High-Dose Primaquine Induces Proximal Tubular Degeneration and Ventricular Cardiomyopathy Linked to Host Cells Mitochondrial Dysregulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Permission
2.2. Experimental Procedure
2.3. Sample Collection and Mitochondrial Extraction
2.4. Electron Microscopic Study
2.5. Histopathological Study
2.6. Immunohistochemical Study
2.7. qRT-PCR
2.8. Statistical Analysis
3. Results
3.1. Clinicohematological Data
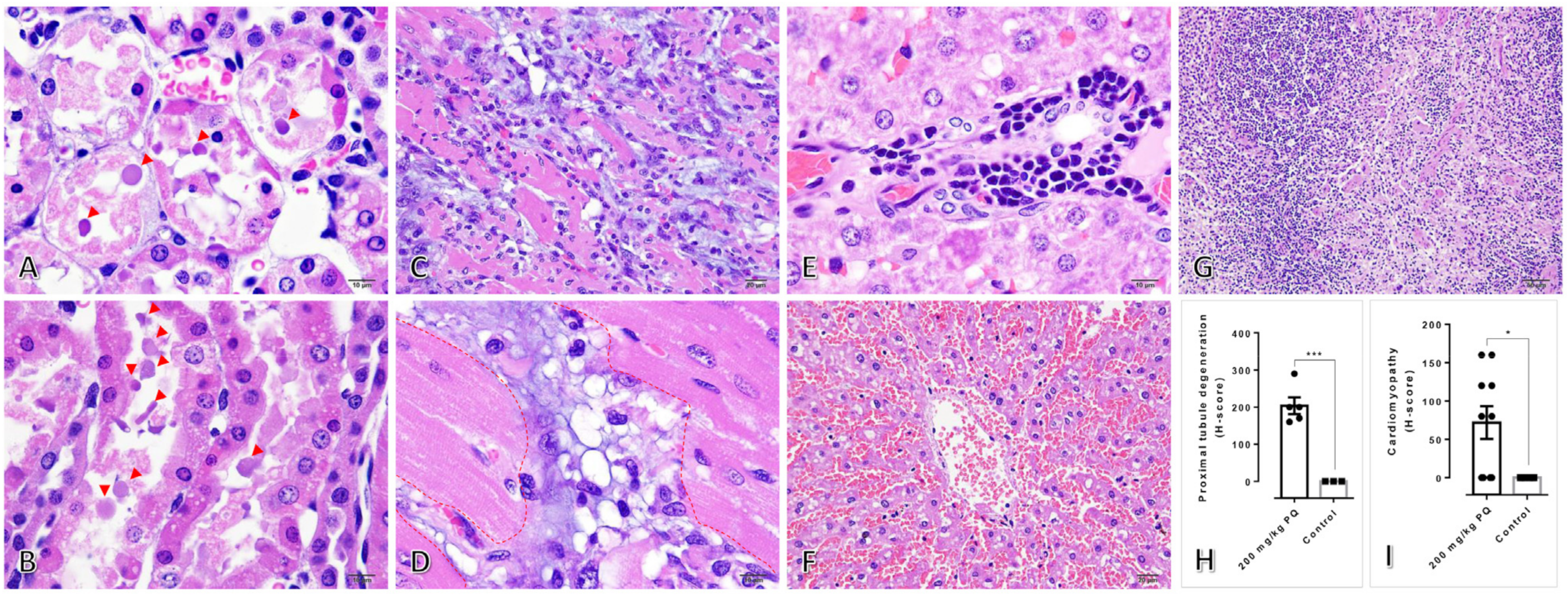
3.2. High Dose of PQ-Induced Renal Degeneration and Ventricular Cardiomyopathy
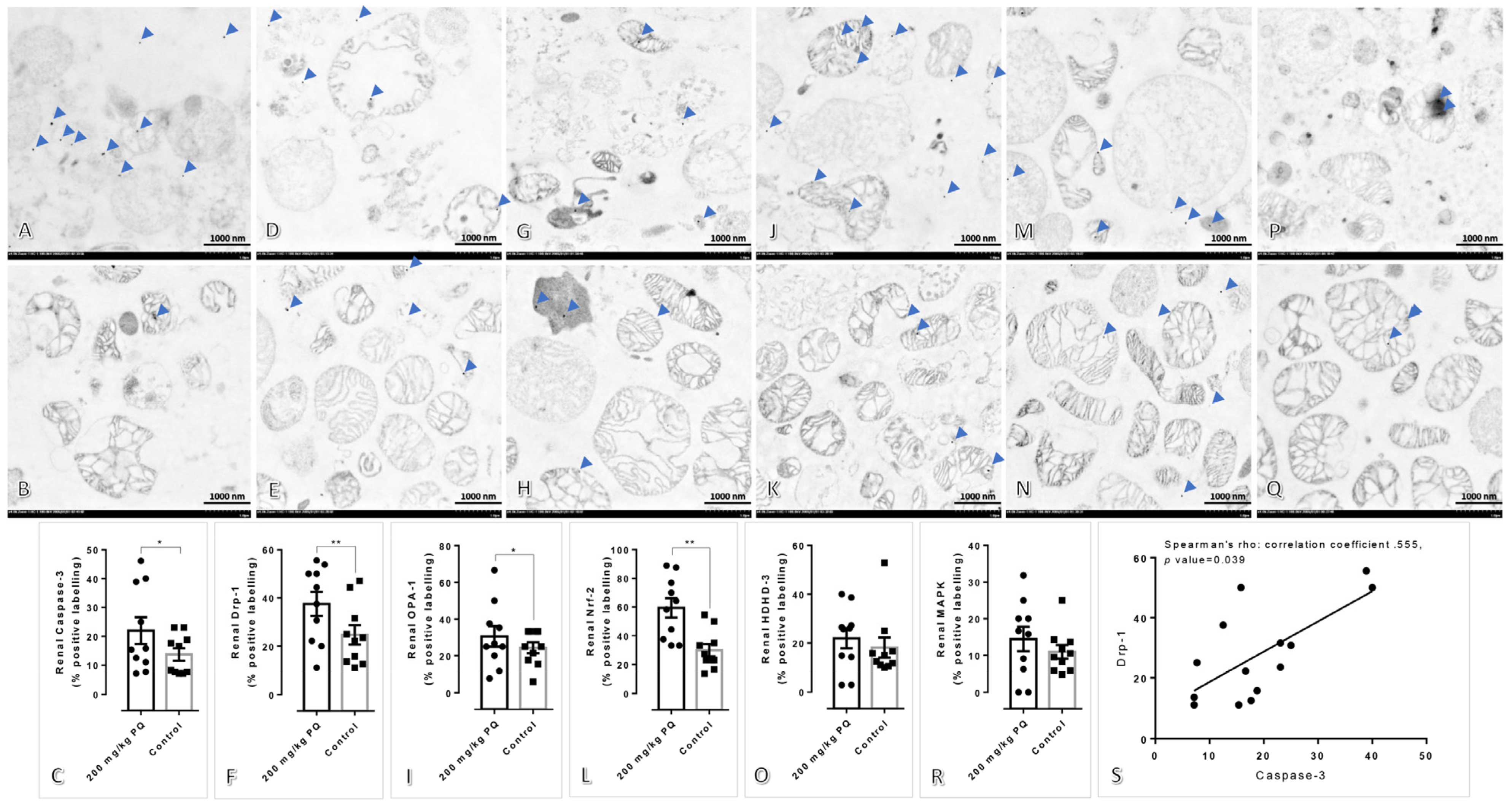
3.3. High Dose of PQ Caused Mitochondrial Alteration in the Kidney and Liver
3.4. Upregulation of Drp-1 and Caspase-3 Were Observed in the Kidney and Liver Mitochondria Induced by a High Dose of PQ
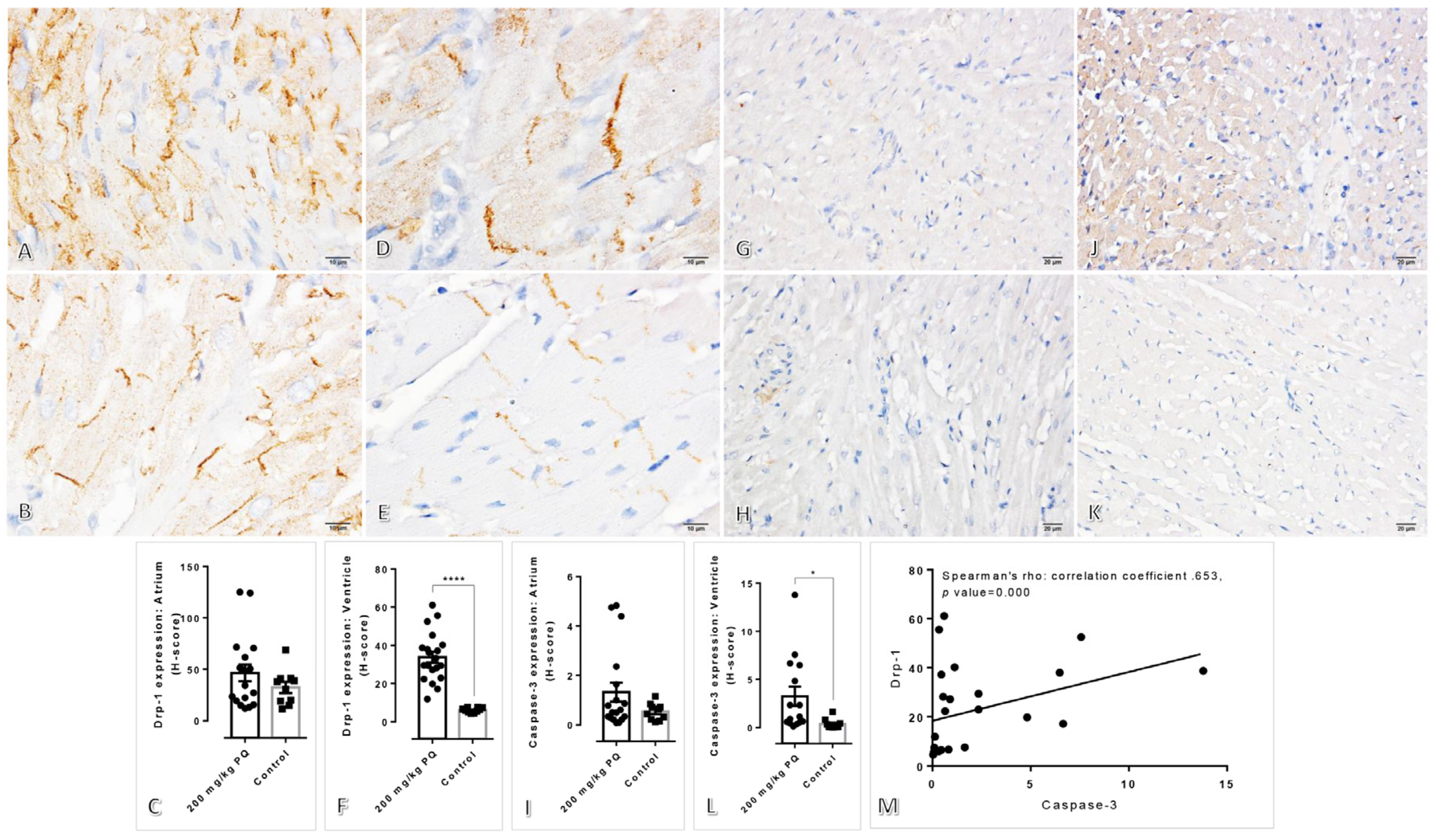
3.5. Upregulation of Drp-1 and Caspase-3 were Observed in the Heart Induced by a High Dose of PQ
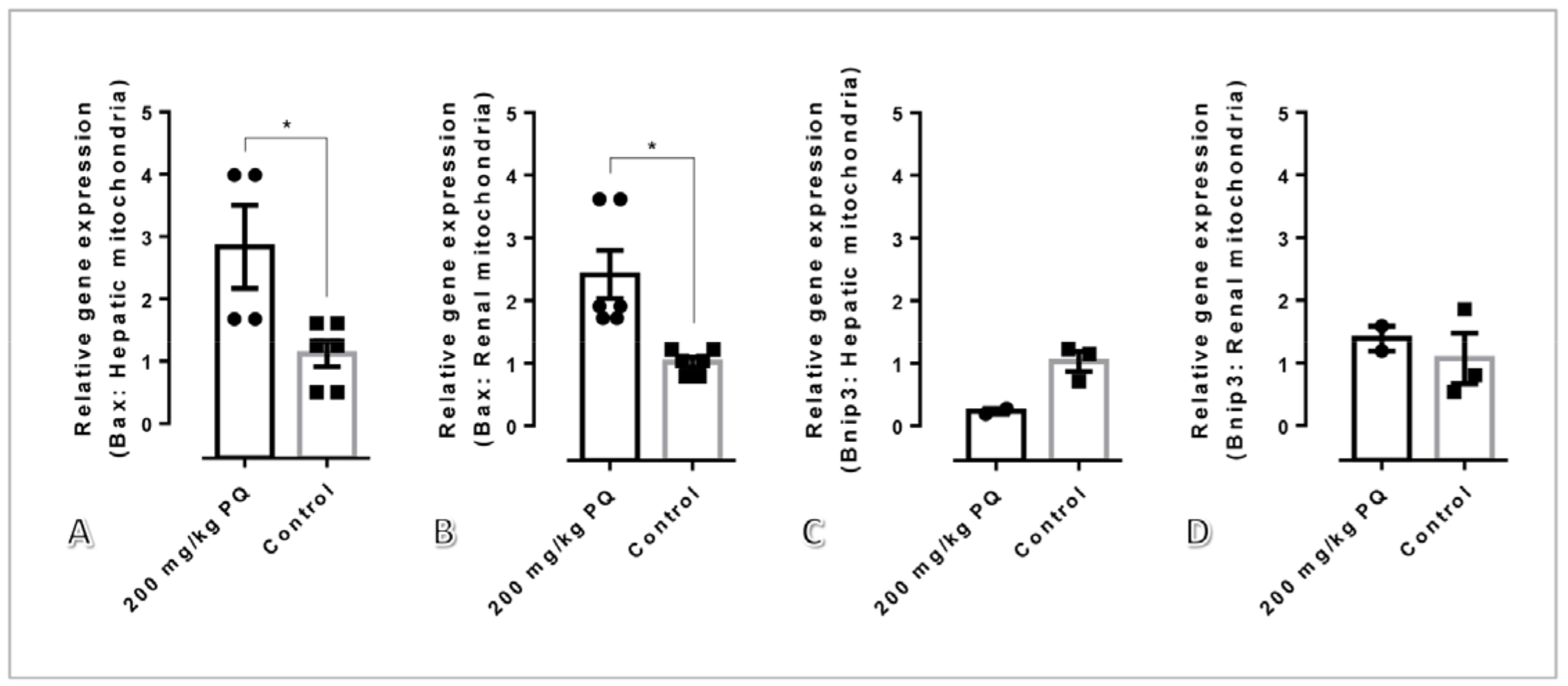
3.6. High Dose of PQ Upregulated Pro-Apoptotic Genes in Association with Mitochondrial Apoptosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Lancet. Malaria in 2022: A year of opportunity. Lancet 2022, 399, 1573. [Google Scholar] [CrossRef] [PubMed]
- Taylor, W.R.; White, N.J. Antimalarial drug toxicity: A review. Drug Saf. 2004, 27, 25–61. [Google Scholar] [CrossRef] [PubMed]
