Physicochemical Transformations of Silver Nanoparticles in the Oro-Gastrointestinal Tract Mildly Affect Their Toxicity to Intestinal Cells In Vitro: An AOP-Oriented Testing Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search and AOP-Wiki Database Examination
2.2. Silver Nanoparticles
2.3. Treatment of Samples with the Simulated Human Digestive Fluids
2.3.1. Preparation of GIT Fluids
2.3.2. In Vitro Digestion of NMs
2.4. Physicochemical Characterization of Silver Nanoparticles
2.5. Cell Culture
2.6. Intracellular Ag Content Determination by Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
2.7. Determination of Cell Viability
2.8. Dihydrorhodamine 123 Assay
2.9. Genotoxicity
2.9.1. Comet Assay
2.9.2. 53BP1 Immunostaining and Foci Count
2.10. Cell Cycle Analysis
2.11. Gene Expression
2.12. Data Management and Statistical Analyses
3. Results
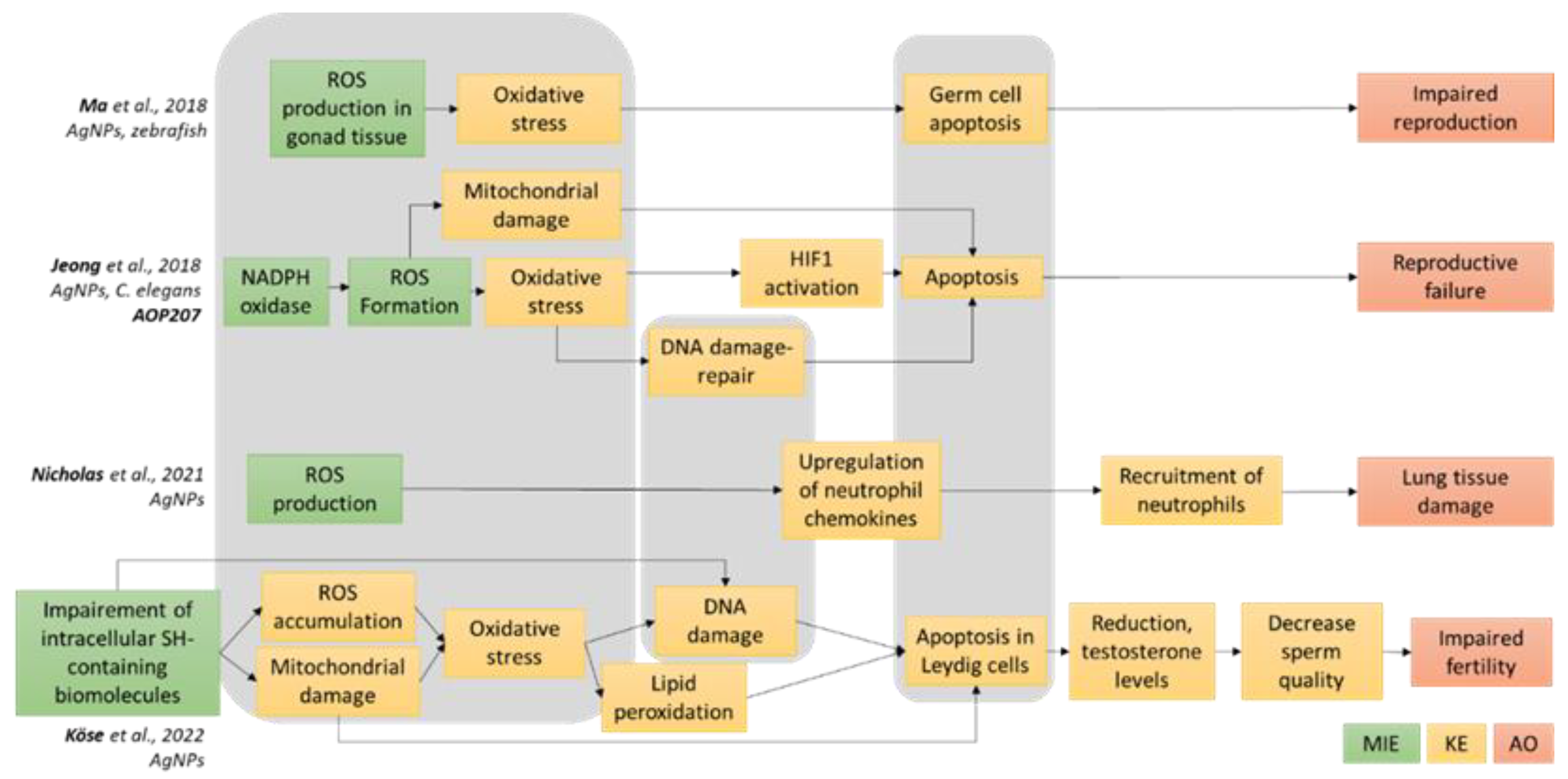
3.1. Definition of the AOP-Oriented Ag NM Toxicity Testing Strategy
3.2. Physicochemical Characteristics of Pristine, Digested and Non-Digested Ag NPs
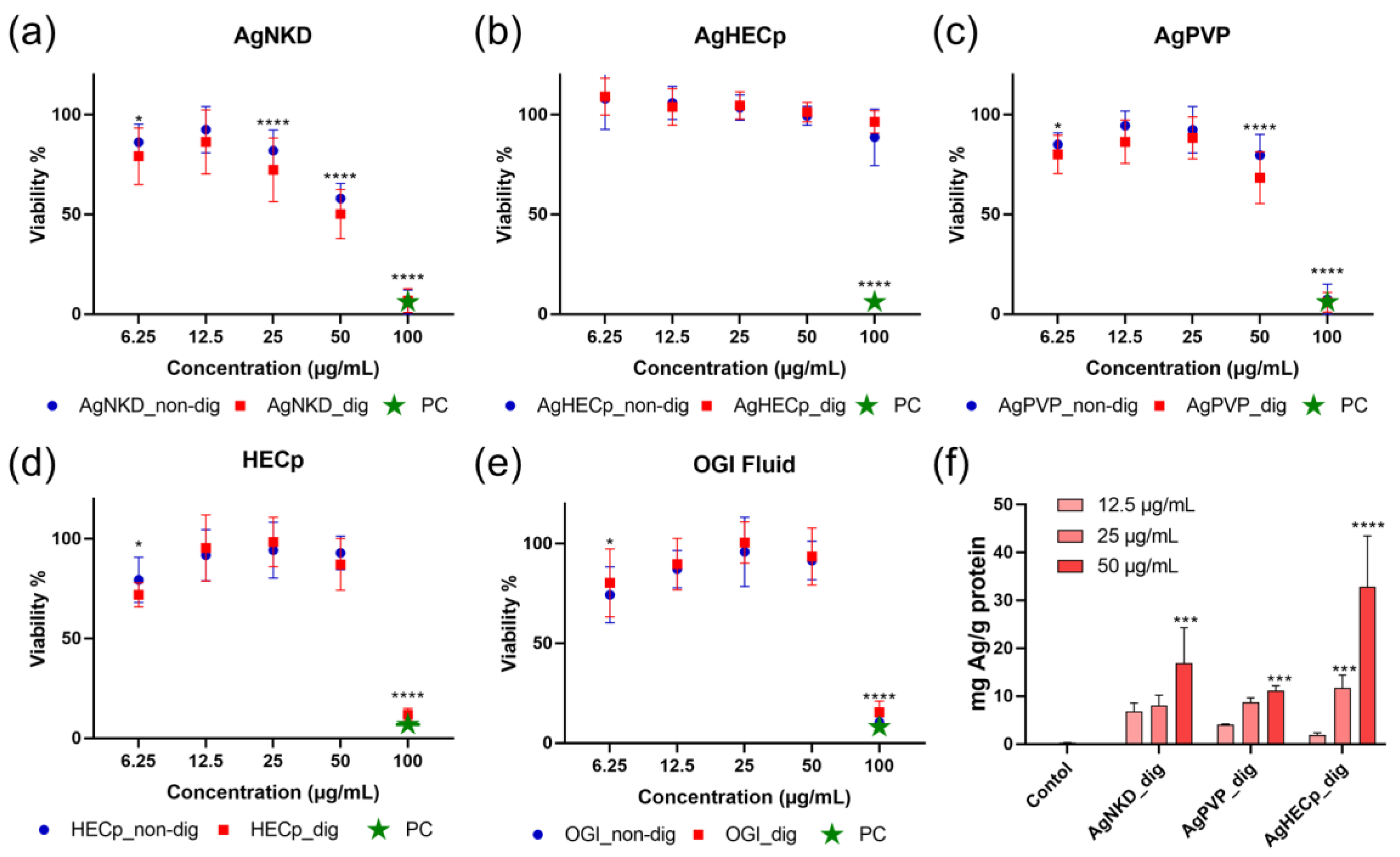
3.3. Cell Viability
3.4. Oxidative Stress
3.5. Genotoxicity
3.6. Cell Cycle and Apoptosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The History of Nanoscience and Nanotechnology: From Chemical-Physical Applications to Nanomedicine. Molecules 2020, 25, 112. [Google Scholar] [CrossRef] [PubMed]
- Nurfatihah, Z.; Siddiquee, S. Nanotechnology: Recent Trends in Food Safety, Quality and Market Analysis. In Nanotechnology: Applications in Energy, Drug and Food; Siddiquee, S., Melvin, G.J.H., Rahman, M.M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 283–293. [Google Scholar]
- Yang, T.X.; Paulose, T.; Redan, B.W.; Mabon, J.C.; Duncan, T.V. Food and Beverage Ingredients Induce the Formation of Silver Nanoparticles in Products Stored within Nanotechnology-Enabled Packaging. ACS Appl. Mater. Interfaces 2021, 13, 1398–1412. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.K.; Ma, Y.; Sun, B.B.; Yang, Y.; Zhang, Y.Q.; Zhu, L.Y. Transport of silver nanoparticles coated with polyvinylpyrrolidone of various molecular sizes in porous media: Interplay of polymeric coatings and chemically heterogeneous surfaces. J. Hazard. Mater. 2022, 429, 128247. [Google Scholar] [CrossRef] [PubMed]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef] [PubMed]
- Loo, Y.Y.; Rukayadi, Y.; Nor-Khaizura, M.A.R.; Kuan, C.H.; Chieng, B.W.; Nishibuchi, M.; Radu, S. In Vitro Antimicrobial Activity of Green Synthesized Silver Nanoparticles Against Selected Gram-negative Foodborne Pathogens. Front. Microbiol. 2018, 9, 1555. [Google Scholar] [CrossRef]
- Lopez-Esparza, J.; Espinosa-Cristobal, L.F.; Donohue-Cornejo, A.; Reyes-Lopez, S.Y. Antimicrobial Activity of Silver Nanoparticles in Polycaprolactone Nanofibers against Gram-Positive and Gram-Negative Bacteria. Ind. Eng. Chem. Res. 2016, 55, 12532–12538. [Google Scholar] [CrossRef]
- Carbone, M.; Donia, D.T.; Sabbatella, G.; Antiochia, R. Silver nanoparticles in polymeric matrices for fresh food packaging. J. King Saud Univ. Sci. 2016, 28, 273–279. [Google Scholar] [CrossRef]
- Krasniewska, K.; Galus, S.; Gniewosz, M. Biopolymers-Based Materials Containing Silver Nanoparticles as Active Packaging for Food Applications—A Review. Int. J. Mol. Sci. 2020, 21, 698. [Google Scholar] [CrossRef]
