Anti-Oxidant, Anti-Mutagenic Activity and Safety Evaluation of Antrocin
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Material
2.2. Safety Evaluations
2.2.1. Ames Test
2.2.2. In Vitro Chromosomal Aberration Test
2.2.3. In Vivo Mammalian Erythrocyte Micronucleus Test
2.2.4. 28-Day Oral Toxicity Test
2.3. Anti-Oxidant Activity
2.3.1. Total Polyphenol Content
2.3.2. Ferric Reducing Ability of Plasma (FRAP)
- B = amount of ferrous ammonium sulfate from standard curve (nmol)
- D = dilution factor
- V = volume of sample added to the well (µL)
2.3.3. Trolox Equivalent Antioxidant Capacity (TEAC)
- Sa = the amount of sample (in nmol) read from the standard curve.
- Sv = the undiluted sample volume added to the well.
2.4. Antimutagenicity Test
- a = number of revertant colonies in presence of sample
- b = number of revertant colonies of positive control (without sample)
- c = spontaneous revertants
2.5. Statistical Analysis
3. Results
3.1. Safety Evaluations
3.1.1. Ames Test
3.1.2. In Vitro Chromosomal Aberration Test
3.1.3. In Vivo Mammalian Erythrocyte Micronucleus Test
3.1.4. 28-Day Oral Toxicity Test
3.2. Anti-Oxidant Activity
3.3. Antimutagenicity Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ozcan, A.; Ogun, M. Biochemistry of reactive oxygen and nitrogen species. In Basic Principles and Clinical Significance of Oxidative Stress; Gowder, S.J.T., Ed.; InTech: Rijeka, Croatia, 2015; Volume 3, pp. 37–58. [Google Scholar]
- Gautam, V.; Sharma, A.; Arora, S.; Bhardwaj, R.; Ahmad, A.; Ahamad, B.; Ahmad, P. In-vitro antioxidant, antimutagenic and cancer cell growth inhibition activities of Rhododendron arboreum leaves and flowers. Saudi J. Biol. Sci. 2020, 27, 1788–1796. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Li, P.C.; Konkimalla, V.S.B.; Kaina, B. From traditional Chinese medicine to rational cancer therapy. Trends Mol. Med. 2007, 13, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Normile, D. The new face of traditional Chinese medicine. Science 2003, 299, 188–190. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.H.; Yeh, S.C.; Tseng, H.C.; Siu, M.L.; Lee, K.T. Chemical quality evaluation of Antrodia cinnamomea fruiting bodies using phytomics similarity index analysis. J. Food Drug Anal. 2016, 24, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Kumar, K.S.; Liao, J.W.; Kuo, Y.H.; Wang, S.Y. Genotoxic, teratotoxic and oral toxic assessments of Antrodia cinnamomea health food product (Leader Deluxe Antrodia cinnamomea®). Toxicol. Rep. 2015, 2, 1409–1417. [Google Scholar] [CrossRef]
- De Silva, D.D.; Rapior, S.; Fons, F.; Bahkali, A.H.; Hyde, K.D. Medicinal mushrooms in supportive cancer therapies: An approach to anti-cancer effects and putative mechanisms of action. Fungal Divers. 2012, 55, 1–35. [Google Scholar] [CrossRef]
- Geethangili, M.; Tzeng, Y.M. Review of pharmacological effects of Antrodia camphorata and its bioactive compounds. Evid. Based Complement. Alternat. Med. 2011, 2011, 212641. [Google Scholar] [CrossRef]
