Magnesium Supplementation Alleviates the Toxic Effects of Silica Nanoparticles on the Kidneys, Liver, and Adrenal Glands in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of SiNPs Suspension
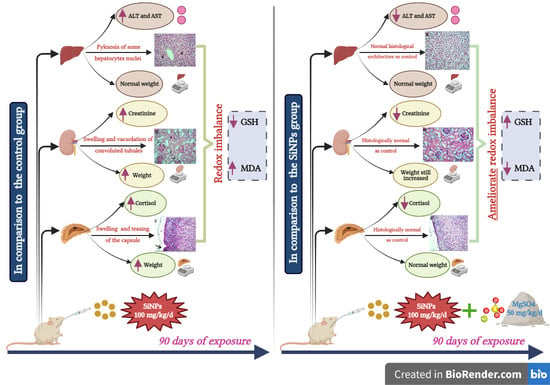
2.3. Animals and Experimental Design
2.4. Quantitative Estimation of Liver Enzymes, Serum Creatinine, and Cortisol Levels
2.5. Assessment of Oxidative Stress Markers
2.6. Histopathological Examination
2.7. Statistical Analysis
3. Results
3.1. Characterization of SiNPs
3.2. Clinical Signs and Mortality
3.3. Hepatic Function Assessment
3.4. Renal Function Assessment
3.5. Adrenal Function Assessment
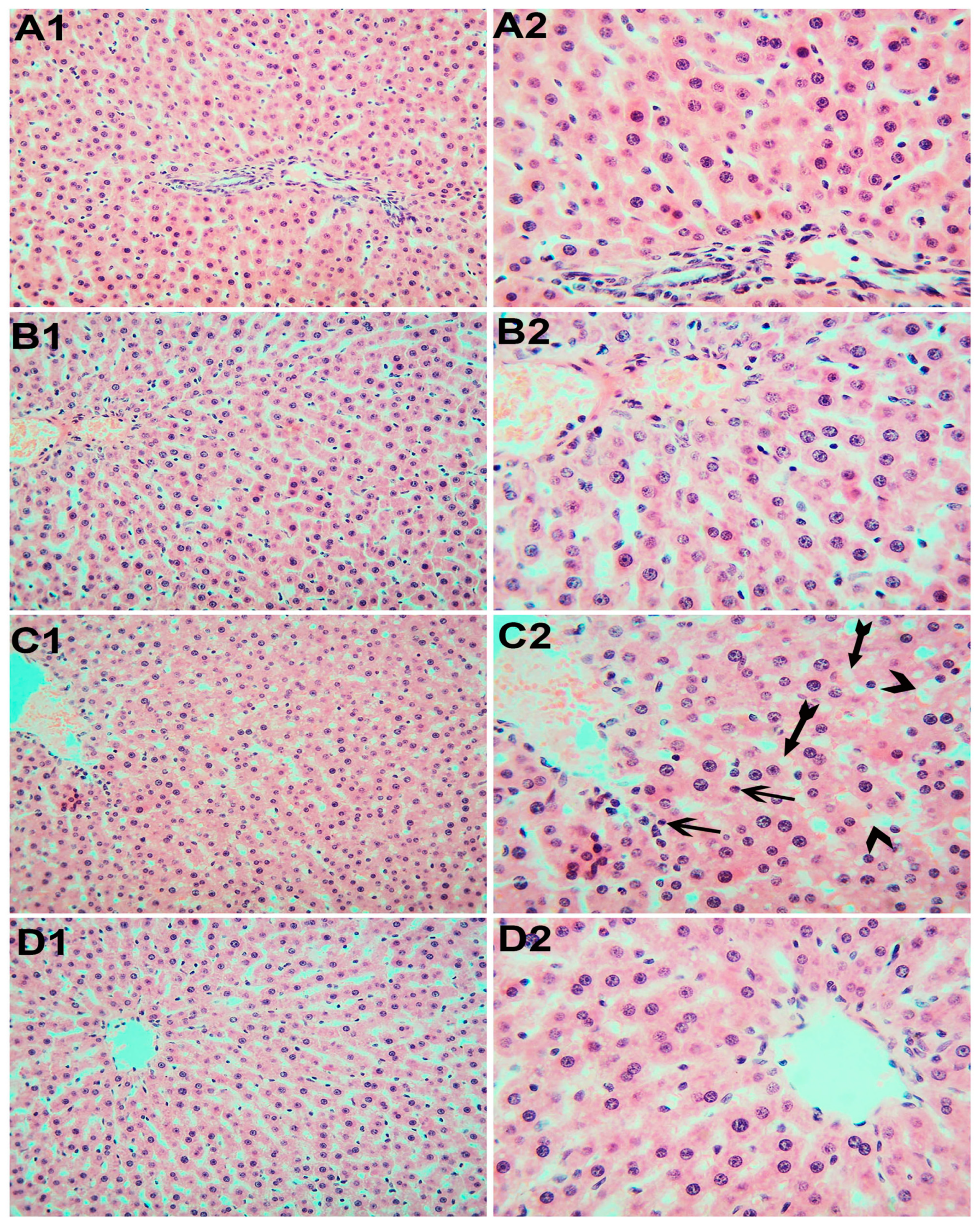
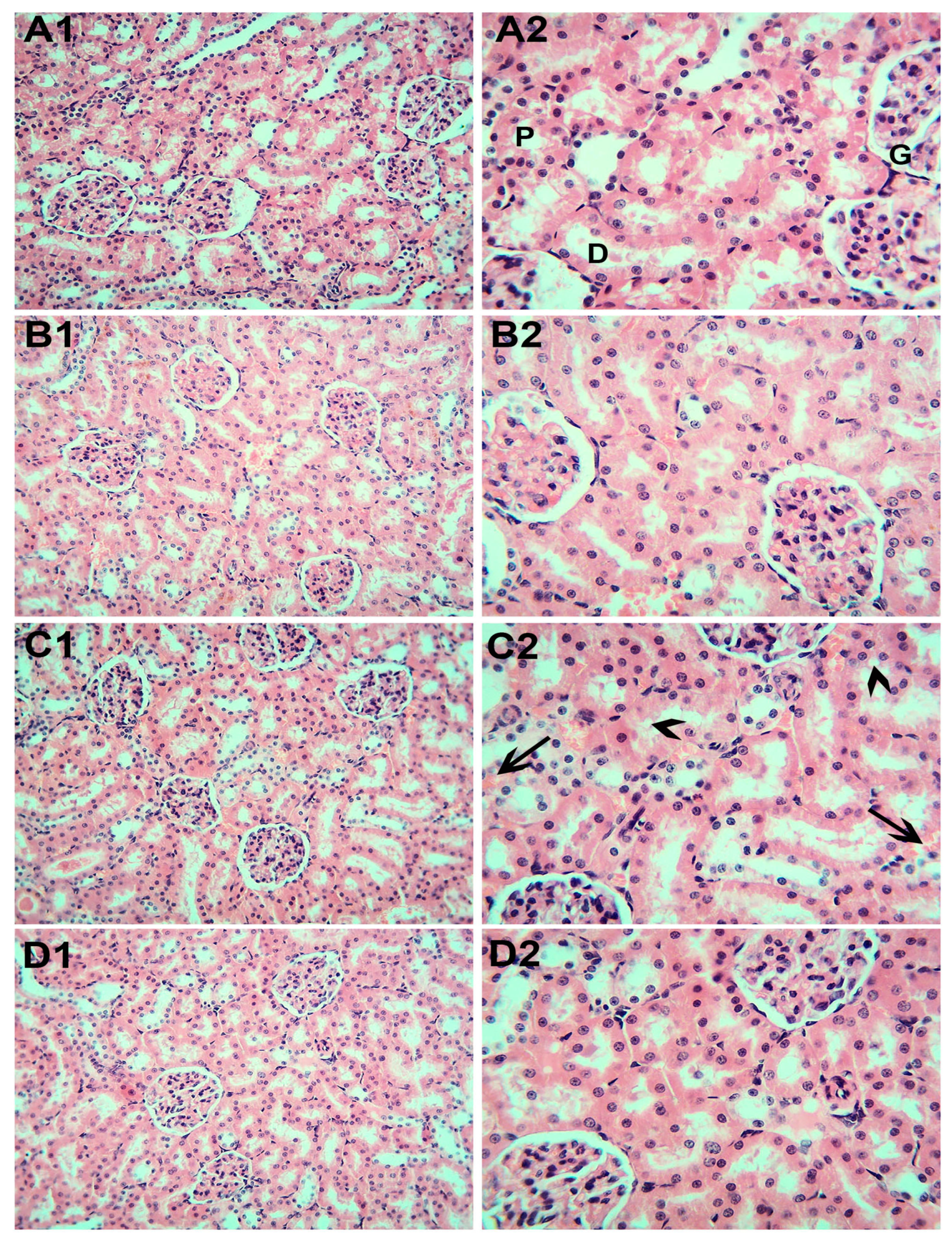
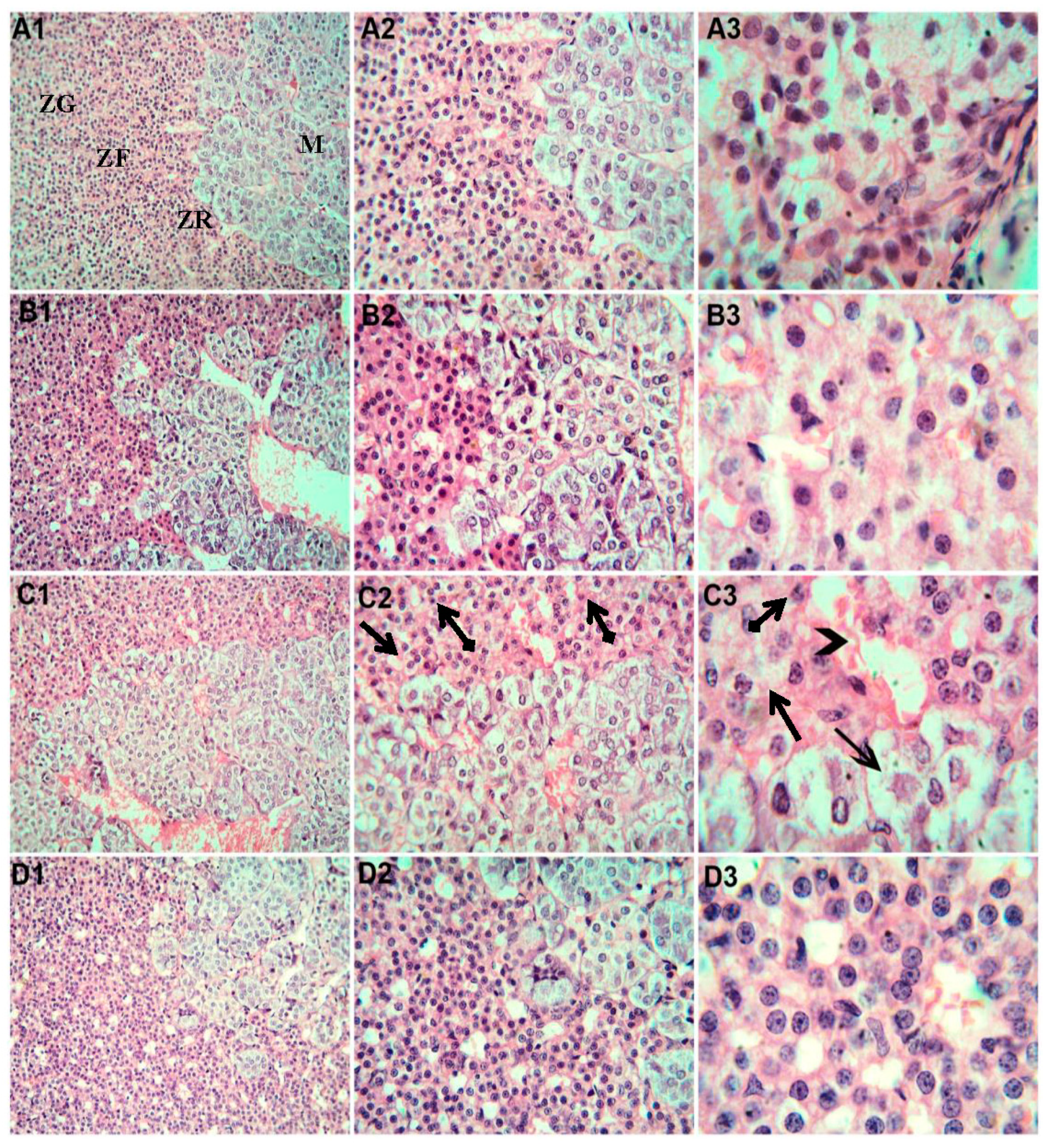
3.6. Histopathological Examination
3.7. Oxidative Stress Assessment
3.8. Weight of the Liver, Kidneys, and Adrenal Glands
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, X.; Liu, R. Recent progress and perspectives on the toxicity of carbon nanotubes at organism, organ, cell, and biomacromolecule levels. Environ. Int. 2012, 40, 244–255. [Google Scholar] [CrossRef]
- Kim, Y.R.; Lee, S.Y.; Lee, E.J.; Park, S.H.; Seong, N.W.; Seo, H.S.; Shin, S.S.; Kim, S.J.; Meang, E.H.; Park, M.K.; et al. Toxicity of colloidal silica nanoparticles administered orally for 90 days in rats. Int. J. Nanomed. 2014, 9, 67–78. [Google Scholar] [CrossRef]
- Bara, N.; Eshwarmoorthy, M.; Subaharan, K.; Kaul, G. Mesoporous silica nanoparticle is comparatively safer than zinc oxide nanoparticle which can cause profound steroidogenic effects on pregnant mice and male offspring exposed in utero. Toxicol. Ind. Health 2018, 34, 507–524. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Mu, Y.; Peng, C.; Lavin, M.F.; Shao, H.; Du, Z. Understanding the mechanisms of silica nanoparticles for nanomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1658. [Google Scholar] [CrossRef]
- van Kesteren, P.C.; Cubadda, F.; Bouwmeester, H.; van Eijkeren, J.C.; Dekkers, S.; de Jong, W.H.; Oomen, A.G. Novel insights into the risk assessment of the nanomaterial synthetic amorphous silica, additive E551, in food. Nanotoxicology 2015, 9, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Faust, J.J.; Schoepf, J.; Hristovski, K.; Capco, D.G.; Herckes, P.; Westerhoff, P. Survey of food-grade silica dioxide nanomaterial occurrence, characterization, human gut impacts and fate across its lifecycle. Sci. Total Environ. 