Abstract
Irradiation resistance is important for adsorbents used in radioactive environments such as high-level liquid waste. In this work, a silica-based composite adsorbent (KAlFe(CN)6/SiO2) was synthesized and γ-irradiated from 10 to 1000 kGy. The angles of the main X-ray diffraction peaks slightly decreased with the increase in irradiation dose, and a minor decomposition of CN− occurred after irradiation to 1000 kGy, indicating that the KAlFe(CN)6/SiO2 adsorbent could preserve structural integrity with a dose below 100 kGy. In 1 to 7 M HNO3, the adsorption ability of the irradiated KAlFe(CN)6/SiO2 remained performant, with a higher Kd than 1625 cm3 g−1. The adsorption equilibrium of Pd(II) in 3 M HNO3 was attained within 45 min before and after irradiation. The maximal adsorption capacity Qe of the irradiated KAlFe(CN)6/SiO2 on Pd(II) ranged from 45.1 to 48.1 mg g−1. A 1.2% relative drop in Qe was observed after 100 kGy irradiation, showing that γ-irradiation lower than 100 kGy insignificantly affected the adsorption capacity of KAlFe(CN)6/SiO2. Calculating and comparing the structures and free energies of different adsorption products via the density functional theory (DFT) method showed that KAlFe(CN)6/SiO2 was more inclined to completely adsorb Pd(II) and spontaneously generate Pd[AlFe(CN)6]2.
1. Introduction
The development of nuclear energy has brought evident benefits in reducing carbon emissions and promoting human health [1,2], although the disposal of spent highly radioactive fuel discharged from nuclear power plants is complex and challenging [3,4,5] partly because of the limited miscibility of platinum group metals (PGMs) [6]. The plutonium uranium extraction (PUREX) process is commonly used to reprocess spent fuel and produces approximately 500 L of radioactive high-level liquid waste (HLLW) per ton of spent fuel. HLLW is dangerous, and has a complex chemical composition and radiation levels of up to 1000 Ci/L [7]. If HLLW is released into the biosphere, radioactive and heavy-metal elements may migrate to the soil and plants, destroying the genetic material of organisms and human organs [8,9]. Therefore, the proper disposal of HLLW, such as by separating high-heat radionuclides, is necessary to protect humans and the biosphere [10,11,12].
Glass solidification is widely used to form a solidified product for HLLW that can envelop radioactive elements and reduce waste volume. This is a type of technology that dissolves the ions in HLLW into amorphous borosilicate glass or phosphate glass to form a new, stable structure. Some studies indicated that the separation of Pd is beneficial for the safety of glass solidification [13,14,15]. Palladium has a low solubility of 0.3 mg g−1 in glass, facilitating precipitation during glass solidification [16]. The precipitated substance has high density, viscosity, and electric conductivity, leading to problems such as blocking the outlet and short-circuiting the electrodes in the melter, threatening production safety. In addition, palladium is a precious strategic resource with distinctive properties that is widely used in areas such as catalytic converters in vehicles and high-pressure turbine blades in jet engines [17,18]. Many scholars have investigated recovering valuable resources such as Pd in HLLW [19]. The long-lived radioisotope of Pd in HLLW is 107Pd, which has a low beta decay energy of 0.033 MeV and is essentially safe for most industrial applications [20]. Adsorbents such as hexacyanoferrate [14], nitrogen-donor-type adsorbents (R-Amine) [21], amino acid-functionalized cellulose microspheres [22], magnetic cross-linking chitosan nanoparticles [23], quaternary ammonium group ion exchange resin [24], bayberry tannin immobilized collagen fiber membranes [25], and ligand-immobilized meso-adsorbents [26] were studied to remove Pd. Among these adsorbents, hexacyanoferrate is expected because of its excellent adsorption capacity and easy accessibility [14,27]. Compared with expensive organic extractants, e.g., TODGA, costing CNY 150–500 (about USD 22–73) per gram, hexacyanoferrate is economical as a commercially available conventional reagent, costing CNY 0.1–0.3 (about USD 0.015–0.045) per gram.
Hexacyanoferrate powder is not suitable for the column, and a solid support is required for better surface area. SiO2 and PAN are commonly used as solid supports for these kinds of systems [28,29,30], as they have better mechanical and hydraulic properties, and a greater surface area. HLLW is a radioactive and heat-releasing material with high requirements for material irradiation resistance. Generally, inorganic adsorbents have better radiation resistance than that of equivalent organic solvent-extraction and ligand systems [31,32]. However, the effects of irradiation on hexacyanoferrate and palladium removal, despite being important for the industry, have rarely been discussed in existing research.
