Exposure to Methylmercury at Juvenile Stage Worsens Autism-like Symptoms in Adult BTBR T+tf/J Mice Due to Lack of Nuclear Factor Erythroid 2-Related Factor 2 Signaling Upregulation in Periphery and Brain
Abstract
1. Introduction
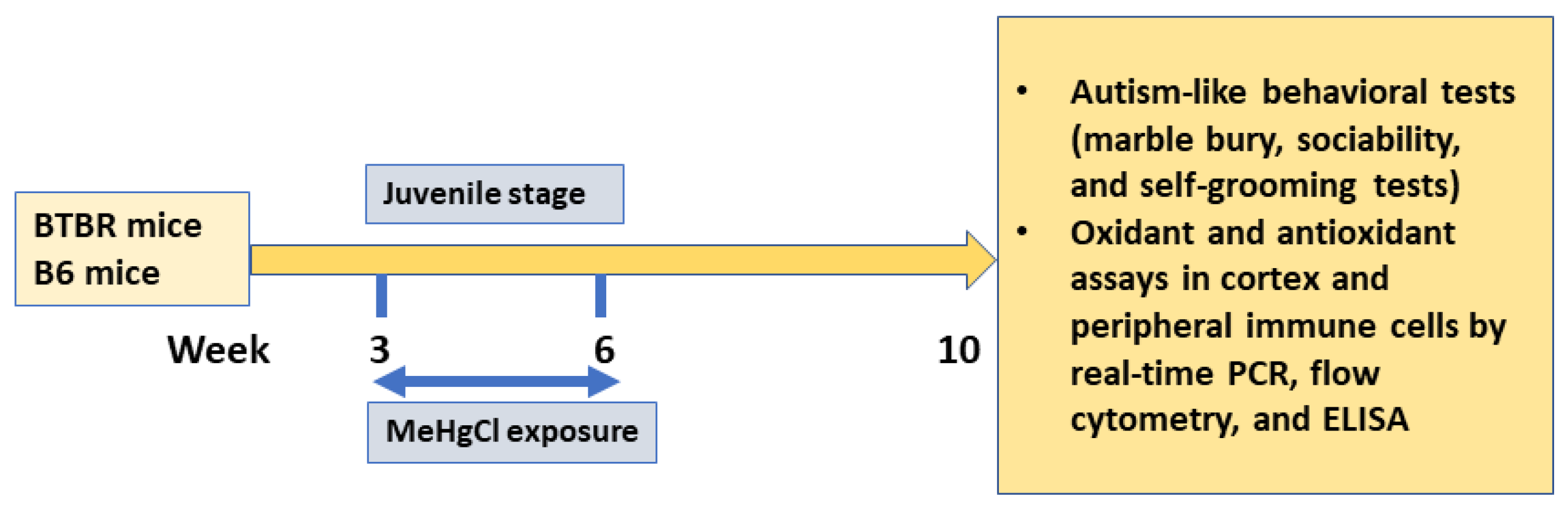
2. Materials and Methods
2.1. Animal Model
2.2. Drug Administration
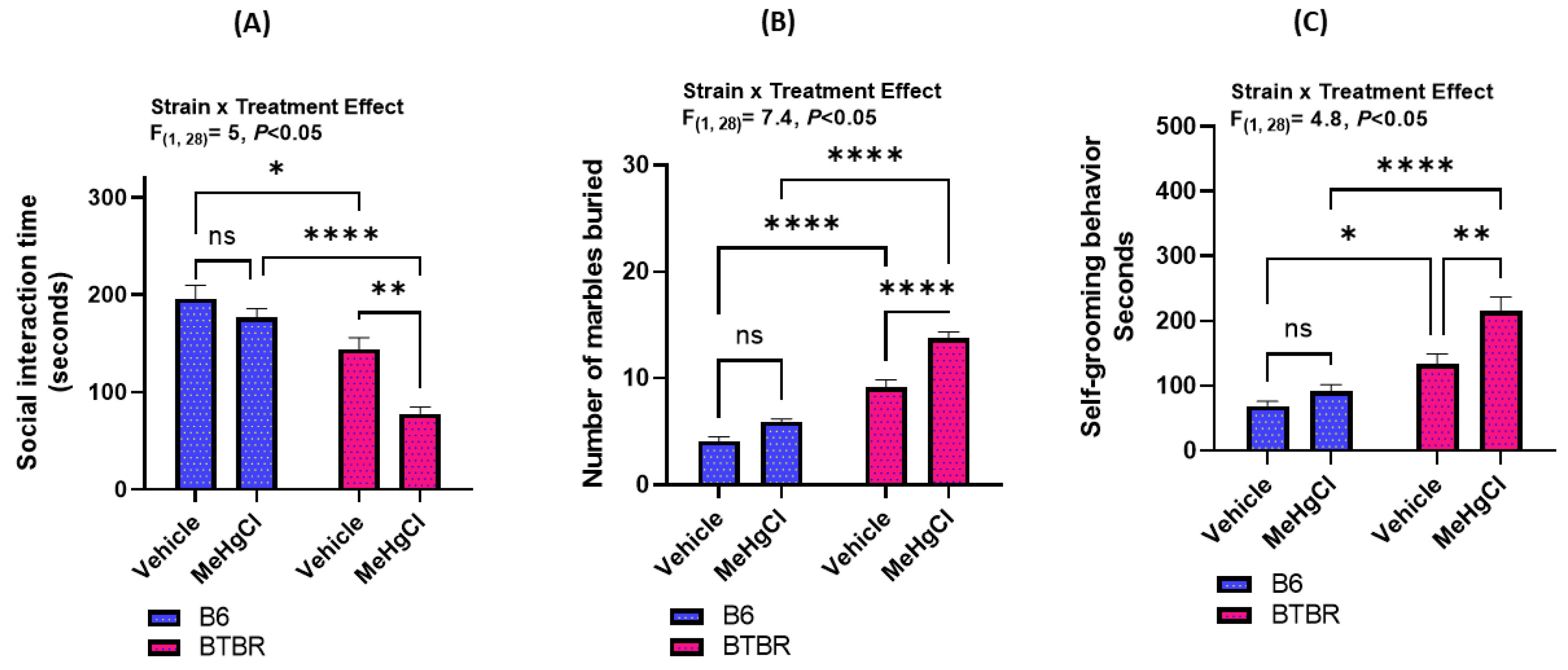
2.3. Marble Burying Test
2.4. Self-Grooming Test
2.5. Three-Chambered Sociability Test
2.6. Flow Cytometry
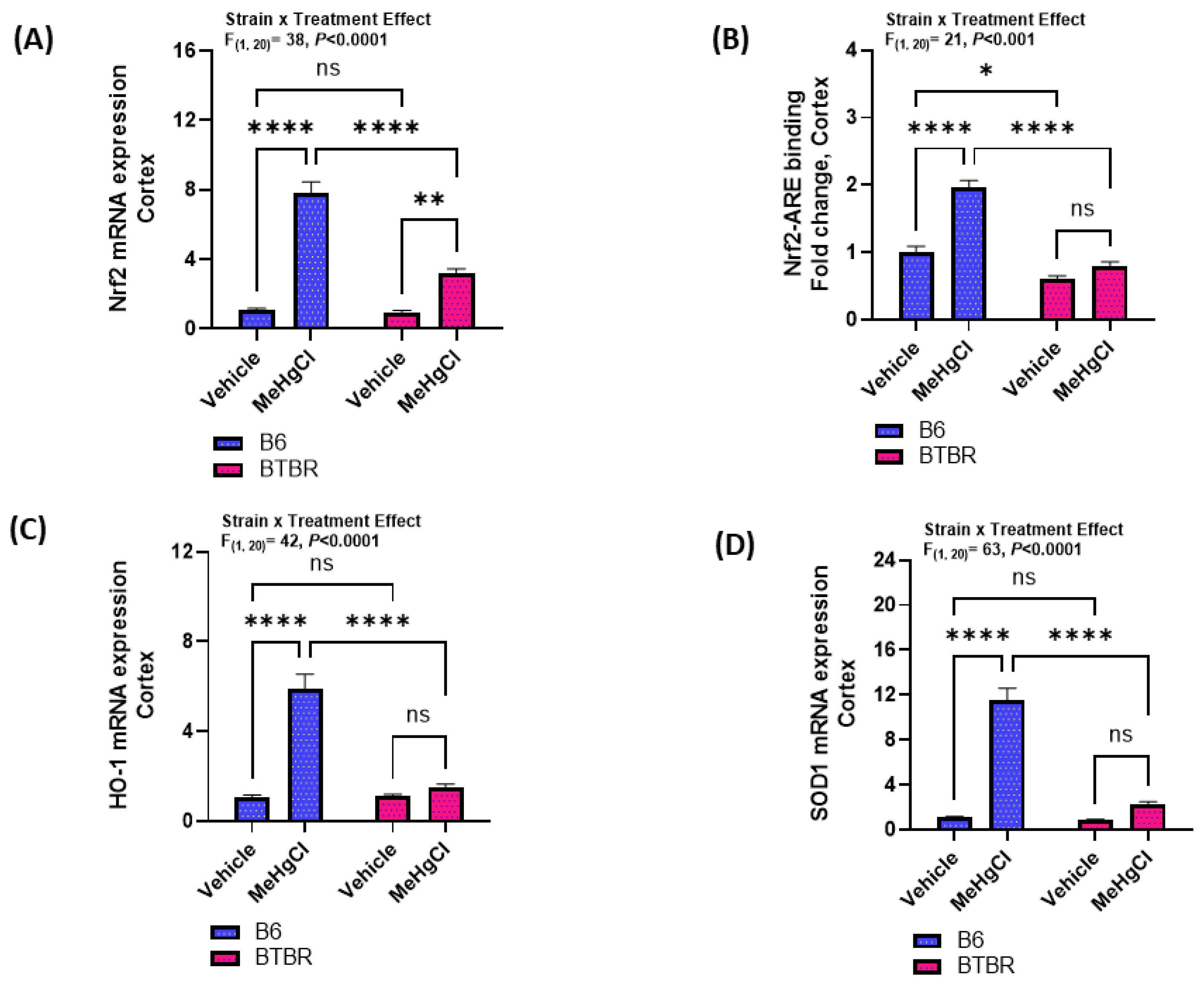
2.7. Trans-Activation ELISA Assay in the Cortex for Nrf2 Binding with Its Antioxidant Response Element
2.8. Real-Time PCR
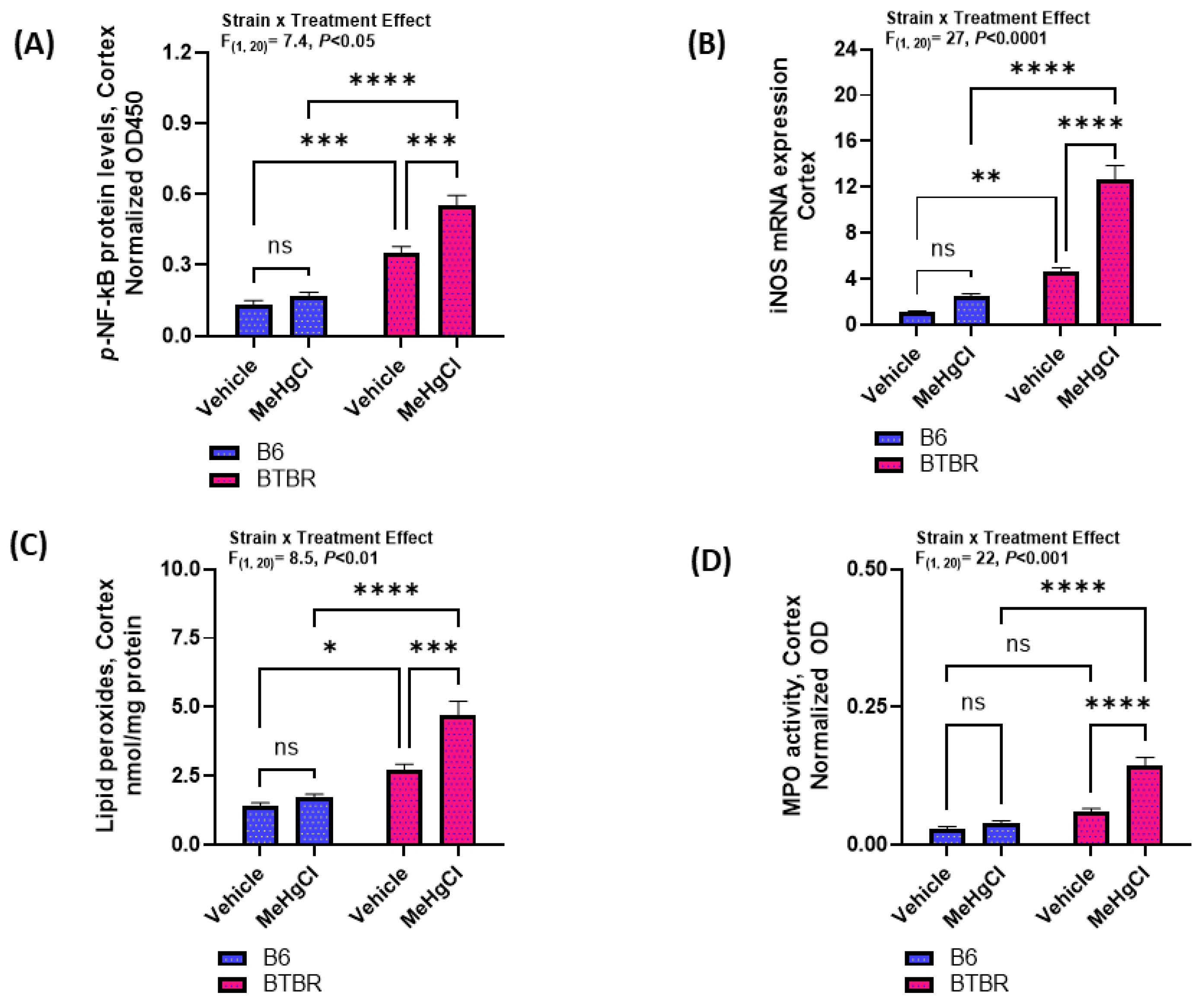
2.9. Lipid Peroxides Measurement in Brain
2.10. Myeloperoxidase (MPO) Activity Measurement in Brain
2.11. Data Analysis
3. Results
3.1. Effect of Methylmercury Chloride Administration during Juvenile Stage on Autism-like Behavior in Adult BTBR and B6 Mice
3.2. Effect of Methylmercury Chloride during Juvenile Stage on Nrf2-Mediated Signaling in Peripheral Neutrophils of Adult BTBR and B6 Mice
3.3. Effect of Methylmercury Chloride during Juvenile Stage on NF-kB-Mediated Signaling in Peripheral Neutrophils of Adult BTBR and B6 Mice
3.4. Effect of Methylmercury Chloride during Juvenile Stage on Nrf2-Mediated Signaling in the Cortex of Adult BTBR and B6 Mice
3.5. Effect of Methylmercury Chloride during Juvenile Stage on NF-kB Mediated Signaling in the Cortex of Adult BTBR and B6 Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tofani, M.; Scarcella, L.; Galeoto, G.; Giovannone, F.; Sogos, C. Behavioral Gender Differences across Pre-School Children with Autism Spectrum Disorders: A Cross-Sectional Study. J. Autism Dev. Disord. 2022, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Arvidsson, O.; Gillberg, C.; Lichtenstein, P.; Lundström, S. Secular Changes in the Symptom Level of Clinically Diagnosed Autism. J Child. Psychol. Psychiatry 2018, 59, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, R.C.; Carter, A.S. State-Level Trends in the Prevalence of Autism Spectrum Disorder (ASD) from 2000 to 2012: A Reanalysis of Findings from the Autism and Developmental Disabilities Network. J. Autism Dev. Disord. 2018, 48, 3086–3092. [Google Scholar] [CrossRef] [PubMed]
