TLR/MyD88-Mediated Inflammation Induced in Porcine Intestinal Epithelial Cells by Ochratoxin A Affects Intestinal Barrier Function
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatment
2.2. qRT-PCR and Western Blotting
2.3. Immunofluorescence
2.4. Transepithelial Electrical Resistance (TEER)
2.5. Statistical Analysis
3. Results
3.1. OTA Exposure Affects TLR Expression in IPEC-J2 Cells
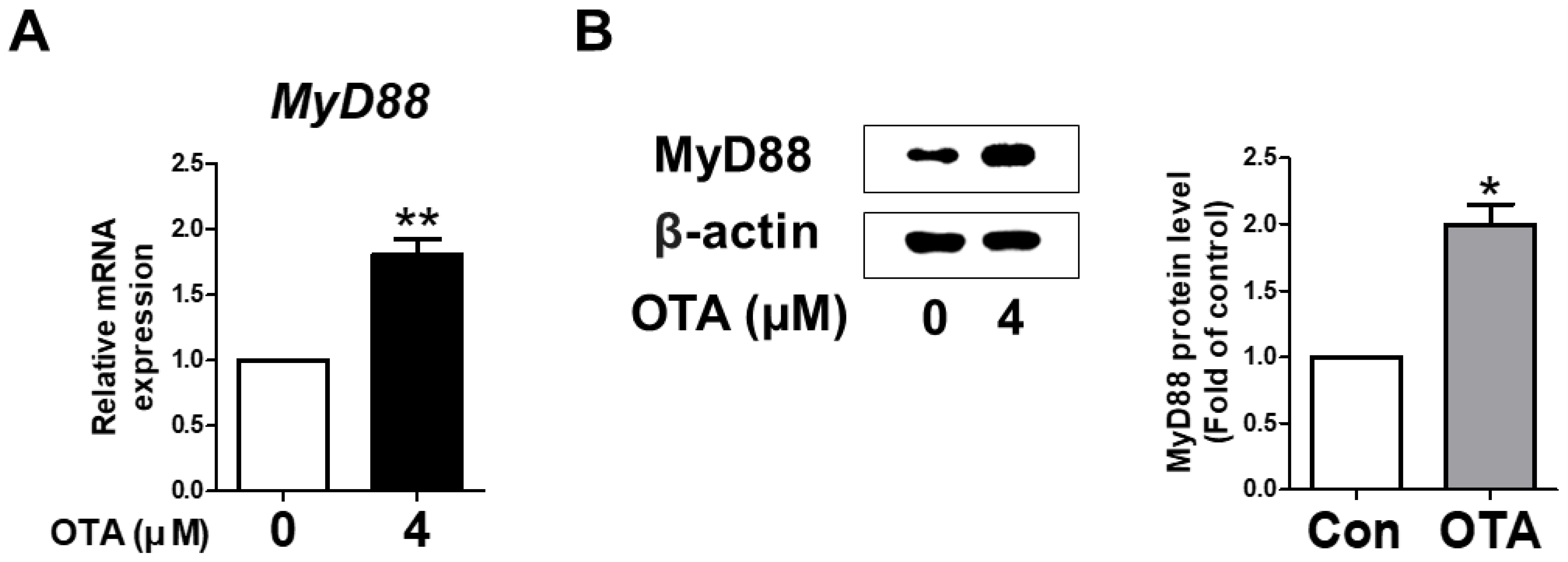
3.2. OTA Exposure Induces MyD88 Activation
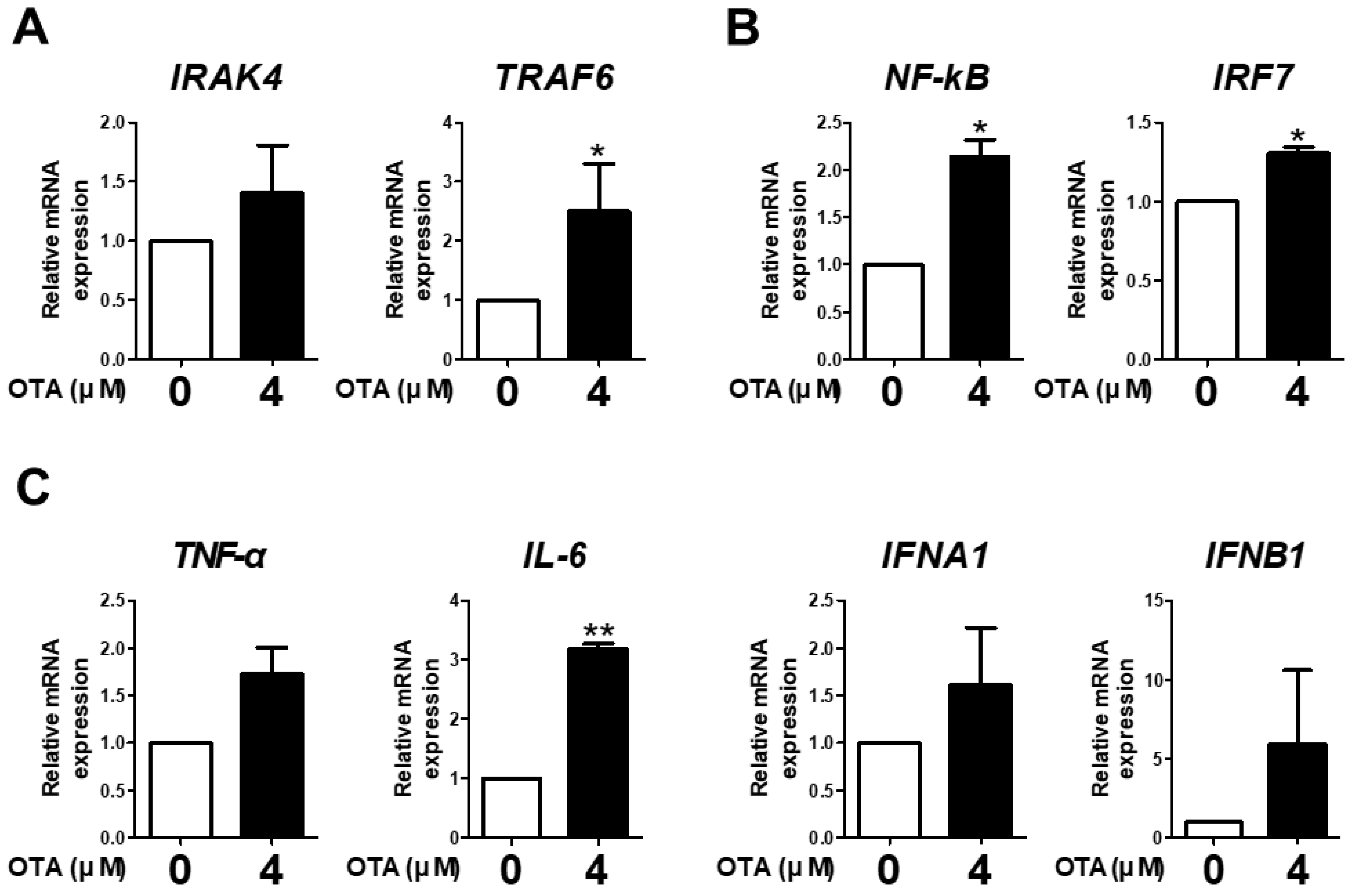
3.3. OTA Exposure Is Related to Downstream TLR/MyD88 Signaling
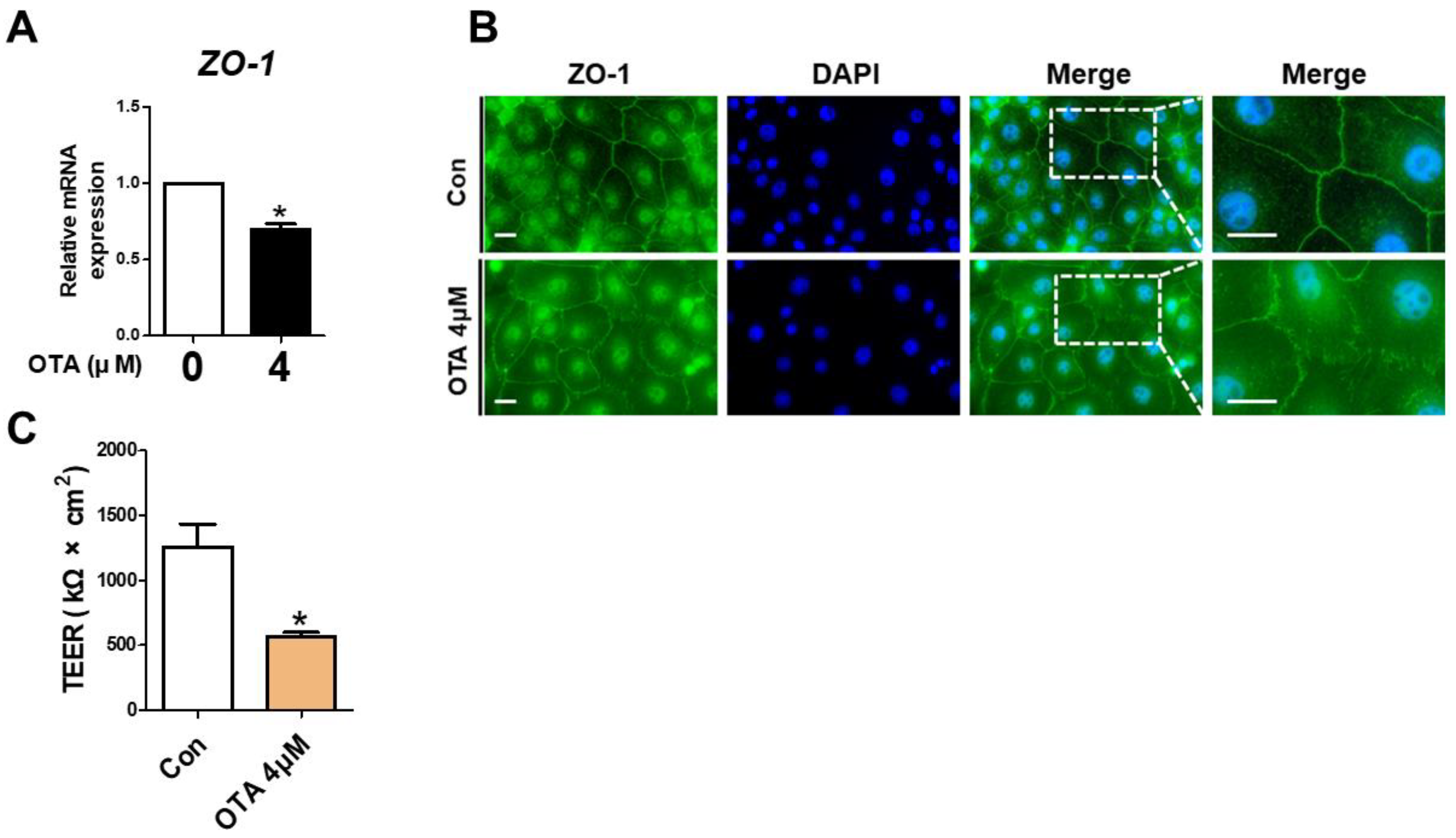
3.4. OTA Exposure Causes Tight Junction Disruption and Intestinal Barrier Damage
3.5. MYD88 Inhibition Alleviates Proinflammatory Cytokine and Intestinal Barrier in OTA Exposure
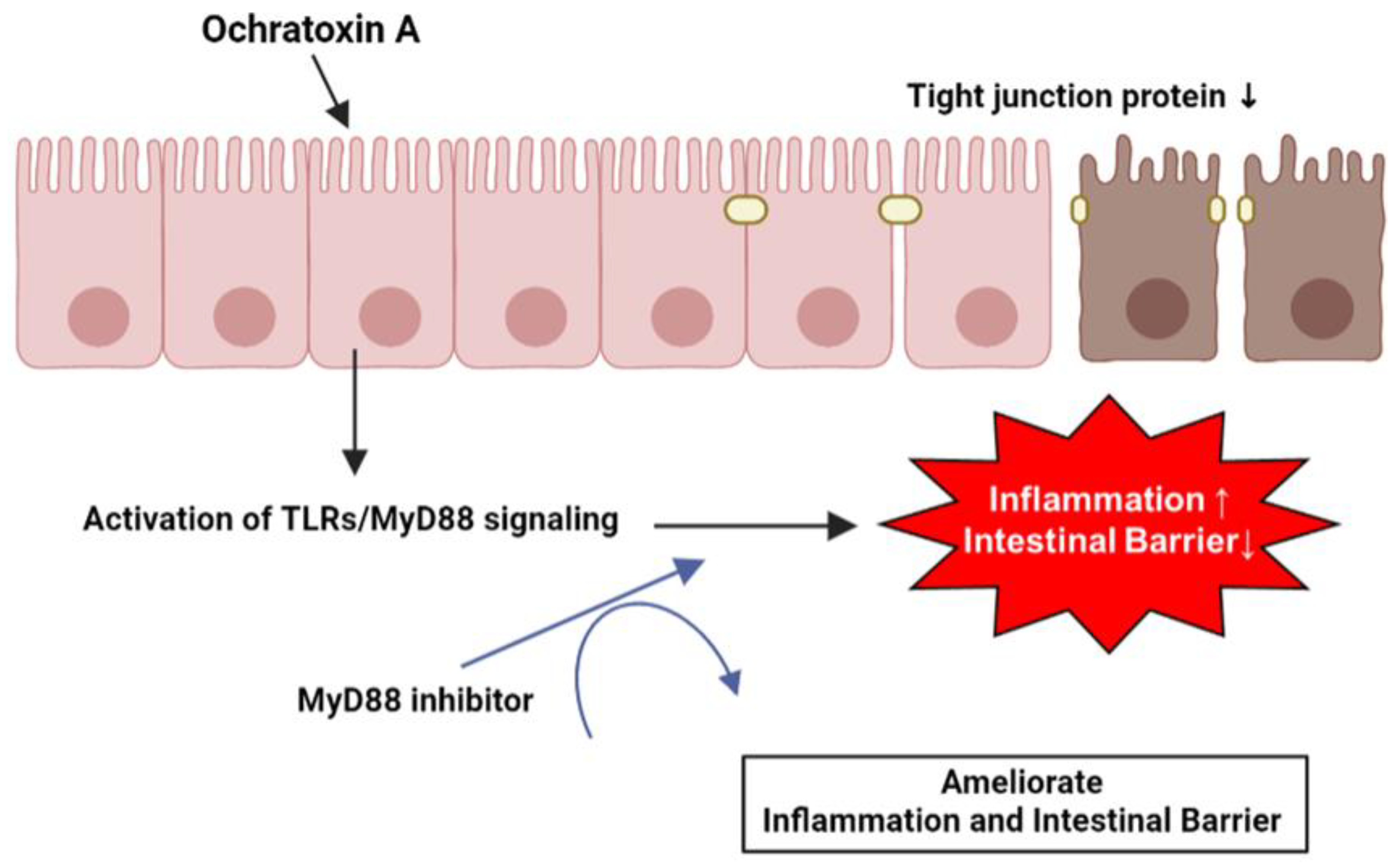
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shao, L.; Serrano, D.; Mayer, L. The role of epithelial cells in immune regulation in the gut. Semin. Immunol. 2001, 13, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Yang, X.; Wang, L.; Gao, K.; Jiang, Z. L-Arginine Inhibited Inflammatory Response and Oxidative Stress Induced by Lipopolysaccharide via Arginase-1 Signaling in IPEC-J2 Cells. Int. J. Mol. Sci. 2019, 20, 1800. [Google Scholar] [CrossRef] [PubMed]
- Duerr, C.U.; Hornef, M.W. The mammalian intestinal epithelium as integral player in the establishment and maintenance of host-microbial homeostasis. Semin. Immunol. 2012, 24, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Allaire, J.M.; Crowley, S.M.; Law, H.T.; Chang, S.Y.; Ko, H.J.; Vallance, B.A. The Intestinal Epithelium: Central Coordinator of Mucosal Immunity. Trends Immunol. 2018, 39, 677–696. [Google Scholar] [CrossRef] [PubMed]