- Fernandez, C.C.; Sokolonski, A.R.; Fonseca, M.S.; Stanisic, D.; Araujo, D.B.; Azevedo, V.; Portela, R.D.; Tasic, L. Applications of Silver Nanoparticles in Dentistry: Advances and Technological Innovation. Int. J. Mol. Sci. 2021, 22, 2485. [Google Scholar] [CrossRef]
- Garcia-Contreras, R.; Argueta-Figueroa, L.; Mejia-Rubalcava, C.; Jimenez-Martinez, R.; Cuevas-Guajardo, S.; Sanchez-Reyna, P.A.; Mendieta-Zeron, H. Perspectives for the use of silver nanoparticles in dental practice. Int. Dent. J. 2011, 61, 297–301. [Google Scholar] [CrossRef]
- Al-Sultan, S.I.; Hereba, A.R.T.; Hassanein, K.M.A.; Abd-Allah, S.M.S.; Mahmoud, U.T.; Abdel-Raheem, S.M. The impact of dietary inclusion of silver nanoparticles on growth performance, intestinal morphology, caecal microflora, carcass traits and blood parameters of broiler chickens. Ital. J. Anim. Sci. 2022, 21, 967–978. [Google Scholar] [CrossRef]
- Dosoky, W.M.; Fouda, M.M.G.; Alwan, A.B.; Abdelsalam, N.R.; Taha, A.E.; Ghareeb, R.Y.; El-Aassar, M.R.; Khafaga, A.F. Dietary supplementation of silver-silica nanoparticles promotes histological, immunological, ultrastructural, and performance parameters of broiler chickens. Sci. Rep. 2021, 11, 4166. [Google Scholar] [CrossRef] [PubMed]
- Tulve, N.S.; Stefaniak, A.B.; Vance, M.E.; Rogers, K.; Mwilu, S.; LeBouf, R.F.; Schwegler-Berry, D.; Willis, R.; Thomas, T.A.; Marr, L.C. Characterization of silver nanoparticles in selected consumer products and its relevance for predicting children’s potential exposures. Int. J. Hyg. Environ. Health 2015, 218, 345–357. [Google Scholar] [CrossRef]
- Amiri, N.; Hadioui, M.; Chalifoux, A.; Wilkinson, K.J. Characterizing silver nanoparticles in beverages and following their release from silver-containing food containers using sector field single particle inductively coupled plasma mass spectrometry (SP-ICP-MS). Meas. Food 2022, 8, 100061. [Google Scholar] [CrossRef]
- Echegoyen, Y.; Nerin, C. Nanoparticle release from nano-silver antimicrobial food containers. Food Chem. Toxicol. 2013, 62, 16–22. [Google Scholar] [CrossRef]
- Huang, Y.M.; Chen, S.X.; Bing, X.; Gao, C.L.; Wang, T.; Yuan, B. Nanosilver Migrated into Food-Simulating Solutions from Commercially Available Food Fresh Containers. Packag. Technol. Sci. 2011, 24, 291–297. [Google Scholar] [CrossRef]
- Gottschalk, F.; Sonderer, T.; Scholz, R.W.; Nowack, B. Modeled Environmental Concentrations of Engineered Nanomaterials (TiO2, ZnO, Ag, CNT, Fullerenes) for Different Regions. Environ. Sci. Technol. 2009, 43, 9216–9222. [Google Scholar] [CrossRef]
- Lowry, G.V.; Espinasse, B.P.; Badireddy, A.R.; Richardson, C.J.; Reinsch, B.C.; Bryant, L.D.; Bone, A.J.; Deonarine, A.; Chae, S.; Therezien, M.; et al. Long-Term Transformation and Fate of Manufactured Ag Nanoparticles in a Simulated Large Scale Freshwater Emergent Wetland. Environ. Sci. Technol. 2012, 46, 7027–7036. [Google Scholar] [CrossRef]
- Ferdous, Z.; Nemmar, A. Health Impact of Silver Nanoparticles: A Review of the Biodistribution and Toxicity Following Various Routes of Exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef]