- Chiang, H.C.; Wu, D.P.; Cherng, I.W.; Ueng, C.H. A sesquiterpene lactone, phenyl and biphenyl compounds from Antrodia cinnamomea. Phytochemistry 1995, 39, 613–616. [Google Scholar] [CrossRef]
- Li, F.Z.; Li, S.; Zhang, P.P.; Huang, Z.H.; Zhang, W.B.; Gong, J.; Yang, Z. A chiral pool approach for asymmetric syntheses of (−)-antrocin,(+)-asperolide C, and (−)-trans-ozic acid. Chem. Commun. 2016, 52, 12426–12429. [Google Scholar] [CrossRef]
- Shi, H.; Fang, L.; Tan, C.; Shi, L.; Zhang, W.; Li, C.C.; Luo, T.; Yang, Z. Total syntheses of drimane-type sesquiterpenoids enabled by a gold-catalyzed tandem reaction. J. Am. Chem. Soc. 2011, 133, 14944–14947. [Google Scholar] [CrossRef]
- Chiu, K.Y.; Wu, C.C.; Chia, C.H.; Hsu, S.L.; Tzeng, Y.M. Inhibition of growth, migration and invasion of human bladder cancer cells by antrocin, a sesquiterpene lactone isolated from Antrodia cinnamomea, and its molecular mechanisms. Cancer Lett. 2016, 373, 174–184. [Google Scholar] [CrossRef]
- Rao, Y.K.; Wu, A.T.; Geethangili, M.; Huang, M.T.; Chao, W.J.; Wu, C.H.; Deng, W.P.; Yeh, C.T.; Tzeng, Y.M. Identification of antrocin from Antrodia camphorata as a selective and novel class of small molecule inhibitor of Akt/mTOR signaling in metastatic breast cancer MDA-MB-231 cells. Chem. Res. Toxicol. 2011, 24, 238–245. [Google Scholar] [CrossRef]
- Yeh, C.T.; Huang, W.C.; Rao, Y.K.; Ye, M.; Lee, W.H.; Wang, L.S.; Tzeng, D.T.; Wu, C.H.; Shieh, Y.S.; Huang, C.Y.F. A sesquiterpene lactone antrocin from Antrodia camphorata negatively modulates JAK2/STAT3 signaling via microRNA let-7c and induces apoptosis in lung cancer cells. Carcinogenesis 2013, 34, 2918–2928. [Google Scholar] [CrossRef]
- Chen, Y.A.; Tzeng, D.T.; Huang, Y.P.; Lin, C.J.; Lo, U.G.; Wu, C.L.; Lin, H.; Hsieh, J.T.; Tang, C.H.; Lai, C.H. Antrocin sensitizes prostate cancer cells to radiotherapy through inhibiting PI3K/AKT and MAPK signaling pathways. Cancers 2018, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N.; Lee, F.D.; Durston, W.E. An improved bacterial test system for the detection and classification of mutagens and carcinogens. Proc. Natl. Acad. Sci. USA 1973, 70, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Mortelmans, K.; Zeiger, E. The Ames Salmonella/microsome mutagenicity assay. Mutat. Res.-Fundam. Mol. Mech. Mutag. 2000, 455, 29–60. [Google Scholar] [CrossRef] [PubMed]
- Registre, M.; Proudlock, R. The in vitro chromosome aberration test. In Genetic Toxicology Testing; Elsevier: Amsterdam, The Netherlands, 2016; pp. 207–267. [Google Scholar]
- OECD. Test No. 474: Mammalian Erythrocyte Micronucleus Test; OECD Publishing: Paris, France, 2016. [Google Scholar]
- OECD. OECD Guideline for Testing of Chemicals. Repeated Dose 28-Day Oral Toxicity in Rodents, Test. No. 407; OECD Publishing: Paris, France, 2008. [Google Scholar]
