.001 *** | 2.486 <0.067 | 31.521 <0.001 *** | 0.742 0.530 | 0.340 0.796 |
| Cd Plant (aboveground) | 2107.398 <0.001 *** | 65.384 <0.001 *** | 8.832 <0.001 *** | 0.08 0.971 | 6320.867 <0.001 *** | 0.365 0.778 | 0.048 0.986 |
| Cd Plant (underground) | 1247.556 <0.001 *** | 71.860 <0.001 *** | 13.292 <0.001 *** | 5.498 0.002 ** | 9897.477 <0.001 *** | 0.814 0.490 | 0.074 0.974 |
| Transport factor | 1.493 0.225 | 6.065 <0.001 *** | 0.920 0.340 | 1.686 0.177 | 6539.869 <0.001 *** | 0.416 0.742 | 0.057 0.982 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, J.; Chang, J.; Xie, J.; Yang, L.; Sheteiwy, M.S.; Moustafa, A.-R.A.; Zaghloul, M.S.; Ren, H. Microorganisms and Biochar Improve the Remediation Efficiency of Paspalum vaginatum and Pennisetum alopecuroides on Cadmium-Contaminated Soil. Toxics 2023, 11, 582. https://doi.org/10.3390/toxics11070582
Liang J, Chang J, Xie J, Yang L, Sheteiwy MS, Moustafa A-RA, Zaghloul MS, Ren H. Microorganisms and Biochar Improve the Remediation Efficiency of Paspalum vaginatum and Pennisetum alopecuroides on Cadmium-Contaminated Soil. Toxics. 2023; 11(7):582. https://doi.org/10.3390/toxics11070582
Chicago/Turabian StyleLiang, Jiahao, Jiechao Chang, Jiayao Xie, Liquan Yang, Mohamed S. Sheteiwy, Abdel-Raouf A. Moustafa, Mohamed S. Zaghloul, and Haiyan Ren. 2023. "Microorganisms and Biochar Improve the Remediation Efficiency of Paspalum vaginatum and Pennisetum alopecuroides on Cadmium-Contaminated Soil" Toxics 11, no. 7: 582. https://doi.org/10.3390/toxics11070582
APA StyleLiang, J., Chang, J., Xie, J., Yang, L., Sheteiwy, M. S., Moustafa, A.-R. A., Zaghloul, M. S., & Ren, H. (2023). Microorganisms and Biochar Improve the Remediation Efficiency of Paspalum vaginatum and Pennisetum alopecuroides on Cadmium-Contaminated Soil. Toxics, 11(7), 582. https://doi.org/10.3390/toxics11070582