In this work, silica-based aluminum hexacyanoferrate was selected as the adsorbent. In addition, we study the γ-irradiation resistance and theoretical Pd(II) adsorption products of KAlFe(CN)6/SiO2. The KAlFe(CN)6/SiO2 adsorbent was prepared and irradiated with 60Co. The effects of different γ-irradiation doses on the crystal structure of adsorbent were analyzed. The adsorption performance of irradiated KAlFe(CN)6/SiO2 on Pd(II) at different HNO3 concentrations was investigated. Adsorption kinetics and adsorption isotherms before and after γ-irradiation were explored. Adsorption products with different adsorption rates and Pd(II) binding sites were calculated by using the DFT method, and their structural characteristics and free energy were compared.
2. Materials and Methods
2.1. Materials
Aluminum chloride hexahydrate (AlCl3∙6H2O) with 99% purity and potassium hexacyanoferrate trihydrate (K4Fe(CN)6∙3H2O) with 99.5% purity were purchased from Shanghai Macklin Inc, Shanghai, China. Palladium nitrate dihydrate (Pd(NO3)2·2H2O) with 99% purity was obtained from Beijing Innochem, Beijing, China. Nitric acid (HNO3) was obtained from Shanghai Sinopharm, Shanghai, China and was of analytical grade. Silica (SiO2) was purchased from Fuji Silysia Chemical Ltd., Kasugai, Japan. The conductivity of deionized water used in the experiments was less than 5 μS cm−1.
2.2. Preparation of KAlFe(CN)6/SiO2
The KAlFe(CN)6/SiO2 adsorbent was synthesized by using a two-step loading method. First, the silica was washed and dried at 90 °C for 1 day. The 1 M AlCl3 solution and 1 M K4Fe(CN)6 solution were successively sucked into the silica pores at a phase ratio of 10 cm3 g−1. Then, the filter flask was left at 25 °C for 1 day to fully react. When the solution had been layered and there was no newly generated precipitate, the product was removed, filtered, washed, and dried at 90 °C for 1 day. According to EDS mapping analysis, the KAlFe(CN)6 accounted for about 30% of the KAlFe(CN)6/SiO2 composite adsorbent in the mass.
2.3. Irradiation Treatment and Characterization
The KAlFe(CN)6/SiO2 adsorbent was γ-irradiated in an air atmosphere at 25 °C. The irradiation source was 60Co (105 Ci), and the irradiation doses were 10, 50, 100, and 1000 kGy calibrated with a Frick dosing agent at a rate of 5 kGy/h. After irradiation, KAlFe(CN)6/SiO2 was characterized. Scanning electron microscopy (SEM) was performed with a Mira 3 (TESCAN ORSAY HOLDING a.s., Brno, Czech Republic). An X-ray diffractometer (XRD, D8 Advance, Brucker Corporation, Billerica, MA, USA) and a Fourier transform infrared spectrometer (FT–IR, Nicolet 6700, Thermo Fisher Scientific, Waltham, MA, USA) were used to analyze the crystal structure and chemical bonds of the adsorbents.
2.4. Batch Experiments
Batch experiments were conducted at 25 °C. In a clean vial, a Pd(II) solution (V = 5 mL) was mixed with KAlFe(CN)6/SiO2 (m = 0.05 g). The supernatant inside the vial was filtered and diluted. The concentration of Pd(II) in the filtrate was obtained by using an inductively coupled plasma (ICP) spectrometer (Shimadzu Corporation, Kyoto, Japan, ICP-7500).
The distribution coefficient of Pd(II) (Kd) was calculated as follows:
where C0 is the concentration of Pd(II) in the preadsorption solution, Ce represents the equilibrium concentration, V is the volume of the Pd(II) solution, and m is the mass of the KAlFe(CN)6/SiO2 adsorbent.
The experimental adsorption capacity of Pd(II) (Qe) is defined as follows:
2.5. DFT Calculations
In exploring the adsorption mechanism of Pd(II) by KAlFe(CN)6, the optimized structures and free energies of the adsorbent and adsorption products were calculated on the basis of DFT. Calculations were performed via VASP 6.2. The modeling and visualization of the hexacyanoferrates were conducted in VESTA [33]. Considering that the adsorption capacity may have been different, the Pd(II) products with adsorption rates of 100% (Pd[AlFe(CN)6]2) and 50% (Pd0.5K[AlFe(CN)6]2) were calculated. The crystal parameters and free-energy changes caused by the difference in Pd(II) adsorption sites in Pd0.5K[AlFe(CN)6]2 were compared. The generalized gradient approximation (GGA) method was selected as the wavefunction optimization potential. Calculations were performed in the inverted space by using the Perdew–Burke–Ernzerhof (PBE) functional [34].
3. Results and Discussion
3.1. XRD Analysis before and after Irradiation
In studying whether irradiation changes the crystal structure of KAlFe(CN)6, XRD characterization was performed on the KAlFe(CN)6 powder sample after irradiation (Figure 1). Table 1 shows the angle of incidence and lattice parameters of the KAlFe(CN)6 powder before and after irradiation.