- McConkey, R. Responding to Autism in Low and Middle Income Countries (Lmic): What to Do and What Not to Do. Brain Sci. 2022, 12, 1475. [Google Scholar] [CrossRef] [PubMed]
- Talantseva, O.I.; Romanova, R.S.; Shurdova, E.M.; Dolgorukova, T.A.; Sologub, P.S.; Titova, O.S.; Kleeva, D.F.; Grigorenko, E.L. The Global Prevalence of Autism Spectrum Disorder: A Three-Level Meta-Analysis. Front. Psychiatry 2023, 14, 1071181. [Google Scholar] [CrossRef] [PubMed]
- Baio, J.; Wiggins, L.; Christensen, D.L.; Maenner, M.J.; Daniels, J.; Warren, Z.; Kurzius-Spencer, M.; Zahorodny, W.; Robinson, C.; Rosenberg, H.; et al. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. MMWR Surveill. Summ. 2018, 67, 1–23. [Google Scholar] [CrossRef]
- Leigh, J.P.; Du, J. Brief Report: Forecasting the Economic Burden of Autism in 2015 and 2025 in the United States. J. Autism Dev. Disord. 2015, 45, 4135–4139. [Google Scholar] [CrossRef]
- Paduraru, E.; Iacob, D.; Rarinca, V.; Plavan, G.; Ureche, D.; Jijie, R.; Nicoara, M. Zebrafish as a Potential Model for Neurodegenerative Diseases: A Focus on Toxic Metals Implications. Int. J. Mol. Sci. 2023, 24, 3428. [Google Scholar] [CrossRef]
- Devalloir, Q.; Fritsch, C.; Bangjord, G.; Bårdsen, B.-J.; Bourgeon, S.; Eulaers, I.; Bustnes, J.O. Long-Term Monitoring of Exposure to Toxic and Essential Metals and Metalloids in the Tawny Owl (Strix Aluco): Temporal Trends and Influence of Spatial Patterns. Sci. Total Environ. 2023, 876, 162710. [Google Scholar] [CrossRef]
- Teng, D.; Mao, K.; Ali, W.; Xu, G.; Huang, G.; Niazi, N.K.; Feng, X.; Zhang, H. Describing the toxicity and sources and the remediation technologies for mercury-contaminated soil. RSC Adv. 2020, 10, 23221–23232. [Google Scholar] [CrossRef]
- Zhang, Y.; Bolivar, V.J.; Lawrence, D.A. Maternal exposure to mercury chloride during pregnancy and lactation affects the immunity and social behavior of offspring. Toxicol. Sci. 2013, 133, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.F.; Bakheet, S.A.; Ansari, M.A.; Nadeem, A.; Alobaidi, A.F.; Attia, S.M.; Alhamed, A.S.; Aldossari, A.A.; Mahmoud, M.A. Methylmercury chloride exposure aggravates