- Wijtten, P.J.; van der Meulen, J.; Verstegen, M.W. Intestinal barrier function and absorption in pigs after weaning: A review. Br. J. Nutr. 2011, 105, 967–981. [Google Scholar] [CrossRef] [PubMed]
- Adil, M.S.; Narayanan, S.P.; Somanath, P.R. Cell-cell junctions: Structure and regulation in physiology and pathology. Tissue Barriers 2021, 9, 1848212. [Google Scholar] [CrossRef]
- Knöpfel, T.; Himmerkus, N.; Günzel, D.; Bleich, M.; Hernando, N.; Wagner, C.A. Paracellular transport of phosphate along the intestine. Am. J. Physiology. Gastrointest. Liver Physiol. 2019, 317, G233–G241. [Google Scholar] [CrossRef]
- Camilleri, M.; Madsen, K.; Spiller, R.; Greenwood-Van Meerveld, B.; Verne, G.N. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2012, 24, 503–512. [Google Scholar] [CrossRef]
- Odenwald, M.A.; Turner, J.R. The intestinal epithelial barrier: A therapeutic target? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 9–21. [Google Scholar] [CrossRef]
- Khalesi, M. Ochratoxin A in liquorice products—A review. Food Addit. Contaminants. Part A Chem. Anal. Control. Expo. Risk Assess. 2015, 32, 2086–2092. [Google Scholar] [CrossRef]
- Al-Anati, L.; Petzinger, E. Immunotoxic activity of ochratoxin A. J. Vet. Pharmacol. Ther. 2006, 29, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Malir, F.; Ostry, V.; Pfohl-Leszkowicz, A.; Malir, J.; Toman, J. Ochratoxin A: 50 Years of Research. Toxins 2016, 8, 191. [Google Scholar] [CrossRef] [PubMed]
