Abstract
Alternative materials for postmortem diagnosis in the case of fatal poisonings are much needed when standard materials, such as blood and urine, are unavailable. The study presents a case of fatal mass methanol intoxication resulting from industrial alcohol consumption. The study aimed to determine methanol and formic acid concentrations in epiglottis cartilage, costal cartilage, and intervertebral disc cartilage and to analyze the correlation between their concentrations in cartilage tissues and the femoral blood. Methanol and formic acid concentrations in samples collected from 17 individuals (n = 17) were estimated using gas chromatography with flame ionization detection (GC-FID). Methanol concentration in the costal cartilage correlated with its concentration in the femoral blood (r = 0.871). Similar correlations were found for epiglottis cartilage (r = 0.822) and intervertebral disc cartilage (r = 0.892). Formic acid concentration in the blood correlated only with its concentration in urine (r = 0.784) and the epiglottis (r = 0.538). Cartilage tissue could serve as an alternative material for methanol analyses in postmortem studies. Formic acid, a methanol metabolite, does not meet the requirements for its presence determination in cartilage tissues.
1. Introduction
In forensic autopsies, blood and urine—classical matrices in forensic toxicology—can be degraded or potentially affected by postmortem redistribution, hence, they are not always available [1]. Therefore, alternative sampling materials are needed. In forensic toxicology, the list of alternative matrices includes oral fluid [2,3], hair [4,5], sweat [6,7], meconium [8,9], breast milk [10,11], vitreous humor [12,13], bile [14,15], and even insects [16,17]. However, the alternative materials present limitations, such as limited xenobiotic accumulation (according to physical–chemical properties), the eventual need for more sensitive analyses, or the inability to correlate xenobiotic concentrations with effects [18].
Cartilage is one of the matrices studied in the context of xenobiotic distribution. The cartilage morphotic elements are embedded in the extracellular matrix, composed of structural elements, such as collagen fibers that protect cellular DNA against environmental factors and proteoglycans that bind water. Both elements ensure cartilage flexibility [19]. Due to these properties, forensic scientists’ interest in cartilage tissues increasingly grows [20]. In forensic genetics, costal cartilage can serve as a DNA source in cases of individual identification [21], and fibrous tissue of the intervertebral disc allows for rapid genetic identification [22,23]. Unfortunately, cartilage hydration affects the ability to determine the levels of water-soluble xenobiotics [19]. However, costal cartilage has been successfully used to detect nitrite ions in fatal sodium nitrite poisoning [24]. Additionally, costal cartilage, ethanol [25], and isopropanol [26] concentrations positively correlated with their concentrations in the blood.
Methanol ingestion and consecutive poisoning is a rising problem, closely associated with high morbidity and mortality [27]. Alcohol dehydrogenase oxidizes methanol to formaldehyde, and subsequently, aldehyde dehydrogenase oxidizes formaldehyde to formic acid, which accounts for the associated anion gap metabolic acidosis and end-organ damage [28]. Pure methanol’s lethal dose ranges from 300 to 1000 mg/kg [29]. Methanol ingestion is usually fatal.
Methanol distribution in different tissues and body fluids after absorption is poorly understood. Our research focused on methanol and formic acid distribution in cartilage tissues sampled from 17 fatal victims of a mass intoxication with industrial alcohol who died between April and June 2022 in the Silesia Region (Poland) [30].
2. Materials and Methods
The samples were collected from 17 individuals who died due to methanol poisoning between April and June 2022 in the Silesia Region (Poland). The sample collection was approved by the Bioethical Commission of the Medical University of Silesia in Katowice (decision no. PCN/CBN/0052/KB/77/22, date of approval: 5 May 2022). Femoral blood, urine, costal cartilage, epiglottis, and fibro-cartilage of the intervertebral disc samples (Figure 1) were collected during medical–legal autopsies commissioned by the Prosecutor’s Office. All analyses were carried out in a certified forensic laboratory immediately after the autopsy.