proinflammatory mediators and Notch-1 signaling in CD14+ and CD40+ cells and is associated with imbalance of neuroimmune function in BTBR T+ Itpr3tf/J mice. Neurotoxicology 2021, 82, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Loan, A.; Leung, J.W.; Cook, D.P.; Ko, C.; Vanderhyden, B.C.; Wang, J.; Chan, H.M. Prenatal low-dose methylmercury exposure causes premature neuronal differentiation and autism-like behaviors in a rodent model. iScience 2023, 26, 106093. [Google Scholar] [CrossRef] [PubMed]
- Cobley, J.N.; Fiorello, M.L.; Bailey, D.M. 13 Reasons Why the Brain Is Susceptible to Oxidative Stress. Redox Biol. 2018, 15, 490–503. [Google Scholar] [CrossRef]
- Teixeira, F.B.; de Oliveira, A.C.A.; Leão, L.K.R.; Fagundes, N.C.F.; Fernandes, R.M.; Fernandes, L.M.P.; da Silva, M.C.F.; Amado, L.L.; Sagica, F.E.S.; de Oliveira, E.H.C.; et al. Exposure to Inorganic Mercury Causes Oxidative Stress, Cell Death, and Functional Deficits in the Motor Cortex. Front. Mol. Neurosci. 2018, 11, 125. [Google Scholar] [CrossRef]
- Goering, P.L.; Morgan, D.L.; Ali, S.F. Effects of Mercury Vapor Inhalation on Reactive Oxygen Species and Antioxidant Enzymes in Rat Brain and Kidney Are Minimal. J. Appl. Toxicol. 2002, 22, 167–172. [Google Scholar] [CrossRef]
- Nadeem, A.; Ahmad, S.F.; Al-Harbi, N.O.; Attia, S.M.; Alshammari, M.A.; Alzahrani, K.S.; Bakheet, S.A. Increased Oxidative Stress in the Cerebellum and Peripheral Immune Cells Leads to Exaggerated Autism-like Repetitive Behavior Due to Deficiency of Antioxidant Response in BTBR T + tf/J Mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 89, 245–253. [Google Scholar] [CrossRef]
- Nadeem, A.; Ahmad, S.F.; Al-Ayadhi, L.Y.; Attia, S.M.; Al-Harbi, N.O.; Alzahrani, K.S.; Bakheet, S.A. Differential Regulation of Nrf2 Is Linked to Elevated Inflammation and Nitrative Stress in Monocytes of Children with Autism. Psychoneuroendocrinology 2020, 113, 104554. [Google Scholar] [CrossRef]
- Nadeem, A.; Ahmad, S.F.; Al-Harbi, N.O.; Attia, S.M.; Bakheet, S.A.; Alsanea, S.; Ali, N.; Albekairi, T.H.; Alsaleh, N.B. Aggravation of Autism-like Behavior in BTBR T+tf/J Mice by Environmental Pollutant, Di-(2-Ethylhexyl) Phthalate: Role of Nuclear Factor Erythroid 2-Related Factor 2 and Oxidative Enzymes in Innate Immune Cells and Cerebellum. Int. Immunopharmacol. 2021, 91, 107323. [Google Scholar] [CrossRef]
