Combined Toxicities of Di-Butyl Phthalate and Polyethylene Terephthalate to Zebrafish Embryos
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Zebrafish Maintenance and Embryo Collection
2.3. Acute Exposure Experiments of PET and DBP
2.4. Measurement of the Acute Toxicity Indicators
2.5. Statistical Analysis
3. Results
3.1. PET Particles Made for Exposure Experiment
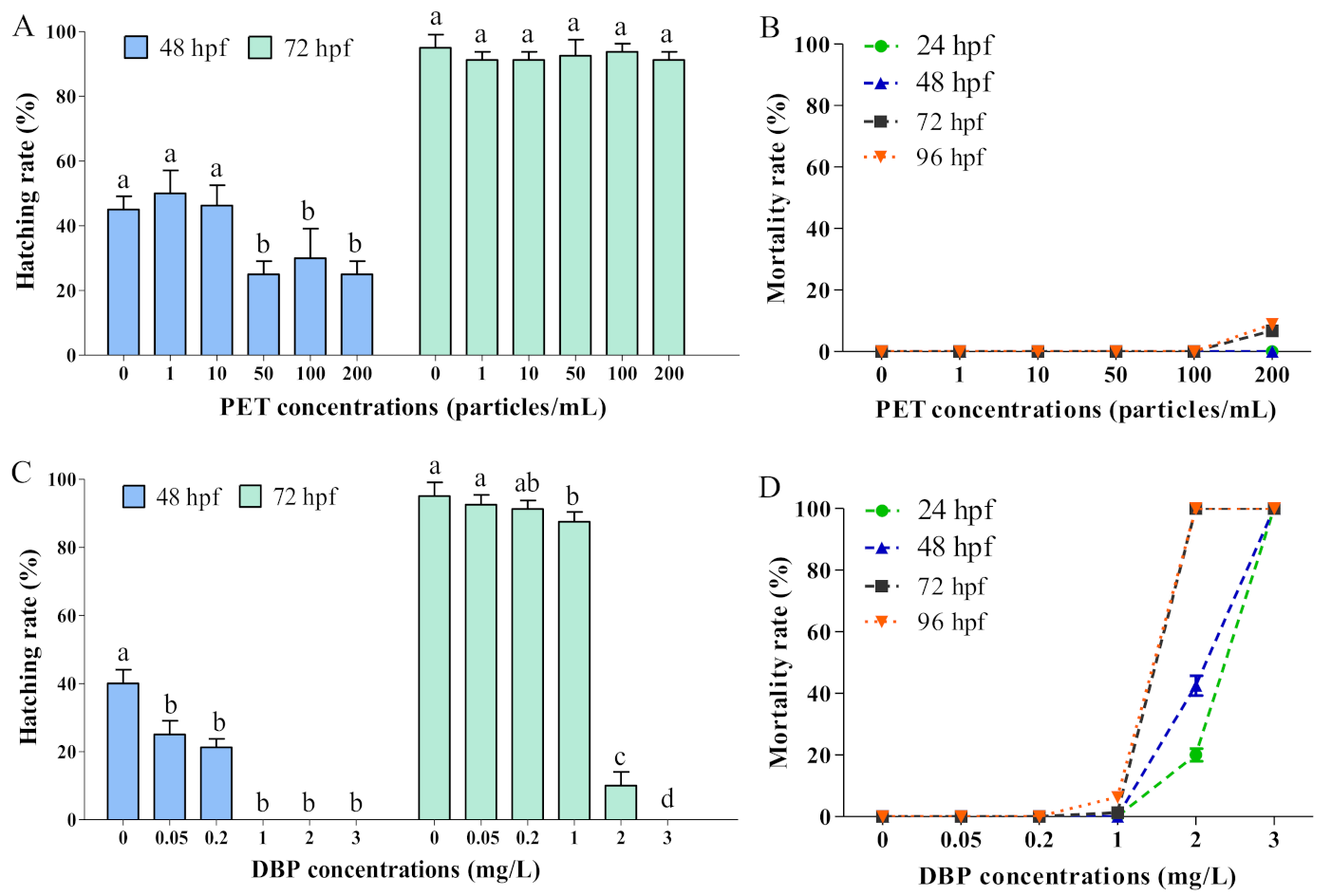
3.2. Single Exposure of PET and DBP
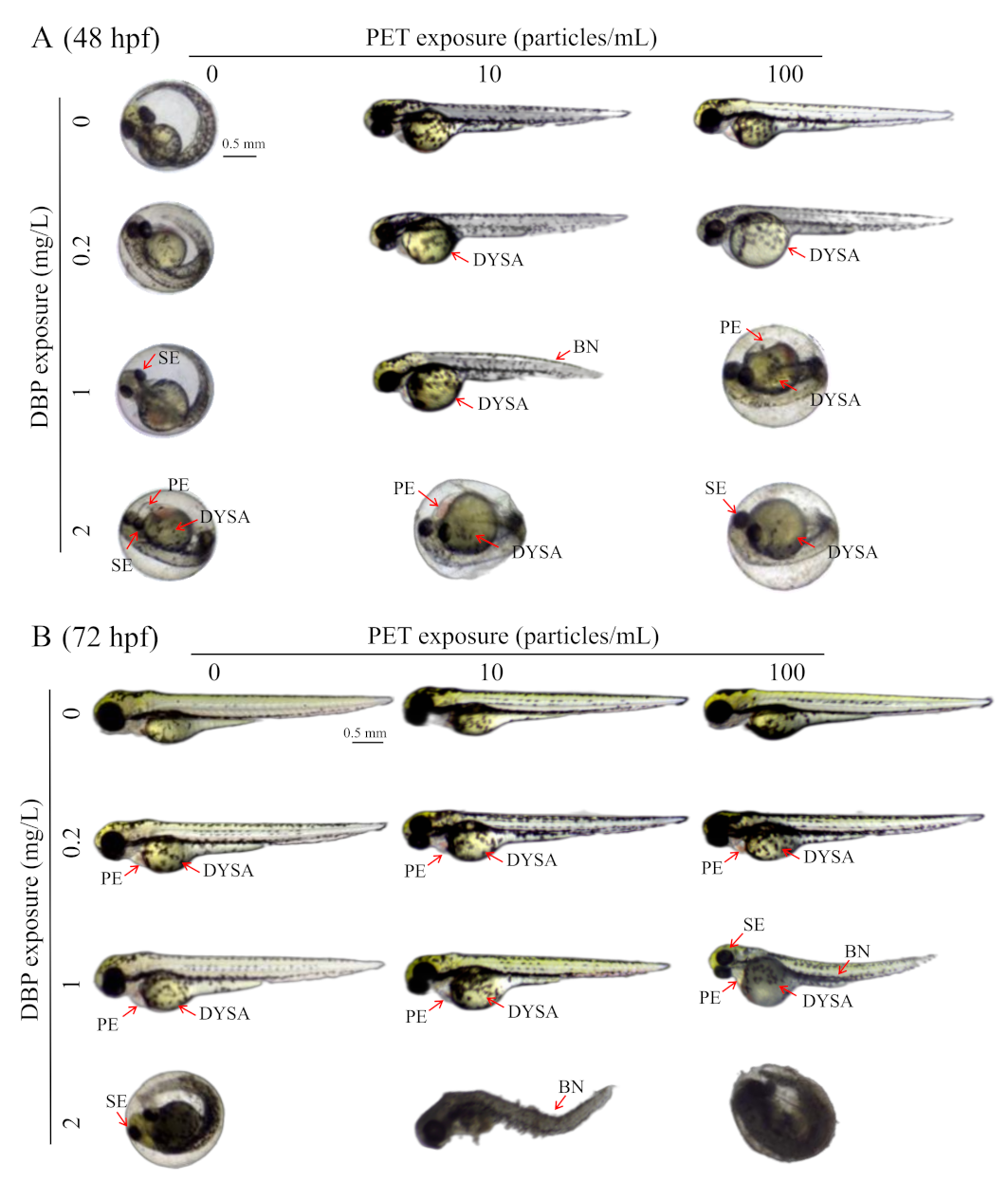
3.3. DBP Toxicities Affected by PET
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gong, J.; Xie, P. Research progress in sources, analytical methods, eco-environmental effects, and control measures of microplastics. Chemosphere 2020, 254, 126790. [Google Scholar] [CrossRef]
- Eriksen, M.; Lebreton, L.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic Pollution in the World’s Oceans: More than 5 Trillion Plastic Pieces Weighing over 250,000 Tons Afloat at Sea. PLoS ONE 2014, 9, e111913. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, M.; Chen, X.P.; Yang, C.M.; Wu, L.L. Combined toxicity of microplastics and cadmium on the zebrafish embryos (Danio rerio). Sci. Total Environ. 2020, 743, 140638. [Google Scholar] [CrossRef]
- He, Y.; Fan, G.J.; Wu, C.E.; Kou, X.H.; Li, T.T.; Tian, F.; Gong, H. Influence of packaging materials on postharvest physiology and texture of garlic cloves during refrigeration storage. Food Chem. 2019, 298, 125019. [Google Scholar] [CrossRef] [PubMed]
- Jemec, A.; Horvat, P.; Kunej, U.; Bele, M.; Krzan, A. Uptake and effects of microplastic textile fibers on freshwater crustacean Daphnia magna. Environ. Pollut. 2016, 219, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Atugoda, T.; Vithanage, M.; Wijesekara, H.; Bolan, N.; Sarmah, A.K.; Bank, M.S.; You, S.M.; Ok, Y.S. Interactions between microplastics, pharmaceuticals and personal care products: Implications for vector transport. Environ. Int. 2021, 149, 106367. [Google Scholar] [CrossRef]
- Holmes, L.A.; Turner, A.; Thompson, R.C. Interactions between trace metals and plastic production pellets under estuarine conditions. Mar. Chem. 2014, 167, 25–32. [Google Scholar] [CrossRef]
- Holmes, L.A.; Turner, A.; Thompson, R.C. Adsorption of trace metals to plastic resin pellets in the marine environment. Environ. Pollut. 2012, 160, 42–48. [Google Scholar] [CrossRef]
- Fonte, E.; Ferreira, P.; Guilhermino, L. Temperature rise and microplastics interact with the toxicity of the antibiotic cefalexin to juveniles of the common goby (Pomatoschistus microps): Post-exposure predatory behaviour, acetylcholinesterase activity and lipid peroxidation. Aquat. Toxicol. 2016, 180, 173–185. [Google Scholar] [CrossRef]
