A Sublethal Concentration of Sulfoxaflor Has Minimal Impact on Buff-Tailed Bumblebee (Bombus terrestris) Locomotor Behaviour under Aversive Conditioning
Abstract
1. Introduction
2. Materials and Methods
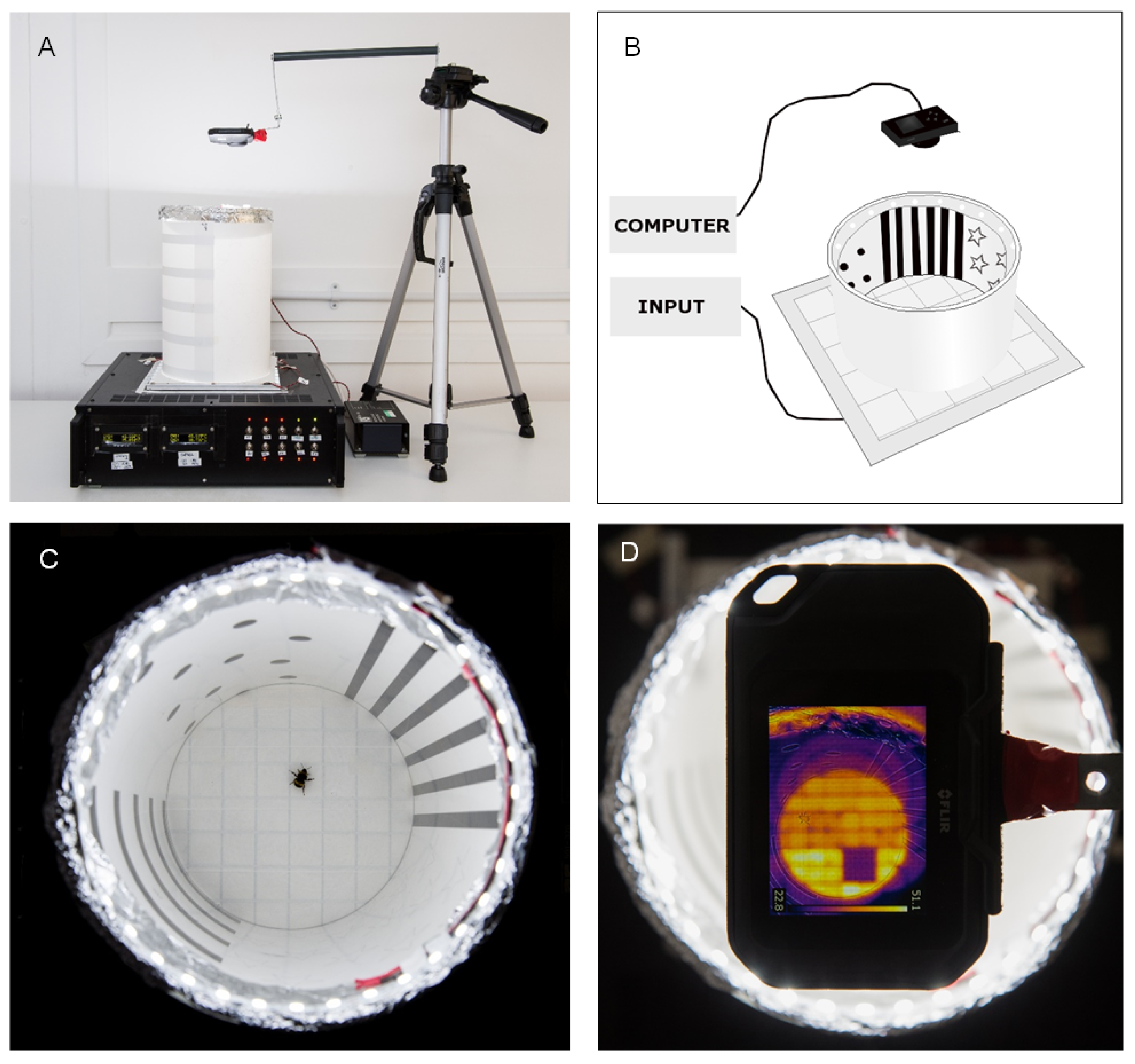
2.1. The Thermal–Visual Arena
2.2. Bee Colonies
2.3. Bee Training Protocol
2.4. Age Cohorts and Marking
2.5. Choice of Pesticide Compounds and Exposure
2.6. Trial Design
2.6.1. Chronic Pesticide Exposure and Temporal Spacing of Trials
2.6.2. Sublethal Concentration Insecticide Trials
2.6.3. Food Consumption Recording
2.6.4. Trial Recording and Video Processing
2.7. Data Analyses
2.7.1. Training Parameters
2.7.2. Power Law Calculation
3. Results
3.1. How Does Chronic Insecticide Exposure Affect Food Consumption?
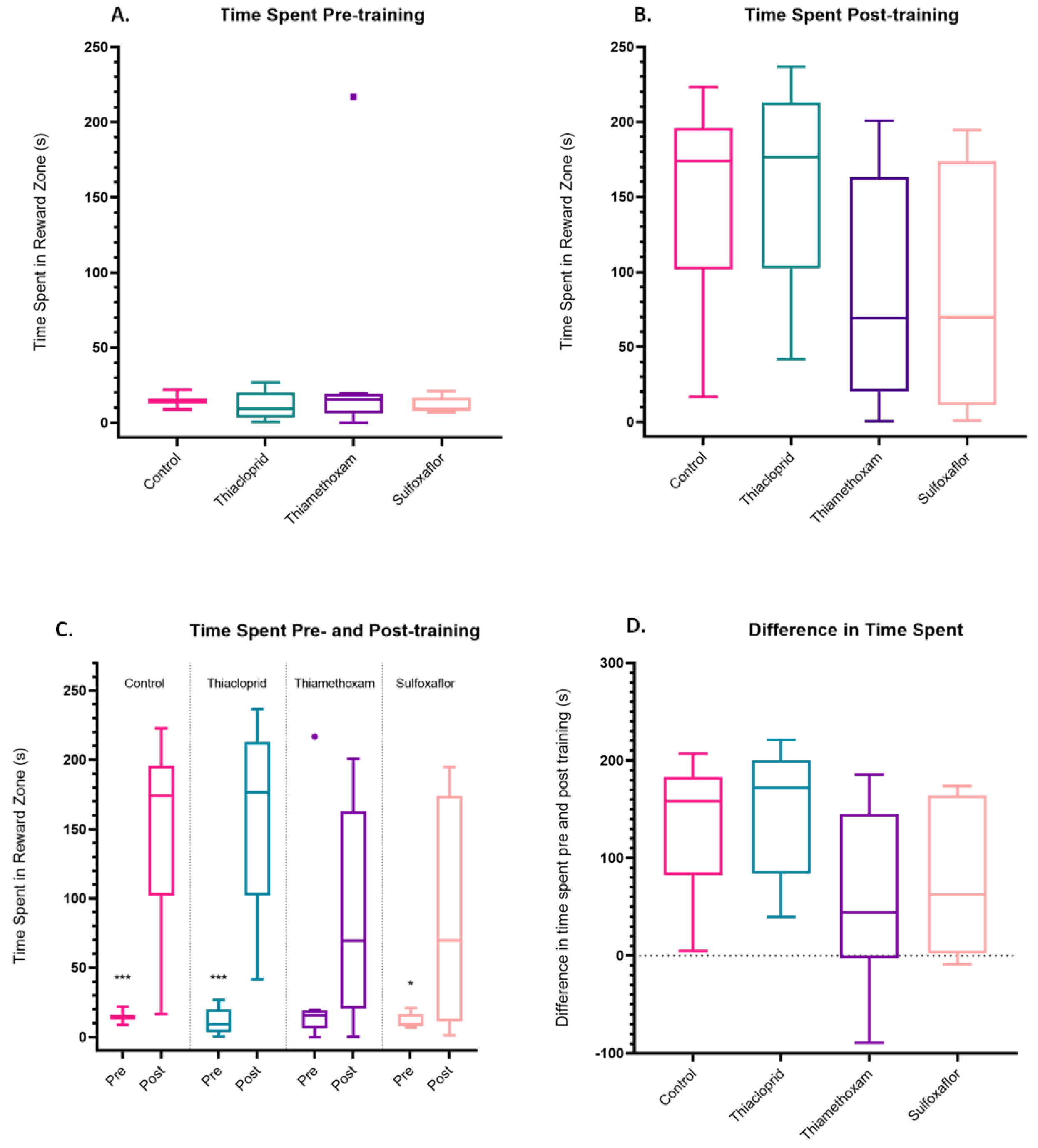
3.2. Pesticide Impacts on Training Parameters
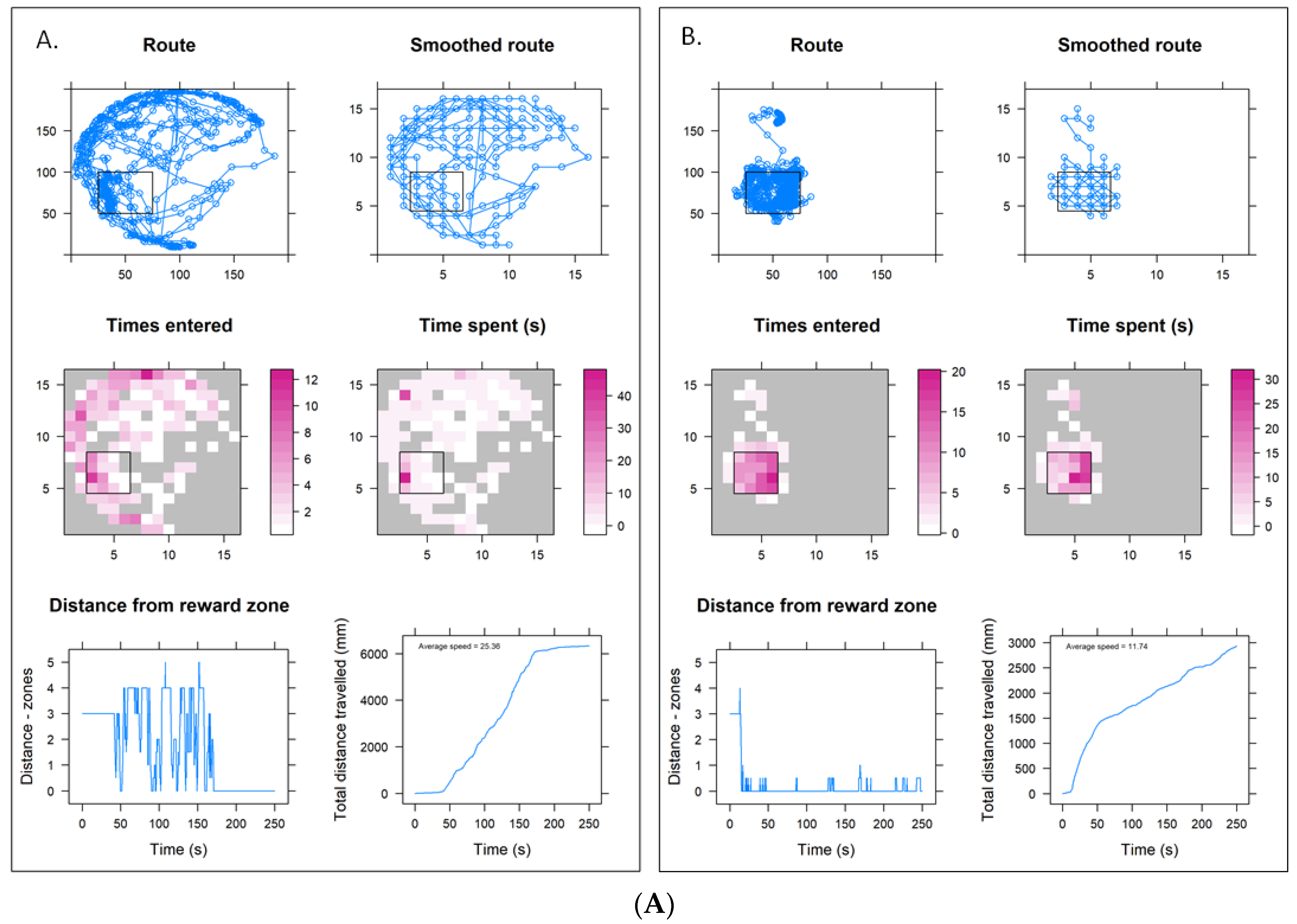
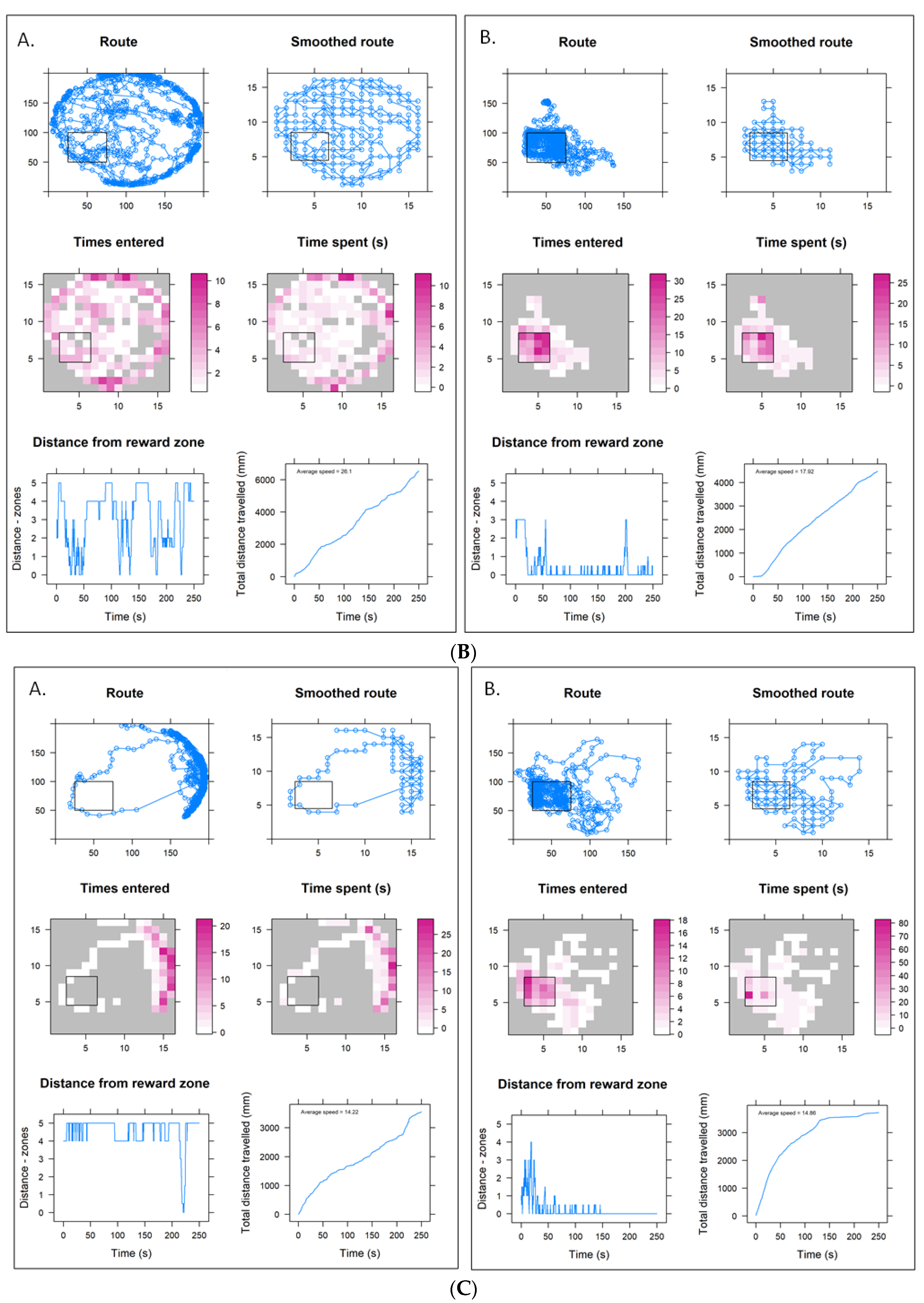
3.3. Lower Concentration Insecticide Trials Data
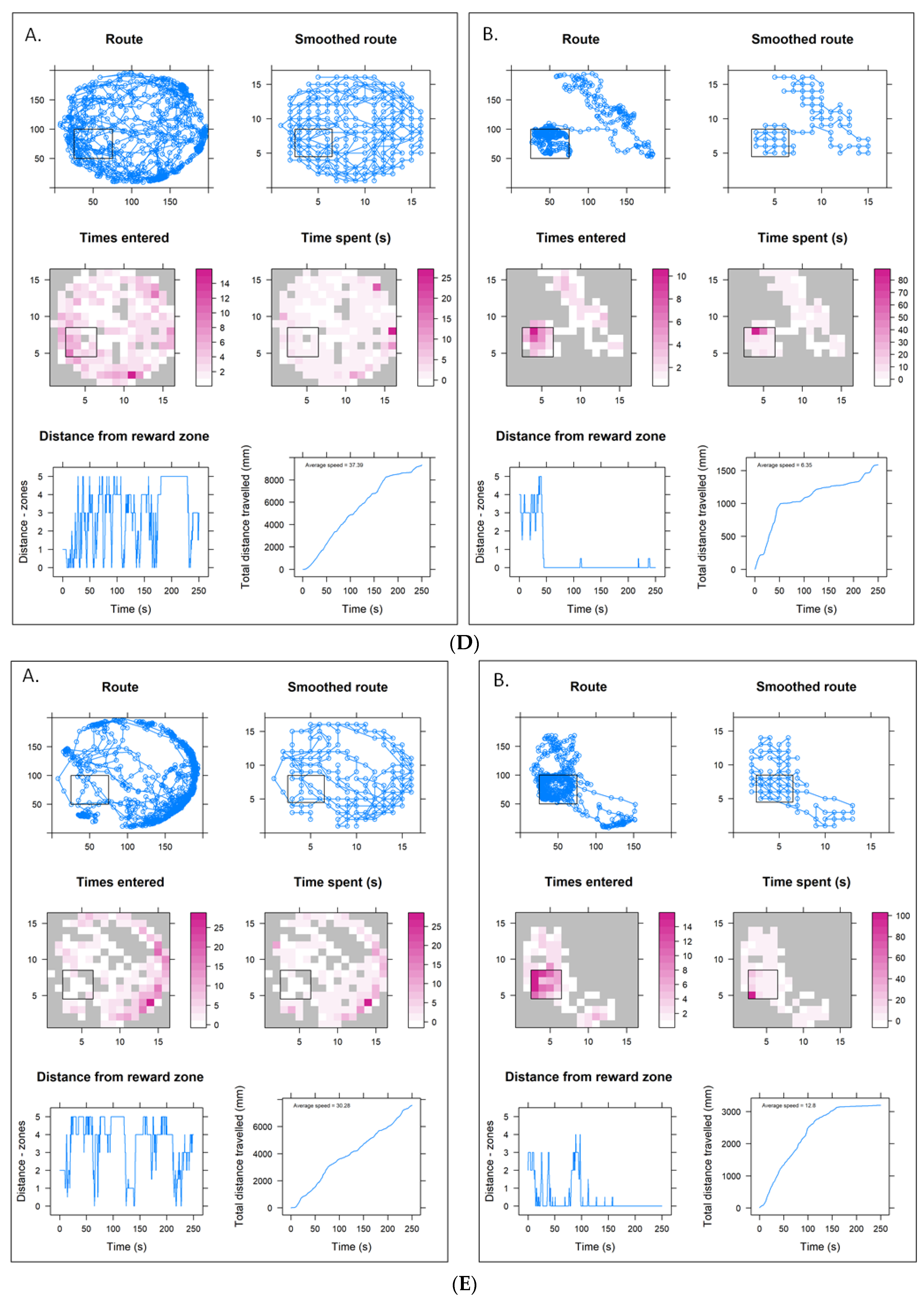