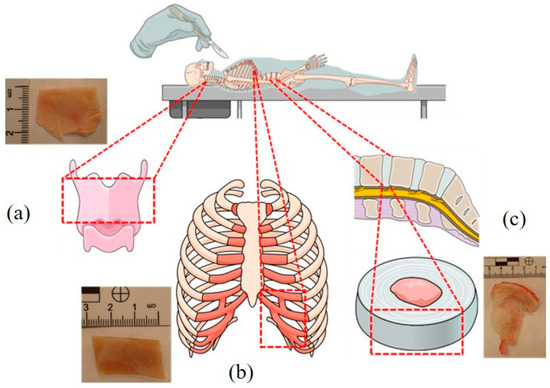
Figure 1.
Anatomical location of the epiglottis (a), costal cartilage (b), and fibro-cartilage of the intervertebral disc (c). The dotted lines indicate the places of material sampling for testing. The diagram was prepared using Mind the Graph software (https://mindthegraph.com/ (accessed on 4 October 2022).
The collected samples, 1 mL of fluid or 1 g of chopped cartilage tissue (devoid of soft tissues—thoroughly cleaned using three sterile scalpels, changed each time after removing the next surface layer, and then they were fragmented into 0.1 × 0.1 × 0.1 cm fragments), and then they were placed in 20 mL glass gas-tight vials, and analyzed as described by Tomsia et al. [25]. An eight-point calibration curve for methanol in mg/mL or mg/g (0; 0.1; 0.2; 0.5; 0.8; 1; 2; 3) was linear in the whole range. The limit of detection (LOD) was determined as 0.05 mg/mL or mg/g, and the limit of quantification (LOQ) was determined as 0.1 mg/mL or mg/g for the entire tested material. Linearity was maintained up to 5000 mg/L (R2 = 0.996).
Formic acid concentration in blood, urine, and tissues was determined using gas chromatography and the method described by Kuo et al. [31] and Abolin et al. [32]. In this method, formic acid was determined in the form of a volatile methyl formate ester. Using the FID detector (Thermo Fisher Scientific Inc., Milan, Italy) ensured the sensitivity of 0.01 mg/mL and reduced the impact of the biological background. The calibration curve for formic acid ranged from 0.1–2.0 mg/mL or mg/g.
The distribution of variables was evaluated using the Shapiro–Wilk test and the quantile–quantile plot. The interval data were expressed as mean values ± standard deviations. The regression analysis was used to determine the relationship between quantitative features. The data with non-normal distribution were log transformed before analysis. Comparisons of the ratios for blood/urine and blood/cartilage alcohol concentration between current and previous results [25] were made using the non-parametric U Mann-Whitney and Kruskal-Wallis tests, respectively. Statistical significance was set at a p < 0.05, and all tests were two-tailed. Statistical analysis was performed using Statistica, version 13.3 (TIBCO Software Inc., Palo Alto, CA, USA, 2017).
3. Results
The study group consisted of three women (18%) and 14 men (82%). Out of 17 victims, only four individuals (18%) were hospitalized. In five cases (30%), methanol in cartilage tissues was not detected. For these cases, the time from death to autopsy (t2) was 8 ± 5 (2–16) days (mean ± SD (min − max). The basic characteristics of quantitative variables are presented in the Table 1.

Table 1.
Descriptive statistics of quantitative variables analyzed in the victims of fatal methanol poisoning (n = 17).
Methanol and formic acid concentrations in the studied cartilage tissues were much lower than in blood and urine. Among the studied cartilage tissues, the lowest concentrations of methanol and formic acid were found in the costal cartilage, and the highest were found in the annulus fibrosis of intervertebral discs. We found that methanol concentration in the costal cartilage (Table 2, Figure 2B) correlated with methanol concentration in the femoral blood (r = 0.871). We found the same correlation type for methanol concentration in the epiglottis (r = 0.822) and the fibro-cartilage of the intervertebral disc (r = 0.892). We also found that the formic acid concentration in the epiglottis cartilage (r = 0.538) correlated with its concentration in the blood (Figure 3).

Table 2.
Analysis of univariate linear regression for methanol concentration in blood and cartilage tissues of fatal methanol poisoning victims.