2016, 565, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Gundert-Remy, U. Re-evaluation of silicon dioxide (E 551) as a food additive. EFSA J. 2018, 16, e05088. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wu, M.; Zhao, J.X. Recent development of silica nanoparticles as delivery vectors for cancer imaging and therapy. Nanomedicine 2014, 10, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.A.; Padavettan, V. Synthesis of silica nanoparticles by sol-gel: Size-dependent properties, surface modification, and applications in silicapolymer nanocomposites—A review. J. Nanomater. 2012, 2012, 8. [Google Scholar] [CrossRef]
- Azouz, R.A.; Korany, R.M.S. Toxic Impacts of Amorphous Silica Nanoparticles on Liver and Kidney of Male Adult Rats: An In Vivo Study. Biol. Trace. Elem. Res. 2021, 199, 2653–2662. [Google Scholar] [CrossRef] [PubMed]
- Hassankhani, R.; Esmaeillou, M.; Tehrani, A.A.; Nasirzadeh, K.; Khadir, F.; Maadi, H. In vivo toxicity of orally administrated silicon dioxide nanoparticles in healthy adult mice. Environ. Sci. Pollut. Res. 2015, 22, 1127–1132. [Google Scholar] [CrossRef]
- Nemmar, A.; Yuvaraju, P.; Beegam, S.; Yasin, J.; Kazzam, E.E.; Ali, B.H. Oxidative stress, inflammation, and DNA damage in multiple organs of mice acutely exposed to amorphous silica nanoparticles. Int. J. Nanomed. 2016, 11, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, J.; Ahamed, M.; Akhtar, M.J.; Alrokayan, S.A.; Siddiqui, M.A.; Musarrat, J.; Al-Khedhairy, A.A. Apoptosis induction by silica nanoparticles mediated through reactive oxygen species in human liver cell line HepG2. Toxicol. Appl. Pharmacol. 2012, 259, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Passagne, I.; Morille, M.; Rousset, M.; Pujalte, I.; L’Azou, B. Implication of oxidative stress in size-dependent toxicity of silica nanoparticles in kidney cells. Toxicology 2012, 299, 112–124. [Google Scholar] [CrossRef]
- Yang, X.; Liu, J.; He, H.; Zhou, L.; Gong, C.; Wang, X.; Yang, L.; Yuan, J.; Huang, H.; He, L.; et al. SiO2 nanoparticles induce cytotoxicity and protein expression alteration in HaCaT cells. Part Fibre Toxicol. 2010, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. 1997, 82, 291–295. [Google Scholar] [CrossRef]
- Heistad, D.D. Oxidative stress and vascular disease: 2005 Duff lecture. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 689–695. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Biochemistry of oxidative stress. Biochem. Soc. Trans. 2007, 35, 1147–1150. [Google Scholar] [CrossRef] [PubMed]