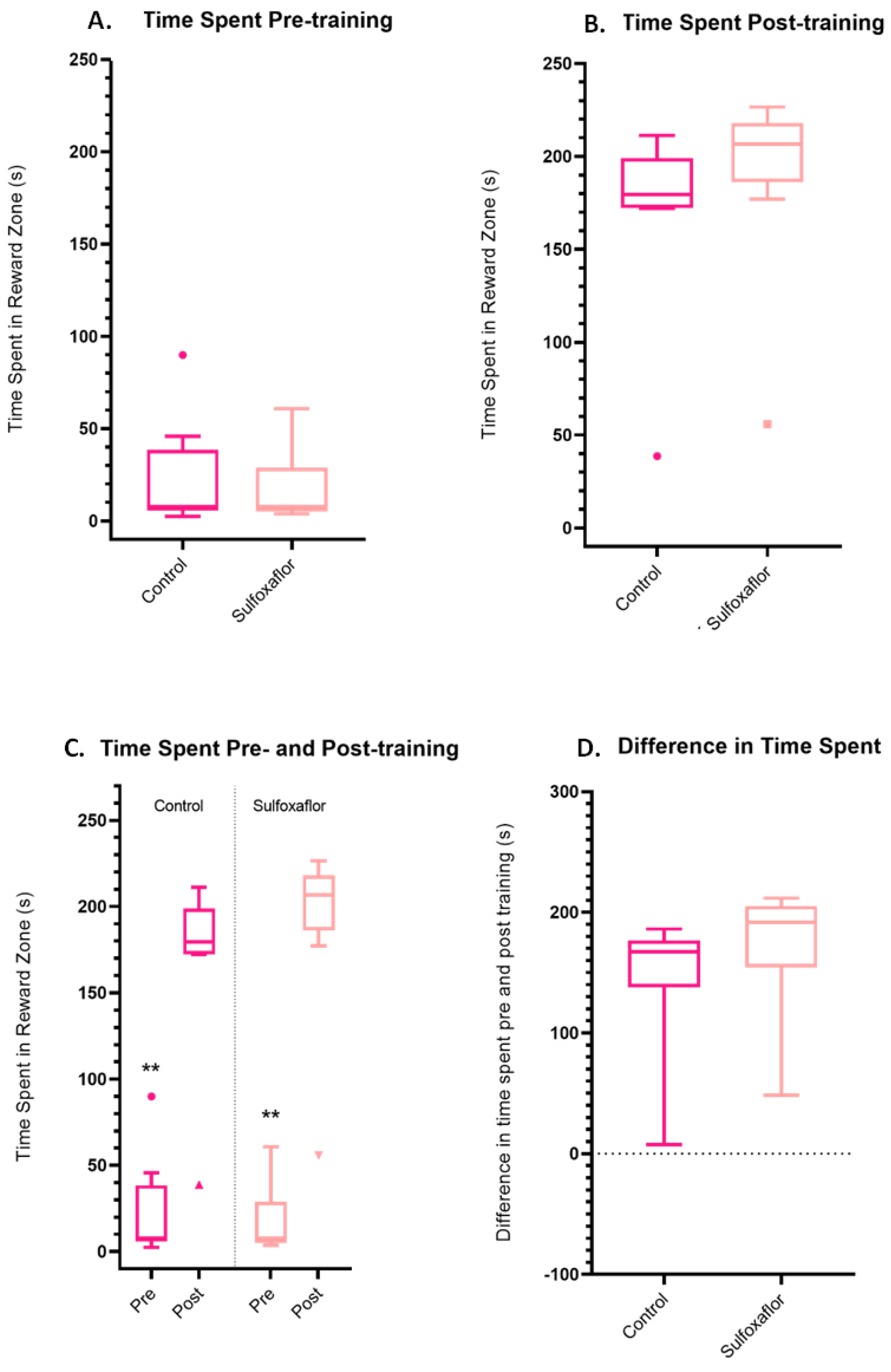
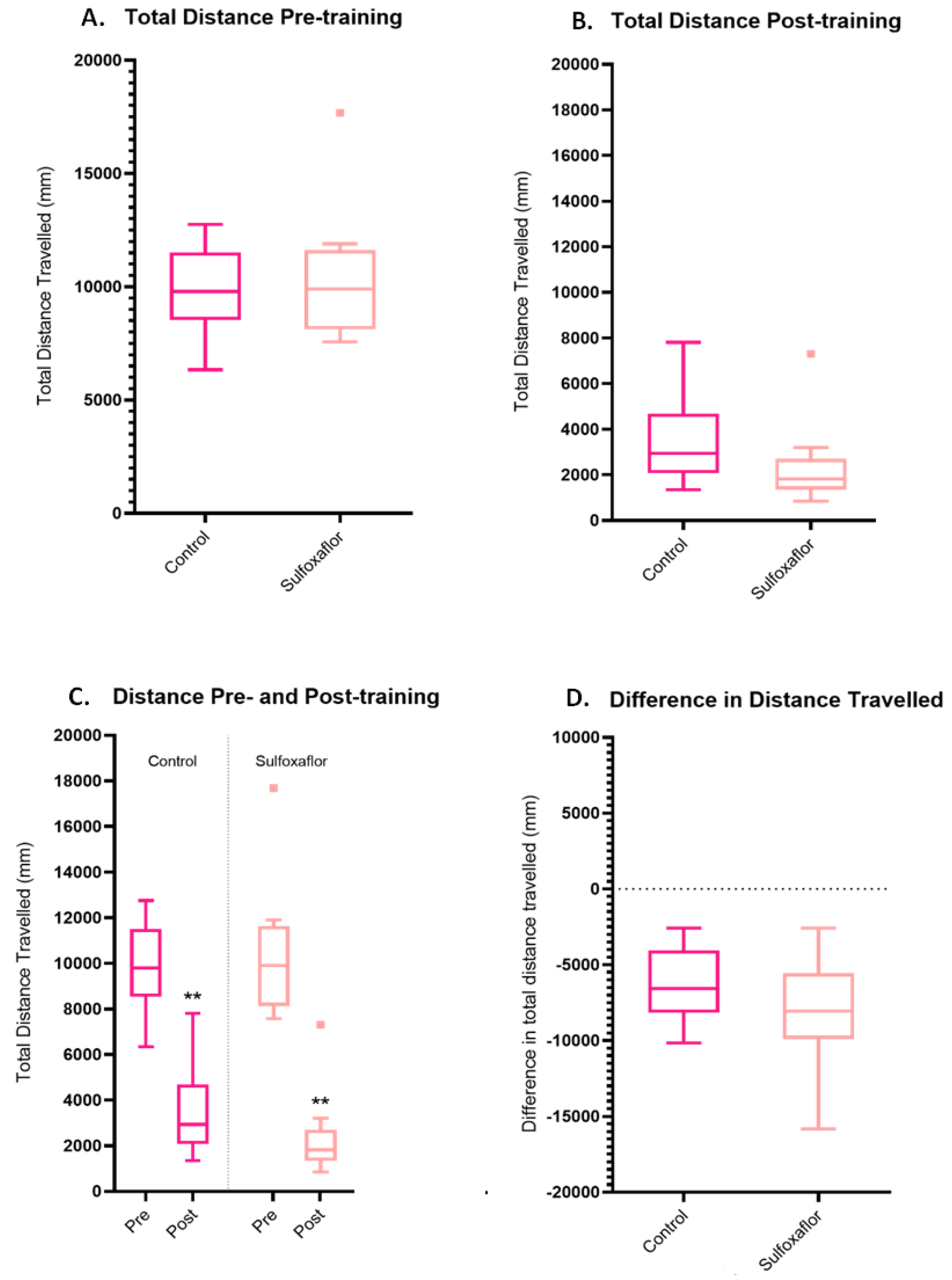
3.4. Higher Concentration Sulfoxaflor Trials Data
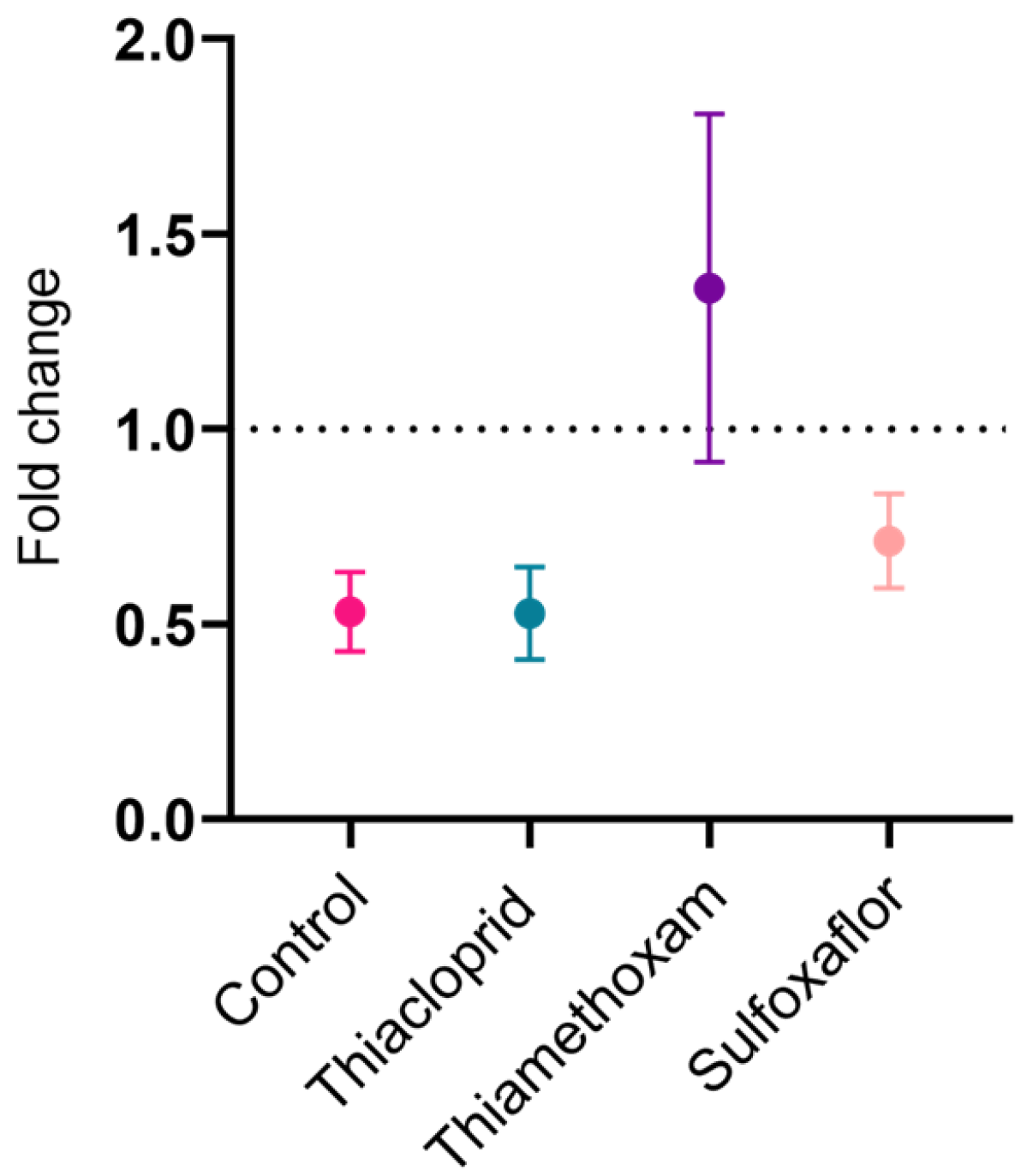
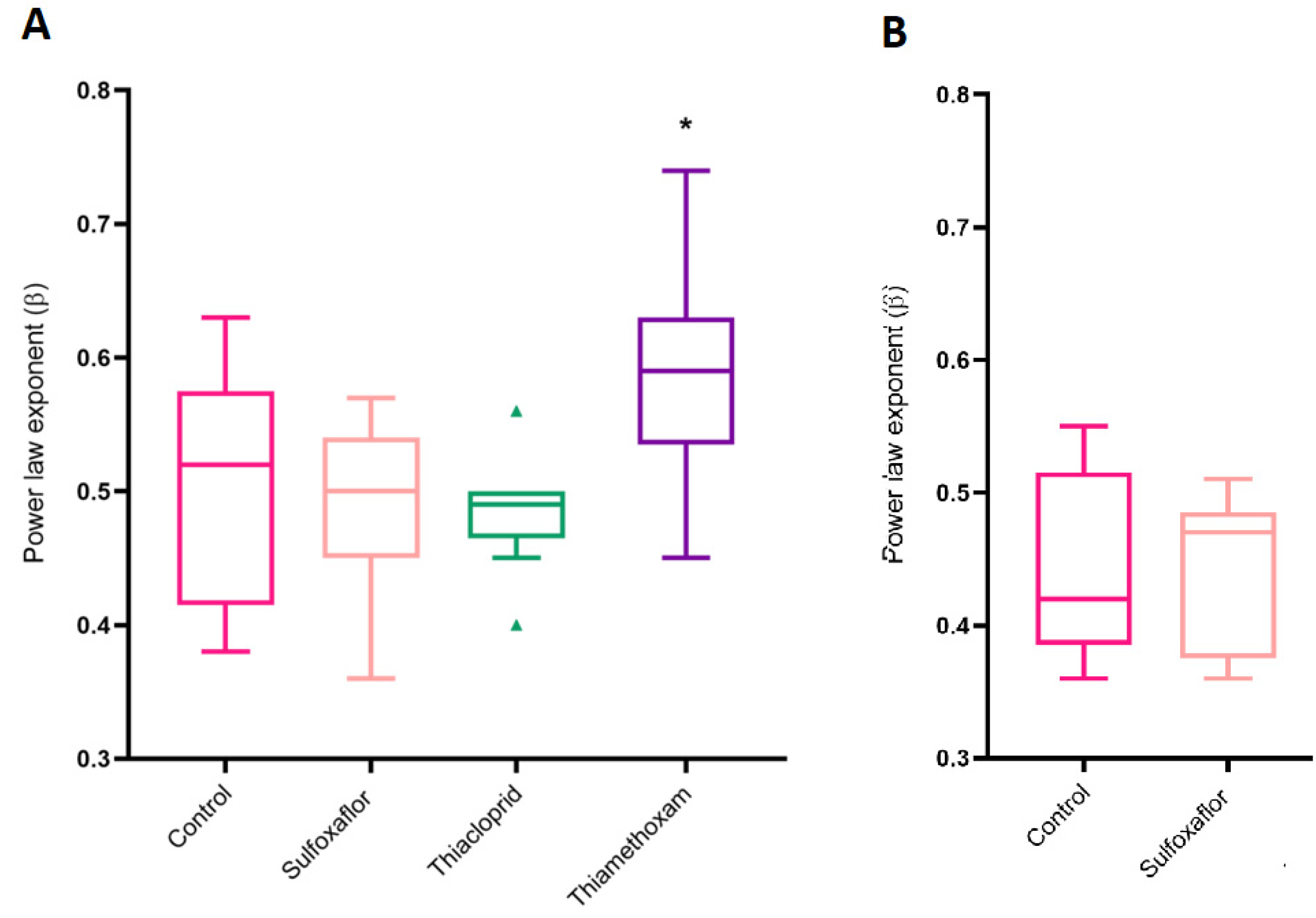
3.5. Speed–Curvature Power Law Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tedjakumala, S.R.; Giurfa, M. Rules and mechanisms of punishment learning in honey bees: The aversive conditioning of the sting extension response. J. Expl. Biol. 2013, 216, 2985–2997. [Google Scholar] [CrossRef] [PubMed]
- James, L.; Davies, T.G.E.; Lim, K.S.; Reynolds, A.M. Do bumblebees have signatures? Demonstrating the existence of a speed-curvature power law in Bombus terrestris locomotion patterns. PLoS ONE 2020, 15, e0226393. [Google Scholar] [CrossRef] [PubMed]
- Sparks, T.C.; Nauen, R. IRAC: Mode of action classification and insecticide resistance management. Pestic. Biochem. Physiol. 2015, 121, 122–128. [Google Scholar] [CrossRef]