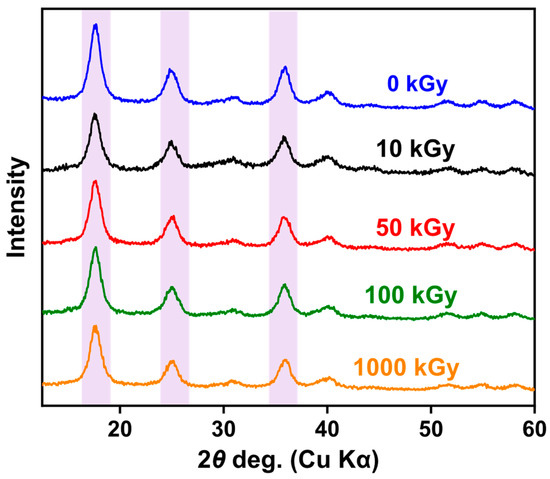
Figure 1.
XRD patterns of KAlFe(CN)6 before and after irradiation.

Table 1.
Angles of incidence of main diffraction peaks and lattice parameters of KAlFe(CN)6 before and after irradiation.
As shown in Figure 1 and Table 1, the lattice of KAlFe(CN)6 expanded slightly after irradiation for 10, 50, 100, or 1000 kGy. The angles of the three main diffraction peaks slightly decreased with the increase in irradiation dose from 17.672°, 25.028°, and 36.051° to 17.482°, 24.840°, and 35.746°, respectively. Nevertheless, the strength and shape of the peaks in the spectrum remained similar throughout irradiation, indicating that irradiation did not cause a notably change in the substance, and the structure of the crystal remained stable. The results can be explained by the action mechanism of irradiation. The effect of γ rays is identical with that of β rays except for the thermal effect [35]. Under γ-ray irradiation, free electrons were formed in the KAlFe(CN)6 structure because of the photoelectric and Compton effects. The electrons interacted with the adsorbent and caused atomic displacements, forming defects such as dislocation loops [36,37].
Further analysis showed that, after irradiation at 1000 kGy, the lattice parameter of the KAlFe(CN)6 adsorbent increased slightly from 10.0155 to 10.0464 Å, and the unit cell remained face-centered cubic. Thus, the irradiation dose from 10 to 1000 kGy hardly affects the lattice structure and irregularity of KAlFe(CN)6, and the radiation resistance of the adsorbent was excellent.
3.2. SEM and FT–IR Analysis before and after Irradiation
The SEM images of KAlFe(CN)6/SiO2 before and after irradiation are shown in Figure 2. The adsorbent particle was spherical with a diameter of about 100 μm before irradiation. It maintained that shape and surface morphology with some minor cracks forming after 50 and 1000 kGy of γ irradiation. The adsorbents did not collapse or disintegrate after irradiation, indicating that KAlFe(CN)6/SiO2 had good radiation resistance.

Figure 2.
SEM images of KAlFe(CN)6/SiO2 before and after (left to right) 0, 50, and 1000 kGy irradiation.
The FT–IR spectra of KAlFe(CN)6/SiO2 before and after irradiation are shown in Figure 3. The peak at 3430 cm−1 was attributed to the O–H stretching vibration peak, and 1621 cm−1 was the H–O–H bending vibration peak [38]; both came from crystal water. The peaks at 1110 and 801 cm−1 were assigned to the Si–O stretching vibration in SiO2, and 473 cm−1 corresponds to the inplane bending vibration of O–Si–O [38]. The peak at 554 cm−1 was assigned to the C–Fe stretching vibration [39]. The peaks at 2126 and 2078 cm−1 correspond to the C≡N stretching vibration [38,40], indicating the presence of hexacyanoferrate. The peak of CN− weakened with the increase in irradiation dose, which indicates that the γ ray destroyed a minor portion of CN− [41,42]. The above analysis shows that KAlFe(CN)6/SiO2 could preserve structural integrity under up to 1000 kGy of irradiation.

Figure 3.
FT–IR patterns of KAlFe(CN)6/SiO2 before and after (top to bottom) 0, 50, 100, and 1000 kGy irradiation.
3.3. Adsorption Properties in Nitric Acid before and after Irradiation
The adsorption performance of the irradiated KAlFe(CN)6/SiO2 was investigated. Figure 4 shows the adsorption effects of different concentrations of HNO3 on Pd(II) after receiving 10 to 1000 kGy of irradiation. Distribution coefficient Kd was slightly reduced after irradiation, and the magnitude of the drop decreased with the increase in HNO3 concentration. In 5 and 7 M HNO3 concentration, Kd remained almost the same from 0 to 100 kGy. In 1 to 3 M HNO3 concentration, Kd was highly sensitive to the adsorption rate because the rate was high. In addition, Kd remained high after 1000 kGy of irradiation, ranging from 1625 to 38,445 cm3 g−1, indicating that the adsorption ability of the irradiated KAlFe(CN)6/SiO2 remained performant in 1 to 7 M HNO3.