- Shackelford, C.; Long, G.; Wolf, J.; Okerberg, C.; Herbert, R. Qualitative and quantitative analysis of nonneoplastic lesions in toxicology studies. Toxicol. Pathol. 2002, 30, 93–96. [Google Scholar] [CrossRef]
- Gutiérrez-Pacheco, S.L.; Valenzuela-Melendres, M.; Hernández-Mendoza, A.; Burgos-Hernández, A.; Robles-Zepeda, R.E.; Peña-Ramos, E.A. Antimutagenic effect of an Asclepias subulata extract against heterocyclic aromatic amines commonly found in cooked meat and its heat stability. Food Chem. 2020, 322, 126725. [Google Scholar] [CrossRef]
- Negi, P.; Jayaprakasha, G.; Jena, B. Antioxidant and antimutagenic activities of pomegranate peel extracts. Food Chem. 2003, 80, 393–397. [Google Scholar] [CrossRef]
- Lo, C.; Chen, Y.; Lin, C.; Kumar, K. Genotoxicity, acute and sub-chronic toxicity studies of solid-state cultivated mycelial powder of Antrodia cinnamomea. ACT 2016, 1, 000105. [Google Scholar]
- Greenwood, S.K.; Hill, R.B.; Sun, J.T.; Armstrong, M.J.; Johnson, T.E.; Gara, J.P.; Galloway, S.M. Population doubling: A simple and more accurate estimation of cell growth suppression in the in vitro assay for chromosomal aberrations that reduces irrelevant positive results. Environ. Mol. Mutagen. 2004, 43, 36–44. [Google Scholar] [CrossRef]
- McInnes, E.F. Wistar and sprague-dawley rats. In Background Lesions in Laboratory Animals; Saunders Elsevier: Edinburgh, UK, 2011; pp. 17–36. [Google Scholar]
- Frazier, K.S.; Seely, J.C.; Hard, G.C.; Betton, G.; Burnett, R.; Nakatsuji, S.; Nishikawa, A.; Durchfeld-Meyer, B.; Bube, A. Proliferative and nonproliferative lesions of the rat and mouse urinary system. Toxicol. Pathol. 2012, 40, 14S–86S. [Google Scholar] [CrossRef] [PubMed]
- Thoolen, B.; Maronpot, R.R.; Harada, T.; Nyska, A.; Rousseaux, C.; Nolte, T.; Malarkey, D.E.; Kaufmann, W.; Küttler, K.; Deschl, U. Proliferative and nonproliferative lesions of the rat and mouse hepatobiliary system. Toxicol. Pathol. 2010, 38, 5S–81S. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.; Carter, C.; Lynch, M.; Lowinger, T.; Dumas, J.; Smith, R.A.; Schwartz, B.; Simantov, R.; Kelley, S. Discovery and development of sorafenib: A multikinase inhibitor for treating cancer. Nat. Rev. Drug Discov. 2006, 5, 835–844. [Google Scholar] [CrossRef] [PubMed]
- EMA. Nexavar: EPAR—Scientific Dicussion; EMA: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Pezzilli, R.; Corinaldesi, R.; Morselli-Labate, A.M. Tyrosine kinase inhibitors and acute pancreatitis. J. Pancreas 2010, 11, 291–293. [Google Scholar]
- Saadati, H.; Saif, M.W. Sorafenib-induced acute pancreatitis. J. Pancreas 2010, 11, 283–284. [Google Scholar]
- Gerber, H.P.; Vu, T.H.; Ryan, A.M.; Kowalski, J.; Werb, Z.; Ferrara, N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat. Med. 1999, 5, 623–628. [Google Scholar] [CrossRef]
- Hall, A.P.; Westwood, F.R.; Wadsworth, P.F. Review of the effects of anti-angiogenic compounds on the epiphyseal growth plate. Toxicol. Pathol. 2006, 34, 131–147. [Google Scholar] [CrossRef]