- Ashton, K.; Holmes, L.; Turner, A. Association of metals with plastic production pellets in the marine environment. Mar. Pollut. Bull. 2010, 60, 2050–2055. [Google Scholar] [CrossRef]
- Mao, Y.; Ai, H.; Chen, Y.; Zhang, Z.; Zeng, P.; Kang, L.; Li, W.; Gu, W.; He, Q.; Li, H. Phytoplankton response to polystyrene microplastics: Perspective from an entire growth period. Chemosphere 2018, 208, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Pan, J.; Li, M.; Li, Y.; Bartlam, M.; Wang, Y. Selective enrichment of bacterial pathogens by microplastic biofilm. Water Res. 2019, 165, 114979. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Qiao, R.; An, H.; Zhang, Y. Influence of microplastics on the accumulation and chronic toxic effects of cadmium in zebrafish (Danio rerio). Chemosphere 2018, 202, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Malafaia, G.; de Souza, A.M.; Pereira, A.C.; Gonçalves, S.; Da Costa Araújo, A.P.; Ribeiro, R.X.; Rocha, T.L. Developmental toxicity in zebrafish exposed to polyethylene microplastics under static and semi-static aquatic systems. Sci. Total Environ. 2020, 700, 134867. [Google Scholar] [CrossRef]
- Qiang, L.; Cheng, J. Exposure to microplastics decreases swimming competence in larval zebrafish (Danio rerio). Ecotox. Environ. Saf. 2019, 176, 226–233. [Google Scholar] [CrossRef]
- Qiao, R.; Sheng, C.; Lu, Y.; Zhang, Y.; Ren, H.; Lemos, B. Microplastics induce intestinal inflammation, oxidative stress, and disorders of metabolome and microbiome in zebrafish. Sci. Total Environ. 2019, 662, 246–253. [Google Scholar] [CrossRef]
- Jin, Y.; Xia, J.; Pan, Z.; Yang, J.; Wang, W.; Fu, Z. Polystyrene microplastics induce microbiota dysbiosis and inflammation in the gut of adult zebrafish. Environ. Pollut. 2018, 235, 322–329. [Google Scholar] [CrossRef]
- Wan, Z.; Wang, C.; Zhou, J.; Shen, M.; Wang, X.; Fu, Z.; Jin, Y. Effects of polystyrene microplastics on the composition of the microbiome and metabolism in larval zebrafish. Chemosphere 2019, 217, 646–658. [Google Scholar] [CrossRef]
- Chen, Q.; Gundlach, M.; Yang, S.; Jiang, J.; Velki, M.; Yin, D.; Hollert, H. Quantitative investigation of the mechanisms of microplastics and nanoplastics toward zebrafish larvae locomotor activity. Sci. Total Environ. 2017, 584–585, 1022–1031. [Google Scholar] [CrossRef]
- Qiao, R.; Lu, K.; Deng, Y.; Ren, H.; Zhang, Y. Combined effects of polystyrene microplastics and natural organic matter on the accumulation and toxicity of copper in zebrafish. Sci. Total Environ. 2019, 682, 128–137. [Google Scholar] [CrossRef]