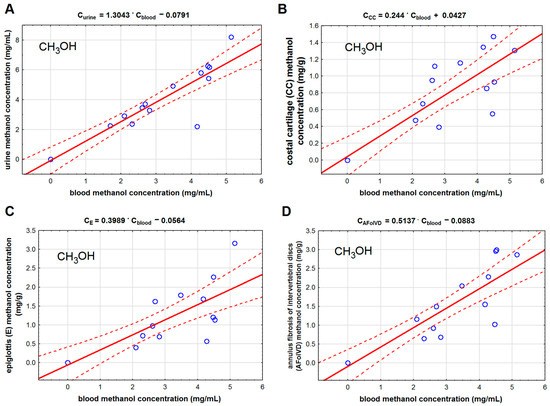
Figure 2.
The ordinary least square regression model for the relationship between methanol (CH3OH) concentration in the blood and in: urine (A), costal cartilage (B), epiglottis (C), and anulus fibrosis of intervertebral discs (D). Legend: AFoIVD—annulus fibrosis of intervertebral discs, CC—costal cartilage, and E—epiglottis. The solid red lines represent regression lines, and the dashed lines indicate 95% confidence intervals.
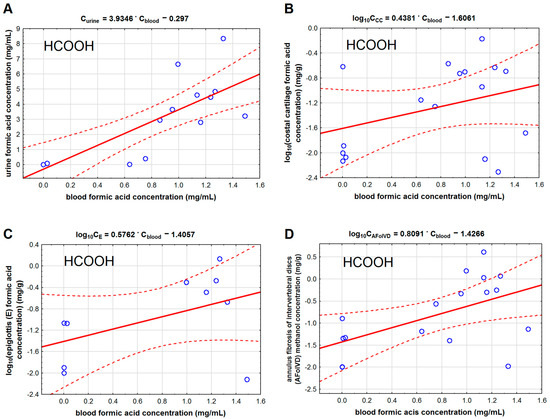
Figure 3.
The ordinary least square regression model for the relationship between formic acid (HCOOH) concentration in the blood and in: urine (A), costal cartilage (B), epiglottis (C), and anulus fibrosis of intervertebral discs (D). Legend: AFoIVD—annulus fibrosis of intervertebral discs, CC—costal cartilage, and E—epiglottis. The solid red lines represent regression lines, and the dashed lines indicate 95% confidence intervals. The data for sections B, C, and D were log transformed before the analysis.
Additionally, methanol and formic acid concentrations in the blood correlated with their concentrations in urine (r = 0.929 and r = 0.784, respectively).
Alcohol Tissue Permeability Comparison
Comparing the results of methanol poisoning cases with cases of ethanol intoxication [25], we found non-statistically significant differences in blood/urine concentration ratios between methanol and ethanol (Table 3). However, we found statistically significant differences in the blood/cartilage concentration ratios for methanol and ethanol (p < 0.001; Table 3). Additional analyses showed no significant differences between the blood/cartilage ratios for methanol and ethanol (p = 1.000) concentrations determined using the UCC method (unground costal cartilage method) and for methanol (determined using the UCC method) and ethanol (p = 0.058) concentration determined using the GCC method (ground costal cartilage method). Comparing the blood/cartilage ratios for ethanol alone, we found significantly lower ratio values for samples prepared using the GCC method (p < 0.001) (Table 3, Figure 4).

Table 3.
Comparison of blood/urine and blood/cartilage concentration ratios for methanol and ethanol poisoning. Data for costal cartilage ethanol concentrations were taken from Tomsia et al. [25] study. Data are presented as medians (lower quartile;upper quartile).
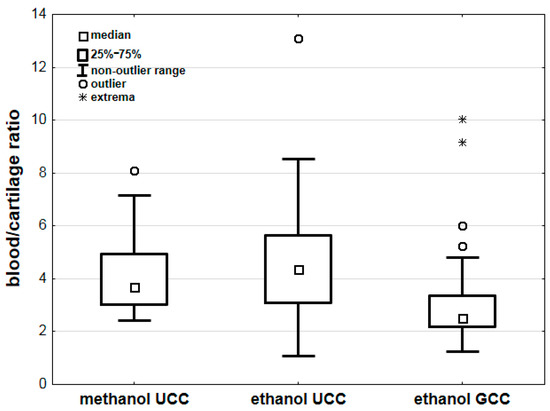
Figure 4.
Comparison of blood/cartilage ratios for methanol and ethanol concentrations. Legend: GCC—costal cartilage prepared with the ground costal cartilage method [25], UCC—costal cartilage prepared with the unground costal cartilage method [25].
