Effects of Multiwall Carbon Nanotubes on Premature Kidney Aging: Biochemical and Histological Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Multi-Walled Carbon Nanotubes
2.2. Transmission Electron Microscopy (TEM)
2.3. Raman Spectroscopy
2.4. Inductively Coupled Plasma-Atomic Emission Spectroscopy (ICP-AES)
2.5. Dosage of PMWCNTs and TMWCNTs
2.6. Animal Experiments and Tracheal Instillation
2.7. Hematoxylin and Eosin (H&E) and Masson’s Trichrome Staining
2.8. Kidney Histology
2.9. Western Blot Analysis
2.10. Serum Analysis of Klotho, Phosphate, and Mouse Bone Metabolism Proteins
2.11. Statistical Analysis
3. Results
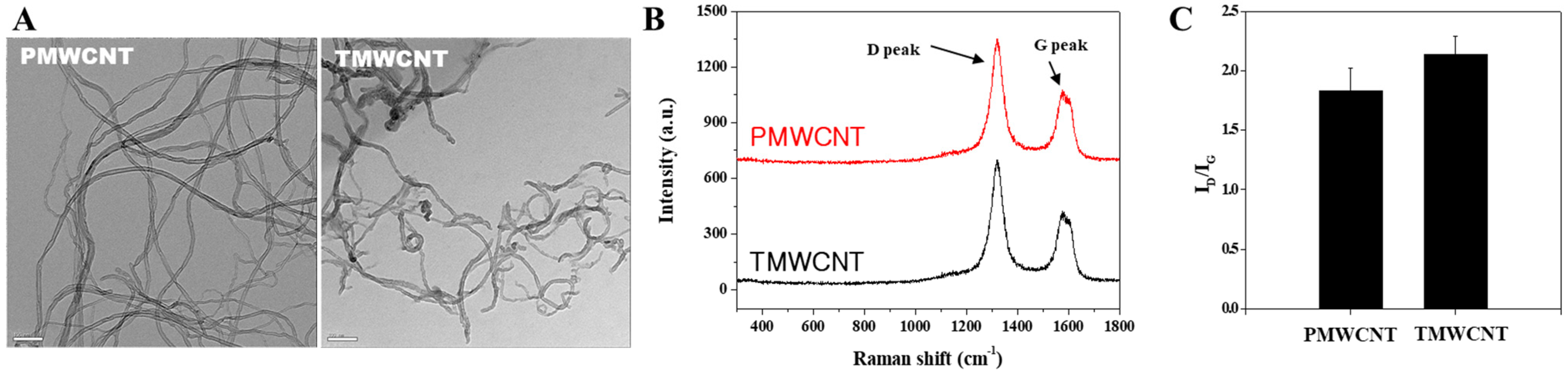
3.1. Characteristics of PMWCNTs and TMWCNTs
3.2. Dosage Determined Using the Maximum Tolerated Dose (MTD) and Animal Experiments
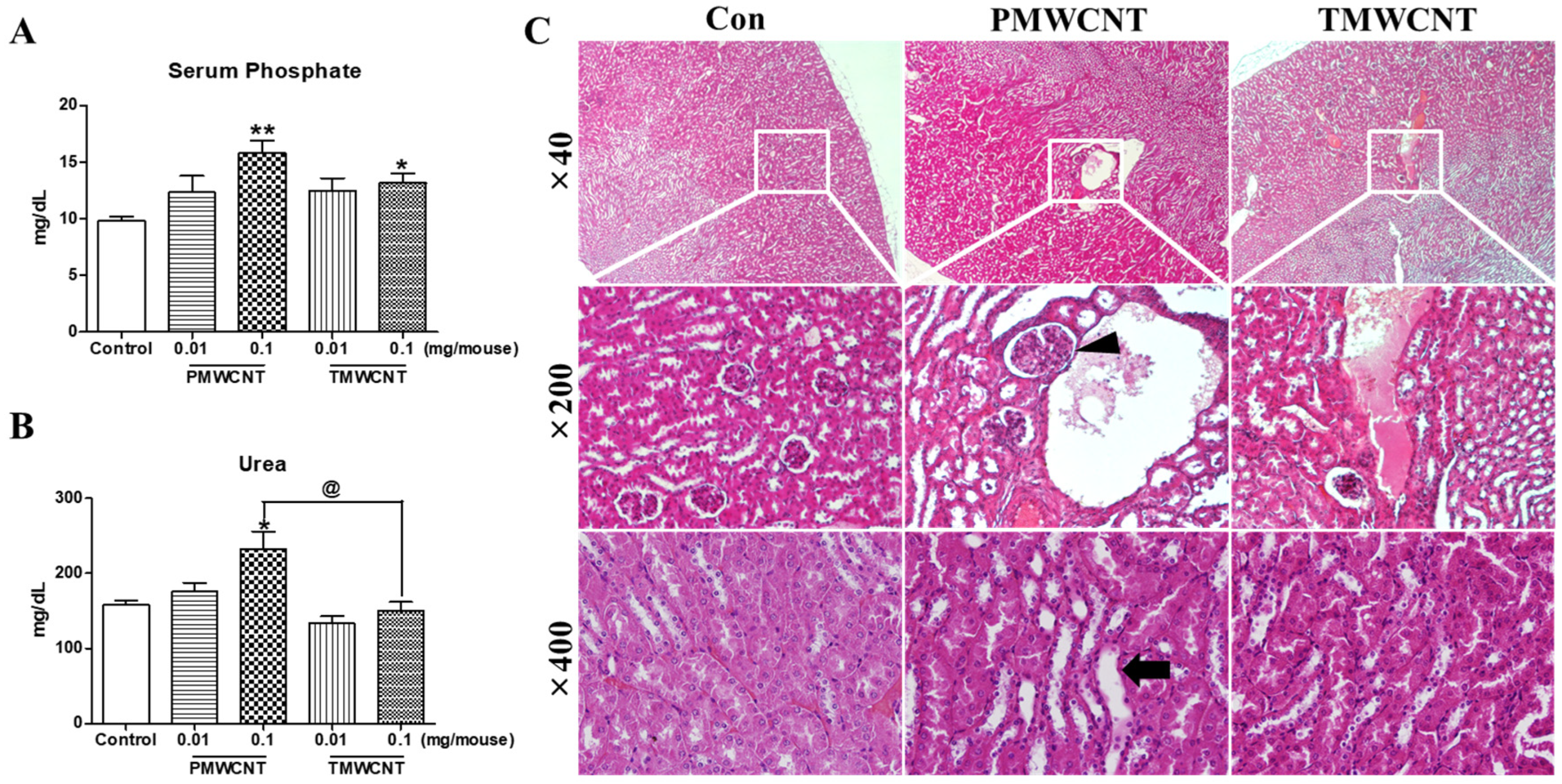
3.3. Renal Dysfunction and Histologic Change at 6 Months Post-Instillation of MWCNTs
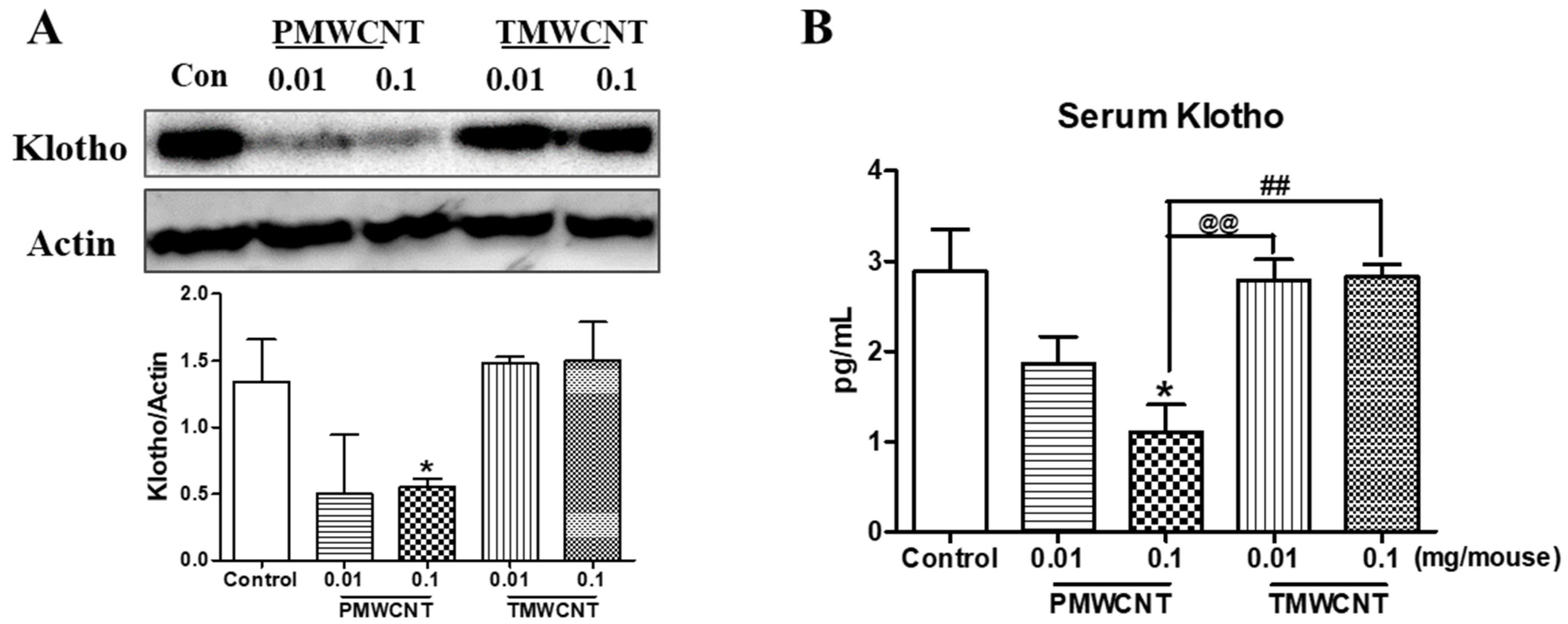
3.4. Kidney and Serum Klotho Levels of Mice at 6 Months Post Instillation of MWCNTs
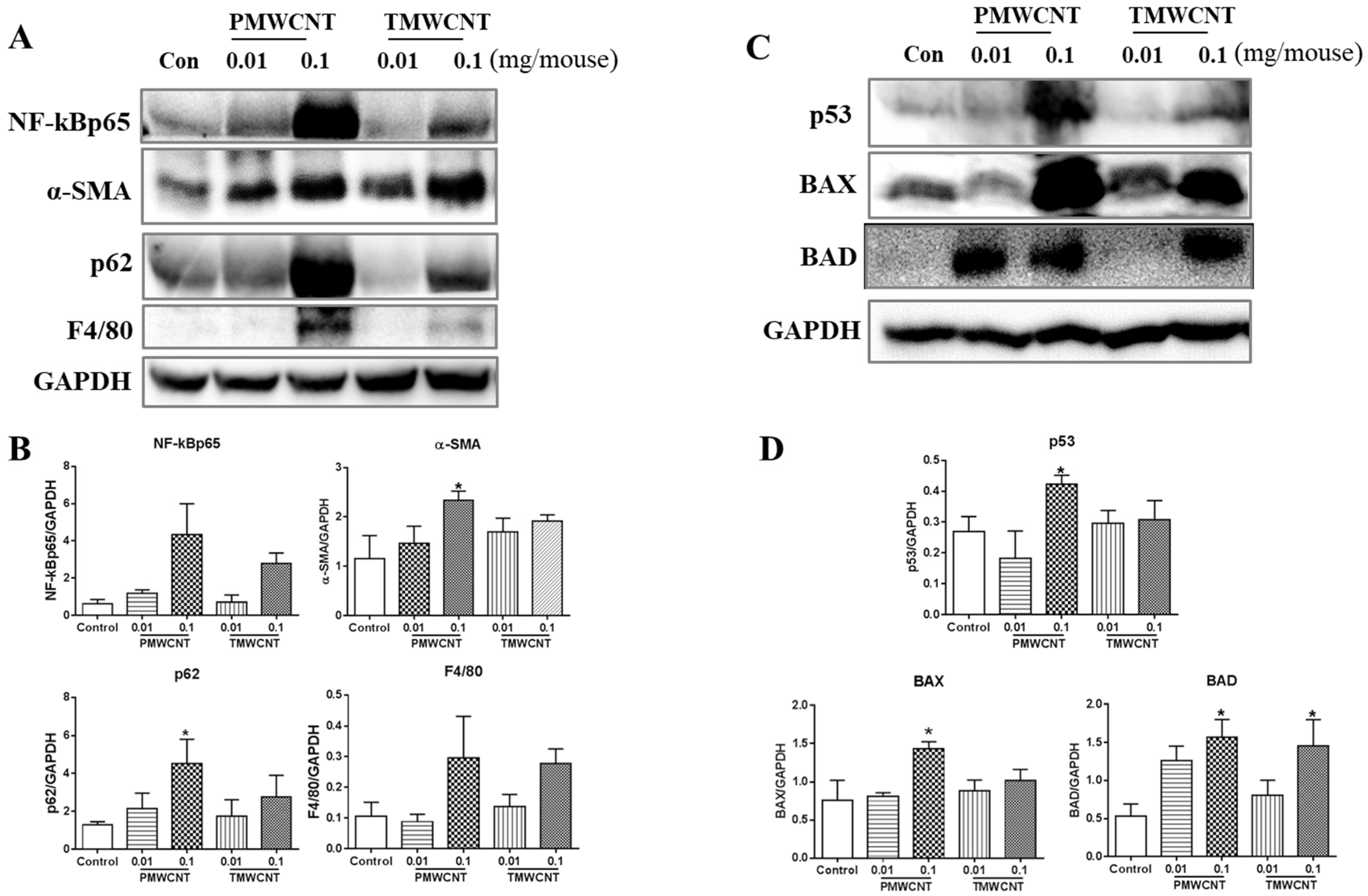
3.5. Activated Inflammatory, Fibrotic and Apoptotic Pathways, and Insufficient Autophagy in the Kidneys of 6-Month Post-Tracheal PMWCNT Instillation Mice
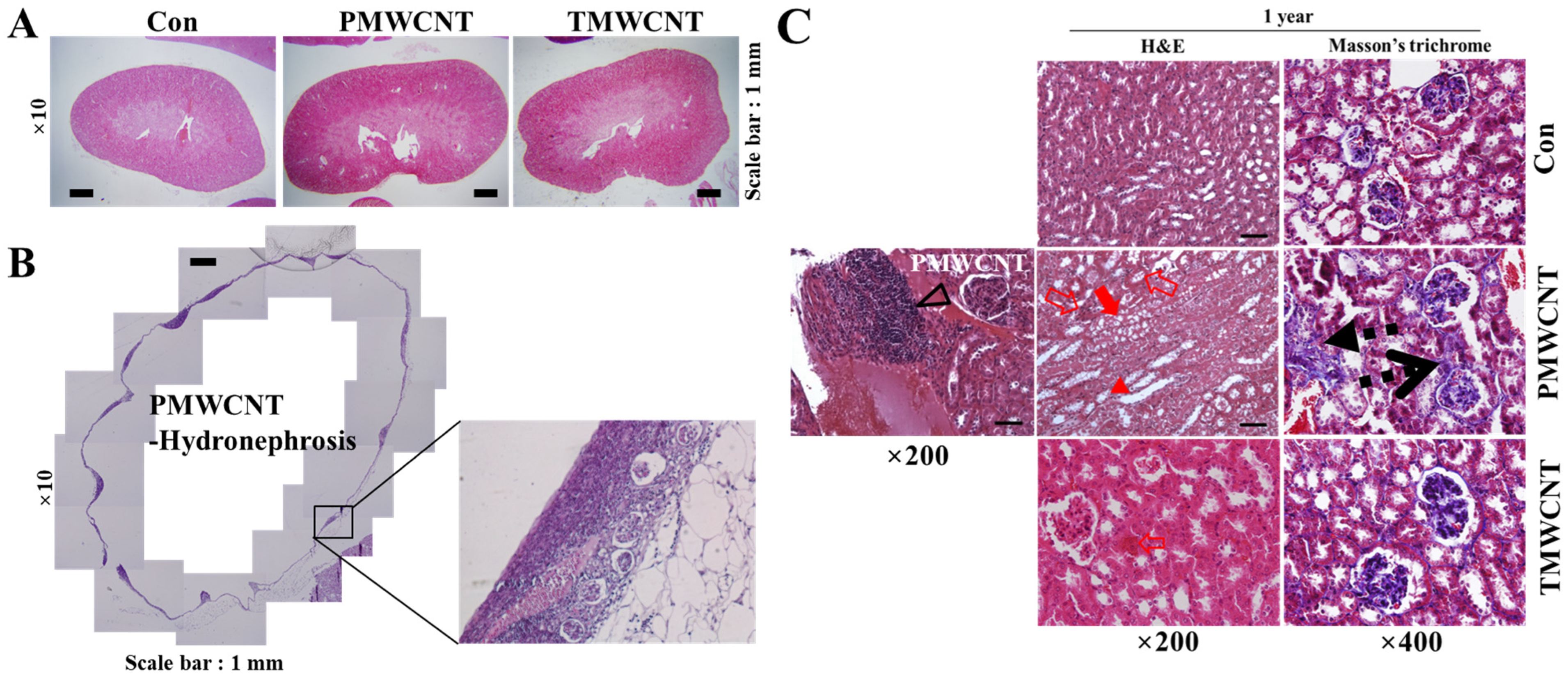
3.6. Histologic Change of Kidney after 1 Year of MWCNTs Instillation
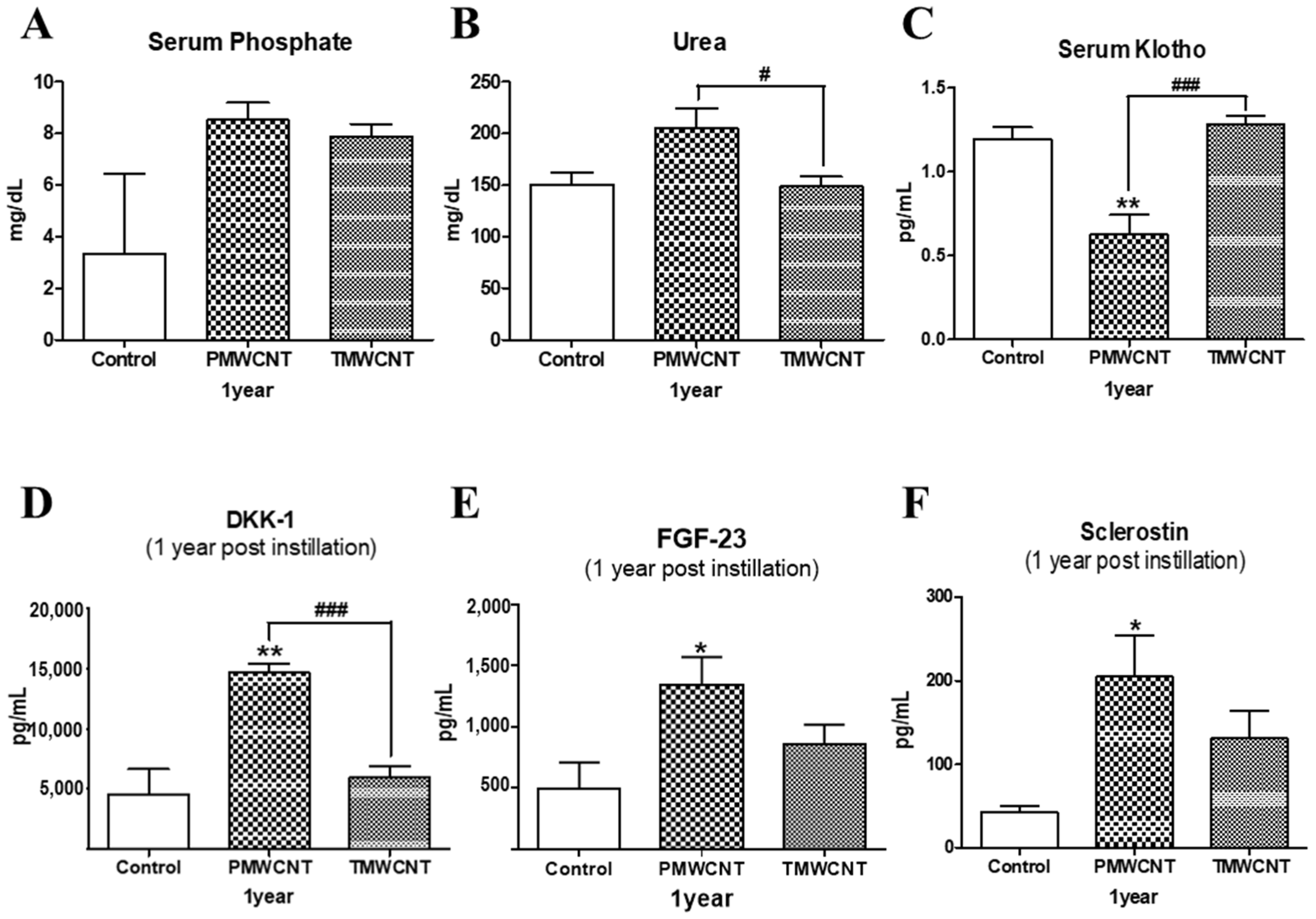
3.7. Changes in Serum Indicators Related to Kidney Function and Aging
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Lam, C.W.; James, J.T.; McCluskey, R.; Arepalli, S.; Hunter, R.L. A review of carbon nanotube toxicity and assessment of potential occupational and environmental health risks. Crit. Rev. Toxicol. 2006, 36, 189–217. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Lim, H.T.; Minai-Tehrani, A.; Kwon, J.T.; Shin, J.Y.; Woo, C.G.; Choi, M.; Baek, J.; Jeong, D.H.; Ha, Y.C.; et al. Toxicity and clearance of intratracheally administered multiwalled carbon nanotubes from murine lung. J. Toxicol. Environ. Health A 2010, 73, 1530–1543. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Lee, S.; Lee, A.Y.; Seo, H.W.; Chae, C.; Cho, M.H. Intratracheal exposure to multi-walled carbon nanotubes induces a nonalcoholic steatohepatitis-like phenotype in C57BL/6J mice. Nanotoxicology 2015, 9, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Aragon, M.J.; Topper, L.; Tyler, C.R.; Sanchez, B.; Zychowski, K.; Young, T.; Herbert, G.; Hall, P.; Erdely, A.; Eye, T. Serum-borne bioactivity caused by pulmonary multiwalled carbon nanotubes induces neuroinflammation via blood–brain barrier impairment. Proc. Natl. Acad. Sci. USA 2017, 114, E1968–E1976. [Google Scholar] [CrossRef]
- Palmer, B.C.; Phelan-Dickenson, S.J.; DeLouise, L.A. Multi-walled carbon nanotube oxidation dependent keratinocyte cytotoxicity and skin inflammation. Part. Fibre Toxicol. 2019, 16, 3. [Google Scholar] [CrossRef]
- Burcham, P.C. Target-Organ Toxicity: Liver and Kidney. In An Introduction to Toxicology; Springer: Berlin, Germany, 2014; pp. 151–187. [Google Scholar]
- Coresh, J.; Selvin, E.; Stevens, L.A.; Manzi, J.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Levey, A.S. Prevalence of chronic kidney disease in the United States. JAMA 2007, 298, 2038–2047. [Google Scholar] [CrossRef]
- Chronopoulos, A.; Cruz, D.N.; Ronco, C. Hospital-acquired acute kidney injury in the elderly. Nat. Rev. Nephrol. 2010, 6, 141–149. [Google Scholar] [CrossRef]
- Melk, A.; Kittikowit, W.; Sandhu, I.; Halloran, K.M.; Grimm, P.; Schmidt, B.M.W.; Halloran, P.F. Cell senescence in rat kidneys in vivo increases with growth and age despite lack of telomere shortening. Kidney Int. 2003, 63, 2134–2143. [Google Scholar] [CrossRef]
- Westhoff, J.H.; Schildhorn, C.; Jacobi, C.; Hömme, M.; Hartner, A.; Braun, H.; Kryzer, C.; Wang, C.; von Zglinicki, T.; Kränzlin, B.; et al. Telomere shortening reduces regenerative capacity after acute kidney injury. J. Am. Soc. Nephrol. 2010, 21, 327–336. [Google Scholar] [CrossRef]
- John, G.B.; Cheng, C.Y.; Kuro-o, M. Role of Klotho in aging, phosphate metabolism, and CKD. Am. J. Kidney Dis. 2011, 58, 127–134. [Google Scholar] [CrossRef]
- Stenvinkel, P.; Larsson, T.E. Chronic kidney disease: A clinical model of premature aging. Am. J. Kidney Dis. 2013, 62, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Mei, C.; Zheng, F. Chronic inflammation potentiates kidney aging. In Seminars in Nephrology; Elsevier: Amsterdam, The Netherlands, 2009; pp. 555–568. [Google Scholar]