- Grillone, G.; Laurino, D.; Manino, A.; Porporato, M. Toxicity of thiametoxam on in vitro reared honey bee brood. Apidologie 2017, 48, 635–643. [Google Scholar] [CrossRef]
- Henry, M.; Béguin, M.; Requier, F.; Rollin, O.; Odoux, J.-F.; Aupinel, P.; Aptel, J.; Tchamitchian, S.; Decourtye, A. A common pesticide decreases foraging success and survival in honey bees. Science 2012, 336, 348–350. [Google Scholar] [CrossRef]
- Tavares, D.A.; Roat, T.C.; Carvalho, S.M.; Silva-Zacarin, E.C.M.; Malaspina, O. In vitro effects of thiamethoxam on larvae of Africanized honey bee Apis mellifera (Hymenoptera: Apidae). Chemosphere 2015, 135, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Tavares, D.A.; Dussaubat, C.; Kretzschmar, A.E.; Carvalho, S.M.; Silva-Zacarin, E.C.M.; Malaspina, O.; Eraldine, G.; Erail, B.; Brunet, J.-L.; Belzunces, L.P. Exposure of larvae to thiamethoxam affects the survival and physiology of the honey bee at post-embryonic stages. Environ. Pollut. 2017, 229, 386–393. [Google Scholar] [CrossRef]