- Schrier, M.S.; Zhang, Y.; Trivedi, M.S.; Deth, R.C. Decreased cortical Nrf2 gene expression in autism and its relationship to thiol and cobalamin status. Biochimie 2022, 192, 1–12. [Google Scholar] [CrossRef]
- Wu, H.; Luan, Y.; Wang, H.; Zhang, P.; Liu, S.; Wang, P.; Cao, Y.; Sun, H.; Wu, L. Selenium inhibits ferroptosis and ameliorates autistic-like behaviors of BTBR mice by regulating the Nrf2/GPx4 pathway. Brain Res. Bull. 2022, 183, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Boomhower, S.R.; Newland, M.C. Adolescent Methylmercury Exposure: Behavioral Mechanisms and Effects of Sodium Butyrate in Mice. Neurotoxicology 2019, 70, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.L.; Tolu, S.S.; Barkan, C.L.; Crawley, J.N. Repetitive Self-Grooming Behavior in the BTBR Mouse Model of Autism Is Blocked by the MGluR5 Antagonist MPEP. Neuropsychopharmacology 2010, 35, 976–989. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Silverman, J.L.; Crawley, J.N. Automated Three-Chambered Social Approach Task for Mice. Curr. Protoc. Neurosci. 2011, 56, 8–26. [Google Scholar] [CrossRef]
- Algahtani, M.M.; Alshehri, S.; Alqarni, S.S.; Ahmad, S.F.; Al-Harbi, N.O.; Alqarni, S.A.; Alfardan, A.S.; Ibrahim, K.E.; Attia, S.M.; Nadeem, A. Inhibition of ITK Signaling Causes Amelioration in Sepsis-Associated Neuroinflammation and Depression-like State in Mice. Int. J. Mol. Sci. 2023, 24, 8101. [Google Scholar] [CrossRef]
- Varghese, M.; Keshav, N.; Jacot-Descombes, S.; Warda, T.; Wicinski, B.; Dickstein, D.L.; Harony-Nicolas, H.; De Rubeis, S.; Drapeau, E.; Buxbaum, J.D.; et al. Autism spectrum disorder: Neuropathology and animal models. Acta Neuropathol. 2017, 134, 537–566. [Google Scholar] [CrossRef]
- Nadeem, A.; Ahmad, S.F.; Al-Harbi, N.O.; Attia, S.; Bakheet, S.; Ibrahim, K.E.; Alqahtani, F.; Alqinyah, M. Nrf2 Activator, Sulforaphane Ameliorates Autism-like Symptoms through Suppression of Th17 Related Signaling and Rectification of Oxidant-Antioxidant Imbalance in Periphery and Brain of BTBR T+tf/J Mice. Behav. Brain Res. 2019, 364, 213–224. [Google Scholar] [CrossRef]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining Roles of Specific Reactive Oxygen Species (ROS) in Cell Biology and Physiology. Nat. Rev. Mol. Cell. Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef]