- Ali, N. Co-occurrence of citrinin and ochratoxin A in rice in Asia and its implications for human health. J. Sci. Food Agric. 2018, 98, 2055–2059. [Google Scholar] [CrossRef]
- Creppy, E.E.; Baudrimont, I.; Betbeder, A.M. Prevention of nephrotoxicity of ochratoxin A, a food contaminant. Toxicol. Lett. 1995, 82–83, 869–877. [Google Scholar] [CrossRef]
- O’Brien, E.; Dietrich, D.R. Ochratoxin A: The continuing enigma. Crit. Rev. Toxicol. 2005, 35, 33–60. [Google Scholar] [CrossRef] [PubMed]
- Duarte, S.C.; Lino, C.M.; Pena, A. Ochratoxin A in feed of food-producing animals: An undesirable mycotoxin with health and performance effects. Vet. Microbiol. 2011, 154, 1–13. [Google Scholar] [CrossRef]
- Battacone, G.; Nudda, A.; Pulina, G. Effects of ochratoxin a on livestock production. Toxins 2010, 2, 1796–1824. [Google Scholar] [CrossRef]
- Yang, C.; Song, G.; Lim, W. Effects of mycotoxin-contaminated feed on farm animals. J. Hazard. Mater. 2020, 389, 122087. [Google Scholar] [CrossRef]
- Zhai, S.; Zhu, Y.; Feng, P.; Li, M.; Wang, W.; Yang, L.; Yang, Y. Ochratoxin A: Its impact on poultry gut health and microbiota, an overview. Poult. Sci. 2021, 100, 101037. [Google Scholar] [CrossRef]
- Tao, Y.; Xie, S.; Xu, F.; Liu, A.; Wang, Y.; Chen, D.; Pan, Y.; Huang, L.; Peng, D.; Wang, X.; et al. Ochratoxin A: Toxicity, oxidative stress and metabolism. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2018, 112, 320–331. [Google Scholar] [CrossRef]
- Wang, H.; Zhai, N.; Chen, Y.; Fu, C.; Huang, K. OTA induces intestinal epithelial barrier dysfunction and tight junction disruption in IPEC-J2 cells through ROS/Ca2+-mediated MLCK activation. Environ. Pollut. 2018, 242, 106–112. [Google Scholar] [CrossRef] [PubMed]