- Tesovnik, T.; Cizelj, I.; Zorc, M.; Čitar, M.; Božič, J.; Glavan, G.; Narat, M. Immune related gene expression in worker honey bee (Apis mellifera carnica) pupae exposed to neonicotinoid thiamethoxam and Varroa mites (Varroa destructor). PLoS ONE 2017, 12, e0187079. [Google Scholar] [CrossRef]
- Tosi, S.; Burgio, G.; Nieh, J.C. A common neonicotinoid pesticide, thiamethoxam, alters honey bee activity, motor functions, and movement to light. Sci. Rep. 2017, 7, 15132. [Google Scholar] [CrossRef]
- Friol, P.S.; Catae, A.F.; Tavares, D.A.; Malaspina, O.; Roat, T.C. Can the exposure of Apis mellifera (Hymenoptera, Apiadae) larvae to a field concentration of thiamethoxam affect newly emerged bees? Chemosphere 2017, 185, 56–66. [Google Scholar] [CrossRef]
- Tesovnik, T.; Zorc, M.; Ristanić, M.; Glavinić, U.; Stevanović, J.; Narat, M.; Stanimirović, Z. Exposure of honey bee larvae to thiamethoxam and its interaction with Nosema ceranae infection in adult honey bees. Environ. Pollut. 2020, 256, 113443. [Google Scholar] [CrossRef] [PubMed]
- Laycock, I.; Cotterell, K.C.; O’Shea-Wheller, T.A.; Cresswell, J.E. Effects of the neonicotinoid pesticide thiamethoxam at field-realistic levels on microcolonies of Bombus terrestris worker bumble bees. Ecotoxicol. Environ. Saf. 2014, 100, 153–158. [Google Scholar] [CrossRef]
- Stanley, D.A.; Smith, K.E.; Raine, N.E. Bumblebee learning and memory is impaired by chronic exposure to a neonicotinoid pesticide. Sci. Rep. 2015, 5, 15508. [Google Scholar] [CrossRef] [PubMed]
- Baron, G.L.; Jansen, V.A.A.; Brown, M.J.F.; Raine, N.E. Pesticide reduces bumblebee colony initiation and increases probability of population extinction. Nat. Ecol. Evol. 2017, 1, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Bayer Crop Science UK. Insecticide Stewardship. Guidelines for Using Insecticides 2020. Available online: https://cropscience.bayer.co.uk/tools-and-services/stewardship-food-and-environment/stewardship/insecticide-stewardship (accessed on 12 January 2023).