- Cheng, L.K.; O’Grady, G.; Du, P.; Egbuji, J.U.; Windsor, J.A.; Pullan, A.J. Gastrointestinal system. Wiley Interdiscip. Rev. Syst. Biol. Med. 2010, 2, 65–79. [Google Scholar] [CrossRef]
- Sohal, I.S.; O’Fallon, K.S.; Gaines, P.; Demokritou, P.; Bello, D. Ingested engineered nanomaterials: State of science in nanotoxicity testing and future research needs. Part. Fibre Toxicol. 2018, 15, 29. [Google Scholar] [CrossRef] [PubMed]
- Bohmert, L.; Girod, M.; Hansen, U.; Maul, R.; Knappe, P.; Niemann, B.; Weidner, S.M.; Thunemann, A.F.; Lampen, A. Analytically monitored digestion of silver nanoparticles and their toxicity on human intestinal cells. Nanotoxicology 2014, 8, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.J.; Guan, R.F.; Lyu, F.; Kang, T.S.; Wu, Y.H.; Chen, X.Q. In vitro cytotoxicity of silver nanoparticles and zinc oxide nanoparticles to human epithelial colorectal adenocarcinoma (Caco-2) cells. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2014, 769, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, D.; Ebmeyer, J.; Knappe, P.; Juling, S.; Bohmert, L.; Selve, S.; Niemann, B.; Braeuning, A.; Thunemann, A.F.; Lampen, A. Impact of food components during in vitro digestion of silver nanoparticles on cellular uptake and cytotoxicity in intestinal cells. Biol. Chem. 2015, 396, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Abdelkhaliq, A.; van der Zande, M.; Undas, A.K.; Peters, R.J.B.; Bouwmeester, H. Impact of in vitro digestion on gastrointestinal fate and uptake of silver nanoparticles with different surface modifications. Nanotoxicology 2020, 14, 111–126. [Google Scholar] [CrossRef]
- Marucco, A.; Prono, M.; Beal, D.; Alasonati, E.; Fisicaro, P.; Bergamaschi, E.; Carriere, M.; Fenoglio, I. Biotransformation of Food-Grade and Nanometric TiO2 in the Oral-Gastro-Intestinal Tract: Driving Forces and Effect on the Toxicity toward Intestinal Epithelial Cells. Nanomaterials 2020, 10, 2132. [Google Scholar] [CrossRef]
- Sohal, I.S.; Cho, Y.K.; O’Fallon, K.S.; Gaines, P.; Demokritou, P.; Bello, D. Dissolution Behavior and Biodurability of Ingested Engineered Nanomaterials in the Gastrointestinal Environment. ACS Nano 2018, 12, 8115–8128. [Google Scholar] [CrossRef]
- Costa, A.; Blosi, M. Process for the Preparation of Nanoparticles of Noble Metals in Hydrogel and Nanoparticles Thus Obtained. WO2016125070, 11 August 2016. [Google Scholar]
- Teodorescu, M.; Bercea, M. Poly(vinylpyrrolidone)—A Versatile Polymer for Biomedical and Beyond Medical Applications. Polym. Plast. Technol. Eng. 2015, 54, 923–943. [Google Scholar] [CrossRef]
- Trabucco, S.; Ortelli, S.; Del Secco, B.; Zanoni, I.; Belosi, F.; Ravegnani, F.; Nicosia, A.; Blosi, M.; Costa, A.L. Monitoring and Optimisation of Ag Nanoparticle Spray-Coating on Textiles. Nanomaterials 2021, 11, 3165. [Google Scholar] [CrossRef]
- Jensen, K.A.; Kembouche, Y.; Christiansen, E.N.R.J.; Wallin, H.; Guit, C.; Spalla, O.; Witschger, O. Final Protocol for Producing Suitable Manufactured Nanomaterial Exposure Media; ANESES: Maisons-Alfort, France, 2011; Available online: https://www.anses.fr/en/system/files/nanogenotox_deliverable_6.pdf (accessed on 12 February 2023).