- Matejić, J.; Šarac, Z.; Ranđelović, V. Pharmacological activity of sesquiterpene lactones. Biotechnol. Biotechnol. Equip. 2010, 24, 95–100. [Google Scholar] [CrossRef]
- Chadwick, M.; Trewin, H.; Gawthrop, F.; Wagstaff, C. Sesquiterpenoids lactones: Benefits to plants and people. Int. J. Mol. Sci. 2013, 14, 12780–12805. [Google Scholar] [CrossRef]
- Ghantous, A.; Gali-Muhtasib, H.; Vuorela, H.; Saliba, N.A.; Darwiche, N. What made sesquiterpene lactones reach cancer clinical trials? Drug Discov. Today 2010, 15, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Youn, U.J.; Miklossy, G.; Chai, X.; Wongwiwatthananukit, S.; Toyama, O.; Songsak, T.; Turkson, J.; Chang, L.C. Bioactive sesquiterpene lactones and other compounds isolated from Vernonia cinerea. Fitoterapia 2014, 93, 194–200. [Google Scholar] [CrossRef] [PubMed]
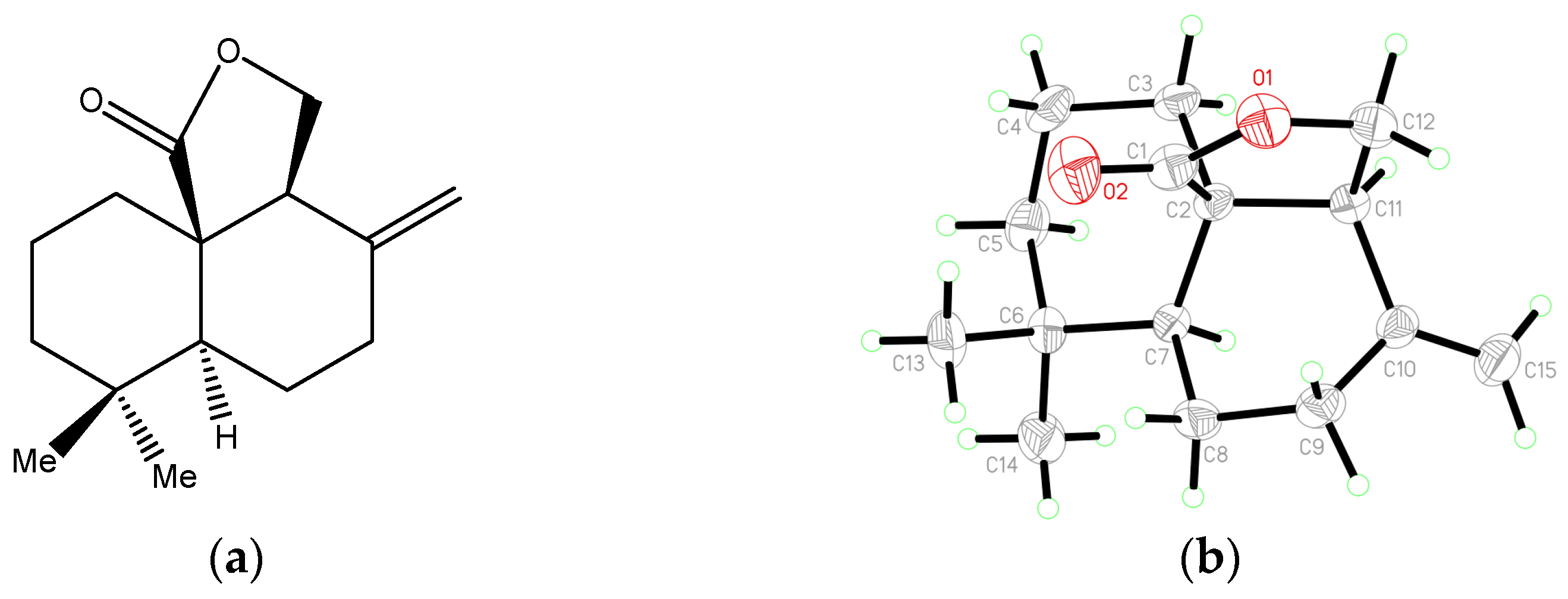
| Empirical formula | C15 H22O2 | |
| Formula weight | 234.32 | |
| Temperature | 200 (2) K | |
| Wavelength | 1.54178 Å | |
| Crystal system | Monoclinic | |
| Space group | P21 | |
| Unit cell dimensions | a = 6.1732 (2) Å | α = 90° |
| b = 15.9936 (4) Å | β = 114.0949(6)° | |
| c = 7.1673 (2) Å | γ = 90° | |
| Volume | 645.98 (3) Å3 | |
| Z | 2 | |
| Density (calculated) | 1.205 Mg/m3 | |
| Absorption coefficient | 0.610 mm−1 | |
| F(000) | 256 | |
| Crystal size | 0.185 × 0.167 × 0.122 mm3 | |
| Theta range for data collection | 8.756 to 74.972°. | |
| Index ranges | −7 ≤ h ≤ 6, −20 ≤ k ≤ 19, −8 ≤ l ≤ 8 | |
| Reflections collected | 4464 | |
| Independent reflections | 2615 [R(int) = 0.0209] | |
| Completeness to theta = 67.679° | 98.9% | |
| Absorption correction | Semi-empirical from equivalents | |
| Max. and min. transmission | 0.7539 and 0.6924 | |
| Refinement method | Full-matrix least-squares on F2 | |
| Data/restraints/parameters | 2615/1/156 | |
| Goodness-of-fit on F2 | 1.049 | |
| Final R indices [I > 2sigma(I)] | R1 = 0.0308, wR2 = 0.0834 | |
| R indices (all data) | R1 = 0.0310, wR2 = 0.0836 | |
| Absolute structure parameter | 0.03(5) | |
| Extinction coefficient | n/a | |
| Largest diff. peak and hole | 0.216 and −0.120 e.Å−3 |
| Group | Number of Revertant (Colony/Plate) | ||||
|---|---|---|---|---|---|
| TA98 | TA100 | TA1535 | TA102 | TA1537 | |
| Without S9 metabolic activation | |||||
| Negative 1 | 271.0 ± 7.8 3 | 168.3 ± 10.5 | 8.0 ± 1.4 | 271.0 ± 7.8 | 9.7 ± 2.9 |
| Positive 2 | 2004.3 ± 604.1 * | 2223.0 ± 53.2 * | 1514.3 ± 63.6 * | 2004.3 ± 604.1 * | 397.3 ± 20.7 * |
| Antrocin | |||||
| 0.003125 | 245.7 ± 10.4 | 170.7 ± 1.2 | 11.7 ± 2.4 | 245.7 ± 10.4 | 14.3 ± 2.6 |
| 0.00625 | 246.0 ± 16.4 | 169.3 ± 8.2 | 14.7 ± 4.0 | 246.0 ± 16.4 | 8.7 ± 0.5 |
| 0.0125 | 279.3 ± 4.5 | 166.7 ± 3.8 | 13.0 ± 2.9 | 279.3 ± 4.5 | 8.0 ± 0.8 |
| 0.025 | 278.3 ± 4.5 | 181.7 ± 5.7 | 10.7 ± 1.2 | 278.3 ± 4.5 | 13.0 ± 2.2 |