- Yu, J.; Chen, L.; Gu, W.; Liu, S.; Wu, B. Heterogeneity effects of nanoplastics and lead on zebrafish intestinal cells identified by single-cell sequencing. Chemosphere 2022, 289, 133133. [Google Scholar] [CrossRef] [PubMed]
- Mak, C.W.; Ching-Fong Yeung, K.; Chan, K.M. Acute toxic effects of polyethylene microplastic on adult zebrafish. Ecotox. Environ. Saf. 2019, 182, 109442. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, C.M.R.; Kelleher, B.M.; Lagarde, R.; Northam, C.; Elebute, O.O.; Cassone, B.J. Transcriptional effects of polyethylene microplastics ingestion in developing zebrafish (Danio rerio). Environ. Pollut. 2018, 243, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhao, Y. Plasticizer Exposure and Reproductive Health: Phthalates and Bisphenol A. In Emerging Chemicals and Human Health; Zhang, Y., Ed.; Springer: Singapore, 2019; pp. 49–67. ISBN 978-981-32-9535-3. [Google Scholar]
- Cormier, B.; Cachot, J.; Blanc, M.; Cabar, M.; Clérandeau, C.; Dubocq, F.; Le Bihanic, F.; Morin, B.; Zapata, S.; Bégout, M.; et al. Environmental microplastics disrupt swimming activity in acute exposure in Danio rerio larvae and reduce growth and reproduction success in chronic exposure in D. rerio and Oryzias melastigma. Environ. Pollut. 2022, 308, 119721. [Google Scholar] [CrossRef]
- Rochman, C.M.; Kurobe, T.; Flores, I.; Teh, S.J. Early warning signs of endocrine disruption in adult fish from the ingestion of polyethylene with and without sorbed chemical pollutants from the marine environment. Sci. Total Environ. 2014, 493, 656–661. [Google Scholar] [CrossRef]
- Burton, G.A. Stressor Exposures Determine Risk: So, Why Do Fellow Scientists Continue To Focus on Superficial Microplastics Risk? Environ. Sci. Technol. 2017, 51, 13515–13516. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Besseling, E.; Foekema, E.; Kooi, M.; Mintenig, S.; Ossendorp, B.C.; Redondo-Hasselerharm, P.E.; Verschoor, A.; van Wezel, A.P.; Scheffer, M. Risks of Plastic Debris: Unravelling Fact, Opinion, Perception, and Belief. Environ. Sci. Technol. 2017, 51, 11513–11519. [Google Scholar] [CrossRef]
- Heudorf, U.; Mersch-Sundermann, V.; Angerer, J. Phthalates: Toxicology and exposure. Int. J. Hyg. Envir. Health 2007, 210, 623–634. [Google Scholar] [CrossRef]
- Schettler, T. Human exposure to phthalates via consumer products. Int. J. Androl. 2006, 29, 134–139. [Google Scholar] [CrossRef]
- Hauser, R.; Duty, S.; Godfrey-Bailey, L.; Calafat, A.M. Medications as a source of human exposure to phthalates. Environ. Health Perspect. 2004, 112, 751–753. [Google Scholar] [CrossRef]