- Ashley, E.A.; Recht, J.; White, N.J. Primaquine: The risks and the benefits. Malar. J. 2014, 13, 418. [Google Scholar] [CrossRef]
- Cohen, R.J.; Sachs, J.R.; Wicker, D.J.; Conrad, M.E. Methemoglobinemia provoked by malarial chemoprophylaxis in Vietnam. N. Engl. J. Med. 1968, 279, 1127–1131. [Google Scholar] [CrossRef]
- Recht, J.; Ashley, E.; White, N.J. Safety of 8-Aminoquinoline Antimalarial Medicines; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Baker, J.K.; Hullihen, J.M.; Pedersen, P.L. Selective toxicity of the antimalarial primaquine-evidence for both uncoupling and inhibitory effects of a metabolite on the energetics of mitochondria and its ATP synthase complex. Pharm. Res. 1986, 3, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Laleve, A.; Vallieres, C.; Golinelli-Cohen, M.P.; Bouton, C.; Song, Z.; Pawlik, G.; Tindall, S.M.; Avery, S.V.; Clain, J.; Meunier, B. The antimalarial drug primaquine targets Fe-S cluster proteins and yeast respiratory growth. Redox Biol. 2016, 7, 21–29. [Google Scholar] [CrossRef]
- Lanners, H.N. Effect of the 8-aminoquinoline primaquine on culture-derived gametocytes of the malaria parasite Plasmodium falciparum. Parasitol. Res. 1991, 77, 478–481. [Google Scholar] [CrossRef]