- Iwasa, T.; Motoyama, N.; Ambrose, J.T.; Roe, R.M. Mechanism for the differential toxicity of neonicotinoid insecticides in the honey bee, Apis mellifera. Crop Prot. 2004, 23, 371–378. [Google Scholar] [CrossRef]
- Tison, L.; Holtz, S.; Adeoye, A.; Kalkan, Ö.; Irmisch, N.S.; Lehmann, N.; Menzel, R. Effects of sublethal doses of thiacloprid and its formulation Calypso® on the learning and memory performance of honey bees. J. Exp. Biol. 2017, 220, 3695–3705. [Google Scholar] [CrossRef] [PubMed]
- Brandt, A.; Grikscheit, K.; Siede, R.; Grosse, R.; Meixner, M.D.; Büchler, R. Immunosuppression in honeybee queens by the neonicotinoids thiacloprid and clothianidin. Sci. Rep. 2017, 7, 4673. [Google Scholar] [CrossRef]
- Orčić, S.M.; Čelić, T.J.; Purać, J.S.; Vukašinović, E.L.; Kojić, D.K. Acute toxicity of sublethal concentrations of thiacloprid and clothianidin to immune response and oxidative status of honey bees. Apidologie 2022, 53, 50. [Google Scholar] [CrossRef]
- Fischer, J.; Müller, T.; Spatz, A.-K.; Greggers, U.; Grünewald, B.; Menzel, R. Neonicotinoids interfere with specific components of navigation in honeybees. PLoS ONE 2014, 9, e91364. [Google Scholar] [CrossRef]
- Tison, L.; Hahn, M.L.; Holtz, S.; Rößner, A.; Greggers, U.; Bischoff, G.; Menzel, R. Honey bees’ behavior is impaired by chronic exposure to the neonicotinoid thiacloprid in the field. Environ. Sci. Technol. 2016, 50, 7218–7227. [Google Scholar] [CrossRef]
- Ellis, C.; Park, K.J.; Whitehorn, P.; David, A.; Goulson, D. The neonicotinoid insecticide thiacloprid impacts upon bumblebee colony development under field conditions. Environ. Sci. Technol. 2017, 51, 1727–1732. [Google Scholar] [CrossRef] [PubMed]
- Havstad, L.T.; Øverland, J.I.; Valand, S.; Aamlid, T.S. Repellency of insecticides and the effect of thiacloprid on bumble bee colony development in red clover (Trifolium pratense L.) seed crops. Acta Agric. Scand. Sect. B Soil Plant Sci. 2019, 69, 439–451. [Google Scholar] [CrossRef]
- Sparks, T.C.; Watson, G.B.; Loso, M.R.; Geng, C.; Babcock, J.M.; Thomas, J.D. Sulfoxaflor and the sulfoximine insecticides: Chemistry, mode of action and basis for efficacy on resistant insects. Pesticide Biochem. Physiol. 2013, 107, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Loso, M.R.; Watson, G.B.; Sparks, T.C.; Rogers, R.B.; Huang, J.X.; Gerwick, B.C.; Babcock, J.M.; Kelley, D.; Hegde, V.B.; et al. Discovery and characterization of sulfoxaflor, a novel insecticide targeting sap-feeding pests. J. Agric. Food Chem. 2011, 59, 2950–2957. [Google Scholar] [CrossRef] [PubMed]
- Siviter, H.; Brown, M.J.F.; Leadbeater, E. Sulfoxaflor exposure reduces bumblebee reproductive success. Nature 2018, 561, 109–112. [Google Scholar] [CrossRef]
- Siviter, H.; Scott, A.; Pasquier, G.; Pull, C.D.; Brown, M.J.F.; Leadbeater, E. No evidence for negative impacts of acute sulfoxaflor exposure on bee olfactory conditioning or working memory. Peer J. 2019, 7, e7208. [Google Scholar] [CrossRef]
- Linguadoca, A.; Rizzi, C.; Villa, S.; Brown, M.J.F. Sulfoxaflor and nutritional deficiency synergistically reduce survival and fecundity in bumblebees. Sci. Total Environ. 2021, 795, 148680. [Google Scholar] [CrossRef]
- Chakrabarti, P.; Carlson, E.A.; Lucas, H.M.; Melathopoulos, A.P.; Sagili, R.R. Field rates of SivantoTM (flupyradifurone) and Transform® (sulfoxaflor) increase oxidative stress and induce apoptosis in honey bees (Apis mellifera L.). PLoS ONE 2020, 15, e0233033. [Google Scholar] [CrossRef]
- Boff, S.; Keller, A.; Raizer, J.; Lupi, D. Decreased efficiency of pollen collection due to sulfoxaflor exposure leads to a reduction in the size of bumble bee workers in late European summer. Front. Ecol. Evol. 2022, 10, 842563. [Google Scholar] [CrossRef]
- Tamburini, G.; Pereira-Peixoto, M.H.; Borth, J.; Lotz, S.; Wintermantel, D.; Allan, M.J.; Dean, R.; Schwarz, J.M.; Knauer, A.; Albrecht, M.; et al. Fungicide and insecticide exposure adversely impacts bumblebees and pollination services under semi-field conditions. Environ. Int. 2021, 157, 106813. [Google Scholar] [CrossRef]
- Siviter, H.; Folly, A.J.; Brown, M.J.F.; Leadbeater, E. Individual and combined impacts of sulfoxaflor and Nosema bombi on bumblebee ( Bombus terrestris ) larval growth. Proc. R. Soc. B Biol. Sci. 2020, 287, 20200935. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Chon, K.; Kim, B.S.; Oh, J.A.; Yoon, C.Y.; Park, H.H. Assessment of acute and chronic toxicity of cyantraniliprole and sulfoxaflor on honey bee (Apis mellifera) larvae. Pest Manag. Sci. 2022, 78, 5402–5412. [Google Scholar] [CrossRef] [PubMed]
- Barascou, L.; Requier, F.; Sené, D.; Crauser, D.; Le Conte, Y.; Alaux, C. Delayed effects of a single dose of a neurotoxic pesticide (sulfoxaflor) on honeybee foraging activity Sci. Total Environ. 2022, 805, 150351. [Google Scholar] [CrossRef]
- Capela, N.; Sarmento, A.; Simoes, S.; Azevedo-Pereira, H.M.V.S.; Sousa, J.P. Sub-lethal doses of sulfoxaflor impair honey bee homing ability. Sci. Total Environ. 2022, 837, 155710. [Google Scholar] [CrossRef]
- von Frisch, K. The Dance Language and Orientation of Bees. Harvard University Press: Cambridge, MA, USA, 1967. [Google Scholar]
- Shafir, S.; Barron, A.B. Optic flow informs distance but not profitability for honeybees. Proc. Biol. Sci. 2010, 277, 1241–1245. [Google Scholar] [CrossRef]
- Cartwright, B.A.; Collett, T.S. How honey bees use landmarks to guide their return to a food source. Nature 1982, 295, 560–564. [Google Scholar] [CrossRef]
- Cartwright, B.A.; Collett, T.S. Landmark learning in bees. J. Comp. Physiol. A 1983, 151, 521–543. [Google Scholar] [CrossRef]
- Collett, M.; Harland, D.; Collett, T.S. The use of landmarks and panoramic context in the performance of local vectors by navigating honeybees. J. Exp. Biol. 2002, 205, 807–814. [Google Scholar] [CrossRef]
- Collett, M.; Chittka, L.; Collett, T.S. Spatial memory in insect navigation. Curr. Biol. 2013, 23, R789–R800. [Google Scholar] [CrossRef]
- Woodgate, J.L.; Makinson, J.C.; Lim, K.S.; Reynolds, A.M.; Chittka, L. Continuous radar tracking illustrates the development of multi-destination routes of bumblebees. Sci. Rep. 2017, 7, 17323. [Google Scholar] [CrossRef]
- Zeil, J.; Kelber, A.; Voss, R. Structure and function of learning flights in bees and wasps. J. Exp. Biol. 1996, 199, 245–252. [Google Scholar] [CrossRef]
- Zhang, S.W.; Bartsch, K.; Srinivasan, M.V. Maze learning by honeybees. Neurobiol. Learn. Mem. 1996, 66, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Chittka, L.; Kunze, J.; Shipman, C.; Buchmann, S.L. The significance of landmarks for path integration in homing honeybee foragers. Naturwissenschaften 1995, 82, 341–343. [Google Scholar] [CrossRef]
- Dyer, F.C.S. 5—Spatial Cognition: Lessons from central-place foraging insects. In Animal Cognition in Nature; Balda, R.P., Pepperberg, I.M., Kamil, A.C., Eds.; Academic Press: Cambridge, MA, USA, 1998; pp. 119–154. ISBN 9780120770304. [Google Scholar] [CrossRef]
- Srinivasan, M.V. Honey bees as a model for vision, perception, and cognition. Annu. Rev. Entomol. 2010, 55, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Si, A.; Pahl, M.; Hayden, B.; Grüter, C. Visually guided decision making in foraging honeybees. Front. Neurosci. 2012, 6, 88. [Google Scholar] [CrossRef] [PubMed]
- Brebner, J.S.; Makinson, J.C.; Bates, O.K.; Rossi, N.; Lim, K.S.; Dubois, T.; Gómez-Moracho, T.; Lihoreau, M.; Chittka, L.; Woodgate, J.L. Bumble bees strategically use ground level linear features in navigation. Anim. Behav. 2021, 179, 147–160. [Google Scholar] [CrossRef]
- Groh, C.; Rgen Tautz, J.; Rö, W. Synaptic organization in the adult honey bee brain is influenced by brood-temperature control during pupal development. Proc. Natl. Acad. Sci. USA 2004, 101, 4268–4273. [Google Scholar] [CrossRef]