4. Discussion
The presented results contribute to the current knowledge of methanol distribution in the human body. A high positive correlation between methanol concentration in all types of studied cartilage and methanol concentration in the blood showed that this type of tissue could serve as an alternative material. The results for formic acid, a methanol metabolite, showed that it does not meet the requirements for its presence determination in cartilage tissues using the applied methods.
Earlier studies [25] showed a statistically significant, strong positive correlation between ethanol concentration in the blood and in cartilage (r = 0.925, p < 0.001) prepared according to the GCC method (ground costal cartilage method). The presented study shows that methanol concentration in the blood also strongly correlates with its concentration in costal cartilage (r = 0.8714, p < 0.001), even though the presented study used the UCC method (unground costal cartilage method), since we found no significant differences between the Pearson’s’ correlation coefficients mentioned above (p = 0.355). Comparing the obtained results with the previous studies [25], we may conclude that, within the UCC method, both methanol and ethanol show similar tissue “permeability”.
So far, few studies about mass methanol poisonings have analyzed the results of postmortem studies [30,33]. The ingestion of the same dose of methanol may result in different clinical symptoms. Therefore, a combination of multiple diagnosis methods may contribute to the forensic diagnosis of methanol poisoning more precisely, and the choice of the diagnostic method should be considered on an individual basis [27]. Using a wide range of alternative materials may help interpret the results and give medical–legal opinions. The high correlation coefficients between the blood and cartilage tissues for methanol suggest that methanol concentration in cartilage can be determined, and methanol behaves similarly to urine. Since the obtained results add another perspective to the distribution of xenobiotics in cartilage tissues, they may also be important in the context of using this type of tissue for regenerative medicine [34] or plastic surgery purposes [35].
5. Conclusions
Methanol presence in costal cartilage, epiglottis, and intervertebral disc cartilage was confirmed for the first time postmortem. Methanol concentration in all types of cartilage appositively correlated with this in femoral blood. Formic acid, a methanol metabolite, does not meet the requirements for its presence determination in cartilage tissues.
Author Contributions
Conceptualization, M.T. and J.N.; Methodology, M.T., J.N. and M.G.; Software, J.N.; Validation, J.N., M.G. and M.T.; Formal analysis, M.T.; Investigation, M.T., J.N. and M.G.; Resources, M.T.; Data curation, J.N.; Writing—original draft preparation, M.T.; Writing—review and editing, M.T. and E.C.; Visualization, M.T. and E.C.; Supervision, M.T. and E.C.; Project administration, M.T.; Funding acquisition, E.C. and M.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Medical University of Silesia (grant numbers: PCN-1-103/N/1/F and PCN-2-119/N/0/O). The APC was funded by the Medical University of Silesia.
Institutional Review Board Statement
Samples from cadavers were taken with the consent of the Bioethical Commission of Medical University of Silesia in Katowice (decision no. PCN/CBN/0052/KB/77/22, date of approval: 05 May 2022).