- Pathai, S.; Bajillan, H.; Landay, A.L.; High, K.P. Is HIV a model of accelerated or accentuated aging? J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Klaassen, C.D. Casarett and Doull’s Toxicology: The Basic Science of Poisons; McGraw-Hill: New York, NY, USA, 2013; Volume 1236. [Google Scholar]
- Hruska, K.A.; Mathew, S.; Lund, R.; Qiu, P.; Pratt, R. Hyperphosphatemia of chronic kidney disease. Kidney Int. 2008, 74, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Moe, S.M.; Drüeke, T.; Lameire, N.; Eknoyan, G. Chronic kidney disease–mineral-bone disorder: A new paradigm. Adv. Chronic. Kidney Dis. 2007, 14, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.J.; Kim, D.H.; Lee, E.K.; Song, C.W.; Yu, B.P.; Chung, H.Y. Oxidative stress induces inactivation of protein phosphatase 2A, promoting proinflammatory NF-κB in aged rat kidney. Free. Radic. Biol. Med. 2013, 61, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Satoh, M.; Fujimoto, S.; Horike, H.; Ozeki, M.; Nagasu, H.; Tomita, N.; Sasaki, T.; Naoki Kashihara, N. Mitochondrial damage-induced impairment of angiogenesis in the aging rat kidney. Lab. Investig. 2011, 91, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, M.C.; Perez-Gomez, M.V.; Sanchez-Niño, M.D.; Sanz, A.B.; Ruiz-Andres, O.; Poveda, J.; Moreno, J.A.; Egido, J.; Ortiz, A. Klotho, phosphate and inflammation/ageing in chronic kidney disease. Nephrol. Dial. Transplant. 2012, 27 (Suppl. S4), iv6–iv10. [Google Scholar] [CrossRef]
- Ohnishi, M.; Nakatani, T.; Lanske, B.; Razzaque, M.S. In vivo genetic evidence for suppressing vascular and soft-tissue calcification through the reduction of serum phosphate levels, even in the presence of high serum calcium and 1, 25-dihydroxyvitamin d levels. Circ. Cardiovasc. Genet. 2009, 2, 583–590. [Google Scholar] [CrossRef]
- Yang, H.; Fogo, A.B. Cell senescence in the aging kidney. J. Am. Soc. Nephrol. 2010, 21, 1436–1439. [Google Scholar] [CrossRef]