- Brun, E.; Barreau, F.; Veronesi, G.; Fayard, B.; Sorieul, S.; Chanéac, C.; Carapito, C.; Rabilloud, T.; Mabondzo, A.; Herlin-Boime, N.; et al. Titanium dioxide nanoparticle impact and translocation through ex vivo, in vivo and in vitro gut epithelia. Part Fibre Toxicol 2014, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low-levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Dussert, F.; Wegner, K.D.; Moriscot, C.; Gallet, B.; Jouneau, P.H.; Reiss, P.; Carriere, M. Evaluation of the Dermal Toxicity of InZnP Quantum Dots Before and After Accelerated Weathering: Toward a Safer-By-Design Strategy. Front. Toxicol. 2021, 3, 636976. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper—Excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Horgan, G.W.; Dempfle, L. Relative expression software tool (REST (c)) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002, 30, e36. [Google Scholar] [CrossRef]
- Ma, Y.B.; Lu, C.J.; Junaid, M.; Jia, P.P.; Yang, L.; Zhang, J.H.; Pei, D.S. Potential adverse outcome pathway (AOP) of silver nanoparticles mediated reproductive toxicity in zebrafish. Chemosphere 2018, 207, 320–328. [Google Scholar] [CrossRef]
- Jeong, J.; Song, T.; Chatterjee, N.; Choi, I.; Cha, Y.K.; Choi, J. Developing adverse outcome pathways on silver nanoparticle-induced reproductive toxicity via oxidative stress in the nematode Caenorhabditis elegans using a Bayesian network model. Nanotoxicology 2018, 12, 1182–1197. [Google Scholar] [CrossRef]
- Nicholas, T.P.; Boyes, W.K.; Scoville, D.K.; Workman, T.W.; Kavanagh, T.J.; Altemeier, W.A.; Faustman, E.M. The effects of gene x environment interactions on silver nanoparticle toxicity in the respiratory system: An adverse outcome pathway. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1708. [Google Scholar] [CrossRef] [PubMed]
- Kose, O.; Mantecca, P.; Costa, A.; Carriere, M. Putative adverse outcome pathways for silver nanoparticle toxicity on mammalian male reproductive system: A literature review. Part. Fibre Toxicol. 2023, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Gliga, A.R.; Skoglund, S.; Wallinder, I.O.; Fadeel, B.; Karlsson, H.L. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: The role of cellular uptake, agglomeration and Ag release. Part. Fibre Toxicol. 2014, 11, 11. [Google Scholar] [CrossRef]
- Hamilton, R.F.; Buckingham, S.; Holian, A. The Effect of Size on Ag Nanosphere Toxicity in Macrophage Cell Models and Lung Epithelial Cell Lines Is Dependent on Particle Dissolution. Int. J. Mol. Sci. 2014, 15, 6815–6830. [Google Scholar] [CrossRef]
- Marassi, V.; Di Cristo, L.; Smith, S.G.J.; Ortelli, S.; Blosi, M.; Costa, A.L.; Reschiglian, P.; Volkov, Y.; Prina-Mello, A. Silver nanoparticles as a medical device in healthcare settings: A five-step approach for candidate screening of coating agents. R. Soc. Open Sci. 2018, 5, 171113. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Bobyk, L.; Tarantini, A.; Beal, D.; Veronesi, G.; Kieffer, I.; Motellier, S.; Valsami-Jones, E.; Lynch, I.; Jouneau, P.H.; Pernet-Gallay, K.; et al. Toxicity and chemical transformation of silver nanoparticles in A549 lung cells: Dose-rate-dependent genotoxic impact. Environ. Sci. Nano 2021, 8, 806–821. [Google Scholar] [CrossRef]
- Kajstura, M.; Halicka, H.D.; Pryjma, J.; Darzynkiewicz, Z. Discontinuous fragmentation of nuclear DNA during apoptosis revealed by discrete “sub-G(1)” peaks on DNA content histograms. Cytom. Part A 2007, 71A, 125–131. [Google Scholar] [CrossRef]