Figure 4.
Kd of KAlFe(CN)6/SiO2 for Pd(II) before and after irradiation in 1–7 M HNO3; phase ratio = 100 cm3 g−1, [Pd] = 200 mg L−1, temperature = 25 °C, and contact time = 24 h.
The adsorption rate is an important factor in judging adsorption performance. Figure 5 shows the effects of contact time on Pd(II) adsorption. The adsorption ratios of the KAlFe(CN)6/SiO2 adsorbent for Pd(II) rapidly increased with time. When the contact time reached 15 min, the adsorption ratios of Pd(II) were over 87%, 86%, and 81% for 0, 50, and 1000 kGy irradiated adsorbents, respectively, indicating excellent adsorption kinetics. Subsequently, the adsorption rate of Pd(II) slowed down because the sites on KAlFe(CN)6/SiO2 tended to be saturated. After 45 min, the adsorption of Pd(II) by KAlFe(CN)6/SiO2 reached equilibrium, and the equilibrium adsorption rates were 99.9%, 99.8%, and 99.8%, respectively.
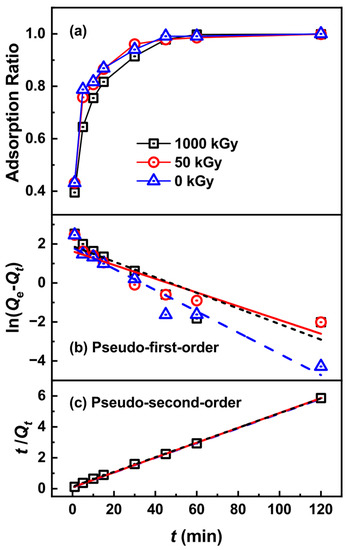
Figure 5.
(a) Adsorption ratio of Pd(II) by KAlFe(CN)6/SiO2 versus adsorption time with a phase ratio = 100 cm3 g−1, [Pd] = 200 mg L−1, temperature = 25 °C, and contact time = 24 h; (b) pseudo-first-order adsorption kinetic model fitting; (c) pseudo-second-order adsorption kinetic model fitting.
Further, the pseudo-first-order kinetic equation (Equation (3)) and pseudo-second-order kinetic equation (Equation (4)) were used for fitting to explore the adsorption rate control steps of KAlFe(CN)6/SiO2 on Pd(II) [43].
where k1 and k2 are fitted parameters related to the adsorption rate.
The pseudo-second-order equation fitted better than the pseudo-first-order equation (Figure 5). For the pseudo-first-order equation, the correlation coefficients were lower than 0.95; the values of the pseudo-second-order equation, on the other hand, were close to 1, indicating good linearity (Table 2). Moreover, the Qe values of KAlFe(CN)6/SiO2 for Pd(II) obtained with the pseudo-second-order equation were 20.95, 20.82, and 20.61 mg g−1 for the irradiated adsorbents with 0, 50, and 1000 kGy, respectively, which values were close to the experimental data. k2 shows that the adsorption rate remained approximately equal before and after 50 kGy irradiation. These results indicate that the pseudo-second-order equation could describe the adsorption kinetics of KAlFe(CN)6/SiO2 well at different nitric acid concentrations, and the adsorption type of Pd(II) through the KAlFe(CN)6/SiO2 adsorbent was controlled via chemical adsorption.

Table 2.
Adsorption kinetic parameters of Pd(II) by KAlFe(CN)6/SiO2 before and after irradiation.
3.4. Adsorption Isotherms before and after Irradiation
In investigating the capacity and mechanism of the adsorption of the KAlFe(CN)6/SiO2 adsorbent on Pd(II), adsorption isotherms were obtained from the experiments, and fitted using the Langmuir (Equation (5)) [44], Freundlich (Equation (6)) [45], and Redlich–Peterson (Equation (7)) [46] models.
where Qmax is the maximal adsorption capacity predicted by the model; KL, 1⁄n, KR, KP, and g are the fitting parameters.
As shown in Figure 6 and Table 3, the coefficients of determination R2 of the Langmuir and Redlich–Peterson models were higher than 0.96, and the fitted parameter g in the Redlich–Peterson model was between 0.96 and 0.995, indicating that the Redlich–Peterson model could be converted into the Langmuir model in these cases. With a smaller irradiation dose than 1000 kGy, the adsorption of KAlFe(CN)6/SiO2 on Pd(II) corresponded to the Langmuir model, suggesting that the adsorption sites on KAlFe(CN)6/SiO2 were on a single layer, and the adsorbed Pd(II) had no internal interaction [44].