- Gutteridge, J.M. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin. Chem. 1995, 41, 1819–1828. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Reactive oxygen species in living systems: Source, biochemistry and role in human disease. Am. J. Med. 1991, 91, 14S–22S. [Google Scholar] [CrossRef] [PubMed]
- Fridovich, I. Fundamental aspects of reactive oxygen species, or what’s matter with oxygen? Ann. N. Y. Acad. Sci. 1999, 893, 13–18. [Google Scholar] [CrossRef]
- Crack, P.J.; Taylor, J.M. Reactive oxygen species and the modulation of stroke. Free Radic. Biol. Med. 2005, 38, 1433–1444. [Google Scholar] [CrossRef] [PubMed]
- Padmaja, S.; Squadrito, G.L.; Pryor, W.A. Inactivation of glutathione peroxidase by peroxynitrite. Arch. Biochem. Biophys. 1998, 349, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Romani, A.M. Cellular magnesium homeostasis. Arch. Biochem. Biophys. 2011, 512, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, Y.; Mollaoglu, H.; Yurumez, Y.; Ucok, K.; Duran, L.; Tünay, K.; Akgün, L. Therapeutic effect of magnesium sulphate on carbon monoxide toxicity-mediated brain lipid peroxidation. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 28–33. [Google Scholar] [PubMed]
- Vernet, P.; Britan, A.; Gueux, E.; Mazur, A.; Drevet, J.R. Dietary magnesium depletion does not promote oxidative stress but targets apical cells within the mouse caput epididymidis. Biochim. Biophys. Acta 2004, 1675, 32–45. [Google Scholar] [CrossRef]
- Shafeeq, S.; Mahboob, T. Magnesium supplementation ameliorates toxic effects of 2,4-dichlorophenoxyacetic acid in rat model. Hum. Exp. Toxicol. 2020, 39, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Kostellow, A.B.; Morrill, G.A. Iron-catalyzed lipid peroxidation in aortic cells in vitro: Protective effect of extracellular magnesium. Atherosclerosis 2004, 175, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, M.; Dominguez, L.J.; Tagliamonte, M.R.; Resnick, L.M.; Paolisso, G. Effects of vitamin E and glutathione on glucose metabolism: Role of magnesium. Hypertension 1999, 34, 1002–1006. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.H.; Miyamoto, M.; Sastre, A.; Schnaar, R.L.; Coyle, J.T. Glutamate toxicity in a neuronal cell line involves inhibition of cystine transport leading to oxidative stress. Neuron 1989, 2, 1547–1558. [Google Scholar] [CrossRef]