- Lu, J.H.; Chou, Y.R.; Deng, Y.H.; Huang, M.S.; Chien, S.T.; Quynh, B.T.N.; Wu, C.Y.; Achtmann, E.A.P.; Cheng, H.C.; Dubey, N.K.; et al. The Novel Herbal Cocktail AGA Alleviates Oral Cancer through Inducing Apoptosis, Inhibited Migration and Promotion of Cell Cycle Arrest at SubG1 Phase. Cancers 2020, 12, 3214. [Google Scholar] [CrossRef]
- Wang, H.M.; Chiu, C.C.; Wu, P.F.; Chen, C.Y. Subamolide E from Cinnamomum subavenium Induces Sub-G1 Cell-Cycle Arrest and Caspase-Dependent Apoptosis and Reduces the Migration Ability of Human Melanoma Cells. J. Agric. Food Chem. 2011, 59, 8187–8192. [Google Scholar] [CrossRef]
- Ahmad, J.; Alhadlaq, H.A.; Siddiqui, M.A.; Saquib, Q.; Al-Khedhairy, A.A.; Musarrat, J.; Ahamed, M. Concentration-Dependent Induction of Reactive Oxygen Species, Cell Cycle Arrest and Apoptosis in Human Liver Cells After Nickel Nanoparticles Exposure. Environ. Toxicol. 2015, 30, 137–148. [Google Scholar] [CrossRef]
- Wang, F.; Gao, F.; Lan, M.B.; Yuan, H.H.; Huang, Y.P.; Liu, J.W. Oxidative stress contributes to silica nanoparticle-induced cytotoxicity in human embryonic kidney cells. Toxicol. Vitr. 2009, 23, 808–815. [Google Scholar] [CrossRef]
- Abraham, R.T. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 2001, 15, 2177–2196. [Google Scholar] [CrossRef]
- Sensoy, I. A review on the food digestion in the digestive tract and the used in vitro models. Curr. Res. Food Sci. 2021, 4, 308–319. [Google Scholar] [CrossRef]
- Laloux, L.; Kastrati, D.; Cambier, S.; Gutleb, A.C.; Schneider, Y.J. The Food Matrix and the Gastrointestinal Fluids Alter the Features of Silver Nanoparticles. Small 2020, 16, 1907687. [Google Scholar] [CrossRef]
- Pindkaova, L.; Kasparkova, V.; Kejlova, K.; Dvorakova, M.; Krsek, D.; Jirova, D.; Kasparova, L. Behaviour of silver nanoparticles in simulated saliva and gastrointestinal fluids. Int. J. Pharm. 2017, 527, 12–20. [Google Scholar] [CrossRef]
- Liu, Y.; Bodmer, W.F. Analysis of P53 mutations and their expression in 56 colorectal cancer cell lines. Proc. Natl. Acad. Sci. USA 2006, 103, 976–981. [Google Scholar] [CrossRef]
- Wijnhoven, S.W.P.; Peijnenburg, W.; Herberts, C.A.; Hagens, W.I.; Oomen, A.G.; Heugens, E.H.W.; Roszek, B.; Bisschops, J.; Gosens, I.; Van de Meent, D.; et al. Nano-silver—A review of available data and knowledge gaps in human and environmental risk assessment. Nanotoxicology 2009, 3, 109–138. [Google Scholar] [CrossRef]
- Vila, L.; Marcos, R.; Hernandez, A. Long-term effects of silver nanoparticles in caco-2 cells. Nanotoxicology 2017, 11, 771–780. [Google Scholar] [CrossRef]
- Halappanavar, S.; van den Brule, S.; Nymark, P.; Gate, L.; Seidel, C.; Valentino, S.; Zhernovkov, V.; Danielsen, P.H.; De Vizcaya, A.; Wolff, H.; et al. Adverse outcome pathways as a tool for the design of testing strategies to support the safety assessment of emerging advanced materials at the nanoscale. Part. Fibre Toxicol. 2020, 17, 16. [Google Scholar] [CrossRef]
- OECD. Guidance Document for the Scientific Review of Adverse Outcome Pathways; OECD: Paris, France, 2021; Available online: https://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=ENV-CBC-MONO(2021)22%20&doclanguage=en (accessed on 12 February 2023).