- Angelova, P.R.; Esteras, N.; Abramov, A.Y. Mitochondria and Lipid Peroxidation in the Mechanism of Neurodegeneration: Finding Ways for Prevention. Med. Res. Rev. 2021, 41, 770–784. [Google Scholar] [CrossRef]
- Hodges, H.; Fealko, C.; Soares, N. Autism Spectrum Disorder: Definition, Epidemiology, Causes, and Clinical Evaluation. Transl. Pediatr. 2020, 9, S55. [Google Scholar] [CrossRef]
- Frazier, T.W.; Thompson, L.; Youngstrom, E.A.; Law, P.; Hardan, A.Y.; Eng, C.; Morris, N. A Twin Study of Heritable and Shared Environmental Contributions to Autism. J. Autism Dev. Disord. 2014, 44, 2013–2025. [Google Scholar] [CrossRef] [PubMed]
- Hallmayer, J.; Cleveland, S.; Torres, A.; Phillips, J.; Cohen, B.; Torigoe, T.; Miller, J.; Fedele, A.; Collins, J.; Smith, K.; et al. Genetic Heritability and Shared Environmental Factors among Twin Pairs with Autism. Arch. Gen. Psychiatry 2011, 68, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, D.A.; Frye, R.E. A Review of Research Trends in Physiological Abnormalities in Autism Spectrum Disorders: Immune Dysregulation, Inflammation, Oxidative Stress, Mitochondrial Dysfunction and Environmental Toxicant Exposures. Mol. Psychiatry 2012, 17, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, D.A.; Genuis, S.J.; Frye, R.E. Environmental Toxicants and Autism Spectrum Disorders: A Systematic Review. Transl. Psychiatry 2014, 4, e360. [Google Scholar] [CrossRef]
- Nadeem, A.; Ahmad, S.F.; Attia, S.M.; AL-Ayadhi, L.Y.; Al-Harbi, N.O.; Bakheet, S.A. Dysregulated Enzymatic Antioxidant Network in Peripheral Neutrophils and Monocytes in Children with Autism. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 88, 352–359. [Google Scholar] [CrossRef]
- Wilson, E.H.; Weninger, W.; Hunter, C.A. Trafficking of Immune Cells in the Central Nervous System. J. Clin. Investig. 2010, 120, 1368–1379. [Google Scholar] [CrossRef]