| 0.05 | 270.0 ± 11.2 | 166.7 ± 4.5 | 12.7 ± 2.9 | 270.0 ± 11.2 | 9.7 ± 0.9 |
| With S9 metabolic activation | |||||
| Negative | 35.3 ± 2.5 | 194.0 ± 5.9 | 16.3 ± 2.6 | 339.7 ± 9.6 | 9.3 ± 2.4 |
| Positive | 1881.3 ± 434.2 * | 2099.7 ± 145.5 * | 535.0 ± 138.6 * | 1532.0 ± 204.2 * | 269.0 ± 21.9 * |
| Antrocin | |||||
| 0.003125 | 33.7 ± 1.2 | 194.3 ± 5.4 | 17.3 ± 0.5 | 352.0 ± 8.5 | 11.7 ± 1.9 |
| 0.00625 | 32.0 ± 1.6 | 185.0 ± 6.5 | 15.7 ± 6.1 | 326.7 ± 5.9 | 6.7 ± 2.4 |
| 0.0125 | 31.3 ± 3.4 | 189.0 ± 5.1 | 15.3 ± 3.3 | 327.3 ± 5.7 | 6.0 ± 0.8 |
| 0.025 | 34.3 ± 3.3 | 197.0 ± 8.8 | 18.0 ± 1.6 | 327.3 ± 7.7 | 10.3 ± 2.1 |
| 0.05 | 36.0 ± 4.3 | 195.3 ± 2.9 | 14.0 ± 2.4 | 322.0 ± 6.2 | 6.0 ± 1.4 |
| Group | Total Aberrations | Frequency of Chromosomal Aberration (%) 1 |
|---|---|---|
| Without S9 (3 h) | ||
| Negative control | 17/300 | 5.7 ± 3.2 2 |
| Mitomycin C (2.5 μg/mL) | 44/300 | 14.7 ± 2.3 * |
| Antrocin (μg/mL) | ||
| 25 | 13/300 | 4.3 ± 3.8 |
| 50 | 13/300 | 4.3 ± 2.9 |
| 100 | 16/300 | 5.3 ± 2.1 |
| With S9 (3 h) | ||
| Negative control | 17/300 | 5.7 ± 0.6 |
| Cyclophosphamide (25 μg/mL) | 46/300 | 15.3 ± 2.3 * |
| Antrocin (μg/mL) | ||
| 25 | 16/300 | 5.3 ± 1.2 |
| 50 | 19/300 | 6.3 ± 2.1 |
| 100 | 19/300 | 6.3 ± 2.1 |
| Without S9 (19 h) | ||
| Negative control | 28/300 | 9.3 ± 1.2 |
| Mitomycin C (2.5 μg/mL) | 86/300 | 32.0 ± 6.0 * |
| Antrocin (μg/mL) | ||
| 25 | 28/300 | 9.3 ± 1.2 |
| 50 | 31/300 | 10.3 ± 2.5 |
| 100 | 22/300 | 7.3 ± 1.5 |
| Group/ | Dose (mg/kg) | RETs/1000RBCs (‰) | Mn-RETs/1000RETs (‰) |
|---|---|---|---|
| Intervals | |||
| Male | |||
| 48 h | |||
| NC 1 | 0 | 19.1 ± 5.2 2 | 2.6 ± 0.8 |
| PC | 60 | 3.6 ± 2.1 * | 22.9 ± 12.2 * |
| Antrocin | 100 | 20.4 ± 2.4 | 2.2 ± 0.9 |
| 125 | 20.8 ± 2.5 | 1.8 ± 0.8 | |
| 250 | 18.8 ± 1.9 | 1.5 ± 0.2 | |
| 72 h | |||
| NC | 0 | 23.7 ± 5.2 | 2.2 ± 0.3 |
| PC | 60 | 3.9 ± 2.1 * | 6.8 ± 3.7 * |
| Antrocin | 100 | 31.0 ± 6.1 | 3.0 ± 1.0 |
| 125 | 28.9 ± 5.0 | 2.1 ± 0.5 | |
| 250 | 26.5 ± 4.6 | 2.2 ± 0.5 |
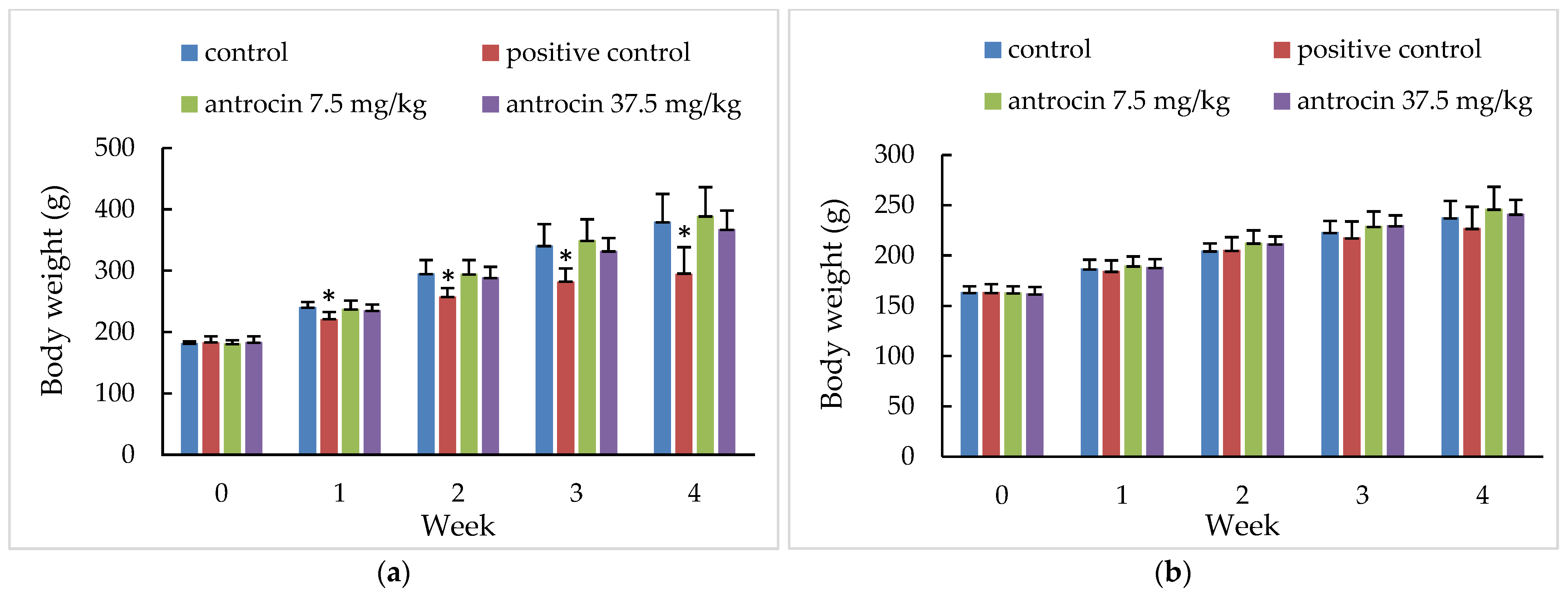
| Sex/Group | Body Weight/Body Weight Gain (g) | ||||
|---|---|---|---|---|---|
| 0-Day | 7-Day 1 | 14-Day 2 | 21-Day 3 | 28-Day 4 | |
| Male | |||||
| NC 5 | 181.8 ± 3.2 6 | 240.8 ± 8.3 | 295.4 ± 22.0 | 341.1 ± 35.0 | 379.8 ± 45.5 |
| 59.1 ± 8.7 | 54.5 ± 14.5 | 45.7 ± 13.7 | 38.7 ± 11.0 | ||
| Sorafenib | |||||
| 7.5 mg/kg | 183.0 ± 9.8 | 221.3 ± 11.8 | 257.5 ± 14.5 | 282.1 ± 21.7 | 295.2 ± 43.3 |
| 38.2 ± 3.7 * | 36.2 ± 7.4 * | 24.6 ± 11.7 * | 13.1 ± 27.7 | ||
| Antrocin | |||||
| 7.5 mg/kg | 181.4 ± 5.1 | 237.9 ± 13.6 | 295.0 ± 22.5 | 349.5 ± 34.3 | 389.6 ± 46.6 |
| 56.5 ± 10.1 | 57.1 ± 8.9 | 54.5 ± 12.4 | 40.1 ± 12.5 | ||
| 37.5 mg/kg | 183.9 ± 9.0 | 235.6 ± 9.6 | 289.0 ± 17.3 | 332.3 ± 21.0 | 367.6 ± 30.6 |
| 51.8 ± 4.7 | 53.4 ± 13.9 | 43.2 ± 7.7 | 35.3 ± 12.9 | ||
| Female | |||||
| NC | 163.7 ± 6.0 | 187.1 ± 8.7 | 205.0 ± 7.1 | 223.4 ± 11.0 | 237.9 ± 16.5 |