- Canesi, L.; Ciacci, C.; Vallotto, D.; Gallo, G.; Marcomini, A.; Pojana, G. In vitro effects of suspensions of selected nanoparticles (C60 fullerene, TiO2, SiO2) on Mytilus hemocytes. Aquat. Toxicol. 2010, 96, 151–158. [Google Scholar] [CrossRef]
- Vasbinder, M.A.; Locke, P. Introduction: Global Laws, Regulations, and Standards for Animals in Research. ILAR J. 2016, 57, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, L.A.; Al-Husseini, A.M. Estimate Toxic Effect of Silica Nanoparticles on Kidney, Liver and Lung Function of Male Albino Rats. Syst. Rev. Pharm. 2021, 12, 570–575. [Google Scholar] [CrossRef]
- Hu, H.; Fan, X.; Guo, Q.; Wei, X.; Yang, D.; Zhang, B.; Liu, J.; Wu, Q.; Oh, Y.; Feng, Y.; et al. Silicon dioxide nanoparticles induce insulin resistance through endoplasmic reticulum stress and generation of reactive oxygen species. Part Fibre Toxicol. 2019, 16, 41. [Google Scholar] [CrossRef] [PubMed]
- Kadu, P.J.; Kushare, S.S.; Thacker, D.D.; Gattani, S.G. Enhancement of oral bioavailability of atorvastatin calcium by self-emulsifying drug delivery systems (SEDDS). Pharm. Dev. Technol. 2011, 16, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, T.; Fu, C.; Tan, L.; Meng, X.; Liu, H. Biodistribution, excretion, and toxicity of mesoporous silica nanoparticles after oral administration depend on their shape. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1915–1924. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.A.; Kim, M.K.; Paek, H.J.; Kim, Y.R.; Kim, M.K.; Lee, J.K.; Jeong, J.; Choi, S.J. Tissue distribution and excretion kinetics of orally administered silica nanoparticles in rats. Int. J. Nanomed. 2014, 9, 251–260. [Google Scholar] [CrossRef]
- Van der Zande, M.; Vandebriel, R.; Groot, M.; Kramer, E.; Herrera Rivera, Z.; Rasmussen, K.; Ossenkoppele, J.; Tromp, P.; Gremmer, E.; Peters, R.; et al. Sub-chronic toxicity study in rats orally exposed to nanostructured silica. Part Fibre Toxicol. 2014, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, L.; Fu, C.; Liu, H.; Chen, D.; Tang, F. Pathological mechanisms of liver injury caused by continuous intraperitoneal injection of silica nanoparticles. Biomaterials 2012, 33, 2399–2407. [Google Scholar] [CrossRef]
- Liu, T.; Li, L.; Teng, X.; Huang, X.; Liu, H.; Chen, D.; Ren, J.; He, J.; Tang, F. Single and repeated dose toxicity of mesoporous hollow silica nanoparticles in intravenously exposed mice. Biomaterials 2011, 32, 1657–1668. [Google Scholar] [CrossRef]