- Schecter, A.; Lorber, M.; Guo, Y.; Wu, Q.; Yun, S.H.; Kannan, K.; Hommel, M.; Imran, N.; Hynan, L.S.; Cheng, D.; et al. Phthalate Concentrations and Dietary Exposure from Food Purchased in New York State. Environ. Health Perspect. 2013, 121, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Li, Y. Exposure to DBP induces the toxicity in early development and adverse effects on cardiac development in zebrafish (Danio rerio). Chemosphere 2019, 218, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Bang, D.Y.; Lee, I.K.; Lee, B. Toxicological Characterization of Phthalic Acid. Toxicol. Res. 2011, 27, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Karlovsky, P. Removal of the endocrine disrupter butyl benzyl phthalate from the environment. Appl. Microbiol. Biot. 2010, 87, 61–73. [Google Scholar] [CrossRef]
- Cotruvo, J.A. 2017 WHO Guidelines for Drinking Water Quality: First Addendum to the Fourth Edition. J. AWWA 2017, 109, 44–51. [Google Scholar] [CrossRef]
- Di Lorenzo, M.; Forte, M.; Valiante, S.; Laforgia, V.; De Falco, M. Interference of dibutylphthalate on human prostate cell viability. Ecotox. Environ. Saf. 2018, 147, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Staples, C.A.; Peterson, D.R.; Parkerton, T.F.; Adams, W.J. The environmental fate of phthalate esters: A literature review. Chemosphere 1997, 35, 667–749. [Google Scholar] [CrossRef]
- Högberg, J.; Hanberg, A.; Berglund, M.; Skerfving, S.; Remberger, M.; Calafat, A.M.; Filipsson, A.F.; Jansson, B.; Johansson, N.; Appelgren, M.; et al. Phthalate Diesters and Their Metabolites in Human Breast Milk, Blood or Serum, and Urine as Biomarkers of Exposure in Vulnerable Populations. Environ. Health Perspect. 2008, 116, 334–339. [Google Scholar] [CrossRef]
- Oehlmann, J.; Oetken, M.; Schulte-Oehlmann, U. A critical evaluation of the environmental risk assessment for plasticizers in the freshwater environment in Europe, with special emphasis on bisphenol A and endocrine disruption. Environ. Res. 2008, 108, 140–149. [Google Scholar] [CrossRef]
- Liu, H.; Cui, K.; Zeng, F.; Chen, L.; Cheng, Y.; Li, H.; Li, S.; Zhou, X.; Zhu, F.; Ouyang, G.; et al. Occurrence and distribution of phthalate esters in riverine sediments from the Pearl River Delta region, South China. Mar. Pollut. Bull. 2014, 83, 358–365. [Google Scholar] [CrossRef]