- Ampawong, S.; Isarangkul, D.; Reamtong, O.; Aramwit, P. Adaptive effect of sericin on hepatic mitochondrial conformation through its regulation of apoptosis, autophagy and energy maintenance: A proteomics approach. Sci. Rep. 2018, 8, 14943. [Google Scholar] [CrossRef]
- Tirawanchai, N.; Kengkoom, K.; Isarangkul, D.; Burana-Osot, J.; Kanjanapruthipong, T.; Chantip, S.; Phattanawasin, P.; Sotanaphun, U.; Ampawong, S. A combination extract of kaffir lime, galangal, and lemongrass maintains blood lipid profiles, hepatocytes, and liver mitochondria in rats with nonalcoholic steatohepatitis. Biomed. Pharmacother. 2020, 124, 109843. [Google Scholar] [CrossRef]
- Fongsodsri, K.; Thaipitakwong, T.; Rujimongkon, K.; Kanjanapruthipong, T.; Ampawong, S.; Reamtong, O.; Aramwit, P. Mulberry-Derived 1-Deoxynojirimycin Prevents Type 2 Diabetes Mellitus Progression via Modulation of Retinol-Binding Protein 4 and Haptoglobin. Nutrients 2022, 14, 4538. [Google Scholar] [CrossRef]
- Ampawong, S.; Isarangkul, D.; Aramwit, P. Sericin ameliorated dysmorphic mitochondria in high-cholesterol diet/streptozotocin rat by antioxidative property. Exp. Biol. Med. 2017, 242, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Ampawong, S.; Isarangkul, D.; Aramwit, P. Sericin improves heart and liver mitochondrial architecture in hypercholesterolaemic rats and maintains pancreatic and