- El Cafsi, I.; Bjeoui, S.; Rabeh, I.; Nechi, S.; Chelbi, E.; El Cafsi, M.; Ghram, A. Effects of ochratoxin A on membrane phospholipids of the intestine of broiler chickens, practical consequences. Anim. Int. J. Anim. Biosci. 2020, 14, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. TLR signaling. Semin. Immunol. 2007, 19, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Deguine, J.; Barton, G.M. MyD88: A central player in innate immune signaling. F1000prime Rep. 2014, 6, 97. [Google Scholar] [CrossRef]
- Tohno, M.; Shimazu, T.; Aso, H.; Kawai, Y.; Saito, T.; Kitazawa, H. Molecular cloning and functional characterization of porcine MyD88 essential for TLR signaling. Cell. Mol. Immunol. 2007, 4, 369–376. [Google Scholar]
- Dai, C.; Sun, L.; Yu, L.; Zhu, G.; Wu, S.; Bao, W. Effects of porcine MyD88 knockdown on the expression of TLR4 pathway-related genes and proinflammatory cytokines. Biosci. Rep. 2016, 36, e00409. [Google Scholar] [CrossRef]
- Yoon, J.W.; Lee, S.I. Gene expression profiling after ochratoxin A treatment in small intestinal epithelial cells from pigs. J. Anim. Sci. Technol. 2022, 64, 842–853. [Google Scholar] [CrossRef]
- Pleadin, J.; Frece, J.; Markov, K. Mycotoxins in food and feed. Adv. Food Nutr. Res. 2019, 89, 297–345. [Google Scholar] [CrossRef]
- Petzinger, E.; Ziegler, K. Ochratoxin A from a toxicological perspective. J. Vet. Pharmacol. Ther. 2000, 23, 91–98. [Google Scholar] [CrossRef]