| 23.5 ± 5.7 | 17.8 ± 4.1 | 18.4 ± 4.0 | 14.5 ± 6.0 | ||
| PC | |||||
| 7.5 mg/kg | 163.7 ± 7.8 | 184.7 ± 10.4 | 205.6 ± 12.5 | 217.9 ± 16.0 | 227.3 ± 21.1 |
| 21.0 ± 5.6 | 20.9 ± 4.1 | 12.3 ± 5.1 | 9.4 ± 5.9 | ||
| Antrocin | |||||
| 7.5 mg/kg | 163.3 ± 6.3 | 190.0 ± 9.0 | 212.9 ± 12.1 | 229.3 ± 14.6 | 246.6 ± 21.7 |
| 26.7 ± 5.6 | 22.8 ± 6.0 | 16.4 ± 5.2 | 17.3 ± 7.9 | ||
| 37.5 mg/kg | 162.4 ± 6.3 | 188.5 ± 7.9 | 212.0 ± 7.0 | 230.1 ± 9.7 | 241.7 ± 13.6 |
| 26.1 ± 4.1 | 23.4 ± 3.6 | 18.1 ± 5.0 | 11.6 ± 6.3 | ||
| Group | NC 2 | Sorafenib 7.5 mg/kg | Antrocin 7.5 mg/kg | Antrocin 37.5 mg/kg | NC 2 | Sorafenib 7.5 mg/kg | Antrocin 7.5 mg/kg | Antrocin 37.5 mg/kg | |
|---|---|---|---|---|---|---|---|---|---|
| Sex | Male | Female | |||||||
| RBC 1 | (106/µL) | 7.4 ± 0.3 3 | 8.4 ± 0.5 * | 7.7 ± 0.1 | 8.1 ± 0.6 | 7.9 ± 0.5 3 | 7.7 ± 0.4 | 7.5 ± 0.6 | 8.0 ± 0.4 |
| HGB | (g/dL) | 14.7 ± 0.8 | 16.4 ± 1.0 * | 15.1 ± 0.4 | 15.7 ± 0.9 | 15.0 ± 0.8 | 15.5 ± 0.6 | 14.7 ± 1.2 | 15.8 ± 0.5 |
| HCT | (%) | 44.6 ± 2.3 | 49.8 ± 2.3 * | 45.8 ± 1.2 | 48.3 ± 3.2 | 46.6 ± 2.6 | 47.7 ± 1.7 | 45.0 ± 3.7 | 48.4 ± 2.0 |
| MCV | (fL) | 59.8 ± 1.0 | 59.2 ± 0.8 | 59.8 ± 1.5 | 59.8 ± 1.3 | 58.7 ± 1.1 | 61.8 ± 1.2 * | 59.7 ± 1.7 | 60.6 ± 1.0 * |
| MCH | (pg) | 19.8 ± 0.4 | 19.5 ± 0.3 | 19.7 ± 0.5 | 19.4 ± 0.6 | 18.9 ± 0.3 | 20.0 ± 0.5 * | 19.5 ± 0.8 | 19.8 ± 0.5 * |
| MCHC | (g/dL) | 33.1 ± 0.5 | 33.0 ± 0.7 | 32.9 ± 0.3 | 32.4 ± 0.6 | 32.2 ± 0.6 | 32.4 ± 0.8 | 32.7 ± 0.3 | 32.7 ± 0.4 |
| PLT | (103/µL) | 940.6 ± 272.7 | 874.0 ± 307.7 | 1235.2 ± 20.9 * | 1372.2 ± 161.8 * | 1166.8 ± 128.0 | 754.2 ± 238.6 * | 606.0 ± 430.1 * | 1065.6 ± 199.8 |
| WBC | (103/µL) | 4.0 ± 2.2 3 | 6.0 ± 2.2 | 5.3 ± 2.0 | 6.4 ± 1.1 | 7.5 ± 4.5 | 7.1 ± 1.1 | 8.7 ± 3.4 | 7.8 ± 4.5 |
| NEUT | (%) | 22.3 ± 4.0 | 35.4 ± 12.0 | 18.9 ± 3.1 | 13.6 ± 3.7 * | 12.9 ± 4.4 | 17.8 ± 4.6 | 9.6 ± 3.3 | 10.9 ± 3.4 |
| LYMPH | (%) | 70.1 ± 2.8 | 59.0 ± 10.7 | 77.2 ± 1.7 * | 83.9 ± 3.9 * | 83.0 ± 4.9 | 77.2 ± 5.7 | 86.1 ± 5.4 | 84.5 ± 4.1 |
| MONO | (%) | 2.9 ± 2.0 | 0.9 ± 0.5 | 2.7 ± 2.4 | 1.8 ± 1.1 | 2.9 ± 2.1 | 3.1 ± 1.7 | 2.3 ± 1.1 | 3.2 ± 2.0 |
| EOSIN | (%) | 4.6 ± 4.8 | 0.1 ± 0.1 | 1.2 ± 0.3 | 0.6 ± 0.4 | 1.1 ± 0.3 | 1.8 ± 1.6 | 2.1 ± 1.7 | 1.2 ± 0.3 |
| BASO | (%) | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.0 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.0 ± 0.1 | 0.2 ± 0.2 |
| Group | NC 2 | Sorafenib 7.5 mg/kg | Antrocin 7.5 mg/kg | Antrocin 37.5 mg/kg | NC 2 | Sorafenib 7.5 mg/kg | Antrocin 7.5 mg/kg | Antrocin 37.5 mg/kg | |
|---|---|---|---|---|---|---|---|---|---|
| Sex | Male | Female | |||||||
| AST 1 | (U/L) | 61.0 ± 9.6 3 | 106.2 ± 2.4 * | 61.4 ± 4.3 | 60.2 ± 3.3 | 74.8 ± 6.1 3 | 94.8 ± 6.4 * | 58.0 ± 8.5 * | 72.6 ± 7.4 |
| ALT | (U/L) | 27.4 ± 4.6 | 84.8 ± 13.7 * | 28.2 ± 3.1 | 27.6 ± 2.1 | 31.0 ± 8.3 | 69.0 ± 7.6 * | 24.2 ± 1.9 | 32.6 ± 6.2 |
| ALP | (U/L) | 257.2 ± 39.2 | 261.0 ± 148.9 | 238.4 ± 43.9 | 256.8 ± 44.0 | 128.2 ± 25.9 | 147.6 ± 53.2 | 147.4 ± 27.6 | 116.4 ± 35.7 |