- Kurosu, H.; Yamamoto, M.; Clark, J.D.; Pastor, J.V.; Nandi, A.; Gurnani, P.; McGuinness, O.P.; Chikuda, H.; Yamaguchi, M.; Kawaguchi, H.; et al. Suppression of aging in mice by the hormone Klotho. Science 2005, 309, 1829–1833. [Google Scholar] [CrossRef]
- Kuro-o, M. Klotho, phosphate and FGF-23 in ageing and disturbed mineral metabolism. Nat. Rev. Nephrol. 2013, 9, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Bolignano, D.; Mattace-Raso, F.; Sijbrands, E.J.; Zoccali, C. The aging kidney revisited: A systematic review. Ageing Res. Rev. 2014, 14, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bonventre, J.V.; Parrish, A.R. The aging kidney: Increased susceptibility to nephrotoxicity. Int. J. Mol. Sci. 2014, 15, 15358–15376. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Bai, X.Y.; Shi, S.; Cui, S.; Hong, Q.; Cai, G.; Chen, X. Age-related changes in the function of autophagy in rat kidneys. Age 2012, 34, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Gasnier, A.; Pedano, M.L.; Gutierrez, F.; Labbé, P.; Rivas, G.A.; Rubianes, M.D. Glassy carbon electrodes modified with a dispersion of multi-wall carbon nanotubes in dopamine-functionalized polyethylenimine: Characterization and analytical applications for nicotinamide adenine dinucleotide quantification. Electrochim. Acta 2012, 71, 73–81. [Google Scholar] [CrossRef]
- Harkema, J.R.; Carey, S.A.; Wagner, J.G. The nose revisited: A brief review of the comparative structure, function, and toxicologic pathology of the nasal epithelium. Toxicol. Pathol. 2006, 34, 252–269. [Google Scholar] [CrossRef]
- Driscoll, K.E.; Costa, D.L.; Hatch, G.; Henderson, R.; Oberdorster, G.; Salem, H.; Schlesinger, R.B. Intratracheal instillation as an exposure technique for the evaluation of respiratory tract toxicity: Uses and limitations. Toxicol. Sci. 2000, 55, 24–35. [Google Scholar] [CrossRef]
- Haschek, W.M.; Rousseaux, C.G.; Wallig, M.A. Fundamentals of Toxicologic Pathology; Academic Press: Cambridge, MA, USA, 2009; pp. 303–316. [Google Scholar]
- Izquierdo, M.C.; Sanz, A.B.; Sánchez-Niño, M.D.; Pérez-Gómez, M.V.; Ruiz-Ortega, M.; Poveda, J.; Ruiz-Andrés, O.; Ramos, A.M.; Moreno, J.A.; Egido, J.; et al. Acute kidney injury transcriptomics unveils a relationship between inflammation and ageing. Nefrologia 2012, 32, 715–723. [Google Scholar]
