Predictive Models for Compound Binding to Androgen and Estrogen Receptors Based on Counter-Propagation Artificial Neural Networks
Abstract
1. Introduction
2. Data
3. Methods
4. Results and Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Diamanti-Kandarakis, E.; Bourguignon, J.-P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-Disrupting Chemicals: An Endocrine Society Scientific Statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef] [PubMed]
- Zoeller, R.T.; Brown, T.R.; Doan, L.L.; Gore, A.C.; Skakkebaek, N.E.; Soto, A.M.; Woodruff, T.J.; Vom Saal, F.S. Endocrine-Disrupting Chemicals and Public Health Protection: A Statement of Principles from The Endocrine Society. Endocrinology 2012, 153, 4097–4110. [Google Scholar] [CrossRef] [PubMed]
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. Executive Summary to EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015, 36, 593–602. [Google Scholar] [CrossRef]
- Amir, S.; Shah, S.T.A.; Mamoulakis, C.; Docea, A.O.; Kalantzi, O.-I.; Zachariou, A.; Calina, D.; Carvalho, F.; Sofikitis, N.; Makrigiannakis, A.; et al. Endocrine Disruptors Acting on Estrogen and Androgen Pathways Cause Reproductive Disorders through Multiple Mechanisms: A Review. Int. J. Environ. Res. Public Health 2021, 18, 1464. [Google Scholar] [CrossRef] [PubMed]
- Pakdel, F. Molecular Pathways of Estrogen Receptor Action. Int. J. Mol. Sci. 2018, 19, 2591. [Google Scholar] [CrossRef]
- Giraldi, T.; Giovannelli, P.; Di Donato, M.; Castoria, G.; Migliaccio, A.; Auricchio, F. Steroid signaling activation and intracellular localization of sex steroid receptors. J. Cell Commun. Signal. 2010, 4, 161–172. [Google Scholar] [CrossRef]
- Fuentes, N.; Silveyra, P. Estrogen receptor signaling mechanisms. Adv. Protein Chem. Struct. Biol. 2019, 116, 135–170. [Google Scholar] [CrossRef]
- Jia, M.; Dahlman-Wright, K.; Gustafsson, J.Å. Estrogen receptor alpha and beta in health and disease. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 557–568. [Google Scholar] [CrossRef]
- Evers, N.M.; Berg, J.H.V.D.; Wang, S.; Melchers, D.; Houtman, R.; de Haan, L.H.; Ederveen, A.G.; Groten, J.P.; Rietjens, I.M. Cell proliferation and modulation of interaction of estrogen receptors with coregulators induced by ERα and ERβ agonists. J. Steroid Biochem. Mol. Biol. 2014, 143, 376–385. [Google Scholar] [CrossRef]
- Lu, N.Z.; Wardell, S.E.; Burnstein, K.L.; Defranco, D.; Fuller, P.J.; Giguere, V.; Hochberg, R.B.; McKay, L.; Renoir, J.-M.; Weigel, N.L.; et al. International Union of Pharmacology. LXV. The Pharmacology and Classification of the Nuclear Receptor Superfamily: Glucocorticoid, Mineralocorticoid, Progesterone, and Androgen Receptors. Pharmacol. Rev. 2006, 58, 782–797. [Google Scholar] [CrossRef]
- Sakkiah, S.; Wang, T.; Zou, W.; Wang, Y.; Pan, B.; Tong, W.; Hong, H. Endocrine Disrupting Chemicals Mediated through Binding Androgen Receptor Are Associated with Diabetes Mellitus. Int. J. Environ. Res. Public Health 2017, 15, 25. [Google Scholar] [CrossRef] [PubMed]
- Baniahmad, A. Inhibition of the Androgen Receptor by Antiandrogens in Spinobulbar Muscle Atrophy. J. Mol. Neurosci. 2015, 58, 343–347. [Google Scholar] [CrossRef]
- Díaz-Sánchez, V.; Morimoto, S.; Morales, A.; Robles-Díaz, G.; Cerbón, M. Androgen Receptor in the Rat Pancreas. Pancreas 1995, 11, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Christopoulos, P.F.; Vlachogiannis, N.; Vogkou, C.T.; Koutsilieris, M. The Role of the Androgen Receptor Signaling in Breast Malignancies. Anticancer. Res. 2017, 37, 6533–6540. [Google Scholar] [CrossRef] [PubMed]