- Jones, J.C.; Helliwell, P.; Beekman, M.; Maleszka, R.; Oldroyd, B.P. The effects of rearing temperature on developmental stability and learning and memory in the honey bee, Apis mellifera. J. Comp. Physiol. A 2005, 191, 1121–1129. [Google Scholar] [CrossRef]
- Tautz, J.; Maier, S.; Groh, C.; Rossler, W.; Brockmann, A. Behavioral performance in adult honey bees is influenced by the temperature experienced during their pupal development. Proc. Natl. Acad. Sci. USA 2003, 100, 7343–7347. [Google Scholar] [CrossRef]
- Dyer, A.G.; Whitney, H.M.; Arnold, S.E.J.; Glover, B.J.; Chittka, L. Bees associate warmth with floral colour. Nature 2006, 442, 525. [Google Scholar] [CrossRef]
- Hammer, T.J.; Hata, C.; Nieh, J.C. Thermal learning in the honeybee, Apis mellifera. J. Exp. Biol. 2009, 212, 3928–3934. [Google Scholar] [CrossRef] [PubMed]
- Harrap, M.J.M.; Rands, S.A.; de Ibarra, N.H.; Whitney, H.M. The diversity of floral temperature patterns, and their use by pollinators. ELife 2017, 6, e31262. [Google Scholar] [CrossRef] [PubMed]
- Norgate, M.; Boyd-Gerny, S.; Simonov, V.; Rosa, M.G.P.; Heard, T.A.; Dyer, A.G. Ambient temperature influences Australian native stingless bee (Trigona carbonaria) preference for warm nectar. PLoS ONE 2010, 5, e12000. [Google Scholar] [CrossRef]
- Whitney, H.M.; Dyer, A.; Chittka, L.; Rands, S.A.; Glover, B.J. The interaction of temperature and sucrose concentration on foraging preferences in bumblebees. Naturwissenschaften 2008, 95, 845–850. [Google Scholar] [CrossRef]
- Junca, P.; Sandoz, J.-C. Heat perception and aversive learning in honey bees: Putative involvement of the thermal/chemical sensor AmHsTRPA. Front. Physiol. 2015, 6, 316. [Google Scholar] [CrossRef] [PubMed]
- Kohno, K.; Sokabe, T.; Tominaga, M.; Kadowaki, T. Honey bee thermal/chemical sensor, AmHsTRPA, reveals neofunctionalization and loss of Tansient Receptor Potential Channel genes. J. Neurosci. 2010, 30, 12219–12229. [Google Scholar] [CrossRef]
- Breed, G.A.; Don Bowen, W.; Leonard, M.L. Behavioral signature of intraspecific competition and density dependence in colony-breeding marine predators. Ecol. Evol. 2013, 3, 3838–3854. [Google Scholar] [CrossRef]
- Breed, G.A.; Severns, P.M.; Edwards, A.M. Apparent power-law distributions in animal movements can arise from intraspecific interactions. J. R. Soc. Interface 2015, 12, 20140927. [Google Scholar] [CrossRef]
- Fagan, W.F.; Lewis, M.A.; Auger-Méthé, M.; Avgar, T.; Benhamou, S.; Breed, G.; Ladage, L.; Schlägel, U.E.; Tang, W.W.; Papastamatiou, Y.P.; et al. Spatial memory and animal movement. Ecol. Lett. 2013, 16, 1316–1329. [Google Scholar] [CrossRef]
- Potts, J.R.; Harris, S.; Giuggioli, L. Quantifying behavioral changes in territorial animals caused by sudden population declines. Am. Nat. 2013, 182, E73–E82. [Google Scholar] [CrossRef]
- Nathan, R.; Giuggioli, L. A milestone for movement ecology research. Mov. Ecol. 2013, 1, 1. [Google Scholar] [CrossRef] [PubMed]
- Block, B.A.; Jonsen, I.D.; Jorgensen, S.J.; Winship, A.J.; Shaffer, S.A.; Bograd, S.J.; Hazen, E.L.; Foley, D.G.; Breed, G.A.; Harrison, A.L.; et al. Tracking apex marine predator movements in a dynamic ocean. Nature 2011, 475, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.P.; Breed, G.A.; Robinson, P.W. New insights into pelagic migrations: Implications for ecology and conservation. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 73–96. [Google Scholar] [CrossRef]
- Reynolds, A.M.; Rhodes, C.J. The Lévy flight paradigm: Random search patterns and mechanisms. Ecology 2009, 90, 877–887. [Google Scholar] [CrossRef]
- Mashanova, A.; Oliver, T.H.; Jansen, V.A.A. Evidence for intermittency and a truncated power law from highly resolved aphid movement data. J. R. Soc. Interface 2010, 7, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Marin, A.; Zago, M.; Lacquaniti, F. The speed–curvature power law in Drosophila larval locomotion. Biol. Lett. 2016, 12, 20160597. [Google Scholar] [CrossRef]
- Sims, D.W.; Southall, E.J.; Humphries, N.E.; Hays, G.C.; Bradshaw, C.J.A.; Pitchford, J.W.; James, A.; Ahmed, M.Z.; Brierley, A.S.; Hindell, M.A.; et al. Scaling laws of marine predator search behaviour. Nature 2008, 451, 1098–1102. [Google Scholar] [CrossRef]
- Zago, M.; Matic, A.; Flash, T.; Gomez-Marin, A.; Lacquaniti, F. The speed-curvature power law of movements: A reappraisal. Exp. Brain Res. 2017, 236, 69–82. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Revised Guidance on the Risk Assessment of Plant Protection Products on Bees (Apis mellifera, Bombus spp. and Solitary Bees). Bees and Pesticides: Draft Guidance Update for Public Consultation. 2022. Available online: https://www.efsa.europa.eu/en/news/bees-and-pesticides-draft-guidance-update-public-consultation (accessed on 6 February 2023).
- MacIntosh, A.J.J.; Alados, C.L.; Huffman, M.A. Fractal analysis of behaviour in a wild primate: Behavioural complexity in health and disease. J. R. Soc. Interface 2011, 8, 1497–1509. [Google Scholar] [CrossRef]
- Macintosh, A.J.J.; Pelletier, L.; Chiaradia, A.; Kato, A.; Ropert-Coudert, Y. Temporal fractals in seabird foraging behaviour: Diving through the scales of time. Sci. Rep. 2013, 3, 1884. [Google Scholar] [CrossRef]
- Viswanathan, G.M.; Peng, C.K.; Stanley, H.E.; Goldberger, A.L. Deviations from uniform power law scaling in nonstationary time series. Phys. Rev. E—Stat. Phys. Plasmas Fluids Relat. Interdiscip. Top. 1997, 55, 845–849. [Google Scholar] [CrossRef] [PubMed]
- Shlesinger, M.F.; Zaslavsky, G.M.; Klafter, J. Strange kinetics. Nature 1993, 363, 31–37. [Google Scholar] [CrossRef]
- Wolf, S.; Nicholls, E.; Reynolds, A.M.; Wells, P.; Lim, K.S.; Paxton, R.J.; Osborne, J.L. Optimal search patterns in honeybee orientation flights are robust against emerging infectious diseases. Sci. Rep. 2016, 6, 32612. [Google Scholar] [CrossRef] [PubMed]
- Viviani, P.; Flash, T. Minimum-jerk, two-thirds power law, and isochrony: Converging approaches to movement planning. J. Exp. Psychol. Hum. Percept. Perform. 1995, 21, 32–53. [Google Scholar] [CrossRef]
- Wann, J.; Nimmo-Smith, I.; Wing, A.M. Relation between velocity and curvature in movement: Equivalence and divergence between a power law and a minimum-jerk model. J. Exp. Psychol. Hum. Percept. Perform. 1988, 14, 622–637. [Google Scholar] [CrossRef] [PubMed]
- Ofstad, T.A.; Zuker, C.S.; Reiser, M.B. Visual place learning in Drosophila melanogaster. Nature 2011, 474, 204–207. [Google Scholar] [CrossRef]
- Inger, R.; Bennie, J.; Davies, T.W.; Gaston, K.J. Potential biological and ecological effects of flickering artificial light. PLoS ONE 2014, 9, e98631. [Google Scholar] [CrossRef]
- Castle, S.J.; Byrne, F.J.; Bi, J.L.; Toscano, N.C. Spatial and temporal distribution of imidacloprid and thiamethoxam in citrus and impact on Homalodisca coagulata populations. Pest Manag. Sci. 2005, 61, 75–84. [Google Scholar] [CrossRef]
- Pohorecka, K.; Skubida, P.; Miszczak, A.; Semkiw, P.; Sikorski, P.; Zagibajło, K.; Teper, D.; Kołtowski, Z.; Skubida, M.; Zdańska, D.; et al. Residues of neonicotinoid insecticides in bee collected plant materials from oilseed rape crops and their effect on bee colonies. J. Apic. Sci. 2013, 56, 115–134. [Google Scholar] [CrossRef]
- Rundlöf, M.; Andersson, G.K.S.; Bommarco, R.; Fries, I.; Hederström, V.; Herbertsson, L.; Jonsson, O.; Klatt, B.K.; Pedersen, T.R.; Yourstone, J.; et al. Seed coating with a neonicotinoid insecticide negatively affects wild bees. Nature 2015, 521, 77–80. [Google Scholar] [CrossRef]
- Sanchez-Bayo, F.; Goka, K. Pesticide residues and bees. A risk assessment. PLoS ONE 2014, 9, e94482. [Google Scholar] [CrossRef] [PubMed]
- Thompson, H.; Harrington, P.; Wilkins, S.; Pietravalle, S.; Sweet, D.; Jones, A. Effects of Neonicotinoid Seed Treatments on Bumble Bee Colonies under Field Conditions; Food Environment Research Agency: York, UK, 2013. Available online: http://data.parliament.uk/DepositedPapers/Files/DEP2013-0598/Fera_bumble_bee_report_PS2371.pdf (accessed on 16 January 2023).