Informed Consent Statement
Not applicable. Material was collected with the written consent of the prosecutor.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gallardo, E.; Queiroz, J.A. The role of alternative specimens in toxicological analysis. Biomed. Chromatogr. 2008, 22, 795–821. [Google Scholar] [CrossRef] [PubMed]
- Reisinger, A.J.; Miller, A.C.; Shaw, L.A.; Champion, J.L.; Neiswonger, M.A. Oral Cavity Fluid as an Investigative Approach for Qualitative and Quantitative Evaluations of Drugs in Postmortem Subjects. J. Anal. Toxicol. 2019, 43, 444–451. [Google Scholar] [CrossRef]
- Truver, M.T.; Palmquist, K.B.; Swortwood, M.J. Oral Fluid and Drug Impairment: Pairing Toxicology with Drug Recognition Expert Observations. J. Anal. Toxicol. 2019, 43, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Kuwayama, K.; Miyaguchi, H.; Kanamori, T.; Tsujikawa, K.; Yamamuro, T.; Segawa, H.; Okada, Y.; Iwata, Y.T. Micro-segmental hair analysis: Detailed procedures and applications in forensic toxicology. Forensic Toxicol. 2022, 40, 215–233. [Google Scholar] [CrossRef]
- Ferreira, C.; Paulino, C.; Quintas, A. Extraction Procedures for Hair Forensic Toxicological Analysis: A Mini-Review. Chem. Res. Toxicol. 2019, 32, 2367–2381. [Google Scholar] [CrossRef]
- Jadoon, S.; Karim, S.; Akram, M.R.; Khan, A.K.; Zia, M.A.; Siddiqi, A.R.; Murtaza, G. Recent Developments in Sweat Analysis and Its Applications. Int. J. Anal. Chem. 2015, 2015, 164974. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Ahmed, A.; Sharma, A.; Arya, S. Graphene and Its Derivatives: Synthesis and Application in the Electrochemical Detection of Analytes in Sweat. Biosensors 2022, 12, 910. [Google Scholar] [CrossRef]
- Palmer, K.L.; Krasowski, M.D. Alternate Matrices: Meconium, Cord Tissue, Hair, and Oral Fluid. Methods Mol. Biol. 2019, 1872, 191–197. [Google Scholar] [CrossRef]
- Hernandez, A.; Lacroze, V.; Doudka, N.; Becam, J.; Pourriere-Fabiani, C.; Lacarelle, B.; Solas, C.; Fabresse, N. Determination of Prenatal Substance Exposure Using Meconium and Orbitrap Mass Spectrometry. Toxics 2022, 10, 55. [Google Scholar] [CrossRef]
- Sempio, C.; Wymore, E.; Palmer, C.; Bunik, M.; Henthorn, T.K.; Christians, U.; Klawitter, J. Detection of Cannabinoids by LC–MS-MS and ELISA in Breast Milk. J. Anal. Toxicol. 2021, 45, 686–692. [Google Scholar] [CrossRef]
- Busardò, F.P.; Bertol, E.; Mannocchi, G.; Tittarelli, R.; Pantano, F.; Vaiano, F.; Baglio, G.; Kyriakou, C.; Marinelli, E. Determination of GHB levels in breast milk and correlation with blood concentrations. Forensic Sci. Int. 2016, 265, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Metushi, I.G.; Fitzgerald, R.L.; McIntyre, I.M. Assessment and Comparison of Vitreous Humor as an Alternative Matrix for Forensic Toxicology Screening by GC–MS. J. Anal. Toxicol. 2016, 40, 243–247. [Google Scholar] [CrossRef]
- Bévalot, F.; Cartiser, N.; Bottinelli, C.; Fanton, L.; Guitton, J. Vitreous humor analysis for the detection of xenobiotics in forensic toxicology: A review. Forensic Toxicol. 2016, 34, 12–40. [Google Scholar] [CrossRef]
- Bévalot, F.; Cartiser, N.; Bottinelli, C.; Guitton, J.; Fanton, L. State of the art in bile analysis in forensic toxicology. Forensic Sci. Int. 2016, 259, 133–154. [Google Scholar] [CrossRef] [PubMed]
- Bierly, J.; Labay, L.M. The Utility of Bile in Postmortem Forensic Toxicology. Acad. Forensic Pathol. 2018, 8, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, M.; Wille, S.M.; Fernandez, M.D.M.R.; Di Fazio, V.; Samyn, N.; De Boeck, G.; Bourel, B. Entomotoxicology, experimental set-up and interpretation for forensic toxicologists. Forensic Sci. Int. 2011, 208, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hodecek, J. Revisiting the concept of entomotoxicology. Forensic Sci. Int. Synerg. 2020, 2, 282–286. [Google Scholar] [CrossRef] [PubMed]