- Helsen, C.; Broeck, T.V.D.; Voet, A.; Prekovic, S.; Van Poppel, H.; Joniau, S.; Claessens, F. Androgen receptor antagonists for prostate cancer therapy. Endocr. Relat. Cancer 2014, 21, T105–T118. [Google Scholar] [CrossRef]
- OECD. Revised Guidance Document 150 on Standardised Test Guidelines for Evaluating Chemicals for Endocrine Disruption; OECD Series on Testing and Assessment; OECD Publishing: Paris, France, 2018; Volume 150. [Google Scholar]
- Wang, Z.; Liu, Y.; Niu, X. Application of artificial intelligence for improving early detection and prediction of therapeutic outcomes for gastric cancer in the era of precision oncology. Semin. Cancer Biol. 2023, 93, 83–96. [Google Scholar] [CrossRef]
- Negassi, M.; Suarez-Ibarrola, R.; Hein, S.; Miernik, A.; Reiterer, A. Application of artificial neural networks for automated analysis of cystoscopic images: A review of the current status and future prospects. World J. Urol. 2020, 38, 2349–2358. [Google Scholar] [CrossRef]
- Nagy, B.; Galata, D.L.; Farkas, A.; Nagy, Z.K. Application of Artificial Neural Networks in the Process Analytical Technology of Pharmaceutical Manufacturing—A Review. AAPS J. 2022, 24, 74. [Google Scholar] [CrossRef]
- Kulichenko, M.; Smith, J.S.; Nebgen, B.; Li, Y.W.; Fedik, N.; Boldyrev, A.I.; Lubbers, N.; Barros, K.; Tretiak, S. The Rise of Neural Networks for Materials and Chemical Dynamics. J. Phys. Chem. Lett. 2021, 12, 6227–6243. [Google Scholar] [CrossRef]
- Taskinen, J.; Yliruusi, J. Prediction of physicochemical properties based on neural network modelling. Adv. Drug Deliv. Rev. 2003, 55, 1163–1183. [Google Scholar] [CrossRef]
- Venko, K.; Drgan, V.; Novič, M. Classification models for identifying substances exhibiting acute contact toxicity in honeybees (Apis mellifera)$. SAR QSAR Environ. Res. 2018, 29, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Fjodorova, N.; Novič, M. Comparison of criteria used to access carcinogenicity in CPANN QSAR models versus the knowledge-based expert system Toxtree. SAR QSAR Environ. Res. 2014, 25, 423–441. [Google Scholar] [CrossRef] [PubMed]
- Fjodorova, N.; Vračko, M.; Novič, M.; Roncaglioni, A.; Benfenati, E. New public QSAR model for carcinogenicity. Chem. Cent. J. 2010, 4 (Suppl. S1), S3. [Google Scholar] [CrossRef] [PubMed]
- Lagares, L.M.; Pérez-Castillo, Y.; Minovski, N.; Novič, M. Structure–Function Relationships in the Human P-Glycoprotein (ABCB1): Insights from Molecular Dynamics Simulations. Int. J. Mol. Sci. 2021, 23, 362. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.J.; Grulke, C.M.; Edwards, J.; McEachran, A.D.; Mansouri, K.; Baker, N.C.; Patlewicz, G.; Shah, I.; Wambaugh, J.F.; Judson, R.S.; et al. The CompTox Chemistry Dashboard: A community data resource for environmental chemistry. J. Cheminform. 2017, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, S.A. β-Lactamase: An ideal reporter system for monitoring gene expression in live eukaryotic cells. Biotechniques 2007, 42, 91–96. [Google Scholar] [CrossRef]
- U.S. EPA Center for Computational Toxicology and Exposure (CCTE). Toxicity Forecaster (TOXCAST) In Vitro Assays Assay Documentation for Non-Guideline In Vitro Test Methods. 2022. Available online: https://clowder.edap-cluster.com/files/6215520fe4b039b22c7a7836 (accessed on 24 May 2023).
- Filer, D.L.; Kothiya, P.; Setzer, R.W.; Judson, R.S.; Martin, M.T. tcpl: The ToxCast pipeline for high-throughput screening data. Bioinformatics 2016, 33, 618–620. [Google Scholar] [CrossRef]
- Talete Srl. Dragon Software for Molecular Descriptor Calculation. 2014. Available online: http://www.talete.mi.it/products/dragon_description.htm (accessed on 10 October 2022).