- AshaRani, P.V.; Mun, G.L.K.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and Genotoxicity of Silver Nanoparticles in Human Cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef]
- Rinna, A.; Magdolenova, Z.; Hudecova, A.; Kruszewski, M.; Refsnes, M.; Dusinska, M. Effect of silver nanoparticles on mitogen-activated protein kinases activation: Role of reactive oxygen species and implication in DNA damage. Mutagenesis 2015, 30, 59–66. [Google Scholar] [CrossRef]
- Chang, X.R.; Wang, X.J.; Li, J.Y.; Shang, M.T.; Niu, S.Y.; Zhang, W.L.; Li, Y.J.; Sun, Z.Y.; Gan, J.Y.; Li, W.H.; et al. Silver nanoparticles induced cytotoxicity in HT22 cells through autophagy and apoptosis via PI3K/AKT/mTOR signaling pathway. Ecotoxicol. Environ. Saf. 2021, 208, 111696. [Google Scholar] [CrossRef]
- Carnovale, C.; Guarnieri, D.; Di Cristo, L.; De Angelis, I.; Veronesi, G.; Scarpellini, A.; Malvindi, M.A.; Barone, F.; Pompa, P.P.; Sabella, S. Biotransformation of Silver Nanoparticles into Oro-Gastrointestinal Tract by Integrated In Vitro Testing Assay: Generation of Exposure-Dependent Physical Descriptors for Nanomaterial Grouping. Nanomaterials 2021, 11, 1587. [Google Scholar] [CrossRef]
- Teubl, B.J.; Stojkovic, B.; Docter, D.; Pritz, E.; Leitinger, G.; Poberaj, I.; Prassl, R.; Stauber, R.H.; Frohlich, E.; Khinast, J.G.; et al. The effect of saliva on the fate of nanoparticles. Clin. Oral Investig. 2018, 22, 929–940. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.J.; Yu, C.H.; Tan, Y.; Hou, Z.; Li, M.; Shao, F.; Lu, X.X. Effects of prenatal exposure to silver nanoparticles on spatial cognition and hippocampal neurodevelopment in rats. Environ. Res. 2015, 138, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Axson, J.L.; Stark, D.I.; Bondy, A.L.; Capracotta, S.S.; Maynard, A.D.; Philbert, M.A.; Bergin, I.L.; Ault, A.P. Rapid Kinetics of Size and pH-Dependent Dissolution and Aggregation of Silver Nanoparticles in Simulated Gastric Fluid. J. Phys. Chem. C 2015, 119, 20632–20641. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.M.; Wu, Y.; Gray, P.; Zheng, J.W.; Cao, G.J.; Zhang, H.; Zhang, X.W.; Boudreau, M.; Croley, T.R.; Chen, C.Y.; et al. Influence of gastrointestinal environment on free radical generation of silver nanoparticles and implications for their cytotoxicity. Nanoimpact 2018, 10, 144–152. [Google Scholar] [CrossRef]
- Kaiser, J.P.; Roesslein, M.; Diener, L.; Wichser, A.; Nowack, B.; Wick, P. Cytotoxic effects of nanosilver are highly dependent on the chloride concentration and the presence of organic compounds in the cell culture media. J. Nanobiotechnol. 2017, 15, 5. [Google Scholar] [CrossRef]
- Ault, A.P.; Stark, D.I.; Axson, J.L.; Keeney, J.N.; Maynard, A.D.; Bergin, I.L.; Philbert, M.A. Protein corona-induced modification of silver nanoparticle aggregation in simulated gastric fluid. Environ. Sci. Nano 2016, 3, 1510–1520. [Google Scholar] [CrossRef]
- Mwilu, S.K.; El Badawy, A.M.; Bradham, K.; Nelson, C.; Thomas, D.; Scheckel, K.G.; Tolaymat, T.; Ma, L.Z.; Rogers, K.R. Changes in silver nanoparticles exposed to human synthetic stomach fluid: Effects of particle size and surface chemistry. Sci. Total Environ. 2013, 447, 90–98. [Google Scholar] [CrossRef]
- Rogers, K.R.; Bradham, K.; Tolaymat, T.; Thomas, D.J.; Hartmann, T.; Ma, L.Z.; Williams, A. Alterations in physical state of silver nanoparticles exposed to synthetic human stomach fluid. Sci. Total Environ. 2012, 420, 334–339. [Google Scholar] [CrossRef]
- Walczak, A.P.; Kramer, E.; Hendriksen, P.J.M.; Helsdingen, R.; van der Zande, M.; Rietjens, I.; Bouwmeester, H. In vitro gastrointestinal digestion increases the translocation of polystyrene nanoparticles in an in vitro intestinal co-culture model. Nanotoxicology 2015, 9, 886–894. [Google Scholar] [CrossRef]
- Motta, G.; Gualtieri, M.; Saibene, M.; Bengalli, R.; Carriere, M.; Mantecca, P. Preliminary toxicological analysis in a Safe-by-design and Adverse outcome pathway driven approach on different silver nanoparticles: Assessment of acute responses in A549. Toxics 2023, 11, 195. [Google Scholar] [CrossRef]