Figure 6.
Adsorption isotherms and model fitting of KAlFe(CN)6/SiO2 for Pd(II) before and after irradiation in 3 M HNO3 with a phase ratio = 100 cm3 g−1, temperature = 25 °C, and contact time = 24 h. (a) before irradiation; after (b) 10 kGy, (c) 50 kGy, (d) 100 kGy, and (e) 1000 kGy irradiation.

Table 3.
Fitted parameters of adsorption isotherms before and after irradiation.
The fitted results of Equation (5) show that, after irradiation with 10, 50, 100, and 1000 kGy, the maximal adsorption capacities Qe of KAlFe(CN)6/SiO2 on Pd(II) were 48.1, 47.8, 47.7, and 45.1 mg g−1, respectively. The relative decrease in the unirradiated sample was only 0.4%, 1.0%, 1.2%, and 6.2%, respectively. The results indicate that the adsorption capacity of KAlFe(CN)6/SiO2 was insignificantly influenced by the irradiation dose and remained virtually unchanged after no more than 100 kGy of irradiation. Thus, KAlFe(CN)6/SiO2 could be used for the separation and recovery of Pd(II) under radioactive conditions.
As shown in Table 4, compared with other adsorbents, the adsorption capacity of KAlFe(CN)6/SiO2 was higher with shorter equilibrium time.

Table 4.
Comparison of adsorption capacity and equilibrium time on Pd(II) with different adsorbents.
3.5. DFT Calculations
The DFT method was applied to analyze the adsorption products of Pd(II) by KAlFe(CN)6. Stable structures with different adsorption rates and positions of adsorption sites were calculated. Figure 7a illustrates the optimized structure of the Pd[AlFe(CN)6]2 (S1) product of saturated adsorption. Figure 7b–f show the optimized structures of the Pd0.5K[AlFe(CN)6]2 product with an adsorption rate of 50%, namely, S2-1 to S2-5, for different adsorption sites. Table 5 lists the lattice parameters of these optimized structures, proving that all six types of products had FCC cells with similar volumes, where the cell of the saturated adsorption of product S1 was a cube, whereas unsaturated product S2 was a cuboid because of its asymmetry in the position of K and Pd atoms.

Figure 7.
Optimized stable structures of (a) Pd[AlFe(CN)6]2 and (b–f) Pd0.5K[AlFe(CN)6]2.

Table 5.
Structural parameters of Pd[AlFe(CN)6]2 and Pd0.5K[AlFe(CN)6]2 with different adsorption positions.
On the basis of the optimized stable structures before and after adsorption, the Helmholtz free energy (F) values were calculated. Thermodynamic calibration was performed via Phonopy [51] and VASPKIT [52], and the change in free energy (ΔF) was calculated as
where F represents the free energy, m is the number of Pd atoms, and n is the number of K atoms.
The results are illustrated in Figure 8, where the free-energy changes of S1 are in the line chart and those of S2s are in the column chart. The ΔF of S1 was lower than that of S2, from 273.15 to 373.15 K, showing that S1 was spontaneously generated. The KAlFe(CN)6 adsorbent tended to completely absorb Pd(II) and become Pd[AlFe(CN)6]2.

Figure 8.
Calculated free-energy changes of different reactions.
S2 structures, on the other hand, were intermediate products of adsorption. The system free-energy changes indicate that S2-2 and S2-3 were likely generated more spontaneously than the others because of the low ΔF. In addition, the free-energy changes varied with temperature, that is, S2-3 was slightly superior, below 323.15 K, whereas S2-2 changed to the dominant structure of above 323.15 K.
4. Conclusions
The crystal structure and adsorption ability of gamma-irradiated KAlFe(CN)6/SiO2 on Pd(II) were investigated. SEM, XRD, and FT–IR characterizations showed that the peak positions and peak intensities of the irradiated adsorbent changed slightly, but its crystal structure was stable and not significantly damaged. After irradiation, the Kd of Pd(II) dropped slightly, and the magnitude of the drop decreased with the increase in HNO3 concentration. For the irradiation dose of 100 kGy, Kd on the adsorbent was higher than 5846 cm3 g−1, and Qe was 48.1 mg g−1, which was only 1.2% lower than that of the unirradiated samples. The adsorption equilibrium was attained within 45 min, and the adsorption of Pd(II) followed the pseudo-second-order kinetic equation, which corresponds to chemical adsorption. The adsorption results indicated that KAlFe(CN)6/SiO2 achieved excellent adsorption performance on Pd(II) with 100 kGy irradiation. The DFT calculations showed that the stablest theoretical Pd(II) adsorption product of KAlFe(CN)6 was Pd[AlFe(CN)6]2, and this adsorption process was spontaneous. When the temperature was lower than 323.15 K, the most dominant intermediate product was Pd0.5K[AlFe(CN)6]2 with a cell volume of 1013.53 Å3, followed by Pd0.5K[AlFe(CN)6]2 with a cell volume of 1013.09 Å3. Therefore, KAlFe(CN)6/SiO2 could be used to separate and recover Pd(II) under radioactive conditions for use in other industries. Because of the low beta decay energy, the recovered Pd is essentially safe for industrial applications. Further study into recovering Pd from adsorbents is required, and there are several ongoing works on this topic.