adrenal cell biosynthesis. Exp. Cell Res. 2017, 358, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Rujimongkon, K.; Ampawong, S.; Isarangkul, D.; Reamtong, O.; Aramwit, P. Sericin-mediated improvement of dysmorphic cardiac mitochondria from hypercholesterolaemia is associated with maintaining mitochondrial dynamics, energy production, and mitochondrial structure. Pharm. Biol. 2022, 60, 708–721. [Google Scholar] [CrossRef] [PubMed]
- Svingen, T.; Letting, H.; Hadrup, N.; Hass, U.; Vinggaard, A.M. Selection of reference genes for quantitative RT-PCR (RT-qPCR) analysis of rat tissues under physiological and toxicological conditions. PeerJ 2015, 3, e855. [Google Scholar] [CrossRef]
- Liang, W.; Cai, A.; Chen, G.; Xi, H.; Wu, X.; Cui, J.; Zhang, K.; Zhao, X.; Yu, J.; Wei, B.; et al. Shikonin induces mitochondria-mediated apoptosis and enhances chemotherapeutic sensitivity of gastric cancer through reactive oxygen species. Sci. Rep. 2016, 6, 38267. [Google Scholar] [CrossRef]
- Gunjan, S.; Singh, S.K.; Sharma, T.; Dwivedi, H.; Chauhan, B.S.; Imran Siddiqi, M.; Tripathi, R. Mefloquine induces ROS mediated programmed cell death in malaria parasite: Plasmodium. Apoptosis 2016, 21, 955–964. [Google Scholar] [CrossRef]
- Sahu, K.; Langeh, U.; Singh, C.; Singh, A. Crosstalk between anticancer drugs and mitochondrial functions. Curr. Res. Pharmacol. Drug Discov. 2021, 2, 100047. [Google Scholar] [CrossRef]