- Cho, M.; Cho, W.S.; Choi, M.; Kim, S.J.; Han, B.S.; Kim, S.H.; Kim, H.O.; Sheen, Y.Y.; Jeong, J. The impact of size on tissue distribution and elimination by single intravenous injection of silica nanoparticles. Toxicol. Lett. 2009, 189, 177–183. [Google Scholar] [CrossRef]
- He, Q.; Zhang, Z.; Gao, F.; Li, Y.; Shi, J. In vivo biodistribution and urinary excretion of mesoporous silica nanoparticles: Effects of particle size and PEGylation. Small 2011, 7, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Nishimori, H.; Kondoh, M.; Isoda, K.; Tsunoda, S.; Tsutsumi, Y.; Yagi, K. Histological analysis of 70-nm silica particles-induced chronic toxicity in mice. Eur. J. Pharm. Biopharm. 2009, 72, 626–629. [Google Scholar] [CrossRef]
- Kim, M.; Park, J.; Jeong, H.; Hong, J.; Choi, W.S.; Lee, B.; Park, C.Y. An Evaluation of the in vivo Safety of Nonporous Silica Nanoparticles: Ocular Topical Administration versus Oral Administration. Sci. Rep. 2017, 7, 8238. [Google Scholar] [CrossRef]
- Kim, I.Y.; Joachim, E.; Choi, H.; Kim, K. Toxicity of silica nanoparticles depends on size, dose, and cell type. Nanomedicine 2015, 11, 1407–1416. [Google Scholar] [CrossRef]
- Almanaa, T.N.; Aref, M.; Kakakhel, M.A.; Elshopakey, G.E.; Mahboub, H.H.; Abdelazim, A.M.; Kamel, S.; Belali, T.M.; Abomughaid, M.M.; Alhujaily, M.; et al. Silica Nanoparticle Acute Toxicity on Male Rattus norvegicus Domestica: Ethological Behavior, Hematological Disorders, Biochemical Analyses, Hepato-Renal Function, and Antioxidant-Immune Response. Front. Bioeng. Biotechnol. 2022, 10, 868111. [Google Scholar] [CrossRef] [PubMed]
- Crawford, A.A.; Soderberg, S.; Kirschbaum, C.; Murphy, L.; Eliasson, M.; Ebrahim, S.; Smith, G.D.; Olsson, T.; Sattar, N.; Lawlor, D.A.; et al. Morning plasma cortisol as a cardiovascular risk factor: Findings from prospective cohort and Mendelian randomization studies. Eur. J. Endocrinol. 2019, 181, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Battocchio, M.; Rebellato, A.; Grillo, A.; Dassie, F.; Maffei, P.; Bernardi, S.; Fabris, B.; Carretta, R.; Fallo, F. Ambulatory Arterial Stiffness Indexes in Cushing’s Syndrome. Horm. Metab. Res. 2017, 49, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Ducat, E.; Ray, B.; Bart, G.; Umemura, Y.; Varon, J.; Ho, A.; Kreek, M.J. Mu-opioid receptor A118G polymorphism in healthy volunteers affects hypothalamic–pituitary–adrenal axis adrenocorticotropic hormone stress response to metyrapone. Addict. Biol. 2013, 18, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Koe, A.S.; Salzberg, M.R.; Morris, M.J.; O’Brien, T.J.; Jones, N.C. Early life maternal separation stress augmentation of limbic epileptogenesis: The role of corticosterone and HPA axis programming. Psychoneuroendocrinology 2014, 42, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Schutten, J.C.; Joris, P.J.; Minović, I.; Post, A.; van Beek, A.P.; de Borst, M.H.; Mensink, R.P.; Bakker, S.J.L. Long-term magnesium supplementation improves glucocorticoid metabolism: A post-hoc analysis of an intervention trial. Clin. Endocrinol. 2021, 94, 150–157. [Google Scholar] [CrossRef]
- Boyle, N.B.; Lawton, C.; Dye, L. The Effects of Magnesium Supplementation on Subjective Anxiety and Stress—A Systematic Review. Nutrients 2017, 9, 429. [Google Scholar] [CrossRef] [PubMed]
- Adly, N.N.; Abd-El-Gawad, W.M. Does magnesium level play a role in the association between major depression and cortisol? Geriatr. Gerontol. Int. 2013, 13, 511–513. [Google Scholar] [CrossRef]
- Athinarayanan, J.; Alshatwi, A.A.; Periasamy, V.S.; Al-Warthan, A.A. Identification of nanoscale ingredients in commercial food products and their induction of mitochondrially mediated cytotoxic effects on human mesenchymal stem cells. J. Food. Sci. 2015, 80, N459–N464. [Google Scholar] [CrossRef] [PubMed]
- Mohammadpour, R.; Yazdimamaghani, M.; Cheney, D.L.; Jedrzkiewicz, J.; Ghandehari, H. Subchronic toxicity of silica nanoparticles as a function of size and porosity. J. Control. Release 2019, 304, 216–232. [Google Scholar] [CrossRef]
- Park, J.; Min, J.S.; Kim, B.; Chae, U.B.; Yun, J.W.; Choi, M.S.; Kong, K.; Chang, K.; Lee, D.S. Mitochondrial ROS govern the LPSinduced pro-inflammatory response in microglia cells by regulating MAPK and NF-κB pathways. Neurosci. Lett. 2015, 584, 191–196. [Google Scholar] [CrossRef]
- Arancibia-Hernández, Y.L.; Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Pedraza-Chaverri, J. Antioxidant/anti-inflammatory effect of Mg2+ in coronavirus disease 2019 (COVID-19). Rev. Med. Virol. 2022, 32, e2348. [Google Scholar] [CrossRef] [PubMed]
- Morais, J.B.S.; Severo, J.S.; Santos, L.R.D.; de Sousa Melo, S.R.; de Oliveira Santos, R.; de Oliveira, A.R.S.; de Oliveira, A.R.; Clímaco Cruz, K.J.; do Nascimento Marreiro, D. Role of magnesium in oxidative stress in individuals with obesity. Biol. Trace. Elem. Res. 2017, 176, 20–26. [Google Scholar] [CrossRef]