Author Contributions
Investigation, visualization, and writing—original draft preparation, Y.W. (Yueying Wen); writing—review and editing and funding acquisition, Y.W. (Yan Wu); resources, L.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (grant numbers 12175143 and 11975082).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- IAEA. Climate Change and Nuclear Power 2018; International Atomic Energy Agency: Vienna, Austria, 2018. [Google Scholar]
- Kharecha, P.A.; Hansen, J.E. Prevented Mortality and Greenhouse Gas Emissions from Historical and Projected Nuclear Power. Environ. Sci. Technol. 2013, 47, 4889–4895. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Kuang, Y.; Tang, D.; Guo, Z.; Xu, S. Risk analysis of high level radioactive waste storage tank based on HAZOP. Ann. Nucl. Energy 2018, 119, 106–116. [Google Scholar] [CrossRef]
- Wu, Y.; Lee, C.-P.; Mimura, H.; Zhang, X.; Wei, Y. Stable solidification of silica-based ammonium molybdophosphate by allophane: Application to treatment of radioactive cesium in secondary solid wastes generated from fukushima. J. Hazard. Mater. 2018, 341, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, X.-X.; Wei, Y.-Z.; Mimura, H. Development of adsorption and solidification process for decontamination of Cs-contaminated radioactive water in Fukushima through silica-based AMP hybrid adsorbent. Sep. Purif. Technol. 2017, 181, 76–84. [Google Scholar] [CrossRef]
- Laurin, C.; Régnier, E.; Gossé, S.; Laplace, A.; Agullo, J.; Mure, S.; Brackx, E.; Toplis, M.; Pinet, O. Redox behavior of ruthenium in nuclear glass melt: Ruthenium dioxide reduction reaction. J. Nucl. Mater. 2021, 545, 152650. [Google Scholar] [CrossRef]
- Wei, Y.; Wu, Y.; Li, H. The Newest Nuclear Fuel Cycle; Shanghai Jiao Tong University Press: Shanghai, China, 2016; pp. 99–125, 225–268, 313–360. [Google Scholar]
- Hassan, I.A.; Saleh, H.M. Biological Effects of Hazardous Waste: Threshold Limits of Anomalies and Protective Approaches. Hazard. Waste Manag. Health Risks 2020, 1, 62. [Google Scholar] [CrossRef]
- Choppin, G.; Liljenzin, J.-O.; Rydberg, J.; Ekberg, C. Chapter 15—Radiation Biology and Radiation Protection. In Radiochemistry and Nuclear Chemistry, 4th ed.; Choppin, G., Liljenzin, J.-O., Rydberg, J., Ekberg, C., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 445–491. [Google Scholar]
- Abdelhamid, A.A.; Badr, M.H.; Mohamed, R.A.; Saleh, H.M. Using Agricultural Mixed Waste as a Sustainable Technique for Removing Stable Isotopes and Radioisotopes from the Aquatic Environment. Sustainability 2023, 15, 1600. [Google Scholar] [CrossRef]
- Dawoud, M.M.A.; Hegazi, M.M.; Saleh, H.M.; El Helew, W.K. Removal of stable and radio isotopes from wastewater by using modified microcrystalline cellulose based on Taguchi L16. Int. J. Environ. Sci. Technol. 2023, 20, 1289–1300. [Google Scholar] [CrossRef]
- Forsberg, C.W. Rethinking High-Level Waste Disposal: Separate Disposal of High-Heat Radionuclides (90Sr and 137Cs). Nucl. Technol. 2000, 131, 252–268. [Google Scholar] [CrossRef]
- Sawai, M. Rokkasho Reprocessing Plant: Vitrification Problems Continue Nuke Info Tokyo No. 125. Available online: https://cnic.jp/english/?p=1233 (accessed on 19 October 2022).