- Fog Nielsen, K. Mycotoxin production by indoor molds. Fungal. Genet. Biol. 2003, 39, 103–117. [Google Scholar] [CrossRef]
- Gao, Y.; Meng, L.; Liu, H.; Wang, J.; Zheng, N. The Compromised Intestinal Barrier Induced by Mycotoxins. Toxins 2020, 12, 619. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Akira, S. TLR signaling pathways. Semin. Immunol. 2004, 16, 3–9. [Google Scholar] [CrossRef]
- Uematsu, S.; Sato, S.; Yamamoto, M.; Hirotani, T.; Kato, H.; Takeshita, F.; Matsuda, M.; Coban, C.; Ishii, K.J.; Kawai, T.; et al. Interleukin-1 receptor-associated kinase-1 plays an essential role for Toll-like receptor (TLR)7- and TLR9-mediated interferon-{alpha} induction. J. Exp. Med. 2005, 201, 915–923. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol. Med. 2007, 13, 460–469. [Google Scholar] [CrossRef]
- Kuo, W.T.; Odenwald, M.A.; Turner, J.R.; Zuo, L. Tight junction proteins occludin and ZO-1 as regulators of epithelial proliferation and survival. Ann. N. Y. Acad. Sci. 2022, 1514, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Van Itallie, C.M. Physiology and function of the tight junction. Cold Spring Harb. Perspect. Biol. 2009, 1, a002584. [Google Scholar] [CrossRef]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef]
- Allam-Ndoul, B.; Castonguay-Paradis, S.; Veilleux, A. Gut Microbiota and Intestinal Trans-Epithelial Permeability. Int. J. Mol. Sci. 2020, 21, 6402. [Google Scholar] [CrossRef]
- González-Castro, A.M.; Martínez, C.; Salvo-Romero, E.; Fortea, M.; Pardo-Camacho, C.; Pérez-Berezo, T.; Alonso-Cotoner, C.; Santos, J.; Vicario, M. Mucosal pathobiology and molecular signature of epithelial barrier dysfunction in the small intestine in irritable bowel syndrome. J. Gastroenterol. Hepatol. 2017, 32, 53–63. [Google Scholar] [CrossRef]