- Kovacs, K.; Vaczy, A.; Fekete, K.; Kovari, P.; Atlasz, T.; Reglodi, D.; Gabriel, R.; Gallyas, F.; Sumegi, B. PARP inhibitor protects against chronic hypoxia/Reoxygenation-induced retinal injury by regulation of MAPKs, HIF1alpha, Nrf2, and NFkappaB. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1478–1490. [Google Scholar] [CrossRef] [PubMed]
- Orhan, C.; Er, B.; Deeh, P.B.D.; Bilgic, A.A.; Ojalvo, S.P.; Komorowski, J.R.; Sahin, K. Different sources of dietary magnesium supplementation reduces oxidative stress by regulation Nrf2 and NF-κB signaling pathways in high-fat diet rats. Biol. Trace. Elem. Res. 2021, 199, 4162–4170. [Google Scholar] [CrossRef] [PubMed]
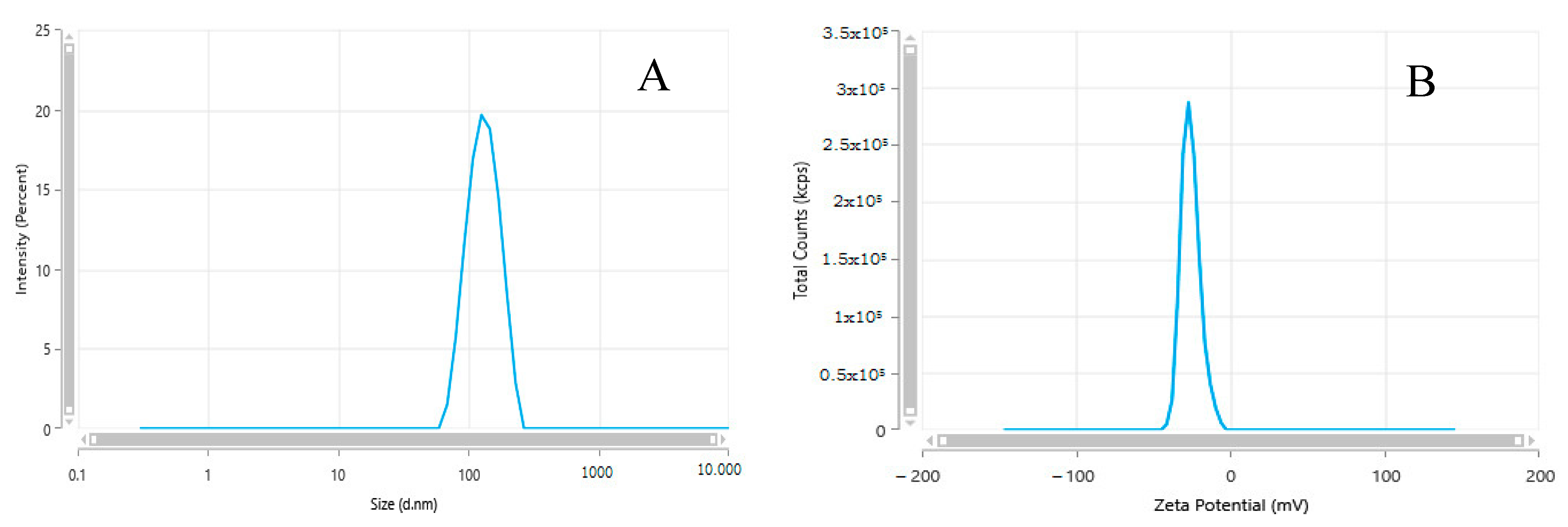
| Appearance (Form) | Surface Area | pH | Solid Content | Particle Size (nm) | Density | Bulk Density | Solvent | Purity |
|---|---|---|---|---|---|---|---|---|
| Nanopowder (spherical, porous) | 590–690 m2/g (TEM) | 3.7–4.7 | 24.0–30.0% | 5–20 nm (TEM) | 2.2–2.6 g/mL at 25 °C | 0.068 g/mL | water | 99.5% (Based on Trace Metals Analysis) |
| Parameter | Study Groups | ANOVA Test p-Value * | |||
|---|---|---|---|---|---|
| Control Group (N = 6) | Mg Group (N = 6) | SiNPs Group (N = 6) | SiNPs + Mg Group (N = 6) | ||
| ALT (U/L) | 24 ± 4.47 | 23.50 ± 5.01 bc | 47.50 ± 3.94 a | 35 ± 4.52 ab | F = 37.975 p < 0.001 ** |
| AST (U/L) | 100.83 ± 11.02 | 94.50 ± 5.39 bc | 152 ± 11.83 a | 133.50 ± 4.85 ab | F = 56.66 p < 0.001 ** |
| Study Groups | ANOVA Test p-Value * | ||||
|---|---|---|---|---|---|
| Control Group (N = 6) | Mg Group (N = 6) | SiNPs Group (N = 6) | SiNPs + Mg Group (N = 6) | ||
| Mean ± SD (mg/dL) | 0.56 ± 0.06 | 0.41 ± 0.08 bc | 1.79 ± 0.20 a | 0.95 ± 0.15 ab | F = 126.730 p < 0.001 ** p < 0.001 ** |
| Study Groups | ANOVA Test p-Value * | ||||
|---|---|---|---|---|---|
| Control Group (N = 6) | Mg Group (N = 6) | SiNPs Group (N = 6) | SiNPs + Mg Group (N = 6) | ||
| Mean ± SD (µg/dL) | 0.25 ± 0.04 | 0.22 ± 0.02 bc | 0.37 ± 0.04 a | 0.30 ± 0.02 ab | F = 28.398 p < 0.001 ** |
| Parameters | Study Groups | ANOVA Test p-Value * | ||||
|---|---|---|---|---|---|---|
| Control Group (N = 6) | Mg Group (N = 6) | SiNPs Group (N = 6) | SiNPs + Mg Group (N = 6) | |||
| Liver | GSH (mean ± SD) | 2.72 ± 0.12 | 2.90 ± 0.07 bc | 1.38 ± 0.19 a | 1.97 ± 0.09 ab | F = 188.405 p < 0.001 ** |
| MDA (mean ± SD) | 5.89 ± 0.37 | 4.92 ± 0.54 bc | 14.80 ± 1.11 a | 10.85 ± 1.07 ab | F = 182.136 p < 0.001 ** | |
| Kidney | GSH (mean ± SD) | 1.58 ± 0.15 | 1.69 ± 0.11 bc | 0.81 ± 0.11 a | 1.18 ± 0.04 ab | F = 81.313 p <0.001 ** |