- Chen, H.; Mao, W.; Shen, Y.; Feng, W.; Mao, G.; Zhao, T.; Yang, L.; Yang, L.; Meng, C.; Li, Y.; et al. Distribution, source, and environmental risk assessment of phthalate esters (PAEs) in water, suspended particulate matter, and sediment of a typical Yangtze River Delta City, China. Environ. Sci. Pollut. Res. 2019, 26, 24609–24619. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Xu, L.; Wang, W.; Liu, W.; Liao, C.; Jiang, G. Occurrence, spatial distribution and ecological risk assessment of phthalate esters in water, soil and sediment from Yangtze River Delta, China. Sci. Total Environ. 2022, 806, 150966. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, J.; Choe, W.; Kim, T.; Lee, J.; Kho, Y.; Choi, K.; Zoh, K. Distribution of phthalate esters in air, water, sediments, and fish in the Asan Lake of Korea. Environ. Int. 2019, 126, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Pu, S.; Hamid, N.; Ren, Y.; Pei, D. Effects of phthalate acid esters on zebrafish larvae: Development and skeletal morphogenesis. Chemosphere 2020, 246, 125808. [Google Scholar] [CrossRef]
- Qian, L.; Liu, J.; Lin, Z.; Chen, X.; Yuan, L.; Shen, G.; Yang, W.; Wang, D.; Huang, Y.; Pang, S.; et al. Evaluation of the spinal effects of phthalates in a zebrafish embryo assay. Chemosphere 2020, 249, 126144. [Google Scholar] [CrossRef]
- Jergensen, T.; Cusmano, D.; Roy, N.M. Di-butyl phthalate (DBP) induces craniofacial defects during embryonic development in zebrafish. Ecotoxicology 2019, 28, 995–1002. [Google Scholar] [CrossRef]
- Mu, X.; Huang, Y.; Li, J.; Yang, K.; Yang, W.; Shen, G.; Li, X.; Lei, Y.; Pang, S.; Wang, C.; et al. New insights into the mechanism of phthalate-induced developmental effects. Environ. Pollut. 2018, 241, 674–683. [Google Scholar] [CrossRef]
- Barbagallo, S.; Baldauf, C.; Orosco, E.; Roy, N.M. Di-butyl phthalate (DBP) induces defects during embryonic eye development in zebrafish. Ecotoxicology 2022, 31, 178–185. [Google Scholar] [CrossRef]
- Cheng, H.; Feng, Y.; Duan, Z.; Duan, X.; Zhao, S.; Wang, Y.; Gong, Z.; Wang, L. Toxicities of microplastic fibers and granules on the development of zebrafish embryos and their combined effects with cadmium. Chemosphere 2021, 269, 128677. [Google Scholar] [CrossRef]
- Godoy, V.; Blázquez, G.; Calero, M.; Quesada, L.; Martín-Lara, M.A. The potential of microplastics as carriers of metals. Environ. Pollut. 2019, 255, 113363. [Google Scholar] [CrossRef]
- Rivera-Hernández, J.R.; Fernández, B.; Santos-Echeandia, J.; Garrido, S.; Morante, M.; Santos, P.; Albentosa, M. Biodynamics of mercury in mussel tissues as a function of exposure pathway: Natural vs microplastic routes. Sci. Total Environ. 2019, 674, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yin, D.; Jia, Y.; Schiwy, S.; Legradi, J.; Yang, S.; Hollert, H. Enhanced uptake of BPA in the presence of nanoplastics can lead to neurotoxic effects in adult zebrafish. Sci. Total Environ. 2017, 609, 1312–1321. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Chen, M.; Song, Y.; Wang, X.; Li, B.; Chen, Z.; Tsang, S.Y.; Cai, Z. Acute exposure to triphenyl phosphate inhibits the proliferation and cardiac differentiation of mouse embryonic stem cells and zebrafish embryos. J. Cell. Physiol. 2019, 234, 21235–21248. [Google Scholar] [CrossRef] [PubMed]