- Bolčič–Tavčar, M.; Vracko, M. Assessing the reproductive toxicity of some (con)azole compounds using a structure–activity relationship approach. SAR QSAR Environ. Res. 2009, 20, 711–725. [Google Scholar] [CrossRef]
- Baldi, P.; Brunak, S.; Chauvin, Y.; Andersen, C.A.F.; Nielsen, H. Assessing the accuracy of prediction algorithms for classification: An overview. Bioinformatics 2000, 16, 412–424. [Google Scholar] [CrossRef]
- Mansouri, K.; Abdelaziz, A.; Rybacka, A.; Roncaglioni, A.; Tropsha, A.; Varnek, A.; Zakharov, A.; Worth, A.; Richard, A.M.; Grulke, C.M.; et al. CERAPP: Collaborative Estrogen Receptor Activity Prediction Project. Environ. Health Perspect. 2016, 124, 1023–1033. [Google Scholar] [CrossRef]
- Vračko, M.; Drgan, V. Grouping of CoMPARA data with respect to compounds from the carcinogenic potency database. SAR QSAR Environ. Res. 2017, 28, 801–813. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, K.; Kleinstreuer, N.; Abdelaziz, A.M.; Alberga, D.; Alves, V.M.; Andersson, P.L.; Andrade, C.H.; Bai, F.; Balabin, I.; Ballabio, D.; et al. CoMPARA: Collaborative Modeling Project for Androgen Receptor Activity. Environ. Health Perspect. 2020, 128, 27002. [Google Scholar] [CrossRef] [PubMed]