- Gao, X.; Yang, Q.; Huang, X.; Yan, Z.; Zhang, S.; Luo, R.; Wang, P.; Wang, W.; Xie, K.; Jiang, T.; et al. Effects of Clostridium perfringens beta2 toxin on apoptosis, inflammation, and barrier function of intestinal porcine epithelial cells. Microb. Pathog. 2020, 147, 104379. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.I.; Kang, K.S. Function of capric acid in cyclophosphamide-induced intestinal inflammation, oxidative stress, and barrier function in pigs. Sci. Rep. 2017, 7, 16530. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Deng, J.; Hu, X.; Zhou, S.; Wu, J.; Xiao, D.; Darko, K.O.; Huang, Y.; Tao, T.; Peng, M.; et al. Vitamin A inhibits the action of LPS on the intestinal epithelial barrier function and tight junction proteins. Food Funct. 2019, 10, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Ajuwon, K.M. Butyrate modifies intestinal barrier function in IPEC-J2 cells through a selective upregulation of tight junction proteins and activation of the Akt signaling pathway. PLoS ONE 2017, 12, e0179586. [Google Scholar] [CrossRef]
- Förster, C. Tight junctions and the modulation of barrier function in disease. Histochem. Cell Biol. 2008, 130, 55–70. [Google Scholar] [CrossRef]


| Genes | Description | Accession No. | Sequence (5′-3′) | |
|---|---|---|---|---|
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | NM_001206359 | Forward Reverse | ACACCGAGCATCTCCTGACT GACGAGGCAGGTCTCCCTAA |