- Careaga, M.; Schwartzer, J.; Ashwood, P. Inflammatory Profiles in the BTBR Mouse: How Relevant Are They to Autism Spectrum Disorders? Brain Behav. Immun. 2015, 43, 11–16. [Google Scholar] [CrossRef]
- Olivito, I.; Avolio, E.; Minervini, D.; Soda, T.; Rocca, C.; Angelone, T.; Iaquinta, F.S.; Bellizzi, D.; De Rango, F.; Bruno, R.; et al. Ketogenic Diet Ameliorates Autism Spectrum Disorders-like Behaviors via Reduced Inflammatory Factors and Microbiota Remodeling in BTBR T+ Itpr3tf/J Mice. Exp. Neurol. 2023, 366, 114432. [Google Scholar] [CrossRef]
- Garg, S.S.; Gupta, J.; Sahu, D.; Liu, C.-J. Pharmacological and Therapeutic Applications of Esculetin. Int. J. Mol. Sci. 2022, 23, 12643. [Google Scholar] [CrossRef]
- Gu, F.; Chauhan, V.; Chauhan, A. Impaired Synthesis and Antioxidant Defense of Glutathione in the Cerebellum of Autistic Subjects: Alterations in the Activities and Protein Expression of Glutathione-Related Enzymes. Free Radic. Biol. Med. 2013, 65, 488–496. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Szabó, C.; Ischiropoulos, H.; Radi, R. Peroxynitrite: Biochemistry, pathophysiology and development of therapeutics. Nat. Rev. Drug. Discov. 2007, 6, 662–680. [Google Scholar] [CrossRef] [PubMed]
- Franco, C.; Gianò, M.; Favero, G.; Rezzani, R. Impairment in the Intestinal Morphology and in the Immunopositivity of Toll-like Receptor-4 and Other Proteins in an Autistic Mouse Model. Int. J. Mol. Sci. 2022, 23, 8731. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.; Chauhan, V.; Brown, W.T.; Cohen, I. Oxidative Stress in Autism: Increased Lipid Peroxidation and Reduced Serum Levels of Ceruloplasmin and Transferrin—The Antioxidant Proteins. Life Sci. 2004, 75, 2539–2549. [Google Scholar] [CrossRef]
- De Felice, A.; Greco, A.; Calamandrei, G.; Minghetti, L. Prenatal Exposure to the Organophosphate Insecticide Chlorpyrifos Enhances Brain Oxidative Stress and Prostaglandin E2 Synthesis in a Mouse Model of Idiopathic Autism. J. Neuroinflammation 2016, 13, 149. [Google Scholar] [CrossRef]
- Nadeem, A.; Ahmad, S.F.; Attia, S.M.; Bakheet, S.A.; Al-Harbi, N.O.; AL-Ayadhi, L.Y. Activation of IL-17 Receptor Leads to Increased Oxidative Inflammation in Peripheral Monocytes of Autistic Children. Brain Behav. Immun. 2018, 67, 335–344. [Google Scholar] [CrossRef]