- Lunghini, F.; Marcou, G.; Azam, P.; Bonachera, F.; Enrici, M.; Van Miert, E.; Varnek, A. Endocrine disruption: The noise in available data adversely impacts the models’ performance. SAR QSAR Environ. Res. 2021, 32, 111–131. [Google Scholar] [CrossRef]
- Huang, R.; Xia, M.; Cho, M.-H.; Sakamuru, S.; Shinn, P.; Houck, K.A.; Dix, D.J.; Judson, R.S.; Witt, K.L.; Kavlock, R.J.; et al. Chemical Genomics Profiling of Environmental Chemical Modulation of Human Nuclear Receptors. Environ. Health Perspect. 2011, 119, 1142–1148. [Google Scholar] [CrossRef]
- Stanojević, M.; Grobelšek, M.V.; Dolenc, M.S. Computational evaluation of endocrine activity of biocidal active substances. Chemosphere 2020, 267, 129284. [Google Scholar] [CrossRef]
- Todorov, M.; Mombelli, E.; Ait-Aissa, S.; Mekenyan, O. Androgen receptor binding affinity: A QSAR evaluation. SAR QSAR Environ. Res. 2011, 22, 265–291. [Google Scholar] [CrossRef] [PubMed]
- Mekenyan, O.G.; Kamenska, V.; Schmieder, P.K.; Ankley, G.T.; Bradbury, S.P. A Computationally Based Identification Algorithm for Estrogen Receptor Ligands: Part 2. Evaluation of a hERalpha Binding Affinity Model. Toxicol. Sci. 2000, 58, 270–281. [Google Scholar] [CrossRef]
- Bhhatarai, B.; Wilson, D.M.; Price, P.S.; Marty, S.; Parks, A.K.; Carney, E. Evaluation of OASIS QSAR Models Using ToxCastTM in Vitro Estrogen and Androgen Receptor Binding Data and Application in an Integrated Endocrine Screening Approach. Environ. Health Perspect. 2016, 124, 1453–1461. [Google Scholar] [CrossRef]
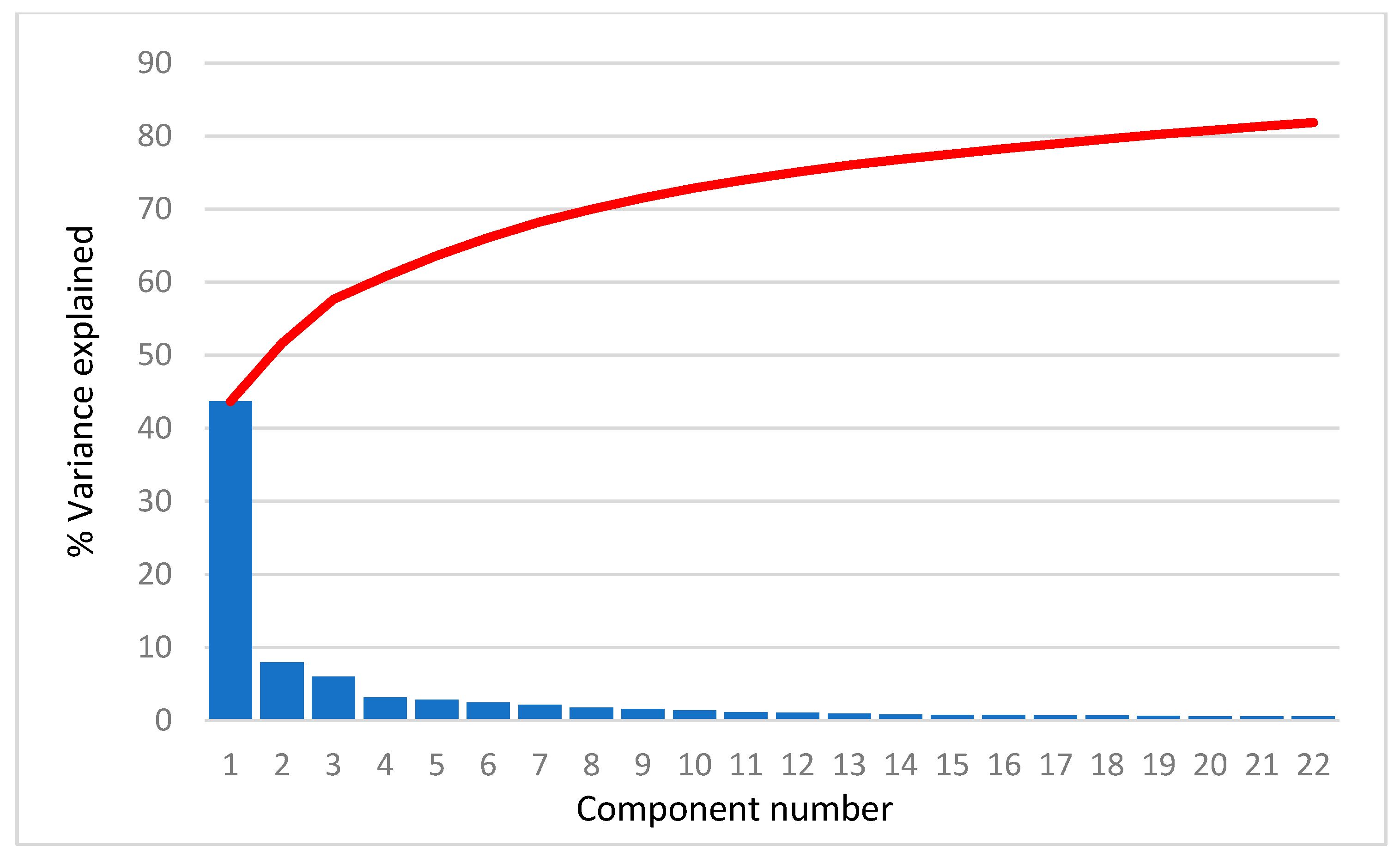


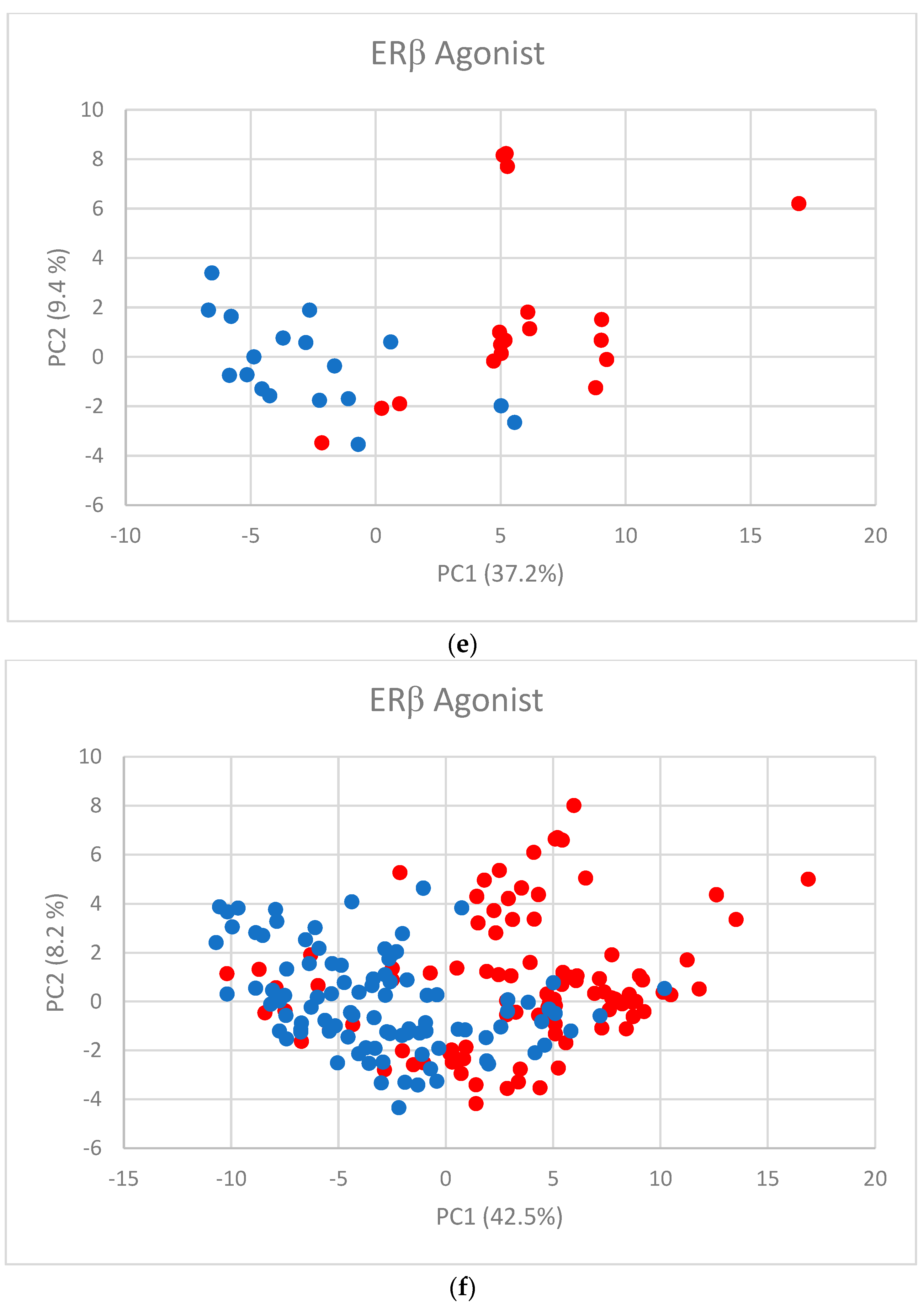
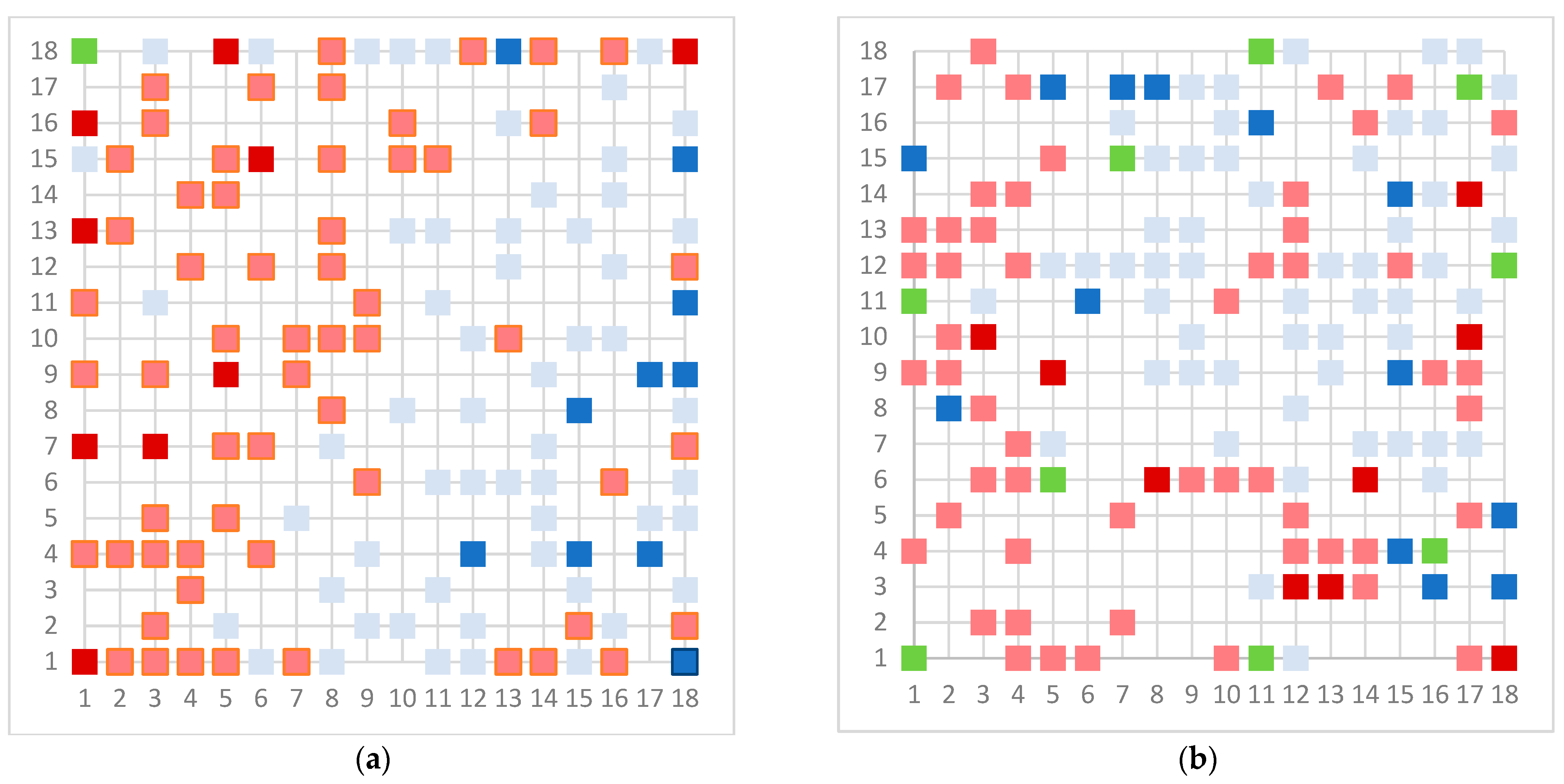
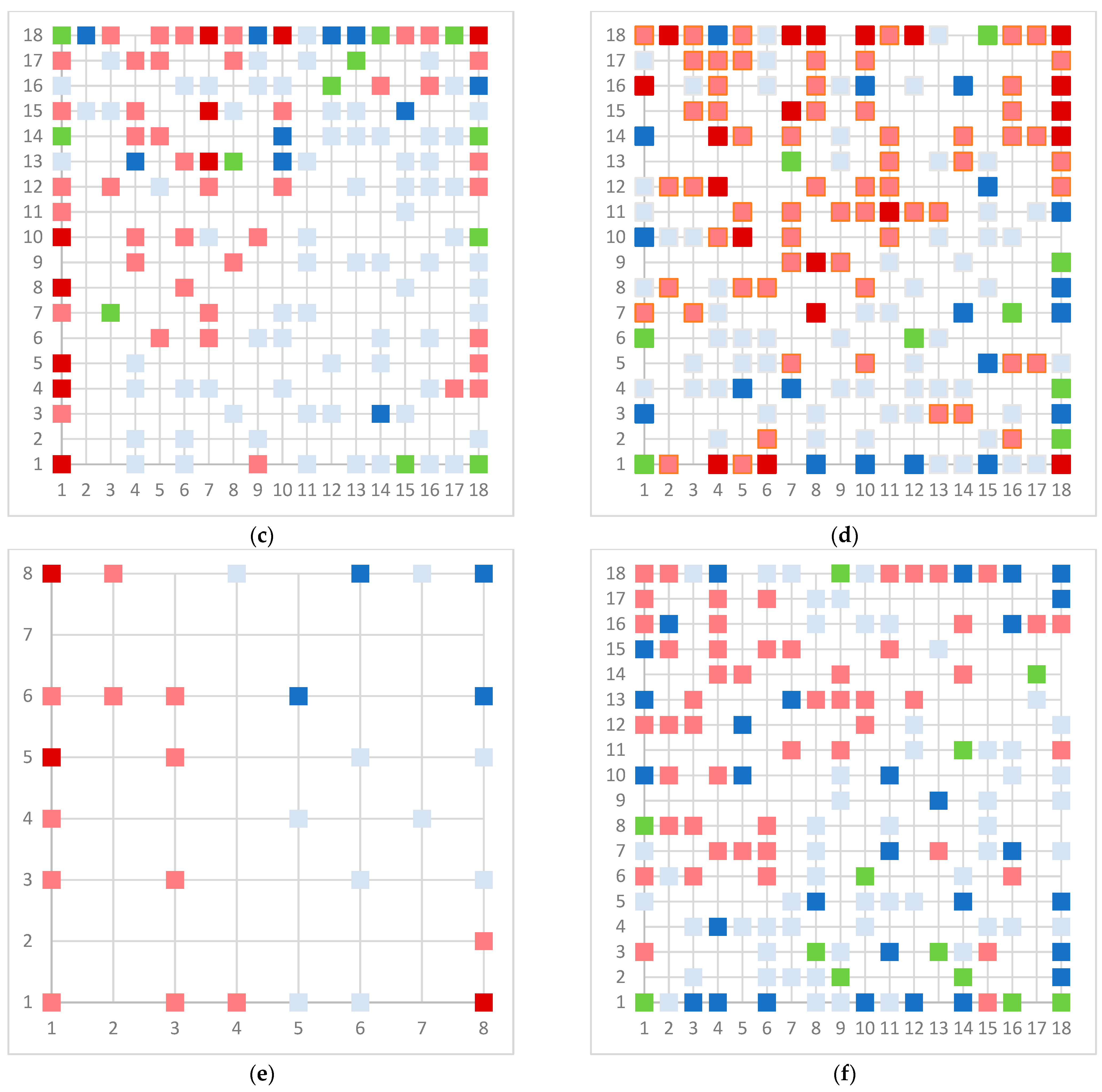
| Assay | Number of Substances in Database | Number of Substances Used for Modeling |
|---|---|---|
| TOX21_AR_BLA_AGONIST_RATIO | 1009 | 156 |
| TOX21_AR_BLA_ANTAGONIST_RATIO | 2004 | 228 |
| TOX21_ERA_BLA_AGONIST_RATIO | 1398 | 123 |
| TOX21_ERA_BLA_ANTAGONIST_RATIO | 1617 | 231 |
| TOX21_ERB_BLA_AGONIST_RATIO | 1740 | 36 |
| TOX21_ERB_BLA_ ANTAGONIST_RATIO | 1966 | 194 |
| Androgen Receptor Agonist | Androgen Receptor Antagonist | |||||
|---|---|---|---|---|---|---|