| TLR1 | Toll-like receptor 1 | NM_001031775 | Forward Reverse | TGTGTTGGAAGAGGTCAGGA TGGCAAAATGGAAGATGCTA |
| TLR2 | Toll-like receptor 2 | NM_213761 | Forward Reverse | CCAGGCAAGTGGATTATTGA AAGAGACGGAAGTGGGAGAA |
| TLR3 | Toll-like receptor 3 | NM_001097444 | Forward Reverse | GTGCCCCATTTGAACTCTTT TGCTCTGGCTGCTTGTCTAT |
| TLR4 | Toll-like receptor 4 | NM_001113039 | Forward Reverse | TGGTCAGCCTCCAAACCTTC GGAATGAAAATGCCCTCTGGG |
| TLR5 | Toll-like receptor 5 | NM_001348771 | Forward Reverse | GGCTTCTGTTGGGATGTTTT CGAGGTGAGCAGGTAAGTCA |
| TLR6 | Toll-like receptor 6 | NM_213760 | Forward Reverse | AGACACGGGCTGGACTTACT GCCACCTCATTTACCTCTGG |
| TLR7 | Toll-like receptor 7 | NM_001097434 | Forward Reverse | CCTTCTTCCTCCTTGCCTCT TTGGCTGATGCTATTTCCTG |
| TLR8 | Toll-like receptor 8 | NM_214187 | Forward Reverse | CTTCCCACATCCCAGACTTT GTCCCTCTCCTCCAAACAGA |
| TLR9 | Toll-like receptor 9 | NM_213958 | Forward Reverse | TCTGACTTCGTCCACCTGTC GGTCGTGATGCTGTTGTAGC |
| MyD88 | Myeloid differentiation primary response 88 | NM_001099923 | Forward Reverse | GTCGGATGGTAGTGGTTGTCT TGCTGGGGAACTCTTTCTTC |
| IRAK4 | Interleukin 1 receptor-associated kinase 4 | NM_001112693 | Forward Reverse | GAACACAACTCTATGCCACCA AATCCTCCCTCTCCCATCTT |
| TRAF6 | TNF receptor-associated factor 6 | NM_001105286 | Forward Reverse | TCTCTGACGGTGAAGTGTCC AAGTTGGCATTTTTGGAAGG |
| NF-κB | Nuclear factor of kappa light polypeptide gene enhancer in B-cells 1 | NM_001048232 | Forward Reverse | TCTCTGACGGTGAAGTGTCC AAGTTGGCATTTTTGGAAGG |
| IRF7 | Interferon regulatory factor 7 | XM_021076250 | Forward Reverse | CGTCCTGGTGAAGTTGGAG GCTCGTCATAGAGGCTGTTG |