- Sajdel-Sulkowska, E.M.; Xu, M.; Koibuchi, N. Increase in Cerebellar Neurotrophin-3 and Oxidative Stress Markers in Autism. Cerebellum 2009, 8, 366–372. [Google Scholar] [CrossRef]
- Ceylan, M.F.; Tural Hesapcioglu, S.; Yavas, C.P.; Senat, A.; Erel, O. Serum Ischemia-Modified Albumin Levels, Myeloperoxidase Activity and Peripheral Blood Mononuclear cells in Autism Spectrum Disorder (ASD). J. Autism Dev. Disord. 2021, 51, 2511–2517. [Google Scholar] [CrossRef]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef]
- Luo, J.; Wang, J.; Zhang, J.; Sang, A.; Ye, X.; Cheng, Z.; Li, X. Nrf2 Deficiency Exacerbated CLP-Induced Pulmonary Injury and Inflammation through Autophagy- and NF-κB/PPARγ-Mediated Macrophage Polarization. Cells 2022, 11, 3927. [Google Scholar] [CrossRef]
- Nadeem, A.; Ahmad, S.F.; Al-Harbi, N.O.; Al-Ayadhi, L.Y.; Alanazi, M.M.; Alfardan, A.S.; Attia, S.M.; Algahtani, M.; Bakheet, S.A. Dysregulated Nrf2 signaling in response to di(2-ethylhexyl) phthalate in neutrophils of children with autism. Int. Immunopharmacol. 2022, 106, 108619. [Google Scholar] [CrossRef]
- Momtazmanesh, S.; Amirimoghaddam-Yazdi, Z.; Moghaddam, H.S.; Mohammadi, M.R.; Akhondzadeh, S. Sulforaphane as an Adjunctive Treatment for Irritability in Children with Autism Spectrum Disorder: A Randomized, Double-blind, Placebo-controlled Clinical Trial. Psychiatry Clin. Neurosci. 2020, 74, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Marchezan, J.; Deckmann, I.; da Fonseca, G.C.; Margis, R.; Riesgo, R.; Gottfried, C. Resveratrol Treatment of Autism Spectrum Disorder—A Pilot Study. Clin. Neuropharmacol. 2022, 45, 122–127. [Google Scholar] [CrossRef]
- Zhong, H.; Xiao, R.; Ruan, R.; Liu, H.; Li, X.; Cai, Y.; Zhao, J.; Fan, X. Neonatal Curcumin Treatment Restores Hippocampal Neurogenesis and Improves Autism-Related Behaviors in a Mouse Model of Autism. Psychopharmacology 2020, 237, 3539–3552. [Google Scholar] [CrossRef]
- Gai, J.; Xing, J.; Wang, Y.; Lei, J.; Zhang, C.; Zhang, J.; Tang, J. Exploration of Potential Targets and Mechanisms of Naringenin in Treating Autism Spectrum Disorder via Network Pharmacology and Molecular Docking. Medicine 2022, 101, e31787. [Google Scholar] [CrossRef] [PubMed]