| 16 × 16 | 18 × 18 | 20 × 20 | 16 × 16 | 18 × 18 | 20 × 20 | |
| TP | 71 | 77 | 77 | 103 | 105 | 109 |
| FP | 0 | 0 | 0 | 2 | 3 | 2 |
| TN | 78 | 78 | 78 | 112 | 111 | 112 |
| FN | 7 | 1 | 1 | 11 | 9 | 5 |
| Se | 0.910 | 0.987 | 0.987 | 0.904 | 0.921 | 0.956 |
| Sp | 1.000 | 1.000 | 1.000 | 0.982 | 0.974 | 0.982 |
| Acc | 0.955 | 0.994 | 0.994 | 0.943 | 0.947 | 0.969 |
| MCC | 0.914 | 0.987 | 0.987 | 0.889 | 0.896 | 0.939 |
| Estrogen receptor alfa agonist | Estrogen receptor alfa antagonist | |||||
| 16 × 16 | 18 × 18 | 20 × 20 | 16 × 16 | 18 × 18 | 20 × 20 | |
| TP | 58 | 59 | 60 | 104 | 109 | 106 |
| FP | 1 | 1 | 1 | 3 | 2 | 0 |
| TN | 60 | 60 | 60 | 112 | 113 | 115 |
| FN | 4 | 3 | 2 | 12 | 7 | 10 |
| Se | 0.935 | 0.952 | 0.968 | 0.897 | 0.940 | 0.914 |
| Sp | 0.984 | 0.984 | 0.984 | 0.974 | 0.983 | 1.000 |
| Acc | 0.959 | 0.967 | 0.976 | 0.935 | 0.961 | 0.957 |
| MCC | 0.920 | 0.935 | 0.951 | 0.873 | 0.923 | 0.917 |
| Estrogen receptor beta agonist | Estrogen receptor beta antagonist | |||||
| 6 × 6 | 8 × 8 | 10 × 10 | 16 × 16 | 18 × 18 | 20 × 20 | |
| TP | 18 | 18 | 18 | 83 | 89 | 90 |
| FP | 0 | 0 | 0 | 3 | 4 | 2 |