- He, X.; Young, S.H.; Schwegler-Berry, D.; Chisholm, W.P.; Fernback, J.E.; Ma, Q. Multiwalled carbon nanotubes induce a fibrogenic response by stimulating reactive oxygen species production, activating NF-κB signaling, and promoting fibroblast-to-myofibroblast transformation. Chem. Res. Toxicol. 2011, 24, 2237–2248. [Google Scholar] [CrossRef]
- Martin, J.E.; Sheaff, M.T. Renal ageing. J. Pathol. 2007, 211, 198–205. [Google Scholar] [CrossRef]
- Li, M.; Nicholls, K.M.; Becker, G.J. Glomerular size and global glomerulosclerosis in normal Caucasian donor kidneys: Effects of aging and gender. J. Nephrol. 2001, 15, 614–619. [Google Scholar]
- Springer, D.A.; Allen, M.; Hoffman, V.; Brinster, L.; Starost, M.F.; Bryant, M.; Eckhaus, M. Investigation and identification of etiologies involved in the development of acquired hydronephrosis in aged laboratory mice with the use of high-frequency ultrasound imaging. Pathobiol. Aging Age-Relat. Dis. 2014, 4, 24932. [Google Scholar] [CrossRef] [PubMed]
- Combet, S.; Geffroy, N.; Berthonaud, V.; Dick, B.; Teillet, L.; Verbavatz, J.M.; Corman, B.; Trinh-Trang-Tan, M.M. Correction of age-related polyuria by dDAVP: Molecular analysis of aquaporins and urea transporters. Am. J. Physiol.-Ren. Physiol. 2003, 284, F199–F208. [Google Scholar] [CrossRef] [PubMed]
- Koh, N.; Fujimori, T.; Nishiguchi, S.; Tamori, A.; Shiomi, S.; Nakatani, T.; Sugimura, K.; Kishimoto, T.; Kinoshita, S.; Kuroki, T.; et al. Severely reduced production of klotho in human chronic renal failure kidney. Biochem. Biophys. Res. Commun. 2001, 280, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Isakova, T.; Wahl, P.; Vargas, G.S.; Gutiérrez, O.M.; Scialla, J.; Xie, H.; Appleby, D.; Nessel, N.; Bellovich, K.; Chen, J.; et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011, 79, 1370–1378. [Google Scholar] [CrossRef]
- Huang, C.L.; Moe, O.W. Klotho: A novel regulator of calcium and phosphorus homeostasis. Pflügers Arch. Eur. J. Physiol. 2011, 462, 185–193. [Google Scholar] [CrossRef]
- Ramírez, R.; Carracedo, J.; Soriano, S.; Jiménez, R.; Martín-Malo, A.; Rodríguez, M.; Blasco, M.; Aljama, P. Stress-induced premature senescence in mononuclear cells from patients on long-term hemodialysis. Am. J. Kidney Dis. 2005, 45, 353–359. [Google Scholar] [CrossRef]