- Avalos, A.; Haza, A.I.; Mateo, D.; Morales, P. Cytotoxicity and ROS production of manufactured silver nanoparticles of different sizes in hepatoma and leukemia cells. J. Appl. Toxicol. 2014, 34, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Carlson, C.; Hussain, S.M.; Schrand, A.M.; Braydich-Stolle, L.K.; Hess, K.L.; Jones, R.L.; Schlager, J.J. Unique Cellular Interaction of Silver Nanoparticles: Size-Dependent Generation of Reactive Oxygen Species. J. Phys. Chem. B 2008, 112, 13608–13619. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, N.; Ranjan, S.; Rajendran, B.; Manickam, V.; Ramalingam, C.; Avadhani, G.S.; Kumar, A. Thermal co-reduction approach to vary size of silver nanoparticle: Its microbial and cellular toxicology. Environ. Sci. Pollut. Res. 2016, 23, 4149–4163. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.C.; Seligy, V.L.; Massarsky, A.; Moon, T.W.; Rippstein, P.; Tan, J.; Tayabali, A.F.; IOP. Comparison of toxicity of uncoated and coated silver nanoparticles. In Proceedings of the 3rd International Conference on Safe Production and Use of Nanomaterials (Nanosafe), Minatec, Grenoble, France, 13–15 November 2012. [Google Scholar]
- Lee, K.J.; Browning, L.M.; Nallathamby, P.D.; Xu, X.H.N. Study of Charge-Dependent Transport and Toxicity of Peptide-Functionalized Silver Nanoparticles Using Zebrafish Embryos and Single Nanoparticle Plasmonic Spectroscopy. Chem. Res. Toxicol. 2013, 26, 904–917. [Google Scholar] [CrossRef] [PubMed]
- El Badawy, A.M.; Silva, R.G.; Morris, B.; Scheckel, K.G.; Suidan, M.T.; Tolaymat, T.M. Surface Charge-Dependent Toxicity of Silver Nanoparticles. Environ. Sci. Technol. 2011, 45, 283–287. [Google Scholar] [CrossRef]
- Samberg, M.E.; Oldenburg, S.J.; Monteiro-Riviere, N.A. Evaluation of Silver Nanoparticle Toxicity in Skin In Vivo and Keratinocytes in Vitro. Environ. Health Perspect. 2010, 118, 407–413. [Google Scholar] [CrossRef]
- Ronavari, A.; Belteky, P.; Boka, E.; Zakupszky, D.; Igaz, N.; Szerencses, B.; Pfeiffer, I.; Konya, Z.; Kiricsi, M. Polyvinyl-Pyrrolidone-Coated Silver Nanoparticles-The Colloidal, Chemical, and Biological Consequences of Steric Stabilization under Biorelevant Conditions. Int. J. Mol. Sci. 2021, 22, 8673. [Google Scholar] [CrossRef]
- Seiffert, J.; Hussain, F.; Wiegman, C.; Li, F.; Bey, L.; Baker, W.; Porter, A.; Ryan, M.P.; Chang, Y.; Gow, A.; et al. Pulmonary Toxicity of Instilled Silver Nanoparticles: Influence of Size, Coating and Rat Strain. PLoS ONE 2015, 10, e119726. [Google Scholar] [CrossRef]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef]
- Jia, M.X.; Zhang, W.J.; He, T.J.; Shu, M.; Deng, J.; Wang, J.H.; Li, W.; Bai, J.; Lin, Q.L.; Luo, F.J.; et al. Evaluation of the Genotoxic and Oxidative Damage Potential of Silver Nanoparticles in Human NCM460 and HCT116 Cells. Int. J. Mol. Sci. 2020, 21, 1618. [Google Scholar] [CrossRef]
- Khan, M.S.; Alomari, A.; Tabrez, S.; Hassan, I.; Wahab, R.; Bhat, S.A.; Alafaleq, N.O.; Altwaijry, N.; Shaik, G.M.; Zaidi, S.K.; et al. Anticancer Potential of Biogenic Silver Nanoparticles: A Mechanistic Study. Pharmaceutics 2021, 13, 707. [Google Scholar] [CrossRef] [PubMed]
- Farah, M.A.; Ali, M.A.; Chen, S.M.; Li, Y.; Al-Hemaid, F.M.; Abou-Tarboush, F.M.; Al-Anazi, K.M.; Lee, J. Silver nanoparticles synthesized from Adenium obesum leaf extract induced DNA damage, apoptosis and autophagy via generation of reactive oxygen species. Colloids Surf. B Biointerfaces 2016, 141, 158–169. [Google Scholar] [CrossRef]