| IL-6 | Interleukin-6 | NM_001252429 | Forward Reverse | GCTTCCAATCTGGGTTCAAT ATTCTTTCCCTTTTGCCTCA |
| TNFα | Tumor necrosis factorα | NM_214022 | Forward Reverse | TTTCTGTGAAAACGGAGCTG CAGCGATGTAGCGACAAGTT |
| IFNA1 | Interferon alpha 1 | NM_214393 | Forward Reverse | GGACCACAGAAGGGACTTTG CTCTCATTCCAGGCAGCAG |
| IFNB1 | Interferon beta 1 | NM_001003923 | Forward Reverse | TACCAACAAAGGAGCAGCAA GGTTTCATTCCAGCCAGTG |
| ZO-1 | Zonula occludens 1 | XM_021098856 | Forward Reverse | GATCCTGACCCGGTGTCTGA TTGGTGGGTTTGGTGGGTTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, J.W.; Shin, S.; Park, J.; Lee, B.R.; Lee, S.I. TLR/MyD88-Mediated Inflammation Induced in Porcine Intestinal Epithelial Cells by Ochratoxin A Affects Intestinal Barrier Function. Toxics 2023, 11, 437. https://doi.org/10.3390/toxics11050437
Yoon JW, Shin S, Park J, Lee BR, Lee SI. TLR/MyD88-Mediated Inflammation Induced in Porcine Intestinal Epithelial Cells by Ochratoxin A Affects Intestinal Barrier Function. Toxics. 2023; 11(5):437. https://doi.org/10.3390/toxics11050437
Chicago/Turabian StyleYoon, Jung Woong, Sangsu Shin, JeongWoong Park, Bo Ram Lee, and Sang In Lee. 2023. "TLR/MyD88-Mediated Inflammation Induced in Porcine Intestinal Epithelial Cells by Ochratoxin A Affects Intestinal Barrier Function" Toxics 11, no. 5: 437. https://doi.org/10.3390/toxics11050437
APA StyleYoon, J. W., Shin, S., Park, J., Lee, B. R., & Lee, S. I. (2023). TLR/MyD88-Mediated Inflammation Induced in Porcine Intestinal Epithelial Cells by Ochratoxin A Affects Intestinal Barrier Function. Toxics, 11(5), 437. https://doi.org/10.3390/toxics11050437