- Wood, T.J.; Goulson, D. The environmental risks of neonicotinoid pesticides: A review of the evidence post 2013. Environ. Sci. Pollut. Res. 2017, 24, 17285–17325. [Google Scholar] [CrossRef] [PubMed]
- Samuelson, E.E.W.; Chen-Wishart, Z.P.; Gill, R.J.; Leadbeater, E. Effect of acute pesticide exposure on bee spatial working memory using an analogue of the radial-arm maze. Sci. Rep. 2016, 6, 38957. [Google Scholar] [CrossRef] [PubMed]
- Stanley, D.A.; Raine, N.E. Chronic exposure to a neonicotinoid pesticide alters the interactions between bumblebees and wild plants. Funct. Ecol. 2016, 30, 1132–1139. [Google Scholar] [CrossRef]
- Elston, C.; Thompson, H.M.; Walters, K.F.A. Sub-lethal effects of thiamethoxam, a neonicotinoid pesticide, and propiconazole, a DMI fungicide, on colony initiation in bumblebee (Bombus terrestris) micro-colonies. Apidologie 2013, 44, 563–574. [Google Scholar] [CrossRef]
- Siviter, H.; Koricheva, J.; Brown, M.J.F.; Leadbeater, E. Quantifying the impact of pesticides on learning and memory in bees. J. Appl. Ecol. 2018, 55, 2812–2821. [Google Scholar] [CrossRef]
- Siviter, H.; Horner, J.; Brown, M.J.F.; Leadbeater, E. Sulfoxaflor exposure reduces egg laying in bumblebees Bombus terrestris. J. Appl. Ecol. 2019, 57, 160–169. [Google Scholar] [CrossRef]
- Pérez-Escudero, A.; Vicente-Page, J.; Hinz, R.C.; Arganda, S.; De Polavieja, G.G. IdTracker: Tracking individuals in a group by automatic identification of unmarked animals. Nat. Methods 2014, 11, 743–748. [Google Scholar] [CrossRef]
- Lihoreau, M.; Chittka, L.; Le Comber, S.C.; Raine, N.E. Bees do not use nearest-neighbour rules for optimization of multi-location routes. Biol. Lett. 2012, 8, 13–16. [Google Scholar] [CrossRef]
- Lihoreau, M.; Chittka, L.; Raine, N.E. Trade-off between travel distance and prioritization of high-reward sites in traplining bumblebees. Funct. Ecol. 2011, 25, 1284–1292. [Google Scholar] [CrossRef]
- Lihoreau, M.; Raine, N.E.; Reynolds, A.M.; Stelzer, R.J.; Lim, K.S.; Smith, A.D.; Osborne, J.L.; Chittka, L. Radar tracking and motion-sensitive cameras on flowers reveal the development of pollinator multi-destination routes over large spatial scales. PLoS Biol. 2012, 10, e1001392. [Google Scholar] [CrossRef]
- Ohashi, K.; Thomson, J.D.; D’Souza, D. Trapline foraging by bumble bees: IV. Optimization of route geometry in the absence of competition. Behav. Ecol. 2007, 18, 1–11. [Google Scholar] [CrossRef]
- Saleh, N.; Chittka, L. Traplining in bumblebees (Bombus impatiens): A foraging strategy’s ontogeny and the importance of spatial reference memory in short-range foraging. Oecologia 2007, 151, 719–730. [Google Scholar] [CrossRef]
- de Oliveira Jacob, C.R.; Zanardi, O.Z.; Malaquias, J.B.; Souza Silva, C.A.; Yamamoto, P.T. The impact of four widely used neonicotinoid insecticides on Tetragonisca angustula (Latreille) (Hymenoptera: Apidae). Chemosphere 2019, 224, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Goldberger, A.L.; Rigney, D.R.; West, B.J. Chaos and fractals in human physiology. Sci. Am. 1990, 262, 42–49. [Google Scholar] [CrossRef]
- West, B.J.; Goldberger, A.L. Physiology in fractal dimensions. Am. Sci. 1987, 75, 354–365. Available online: http://www.jstor.org/stable/27854715 (accessed on 17 January 2023).
- Mishima, M.; Hirai, T.; Itoh, H.; Nakano, Y.; Sakai, H.; Muro, S.; Nishimura, K.; Oku, Y.; Chin, K.; Ohi, M.; et al. Complexity of terminal airspace geometry assessed by lung computed tomography in normal subjects and patients with chronic obstructive pulmonary disease. Proc. Natl. Acad. Sci. USA 1999, 96, 8829–8834. [Google Scholar] [CrossRef]
- Escós, J.M.; Alados, C.L.; Emlen, J.M.; Escos, J.M. Fractal structures and fractal functions as disease indicators. Oikos 1995, 74, 310–314. [Google Scholar] [CrossRef]
- OECD (Organization for Economic Co-Operation and Development). Proposal of a New OECD Guideline for the Testing of Chemicals; Honey Bee (Apis mellifera L.) Homing Flight Test, using Single Oral Exposure to Sublethal Doses of Test Chemical OECD Test Guidelines for Chemicals. 2020. Available online: https://www.oecd.org/chemicalsafety/testing/Draft%20OECD%20TG_Homing%20flight%20test_honeybees_18%20May.pdf (accessed on 6 February 2023).
- OECD (Organization for Economic Co-Operation and Development). Guidance Document on Honey Bee (Apis mellifera L.) Homing Flight Test, using Single Oral Exposure to Sublethal Doses of Test Chemical. Environment Directorate Chemicals and Biotechnology Committee. 2021. Available online: https://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=ENV-CBC-MONO(2021)7%20&doclanguage=en (accessed on 6 February 2023).
- EFSA (European Food Safety Authority); Abdourahime, H.; Anastassiadou, M.; Arena, M.; Auteri, D.; Barmaz, S.; Brancato, A.; Brocca, D.; Bura, L.; Carrasco Cabrera, L.; et al. Conclusion on the peer review of the pesticide risk assessment of the active substance thiacloprid. EFSA J. 2019, 17, 5595. [Google Scholar] [CrossRef]
- Forbes, V.E.; Calow, P. Is the per capita rate of increase a good measure of population-level effects in ecotoxicology? Environ. Toxicol. Chem. 1999, 18, 1544–1556. [Google Scholar] [CrossRef]
- Walthall, W.K.; Stark, J.D. A comparison of acute mortality and population growth rate as endpoints of toxicological effect. Ecotoxicol. Environ. Saf. 1997, 37, 45–52. [Google Scholar] [CrossRef] [PubMed]



| Treatment | Control | Thiacloprid | Thiamethoxam | Sulfoxaflor |
|---|---|---|---|---|
| Mean | −3741 | −3648 | −334.4 | −2422 |
| Std. Deviation | 2539 | 3209 | 4204 | 3328 |
| Std. Error of Mean | 846.5 | 1070 | 1401 | 1109 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
James, L.; Reynolds, A.M.; Mellor, I.R.; Davies, T.G.E. A Sublethal Concentration of Sulfoxaflor Has Minimal Impact on Buff-Tailed Bumblebee (Bombus terrestris) Locomotor Behaviour under Aversive Conditioning. Toxics 2023, 11, 279. https://doi.org/10.3390/toxics11030279
James L, Reynolds AM, Mellor IR, Davies TGE. A Sublethal Concentration of Sulfoxaflor Has Minimal Impact on Buff-Tailed Bumblebee (Bombus terrestris) Locomotor Behaviour under Aversive Conditioning. Toxics. 2023; 11(3):279. https://doi.org/10.3390/toxics11030279
Chicago/Turabian StyleJames, Laura, Andrew M. Reynolds, Ian R. Mellor, and T. G. Emyr Davies. 2023. "A Sublethal Concentration of Sulfoxaflor Has Minimal Impact on Buff-Tailed Bumblebee (Bombus terrestris) Locomotor Behaviour under Aversive Conditioning" Toxics 11, no. 3: 279. https://doi.org/10.3390/toxics11030279
APA StyleJames, L., Reynolds, A. M., Mellor, I. R., & Davies, T. G. E. (2023). A Sublethal Concentration of Sulfoxaflor Has Minimal Impact on Buff-Tailed Bumblebee (Bombus terrestris) Locomotor Behaviour under Aversive Conditioning. Toxics, 11(3), 279. https://doi.org/10.3390/toxics11030279