- de Campos, E.G.; da Costa, B.R.B.; dos Santos, F.S.; Monedeiro, F.; Alves, M.N.R.; Junior, W.J.R.S.; De Martinis, B.S. Alternative matrices in forensic toxicology: A critical review. Forensic Toxicol. 2022, 40, 1–18. [Google Scholar] [CrossRef]
- Eschweiler, J.; Horn, N.; Rath, B.; Betsch, M.; Baroncini, A.; Tingart, M.; Migliorini, F. The Biomechanics of Cartilage—An Overview. Life 2021, 11, 302. [Google Scholar] [CrossRef]
- Tomsia, M.; Cieśla, J.; Pilch-Kowalczyk, J.; Banaszek, P.; Chełmecka, E. Cartilage Tissue in Forensic Science—State of the Art and Future Research Directions. Processes 2022, 10, 2456. [Google Scholar] [CrossRef]
- Tomsia, M.; Droździok, K.; Javan, G.; Skowronek, R.; Szczepański, M.; Chełmecka, E. Costal cartilage ensures low degradation of DNA needed for genetic identification of human remains retrieved at different decomposition stages and different post-mortem intervals. Postepy Hig. Med. Dosw. 2021, 75, 852–858. [Google Scholar] [CrossRef]
- Becker, J.; Mahlke, N.S.; Ritz-Timme, S.; Boehme, P. The human intervertebral disc as a source of DNA for molecular identi-fication. Forensic Sci. Med. Pathol. 2021, 17, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Tomsia, M.; Droździok, K.; Banaszek, P.; Szczepański, M.; Pałasz, A.; Chełmecka, E. The intervertebral discs’ fibrocartilage as a DNA source for genetic identification in severely charred cadavers. Forensic Sci. Med. Pathol. 2022, 18, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Tomsia, M.; Głaz, M.; Nowicka, J.; Szczepański, M. Sodium nitrite detection in costal cartilage and vitreous humor—Case report of fatal poisoning with sodium nitrite. J. Forensic Leg. Med. 2021, 81, 102186. [Google Scholar] [CrossRef]
- Tomsia, M.; Nowicka, J.; Skowronek, R.; Woś, M.; Wójcik, J.; Droździok, K.; Zorychta, M.; Javan, G.T.; Chełmecka, E. A Comparative Study of Ethanol Concentration in Costal Cartilage in Relation to Blood and Urine. Processes 2020, 8, 1637. [Google Scholar] [CrossRef]
- Tomsia, M.; Nowicka, J.; Skowronek, R.; Javan, G.T.; Chełmecka, E. Concentrations of volatile substances in costal cartilage in relation to blood and urine–preliminary studies. Arch. Forensic Med. Criminol. 2021, 71, 38–46. [Google Scholar] [CrossRef]
- Tian, M.; He, H.; Liu, Y.; Li, R.; Zhu, B.; Cao, Z. Fatal methanol poisoning with different clinical and autopsy findings: Case report and literature review. Leg. Med. Tokyo 2022, 54, 101995. [Google Scholar] [CrossRef]
- Liesivuori, J.; Savolainen, H. Methanol and Formic Acid Toxicity: Biochemical Mechanisms. Pharmacol. Toxicol. 1991, 69, 157–163. [Google Scholar] [CrossRef]
- International Programme on Chemical Safety (IPCS). Methanol; Environmental Health Criteria 196; World Health Organization: Geneva, Switzerland, 1997. [Google Scholar]
- Tomsia, M.; Głaz, M.; Nowicka, J.; Cieśla, J.; Sosnowski, M.; Chełmecka, E. Fatal Methanol Poisoning Caused by Drinking Industrial Alcohol: Silesia Region, Poland, April–June. Toxics 2022, 10, 800. [Google Scholar] [CrossRef]
- Abolin, C.; McRae, J.D.; Tozer, T.N.; Takki, S. Gas chromatographic head-space assay of formic acid as methyl formate in biologic fluids: Potential application to methanol poisoning. Biochem. Med. 1980, 23, 209–218. [Google Scholar] [CrossRef]
- Kuo, T. The effects of ethanol on methanol intoxication I. A simple headspace gas chromatography for the determination of blood formic acid. Jpn. J. Leg. Med. 1982, 36, 669–675. [Google Scholar]
- Buszewicz, G.; Teresiński, G.; Mądro, R. Distribution of methanol in biological specimens of victims of a fatal group poisoning. Probl. Forensic Sci. 2004, 59, 115–126. [Google Scholar]
- Cieśla, J.; Tomsia, M. Cadaveric Stem Cells: Their Research Potential and Limitations. Front. Genet. 2021, 12, 798161. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.-V.T.; Sykes, K.; Kriet, J.D.; Humphrey, C. Cartilage Graft Donor Site Morbidity following Rhinoplasty and Nasal Reconstruction. Craniomaxillofacial Trauma Reconstr. 2017, 11, 278–284. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).