- Giovanella, F.; Ferreira, G.K.; de Pra, S.D.; Carvalho-Silva, M.; Gomes, L.M.; Scaini, G.; Goncalves, R.C.; Michels, M.; Galant, L.S.; Longaretti, L.M.; et al. Effects of primaquine and chloroquine on oxidative stress parameters in rats. An. Acad. Bras. Cienc. 2015, 87, 1487–1496. [Google Scholar] [CrossRef]
- Katewa, S.D.; Katyare, S.S. Treatment with antimalarials adversely affects the oxidative energy metabolism in rat liver mitochondria. Drug Chem. Toxicol. 2004, 27, 41–53. [Google Scholar] [CrossRef]
- Marchi, S.; Guilbaud, E.; Tait, S.W.G.; Yamazaki, T.; Galluzzi, L. Mitochondrial control of inflammation. Nat. Rev. Immunol. 2022, 23, 1–15. [Google Scholar] [CrossRef]
- Wang, C.; Youle, R.J. The role of mitochondria in apoptosis*. Annu. Rev. Genet. 2009, 43, 95–118. [Google Scholar] [CrossRef]
- Bras, M.; Yuste, V.J.; Roue, G.; Barbier, S.; Sancho, P.; Virely, C.; Rubio, M.; Baudet, S.; Esquerda, J.E.; Merle-Beral, H.; et al. Drp1 mediates caspase-independent type III cell death in normal and leukemic cells. Mol. Cell Biol. 2007, 27, 7073–7088. [Google Scholar] [CrossRef]
- Jenner, A.; Pena-Blanco, A.; Salvador-Gallego, R.; Ugarte-Uribe, B.; Zollo, C.; Ganief, T.; Bierlmeier, J.; Mund, M.; Lee, J.E.; Ries, J.; et al. DRP1 interacts directly with BAX to induce its activation and apoptosis. EMBO J. 2022, 41, e108587. [Google Scholar] [CrossRef] [PubMed]