| MDA (mean ± SD) | 4.76 ± 0.26 | 4.02 ± 0.14 bc | 11.13 ± 0.98 a | 7.59 ± 0.85 ab | F = 141.260 p < 0.001 ** | |
| Adrenal | GSH (mean ± SD) | 1.47 ± 0.10 | 1.55 ± 0.05 bc | 0.70 ± 0.09 a | 8 ± 0.46 a | F = 127.623 p < 0.001 ** |
| MDA (mean ± SD) | 3.92 ± 0.30 | 3.53 ± 0.25 bc | 1.09 ± 0.09 ab | 6.22 ± 0.16 ab | F = 269.944 p < 0.001 ** | |
| Parameters | Study Groups | ANOVA Test p-Value * | |||
|---|---|---|---|---|---|
| Control Group (N = 6) | Mg Group (N = 6) | SiNPs Group (N = 6) | SiNPs + Mg Group (N = 6) | ||
| Liver weight (g) | 5.43 ± 1.14 | 4.81 ± 0.14 bc | 6.36 ± 0.23 | 5.87 ± 0.33 | F = 7.085 p = 0.002 * |
| Kidney weight (g) | 1.19 ± 0.11 | 1.05 ± 0.05 abc | 1.42 ± 0.09 a | 1.32 ± 0.04 a | F = 25.510 p < 0.001 ** |
| Adrenal weight (g) | 0.06 ± 0.01 | 0.05 ± 0.01 bc | 0.08 ± 0.01 a | 0.07 ± 0.01 ab | F = 31 p < 0.001 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badawy, M.M.; Sayed-Ahmed, M.Z.; Almoshari, Y.; Alqahtani, S.S.; Alshahrani, S.; Mabrouk, H.A.A.; Abd-Elsalam, M.M.; Alkashif, K.; Ahmad, S.; El-Sebaey, A.M.; et al. Magnesium Supplementation Alleviates the Toxic Effects of Silica Nanoparticles on the Kidneys, Liver, and Adrenal Glands in Rats. Toxics 2023, 11, 381. https://doi.org/10.3390/toxics11040381
Badawy MM, Sayed-Ahmed MZ, Almoshari Y, Alqahtani SS, Alshahrani S, Mabrouk HAA, Abd-Elsalam MM, Alkashif K, Ahmad S, El-Sebaey AM, et al. Magnesium Supplementation Alleviates the Toxic Effects of Silica Nanoparticles on the Kidneys, Liver, and Adrenal Glands in Rats. Toxics. 2023; 11(4):381. https://doi.org/10.3390/toxics11040381
Chicago/Turabian StyleBadawy, Mohamed Moharram, Mohamed Z. Sayed-Ahmed, Yosif Almoshari, Saad S. Alqahtani, Saeed Alshahrani, Heba Allah Ali Mabrouk, Marwa M. Abd-Elsalam, Khalid Alkashif, Sarfaraz Ahmad, Ahmed M. El-Sebaey, and et al. 2023. "Magnesium Supplementation Alleviates the Toxic Effects of Silica Nanoparticles on the Kidneys, Liver, and Adrenal Glands in Rats" Toxics 11, no. 4: 381. https://doi.org/10.3390/toxics11040381
APA StyleBadawy, M. M., Sayed-Ahmed, M. Z., Almoshari, Y., Alqahtani, S. S., Alshahrani, S., Mabrouk, H. A. A., Abd-Elsalam, M. M., Alkashif, K., Ahmad, S., El-Sebaey, A. M., Hamama, M. G., & Ahmed, D. A. M. (2023). Magnesium Supplementation Alleviates the Toxic Effects of Silica Nanoparticles on the Kidneys, Liver, and Adrenal Glands in Rats. Toxics, 11(4), 381. https://doi.org/10.3390/toxics11040381