- Kuro-o, M. Klotho as a regulator of oxidative stress and senescence. Biol. Chem. 2008, 389, 233–241. [Google Scholar] [CrossRef]
- Liu, F.; Wu, S.; Ren, H.; Gu, J. Klotho suppresses RIG-I-mediated senescence-associated inflammation. Nat. Cell Biol. 2011, 13, 254–262. [Google Scholar] [CrossRef]
- Gutiérrez, O.M.; Wolf, M.; Taylor, E.N. Fibroblast growth factor 23, cardiovascular disease risk factors, and phosphorus intake in the health professionals follow-up study. Clin. J. Am. Soc. Nephrol. 2011, 6, 2871–2878. [Google Scholar] [CrossRef]
- Moe, S.M. Klotho: A master regulator of cardiovascular disease? Circulation 2012, 125, 2181–2183. [Google Scholar] [CrossRef] [PubMed]
- Kuro-o, M.; Matsumura, Y.; Aizawa, H.; Kawaguchi, H.; Suga, T.; Utsugi, T.; Ohyama, Y.; Kurabayashi, M.; Kaname, T.; Kume, E.; et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997, 390, 45–51. [Google Scholar] [CrossRef]
- Shimada, T.; Hasegawa, H.; Yamazaki, Y.; Muto, T.; Hino, R.; Takeuchi, Y.; Fujita, T.; Nakahara, K.; Fukumoto, S.; Yamashita, T. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J. Bone Miner. Res. 2004, 19, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Franki, N.; Kapasi, A.A.; Reddy, K.; Gibbons, N.; Singhal, P.C. Tubular cell senescence and expression of TGF-β1 and p21 WAF1/CIP1 in tubulointerstitial fibrosis of aging rats. Exp. Mol. Pathol. 2001, 70, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.H.; Kim, E.N.; Kim, M.Y.; Chung, S.; Shin, S.J.; Kim, H.W.; Yang, C.W.; Kim, Y.S.; Chang, Y.S.; Park, C.W.; et al. Age-associated molecular changes in the kidney in aged mice. Oxidative Med. Cell. Longev. 2012, 2012, 171383. [Google Scholar] [CrossRef] [PubMed]
- Huber, T.B.; Edelstein, C.L.; Hartleben, B.; Inoki, K.; Jiang, M.; Koya, D.; Kume, S.; Lieberthal, W.; Pallet, N.; Quiroga, A.; et al. Emerging role of autophagy in kidney function, diseases and aging. Autophagy 2012, 8, 1009–1031. [Google Scholar] [CrossRef]