- Belanger, S.E.; Rawlings, J.M.; Carr, G.J. Use of fish embryo toxicity tests for the prediction of acute fish toxicity to chemicals. Environ. Toxicol. Chem. 2013, 32, 1768–1783. [Google Scholar] [CrossRef]
- Lammer, E.; Carr, G.J.; Wendler, K.; Rawlings, J.M.; Belanger, S.E.; Braunbeck, T. Is the fish embryo toxicity test (FET) with the zebrafish (Danio rerio) a potential alternative for the fish acute toxicity test? Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2009, 149, 196–209. [Google Scholar] [CrossRef]
- Aldavood, S.J.; Abbott, L.C.; Evans, Z.R.; Griffin, D.J.; Lee, M.D.; Quintero-Arevalo, N.M.; Villalobos, A.R. Effect of Cadmium and Nickel Exposure on Early Development in Zebrafish (Danio rerio) Embryos. Water-Sui. 2020, 12, 3005. [Google Scholar] [CrossRef]
- Buschmann, J. The OECD Guidelines for the Testing of Chemicals and Pesticides. In Teratogenicity Testing: Methods and Protocols; Barrow, P.C., Ed.; Humana Press: Totowa, NJ, USA, 2013; pp. 37–56. ISBN 978-1-62703-131-8. [Google Scholar]
- Su, L.; Xue, Y.; Li, L.; Yang, D.; Kolandhasamy, P.; Li, D.; Shi, H. Microplastics in Taihu Lake, China. Environ. Pollut. 2016, 216, 711–719. [Google Scholar] [CrossRef]
- Silva Brito, R.; Canedo, A.; Farias, D.; Rocha, T.L. Transgenic zebrafish (Danio rerio) as an emerging model system in ecotoxicology and toxicology: Historical review, recent advances, and trends. Sci. Total Environ. 2022, 848, 157665. [Google Scholar] [CrossRef]
- Mu, X.Y.; Qi, S.Z.; Liu, J.; Yuan, L.L.; Huang, Y.; Xue, J.Y.; Qian, L.; Wang, C.J.; Li, Y.R. Toxicity and behavioral response of zebrafish exposed to combined microplastic and bisphenol analogues. Environ. Chem. Lett. 2022, 20, 41–48. [Google Scholar] [CrossRef]
- Duan, Z.; Duan, X.; Zhao, S.; Wang, X.; Wang, J.; Liu, Y.; Peng, Y.; Gong, Z.; Wang, L. Barrier function of zebrafish embryonic chorions against microplastics and nanoplastics and its impact on embryo development. J. Hazard. Mater. 2020, 395, 122621. [Google Scholar] [CrossRef]
- Pitt, J.A.; Kozal, J.S.; Jayasundara, N.; Massarsky, A.; Trevisan, R.; Geitner, N.; Wiesner, M.; Levin, E.D.; Di Giulio, R.T. Uptake, tissue distribution, and toxicity of polystyrene nanoparticles in developing zebrafish (Danio rerio). Aquat. Toxicol. 2018, 194, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, J.; Zang, L.; Nishimura, N.; Shimada, Y. Zebrafish: An emerging model to study microplastic and nanoplastic toxicity. Sci. Total Environ. 2020, 728, 138707. [Google Scholar] [CrossRef] [PubMed]