- Wang, Q.; Sang, H.; Chen, L.; Wu, Y.; Wei, Y. Selective separation of Pd(II) through ion exchange and oxidation-reduction with hexacyanoferrates from high-level liquid waste. Sep. Purif. Technol. 2020, 231, 115932. [Google Scholar] [CrossRef]
- Xu, K. International research progress on nuclear waste vitrification: A review. Mater. China 2016, 35, 481–488+517. [Google Scholar] [CrossRef]
- Lu, J.; Lin, P.; Li, L.; Zhao, X.; Wang, Y.; Weng, H.; Lin, M. Solubility of typical PGMs in HLLW glass and influence of PGMs on the properties of HLLW glass. Radiat. Prot. 2023, 43, 17–30. [Google Scholar]
- Yadav, M.; Misra, A.; Malhotra, A.; Kumar, N. Design and analysis of a high-pressure turbine blade in a jet engine using advanced materials. Mater. Today Proc. 2020, 25, 639–645. [Google Scholar] [CrossRef]
- Yakoumis, I. PROMETHEUS: A Copper-Based Polymetallic Catalyst for Automotive Applications. Part I: Synthesis and Characterization. Materials 2021, 14, 622. [Google Scholar] [CrossRef]
- Holdsworth, A.F.; Eccles, H.; Sharrad, C.A.; George, K. Spent Nuclear Fuel—Waste or Resource? The Potential of Strategic Materials Recovery during Recycle for Sustainability and Advanced Waste Management. Waste 2023, 1, 249–263. [Google Scholar] [CrossRef]
- Venkatesan, K.A.; Selvan, B.R.; Antony, M.P.; Srinivasan, T.G.; Rao, P.R.V. Extraction of palladium from nitric acid medium by commercial resins with phosphinic acid, methylene thiol and isothiouronium moieties attached to polystyrene-divinylbenzene. J. Radioanal. Nucl. Chem. 2005, 266, 431–440. [Google Scholar] [CrossRef]
- Suzuki, T.; Otsubo, U.; Ogata, T.; Shiwaku, H.; Kobayashi, T.; Yaita, T.; Matsuoka, M.; Murayama, N.; Narita, H. Selective adsorption of Pd(II) over Ag(I) in nitric acid solutions using nitrogen-donor-type adsorbents. Sep. Purif. Technol. 2023, 308, 122943. [Google Scholar] [CrossRef]
- Peng, L.; Zhang, M.; Dong, Z.; Qi, W.; Zhai, M.; Zhao, L. Efficient and selective adsorption of Pd(II) by amino acid-functionalized cellulose microspheres and their applications in palladium recovery from PCBs leaching solution. Sep. Purif. Technol. 2022, 301, 122037. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, J.; Liang, X.; Liu, Z. Adsorption of platinum(IV) and palladium(II) from aqueous solution by magnetic cross-linking chitosan nanoparticles modified with ethylenediamine. J. Hazard. Mater. 2010, 182, 518–524. [Google Scholar] [CrossRef]
- Els, E.R.; Lorenzen, L.; Aldrich, C. The adsorption of precious metals and base metals on a quaternary ammonium group ion exchange resin. Miner. Eng. 2000, 13, 401–414. [Google Scholar] [CrossRef]
- Ma, H.-W.; Liao, X.-P.; Liu, X.; Shi, B. Recovery of platinum(IV) and palladium(II) by bayberry tannin immobilized collagen fiber membrane from water solution. J. Membr. Sci. 2006, 278, 373–380. [Google Scholar] [CrossRef]
- Awual, M.R.; Khaleque, M.A.; Ratna, Y.; Znad, H. Simultaneous ultra-trace palladium(II) detection and recovery from wastewater using new class meso-adsorbent. J. Ind. Eng. Chem. 2015, 21, 405–413. [Google Scholar] [CrossRef]
- Tang, Y.; Li, W.; Feng, P.; Zhou, M.; Wang, K.; Jiang, K. Investigation of alkali-ion (Li, Na and K) intercalation in manganese hexacyanoferrate KxMnFe(CN)6 as cathode material. Chem. Eng. J. 2020, 396, 125269. [Google Scholar] [CrossRef]
- Hamoud, M.A.; Allan, K.F.; Sanad, W.A.; Saad, E.A.; Mahmoud, M.R. Synthesis of PAN/ferrocyanide composite incorporated with cetrimonium bromide and its employment as a bifunctional adsorbent for coremoval of Cs+ and HCrO4− from aqueous solutions. J. Radioanal. Nucl. Chem. 2020, 324, 647–661. [Google Scholar] [CrossRef]
- Nilchi, A.; Saberi, R.; Moradi, M.; Azizpour, H.; Zarghami, R. Adsorption of cesium on copper hexacyanoferrate–PAN composite ion exchanger from aqueous solution. Chem. Eng. J. 2011, 172, 572–580. [Google Scholar] [CrossRef]