- Fang, Y.; Ginsberg, C.; Sugatani, T.; Monier-Faugere, M.C.; Malluche, H.; Hruska, K.A. Early chronic kidney disease–mineral bone disorder stimulates vascular calcification. Kidney Int. 2014, 85, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.; Rawadi, G. Targeting the Wnt/β-catenin pathway to regulate bone formation in the adult skeleton. Endocrinology 2007, 148, 2635–2643. [Google Scholar] [CrossRef]
- Cejka, D.; Herberth, J.; Branscum, A.J.; Fardo, D.W.; Monier-Faugere, M.C.; Diarra, D.; Haas, M.; Malluche, H.H. Sclerostin and Dickkopf-1 in renal osteodystrophy. Clin. J. Am. Soc. Nephrol. 2011, 6, 877–882. [Google Scholar] [CrossRef]
- Evenepoel, P.; D’Haese, P.; Brandenburg, V. Sclerostin and DKK1: New players in renal bone and vascular disease. Kidney Int. 2015, 88, 235–240. [Google Scholar] [CrossRef]
- Weitzmann, M.N.; Ha, S.W.; Vikulina, T.; Roser-Page, S.; Lee, J.K.; Beck, G.R. Bioactive silica nanoparticles reverse age-associated bone loss in mice. Nanomedicine 2015, 11, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Fergusson, M.M.; Castilho, R.M.; Liu, J.; Cao, L.; Chen, J.; Malide, D.; Rovira, I.I.; Schimel, D.; Kuo, C.J.; et al. Augmented Wnt signaling in a mammalian model of accelerated aging. Science 2007, 317, 803–806. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, L.; Liabeuf, S.; Renard, C.; Lenglet, A.; Lemke, H.D.; Choukroun, G.; Drueke, T.B.; Massy, Z.A.; European Uremic Toxin (EUTox) Work Group. FGF23 is independently associated with vascular calcification but not bone mineral density in patients at various CKD stages. Osteoporos. Int. 2012, 23, 2017–2025. [Google Scholar] [CrossRef] [PubMed]
- Flipon, E.; Liabeuf, S.; Fardellone, P.; Mentaverri, R.; Ryckelynck, T.; Grados, F.; Kamel, S.; Massy, Z.A.; Dargent-Molina, P.; Brazier, M. Is vascular calcification associated with bone mineral density and osteoporotic fractures in ambulatory, elderly women? Osteoporos. Int. 2012, 23, 1533–1539. [Google Scholar] [CrossRef]
- Sayes, C.M.; Liang, F.; Hudson, J.L.; Mendez, J.; Guo, W.; Beach, J.M.; Moore, V.C.; Doyle, C.D.; West, J.L.; Billups, W.E.; et al. Functionalization density dependence of single-walled carbon nanotubes cytotoxicity in vitro. Toxicol. Lett. 2006, 161, 135–142. [Google Scholar] [CrossRef]