- Dolicka, D.; Sobolewski, C.; Correia de Sousa, M.; Gjorgjieva, M.; Foti, M. mRNA Post-Transcriptional Regulation by AU-Rich Element-Binding Proteins in Liver Inflammation and Cancer. Int. J. Mol. Sci. 2020, 21, 6648. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, S.; Ricci, E.P.; Mercier, B.C.; Moore, M.J.; Fitzgerald, K.A. Post-transcriptional regulation of gene expression in innate immunity. Nat. Rev. Immunol. 2014, 14, 361–376. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Algahtani, M.M.; Ahmad, S.F.; Alkharashi, L.A.; Al-Harbi, N.O.; Alanazi, W.A.; Alhamed, A.S.; Attia, S.M.; Bakheet, S.A.; Ibrahim, K.E.; Nadeem, A. Exposure to Methylmercury at Juvenile Stage Worsens Autism-like Symptoms in Adult BTBR T+tf/J Mice Due to Lack of Nuclear Factor Erythroid 2-Related Factor 2 Signaling Upregulation in Periphery and Brain. Toxics 2023, 11, 546. https://doi.org/10.3390/toxics11060546
Algahtani MM, Ahmad SF, Alkharashi LA, Al-Harbi NO, Alanazi WA, Alhamed AS, Attia SM, Bakheet SA, Ibrahim KE, Nadeem A. Exposure to Methylmercury at Juvenile Stage Worsens Autism-like Symptoms in Adult BTBR T+tf/J Mice Due to Lack of Nuclear Factor Erythroid 2-Related Factor 2 Signaling Upregulation in Periphery and Brain. Toxics. 2023; 11(6):546. https://doi.org/10.3390/toxics11060546
Chicago/Turabian StyleAlgahtani, Mohammad M., Sheikh F. Ahmad, Layla A. Alkharashi, Naif O. Al-Harbi, Wael A. Alanazi, Abdullah S. Alhamed, Sabry M. Attia, Saleh A. Bakheet, Khalid E. Ibrahim, and Ahmed Nadeem. 2023. "Exposure to Methylmercury at Juvenile Stage Worsens Autism-like Symptoms in Adult BTBR T+tf/J Mice Due to Lack of Nuclear Factor Erythroid 2-Related Factor 2 Signaling Upregulation in Periphery and Brain" Toxics 11, no. 6: 546. https://doi.org/10.3390/toxics11060546
APA StyleAlgahtani, M. M., Ahmad, S. F., Alkharashi, L. A., Al-Harbi, N. O., Alanazi, W. A., Alhamed, A. S., Attia, S. M., Bakheet, S. A., Ibrahim, K. E., & Nadeem, A. (2023). Exposure to Methylmercury at Juvenile Stage Worsens Autism-like Symptoms in Adult BTBR T+tf/J Mice Due to Lack of Nuclear Factor Erythroid 2-Related Factor 2 Signaling Upregulation in Periphery and Brain. Toxics, 11(6), 546. https://doi.org/10.3390/toxics11060546