- Holdsworth, A.F.; Eccles, H.; Rowbotham, D.; Brookfield, A.; Collison, D.; Bond, G.; Kavi, P.C.; Edge, R. The Effect of Gamma Irradiation on the Physiochemical Properties of Caesium-Selective Ammonium Phosphomolybdate–Polyacrylonitrile (AMP–PAN) Composites. Clean Technol. 2019, 1, 294–310. [Google Scholar] [CrossRef]
- Holdsworth, A.F.; Eccles, H.; Rowbotham, D.; Bond, G.; Kavi, P.C.; Edge, R. The Effect of Gamma Irradiation on the Ion Exchange Properties of Caesium-Selective Ammonium Phosphomolybdate-Polyacrylonitrile (AMP-PAN) Composites under Spent Fuel Recycling Conditions. Separations 2019, 6, 23. [Google Scholar] [CrossRef]
- Tranter, T.J.; Herbst, R.S.; Todd, T.A.; Olson, A.L.; Eldredge, H.B. Evaluation of ammonium molybdophosphate-polyacrylonitrile (AMP-PAN) as a cesium selective sorbent for the removal of 137Cs from acidic nuclear waste solutions. Adv. Environ. Res. 2002, 6, 107–121. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Mao, J.; Wang, T.; Zhang, X.; Sun, M.; Wang, T.; Peng, H. Equivalence analysis of electron and γ-ray irradiation effects on borosilicate glass. Mod. Appl. Phys. 2021, 12, 116–122. [Google Scholar]
- Was, G.S. Fundamentals of Radiation Materials Science; Springer: New York, NY, USA, 2017; pp. 167–181. [Google Scholar]
- Jing, T. Study on radiation damage of electron and γ-rays and mechanism of nuclear hardening. China Nucl. Sci. Technol. Rep. 2000, 00, 1284–1294. [Google Scholar]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 4th ed.; Chemical Industry Press: Beijing, China, 1991. [Google Scholar]
- Roque-Malherbe, R.; Carballo, E.; Polanco, R.; Lugo, F.; Lozano, C. Structure and adsorption properties of a porous cooper hexacyanoferrate polymorph. J. Phys. Chem. Solids 2015, 86, 65–73. [Google Scholar] [CrossRef]
- Nishioka, H.; Nishikawa, M.; Katagi, M.; Tatsuno, M.; Tsuchihashi, H. Analysis of Cyanide Compounds Using Fourier Transform Raman Spectroscopy. Jpn. J. Sci. Technol. Identif. 2000, 4, 61–66. [Google Scholar] [CrossRef]
- Hu, Y.; Hu, H.; Yang, M.; Huang, H.; Feng, H. Radiolysis of copper cyanide solution by electron-beam irradiation. Environ. Pollut. Prev. 2009, 31, 31–34. [Google Scholar]
- Liu, Y.; Hu, H.; Yang, M.; Liu, J.; Dang, J.; Wu, Y. Study on the degradation of zinc-cynide complex in gold-leaching wastewater by the E-beam radiolysis. Ind. Saf. Environ. Prot. 2011, 37, 4–6. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Laskar, I.I.; Hashisho, Z. Insights into modeling adsorption equilibria of single and multicomponent systems of organic and water vapors. Sep. Purif. Technol. 2020, 241, 116681. [Google Scholar] [CrossRef]
- Redlich, O.; Peterson, D.L. A Useful Adsorption Isotherm. J. Phys. Chem. 1959, 63, 1024. [Google Scholar] [CrossRef]
- Muslu, N.; Gülfen, M. Selective separation and concentration of Pd(II) from Fe(III), Co(II), Ni(II), and Cu(II) ions using thiourea-formaldehyde resin. J. Appl. Polym. Sci. 2011, 120, 3316–3324. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, C.; Wang, S.; Zhang, L.; Zhang, B. Augmenting the adsorption parameters of palladium onto pyromellitic acid-functionalized nanosilicas from aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2019, 578, 123581. [Google Scholar] [CrossRef]
- Kasaini, H.; Goto, M.; Furusaki, S. Selective Separation of Pd(II), Rh(III), and Ru(III) Ions from a Mixed Chloride Solution Using Activated Carbon Pellets. Sep. Sci. Technol. 2000, 35, 1307–1327. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, A.; Pu, N.; Xu, C.; Chen, J. Development of Two novel silica based symmetric triazine-ring opening N-donor ligands functional adsorbents for highly efficient separation of palladium from HNO3 solution. J. Hazard. Mater. 2019, 376, 188–199. [Google Scholar] [CrossRef]
- Togo, A.; Tanaka, I. First principles phonon calculations in materials science. Scr. Mater. 2015, 108, 1–5. [Google Scholar] [CrossRef]
- Wang, V.; Xu, N.; Liu, J.-C.; Tang, G.; Geng, W.-T. VASPKIT: A user-friendly interface facilitating high-throughput computing and analysis using VASP code. Comput. Phys. Commun. 2021, 267, 108033. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).