- Wick, P.; Manser, P.; Limbach, L.K.; Dettlaff-Weglikowska, U.; Krumeich, F.; Roth, S.; Stark, W.J.; Bruinink, A. The degree and kind of agglomeration affect carbon nanotube cytotoxicity. Toxicol. Lett. 2007, 168, 121–131. [Google Scholar] [CrossRef]
- Qu, G.; Bai, Y.; Zhang, Y.; Jia, Q.; Zhang, W.; Yan, B. The effect of multiwalled carbon nanotube agglomeration on their accumulation in and damage to organs in mice. Carbon 2009, 47, 2060–2069. [Google Scholar] [CrossRef]
- van Swelm, R.P.L.; Wetzels, J.F.M.; Swinkels, D.W. The multifaceted role of iron in renal health and disease. Nat. Rev. Nephrol. 2020, 16, 77–98. [Google Scholar] [CrossRef]
- Rahimzadeh, M.R.; Rahimzadeh, M.R.; Kazemi, S.; Amiri, R.J.; Pirzadeh, M.; Moghadamnia, A.A. Aluminum poisoning with emphasis on its mechanism and treatment of intoxication. Emerg. Med. Int. 2022, 2022, 1480553. [Google Scholar] [CrossRef]

| PMWCNT (ppm) | TMWCNT (ppm) | |
|---|---|---|
| Mn | nd | nd |
| Co | nd | nd |
| Ni | nd | nd |
| Cu | nd | nd |
| Zn | nd | 8.96 |
| Al | 8973.5 | 613.28 |
| Fe | 12,018.0 | 1082.64 |
| Ti | nd | nd |
| Pt | nd | nd |
| Con | PMWCNT | TMWCNT | |
|---|---|---|---|
| Tubular injury | |||
| Tubular degeneration | 0.21 ± 0.021 | 1.833 ± 0.167 * | 0.900 ± 0.233 |
| Mononuclear/lymphocytic infiltrates | 0.00 ± 0.00 | 0.833 ± 0.401 * | 0.32 ± 0.100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-E.; Cho, M.-H. Effects of Multiwall Carbon Nanotubes on Premature Kidney Aging: Biochemical and Histological Analysis. Toxics 2023, 11, 373. https://doi.org/10.3390/toxics11040373
Kim J-E, Cho M-H. Effects of Multiwall Carbon Nanotubes on Premature Kidney Aging: Biochemical and Histological Analysis. Toxics. 2023; 11(4):373. https://doi.org/10.3390/toxics11040373
Chicago/Turabian StyleKim, Ji-Eun, and Myung-Haing Cho. 2023. "Effects of Multiwall Carbon Nanotubes on Premature Kidney Aging: Biochemical and Histological Analysis" Toxics 11, no. 4: 373. https://doi.org/10.3390/toxics11040373
APA StyleKim, J.-E., & Cho, M.-H. (2023). Effects of Multiwall Carbon Nanotubes on Premature Kidney Aging: Biochemical and Histological Analysis. Toxics, 11(4), 373. https://doi.org/10.3390/toxics11040373