| CK | (U/L) | 173.2 ± 37.7 | 229.2 ± 49.7 | 185.6 ± 96.1 | 156.4 ± 31.1 | 109.0 ± 15.2 | 137.2 ± 16.7 * | 100.2 ± 30.8 | 129.8 ± 59.5 |
| BUN | (mg/dL) | 11.8 ± 1.3 | 14.8 ± 1.8 * | 11.2 ± 1.3 | 11.6 ± 1.8 | 12.4 ± 2.2 | 14.0 ± 1.2 | 12.0 ± 1.0 | 11.0 ± 0.7 |
| Creatinine | (mg/dL) | 0.4 ± 0.0 | 0.4 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.0 | 0.3 ± 0.1 |
| Cholesterol | (mg/dL) | 43.4 ± 9.8 3 | 100.8 ± 13.9 * | 41.2 ± 6.3 | 44.0 ± 2.5 | 41.2 ± 8.6 | 76.2 ± 7.0 * | 51.2 ± 9.8 | 47.0 ± 11.8 |
| Amylase | (U/L) | 1994.2 ± 191.5 | 1432.0 ± 348.8 * | 1931.0 ± 241.1 | 2016.4 ± 289.7 | 1311.8 ± 143.3 | 1076.4 ± 197.5 | 1280.6 ± 111.3 | 1240.4 ± 239.9 |
| Glucose | (mg/dL) | 212.4 ± 37.6 | 176.6 ± 19.4 | 178.0 ± 25.7 | 178.0 ± 14.5 | 179.8 ± 19.9 | 143.0 ± 19.1 * | 182.4 ± 42.9 | 138.2 ± 21.7 * |
| GGT | (U/L) | <1 | <1 | <1 | <1 | 1.6 | <1 | 1–2 | 1–2 |
| LDH | (U/L) | 164.2 ± 24.6 | 229.6 ± 108.3 | 219.6 ± 139.5 | 109.8 ± 33.5 * | 82.4 ± 23.4 | 81.8 ± 23.8 | 92.4 ± 73.8 | 125.4 ± 96.2 |
| TB | (mg/dL) | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| UA | (mg/dL) | 2.0 ± 0.6 | 1.6 ± 0.4 | 1.5 ± 0.1 | 1.6 ± 0.3 | 1.5 ± 0.9 | <0.9 | 0.9 ± 0.1 | 1.3 ± 0.6 |
| Globulin | (g/dL) | 1.9 ± 0.1 3 | 1.6 ± 0.3 | 1.9 ± 0.1 | 2.0 ± 0.2 | 2.1 ± 0.3 | 2.0 ± 0.2 | 1.9 ± 0.1 | 2.2 ± 0.1 |
| Albumin | (g/dL) | 3.5 ± 0.1 | 3.1 ± 0.4 | 3.4 ± 0.1 | 3.5 ± 0.2 | 3.7 ± 0.2 | 3.5 ± 0.3 | 3.7 ± 0.2 | 3.8 ± 0.2 |
| A/G | 1.8 ± 0.1 | 1.9 ± 0.3 | 1.8 ± 0.1 | 1.8 ± 0.1 | 1.8 ± 0.1 | 1.8 ± 0.1 | 1.9 ± 0.2 | 1.7 ± 0.1 | |
| HDL-C | (mg/dL) | 12.8 ± 1.3 | 25.2 ± 1.5 * | 12.8 ± 1.5 | 15.2 ± 2.8 | 12.8 ± 3.3 | 23.2 ± 1.9 * | 16.0 ± 3.4 | 14.6 ± 3.8 |
| TP | (mg/dL) | 5.4 ± 0.1 | 4.7 ± 0.7 | 5.3 ± 0.2 | 5.5 ± 0.4 | 5.8 ± 0.5 | 5.5 ± 0.4 | 5.6 ± 0.2 | 6.0 ± 0.3 |
| TG | (mg/dL) | 32.0 ± 7.1 | 181.4 ± 202.0 | 33.0 ± 8.1 | 46.2 ± 8.3 * | 27.2 ± 2.9 | 59.6 ± 23.4 * | 32.8 ± 5.2 | 27.2 ± 8.1 |
| Ca2+ | (mg/dL) | 9.8 ± 0.4 2 | 8.9 ± 0.4 * | 9.7 ± 0.2 | 9.9 ± 0.4 | 10.0 ± 0.3 | 9.4 ± 0.6 | 9.9 ± 0.1 | 10.2 ± 0.2 |
| Cl− | (mEq/dL) | 105.0 ± 4.5 | 103.6 ± 2.3 | 106.4 ± 1.3 | 104.6 ± 2.1 | 103.8 ± 1.5 | 103.2 ± 1.6 | 105.6 ± 0.5 * | 104.2 ± 1.3 |
| K+ | (mEq/dL) | 7.6 ± 1.0 | 7.6 ± 0.7 | 6.5 ± 0.3 * | 6.4 ± 0.6 | 5.2 ± 0.4 | 5.1 ± 0.1 | 5.2 ± 0.2 | 5.0 ± 0.7 |
| Mg2+ | (mg/L) | 2.1 ± 0.3 | 2.2 ± 0.2 | 2.0 ± 0.1 | 2.1 ± 0.2 | 2.4 ± 0.2 | 2.3 ± 0.2 | 2.1 ± 0.1 * | 2.4 ± 0.3 |
| Na+ | (mEq/dL) | 140.6 ± 1.1 | 138.6 ± 0.9 * | 141.8 ± 0.8 | 141.4 ± 2.3 | 141.2 ± 1.1 | 140.4 ± 2.1 | 139.2 ± 1.6 | 142.6 ± 1.1 |
| Phosphate | (mg/dL) | 8.7 ± 1.1 | 7.1 ± 1.5 | 9.1 ± 0.9 | 9.6 ± 1.6 | 9.3 ± 0.7 | 7.3 ± 1.1 * | 8.5 ± 1.0 | 8.5 ± 0.5 |
| Sex/Group | Brain (g) | Heart (g) | Thymus (g) | Liver (g) | Kidney (g) | Adrenal (g) | Spleen (g) | Testis/Ovary (g) |
|---|---|---|---|---|---|---|---|---|
| Male | ||||||||