| TN | 18 | 18 | 18 | 95 | 94 | 96 |
| FN | 0 | 0 | 0 | 13 | 7 | 6 |
| Se | 1 | 1 | 1 | 0.865 | 0.927 | 0.938 |
| Sp | 1 | 1 | 1 | 0.969 | 0.959 | 0.980 |
| Acc | 1 | 1 | 1 | 0.918 | 0.943 | 0.959 |
| MCC | 1 | 1 | 1 | 0.839 | 0.887 | 0.918 |
| Compound | Structure | AR Antagonist Classification | |
|---|---|---|---|
| In Vitro | In Silico | ||
| 1-Butyl-4-methylpyridinium |  | binding | non-binding |
| 1-Butylpyridinium | 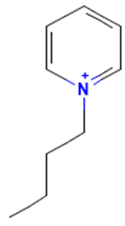 | non-binding | non-binding |
| 1-Butyl-3-methylimidazolium |  | non-binding | non-binding |
| 1,8-Diazabicyclo [5.4.0]undec-7-ene |  | non-binding | non-binding |
| Compound | Structure | AR Antagonist Classification | |
|---|---|---|---|
| In Vitro | In Silico | ||
| 1-Butyl-4-methylpyridinium | 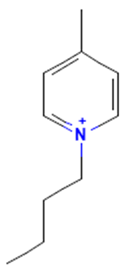 | non-binding | non-binding |
| 1-Butyl-2-methylpyridinium | 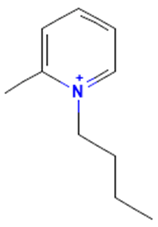 | binding | non-binding |
| Compound | Structure | ERβ Antagonist Classification | |
|---|---|---|---|
| In Vitro | In Silico | ||
| Fenticlor | 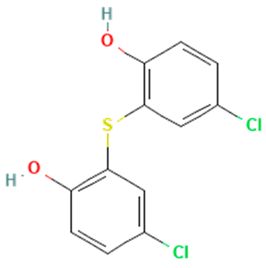 | non-binding | binding |
| Bithionol |  | binding | binding |
| Phenkapton |  | binding | binding |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanojević, M.; Sollner Dolenc, M.; Vračko, M. Predictive Models for Compound Binding to Androgen and Estrogen Receptors Based on Counter-Propagation Artificial Neural Networks. Toxics 2023, 11, 486. https://doi.org/10.3390/toxics11060486
Stanojević M, Sollner Dolenc M, Vračko M. Predictive Models for Compound Binding to Androgen and Estrogen Receptors Based on Counter-Propagation Artificial Neural Networks. Toxics. 2023; 11(6):486. https://doi.org/10.3390/toxics11060486
Chicago/Turabian StyleStanojević, Mark, Marija Sollner Dolenc, and Marjan Vračko. 2023. "Predictive Models for Compound Binding to Androgen and Estrogen Receptors Based on Counter-Propagation Artificial Neural Networks" Toxics 11, no. 6: 486. https://doi.org/10.3390/toxics11060486
APA StyleStanojević, M., Sollner Dolenc, M., & Vračko, M. (2023). Predictive Models for Compound Binding to Androgen and Estrogen Receptors Based on Counter-Propagation Artificial Neural Networks. Toxics, 11(6), 486. https://doi.org/10.3390/toxics11060486