- Hackenberg, S.; Scherzed, A.; Kessler, M.; Hummel, S.; Technau, A.; Froelich, K.; Ginzkey, C.; Koehler, C.; Hagen, R.; Kleinsasser, N. Silver nanoparticles: Evaluation of DNA damage, toxicity and functional impairment in human mesenchymal stem cells. Toxicol. Lett. 2011, 201, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Juarez-Moreno, K.; Gonzalez, E.B.; Giron-Vazquez, N.; Chavez-Santoscoy, R.A.; Mota-Morales, J.D.; Perez-Mozqueda, L.L.; Garcia-Garcia, M.R.; Pestryakov, A.; Bogdanchikova, N. Comparison of cytotoxicity and genotoxicity effects of silver nanoparticles on human cervix and breast cancer cell lines. Hum. Exp. Toxicol. 2017, 36, 931–948. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Li, T.Z.; Su, X.R.; Li, J.Y.; Li, W.H.; Gan, J.Y.; Wu, T.S.; Kong, L.; Zhang, T.; Tang, M.; et al. Genotoxic effects of silver nanoparticles with/without coating in human liver HepG2 cells and in mice. J. Appl. Toxicol. 2019, 39, 908–918. [Google Scholar] [CrossRef] [PubMed]





| AgNKD | AgPVP | AgHEC | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Size (nm) | PdI | ZP (mV) | Size (nm) | PdI | ZP (mV) | Size (nm) | PdI | ZP (mV) | |
| Pristine | |||||||||
| DI H2O | 371 ± 36 | 0.47 ± 0.09 | −21.8 ± 0.7 | 213 ± 29 | 0.50 ± 0.10 | −31.6 ± 2.2 | 202 ± 7 | 0.42 ± 0.01 | 11.0 ± 0.4 |
| Non-digested | |||||||||
| Saliva | 402 ± 4 | 0.42 ± 0.06 | −29.1 ± 0.9 | 210± 10 | 0.62± 0.02 | −17.6 ± 1.9 | 587 ± 21 | 0.58 ± 0.11 | 14.9 ±0.4 |
| Gastric Fluid | 1526 ± 242 | 0.62 ± 0.09 | −2.0 ± 0.1 | 498 ± 56 | 0.50± 0.05 | −2.7 ± 0.1 | 381 ± 29 | 0.57 ± 0.02 | 11.4 ± 0.1 |
| Intestinal Fluid | 1548 ± 107 | 0.40 ± 0.11 | −20.7 ± 0.5 | 590 ± 21 | 0.43 ± 0.07 | −17.6 ± 1.4 | 317 ± 7 | 0.38 ± 0.03 | 7.8 ± 0.4 |
| Culture Medium t 0 h | 1235 ± 205 | 0.63 ± 0.06 | −9.4 ± 0.2 | 358 ± 35 | 0.46 ± 0.09 | −7.3 ± 0.6 | 100 ± 7 | 0.88 ± 0.01 | 1.7 ± 0.4 |
| Culture Medium t 24 h | 1024 ± 277 | 0.72 ± 0.11 | −7.1 ± 1.2 | 395 ± 51 | 0.48 ± 0.05 | −9.5 ± 1.2 | 1010 ± 62 | 0.85 ± 0.03 | 1.6 ± 0.5 |
| Digested | |||||||||
| Saliva | 534 ± 44 | 0.52 ± 0.03 | −28.2 ± 0.78 | 315± 25 | 0.59± 0.08 | −20.1 ± 1.3 | 293 ± 2 | 0.26 ± 0.01 | 12.1 ± 0.7 |
| Gastric Fluid | 1992 ± 292 | 0.59 ± 0.13 | −2.4 ± 0.4 | 1742 ± 505 | 0.65 ± 0.23 | −2.8 ± 0.3 | 701 ± 37 | 0.50 ± 0.12 | 3.4 ± 0.3 |
| Intestinal Fluid | 341 ± 37 | 0.61 ± 0.06 | −25.6 ± 0.9 | 230 ± 23 | 0.58 ± 0.10 | −10.8 ± 0.4 | 530 ± 11 | 0.38 ± 0.01 | 10.8 ± 0.4 |
| Culture Medium t 0 h | 618 ± 80 | 0.62 ± 0.05 | −7.6 ± 0.5 | 251 ± 12 | 0.59 ± 0.02 | −10.7 ± 0.4 | 174 ± 13 | 0.72 ± 0.05 | 1.8 ± 0.3 |
| Culture Medium t 24 h | 437 ± 100 | 0.72 ± 0.03 | −8.9 ± 0.9 | 271 ± 18 | 0.51 ± 0.09 | −12.0 ± 0.6 | 236 ± 2 | 0.37 ± 0.01 | 0.9 ± 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kose, O.; Béal, D.; Motellier, S.; Pelissier, N.; Collin-Faure, V.; Blosi, M.; Bengalli, R.; Costa, A.; Furxhi, I.; Mantecca, P.; et al. Physicochemical Transformations of Silver Nanoparticles in the Oro-Gastrointestinal Tract Mildly Affect Their Toxicity to Intestinal Cells In Vitro: An AOP-Oriented Testing Approach. Toxics 2023, 11, 199. https://doi.org/10.3390/toxics11030199
Kose O, Béal D, Motellier S, Pelissier N, Collin-Faure V, Blosi M, Bengalli R, Costa A, Furxhi I, Mantecca P, et al. Physicochemical Transformations of Silver Nanoparticles in the Oro-Gastrointestinal Tract Mildly Affect Their Toxicity to Intestinal Cells In Vitro: An AOP-Oriented Testing Approach. Toxics. 2023; 11(3):199. https://doi.org/10.3390/toxics11030199
Chicago/Turabian StyleKose, Ozge, David Béal, Sylvie Motellier, Nathalie Pelissier, Véronique Collin-Faure, Magda Blosi, Rossella Bengalli, Anna Costa, Irini Furxhi, Paride Mantecca, and et al. 2023. "Physicochemical Transformations of Silver Nanoparticles in the Oro-Gastrointestinal Tract Mildly Affect Their Toxicity to Intestinal Cells In Vitro: An AOP-Oriented Testing Approach" Toxics 11, no. 3: 199. https://doi.org/10.3390/toxics11030199
APA StyleKose, O., Béal, D., Motellier, S., Pelissier, N., Collin-Faure, V., Blosi, M., Bengalli, R., Costa, A., Furxhi, I., Mantecca, P., & Carriere, M. (2023). Physicochemical Transformations of Silver Nanoparticles in the Oro-Gastrointestinal Tract Mildly Affect Their Toxicity to Intestinal Cells In Vitro: An AOP-Oriented Testing Approach. Toxics, 11(3), 199. https://doi.org/10.3390/toxics11030199