| NC 1 | 2.1 ± 0.1 2 | 1.3 ± 0.1 | 0.5 ± 0.1 | 12.1 ± 2.2 | 3.0 ± 0.5 | 0.05 ± 0.01 | 0.6 ± 0.1 | 3.2 ± 0.4 |
| Sorafenib 7.5 mg/kg | 2.0 ± 0.1 | 0.9 ± 0.2 * | 0.4 ± 0.3 | 7.9 ± 1.4 * | 2.1 ± 0.4 * | 0.05 ± 0.01 | 0.4 ± 0.1 * | 2.7 ± 0.2 * |
| Antrocin | ||||||||
| 7.5 mg/kg | 2.1 ± 0.2 | 1.3 ± 0.2 | 0.6 ± 0.1 | 12.2 ± 1.9 | 2.8 ± 0.4 | 0.05 ± 0.01 | 0.6 ± 0.1 | 3.1 ± 0.2 |
| 37.5 mg/kg | 2.0 ± 0.1 | 1.3 ± 0.2 | 0.4 ± 0.1 | 11.1 ± 1.2 | 2.7 ± 0.2 | 0.06 ± 0.00 | 0.6 ± 0.1 | 3.2 ± 0.2 |
| Female | ||||||||
| NC | 1.9 ± 0.2 | 0.8 ± 0.1 | 0.4 ± 0.1 | 6.8 ± 0.4 | 1.6 ± 0.1 | 0.06 ± 0.01 | 0.5 ± 0.1 | 0.10 ± 0.02 |
| Sorafenib 7.5 mg/kg | 2.0 ± 0.1 | 0.7 ± 0.0 | 0.5 ± 0.1 | 7.3 ± 0.1* | 1.7 ± 0.1 | 0.06 ± 0.01 | 0.4 ± 0.0 | 0.09 ± 0.01 |
| Antrocin | ||||||||
| 7.5 mg/kg | 1.9 ± 0.1 | 0.9 ± 0.1 | 0.4 ± 0.1 | 8.5 ± 0.7* | 1.8 ± 0.1* | 0.05 ± 0.01 | 0.4 ± 0.1 | 0.07 ± 0.02 |
| 37.5 mg/kg | 2.0 ± 0.1 | 1.0 ± 0.1 | 0.4 ± 0.1 | 7.8 ± 0.5* | 1.7 ± 0.1 | 0.05 ± 0.01 | 0.4 ± 0.0 | 0.10 ± 0.01 |
| Components | Total Phenolic Contents (mM Catechin Equivalents) | Ferric Reducing Ability of Plasma (mM Ferrous Equivalent) | Trolox Equivalent Antioxidant Capacity (mM Trolox Equivalents) |
|---|---|---|---|
| Antrocin | 0.016 ± 0.000 1 | 0.218 ± 0.004 | 0.508 ± 0.003 |
| Group | Number of Revertant (Colony/Plate) | |
|---|---|---|
| TA98 | TA1535 | |
| Without S9 metabolic activation | ||
| Negative 1 | 20.3 ± 5.7 3 | 10.7 ± 2.5 |
| Positive 2 | 117.0 ± 7.2 * | 1313.0 ± 42.0 * |
| Antrocin | ||
| 0.0125 | 83.0 ± 3.6 (35.17%) | 1255.7 ± 47.7 (4.40%) |
| 0.025 | 67.7 ± 2.5 (51.03%) | 1095.0 ± 28.0 (16.74%) |
| 0.05 | 21.7 ± 1.5 (98.62%) | 977.0 ± 18.1 (25.80%) |
| With S9 metabolic activation | ||
| Negative | 22.3 ± 4.2 | 11.7 ± 2.1 |
| Positive | 6055.0 ± 661.0 * | 270.3 ± 7.5 * |
| Antrocin | ||
| 0.0125 | 5680.3 ± 640.7 (6.21%) | 248.0 ± 9.5 (8.63%) |
| 0.025 | 4959.0 ± 739.1 (18.17%) | 217.3 ± 10.2 (20.49%) |
| 0.05 | 4024.0 ± 251.1 (33.67%) | 184.3 ± 3.8 (33.25%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Y.-H.; Wu, J.-S.; Dai, Y.-Z.; Chen, Y.-T.; Lin, Y.-X.; Tzeng, Y.-M.; Liao, J.-W. Anti-Oxidant, Anti-Mutagenic Activity and Safety Evaluation of Antrocin. Toxics 2023, 11, 547. https://doi.org/10.3390/toxics11060547
Su Y-H, Wu J-S, Dai Y-Z, Chen Y-T, Lin Y-X, Tzeng Y-M, Liao J-W. Anti-Oxidant, Anti-Mutagenic Activity and Safety Evaluation of Antrocin. Toxics. 2023; 11(6):547. https://doi.org/10.3390/toxics11060547
Chicago/Turabian StyleSu, Yi-Hui, Jia-Shuan Wu, Yan-Zhen Dai, Yng-Tay Chen, Yan-Xiu Lin, Yew-Min Tzeng, and Jiunn-Wang Liao. 2023. "Anti-Oxidant, Anti-Mutagenic Activity and Safety Evaluation of Antrocin" Toxics 11, no. 6: 547. https://doi.org/10.3390/toxics11060547
APA StyleSu, Y.-H., Wu, J.-S., Dai, Y.-Z., Chen, Y.-T., Lin, Y.-X., Tzeng, Y.-M., & Liao, J.-W. (2023). Anti-Oxidant, Anti-Mutagenic Activity and Safety Evaluation of Antrocin. Toxics, 11(6), 547. https://doi.org/10.3390/toxics11060547