- Sökmen, T.Ö.; Sulukan, E.; Türkoğlu, M.; Baran, A.; Özkaraca, M.; Ceyhun, S.B. Polystyrene nanoplastics (20 nm) are able to bioaccumulate and cause oxidative DNA damages in the brain tissue of zebrafish embryo (Danio rerio). Neurotoxicology 2020, 77, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Cheng, J.P.; Xin, Q. Effects of tetracycline on developmental toxicity and molecular responses in zebrafish (Danio rerio) embryos. Ecotoxicology 2015, 24, 707–719. [Google Scholar] [CrossRef]
- Sun, G.; Liu, K. Developmental toxicity and cardiac effects of butyl benzyl phthalate in zebrafish embryos. Aquat. Toxicol. 2017, 192, 165–170. [Google Scholar] [CrossRef]
- Mato, Y.; Isobe, T.; Takada, H.; Kanehiro, H.; Ohtake, C.; Kaminuma, T. Plastic Resin Pellets as a Transport Medium for Toxic Chemicals in the Marine Environment. Environ. Sci. Technol. 2001, 35, 318–324. [Google Scholar] [CrossRef]
- Fu, L.; Li, J.; Wang, G.; Luan, Y.; Dai, W. Adsorption behavior of organic pollutants on microplastics. Ecotox. Environ. Saf. 2021, 217, 112207. [Google Scholar] [CrossRef]
- Teuten, E.L.; Saquing, J.M.; Knappe, D.R.U.; Barlaz, M.A.; Jonsson, S.; Björn, A.; Rowland, S.J.; Thompson, R.C.; Galloway, T.S.; Yamashita, R.; et al. Transport and release of chemicals from plastics to the environment and to wildlife. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2027–2045. [Google Scholar] [CrossRef]
- Cao, Y.; Lin, H.; Zhang, K.; Xu, S.; Yan, M.; Leung, K.M.Y.; Lam, P.K.S. Microplastics: A major source of phthalate esters in aquatic environments. J. Hazard. Mater. 2022, 432, 128731. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Ma, W.; Zhu, J. Combined Toxicities of Di-Butyl Phthalate and Polyethylene Terephthalate to Zebrafish Embryos. Toxics 2023, 11, 469. https://doi.org/10.3390/toxics11050469
Zhang Q, Ma W, Zhu J. Combined Toxicities of Di-Butyl Phthalate and Polyethylene Terephthalate to Zebrafish Embryos. Toxics. 2023; 11(5):469. https://doi.org/10.3390/toxics11050469
Chicago/Turabian StyleZhang, Qiang, Wenjie Ma, and Jingmin Zhu. 2023. "Combined Toxicities of Di-Butyl Phthalate and Polyethylene Terephthalate to Zebrafish Embryos" Toxics 11, no. 5: 469. https://doi.org/10.3390/toxics11050469
APA StyleZhang, Q., Ma, W., & Zhu, J. (2023). Combined Toxicities of Di-Butyl Phthalate and Polyethylene Terephthalate to Zebrafish Embryos. Toxics, 11(5), 469. https://doi.org/10.3390/toxics11050469