- Gui, C.; Ren, Y.; Chen, J.; Wu, X.; Mao, K.; Li, H.; Yu, H.; Zou, F.; Li, W. p38 MAPK-DRP1 signaling is involved in mitochondrial dysfunction and cell death in mutant A53T alpha-synuclein model of Parkinson’s disease. Toxicol. Appl. Pharmacol. 2020, 388, 114874. [Google Scholar] [CrossRef]
- Luna-Sanchez, M.; Beninca, C.; Cerutti, R.; Brea-Calvo, G.; Yeates, A.; Scorrano, L.; Zeviani, M.; Viscomi, C. Opa1 Overexpression Protects from Early-Onset Mpv17(-/-)-Related Mouse Kidney Disease. Mol. Ther. 2020, 28, 1918–1930. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Tian, C.; Puszyk, W.M.; Ogunwobi, O.O.; Cao, M.; Wang, T.; Cabrera, R.; Nelson, D.R.; Liu, C. OPA1 downregulation is involved in sorafenib-induced apoptosis in hepatocellular carcinoma. Lab. Investig. 2013, 93, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Nezu, M.; Suzuki, N. Roles of Nrf2 in Protecting the Kidney from Oxidative Damage. Int. J. Mol. Sci. 2020, 21, 2951. [Google Scholar] [CrossRef]
- Jokinen, M.P.; Lieuallen, W.G.; Boyle, M.C.; Johnson, C.L.; Malarkey, D.E.; Nyska, A. Morphologic aspects of rodent cardiotoxicity in a retrospective evaluation of National Toxicology Program studies. Toxicol. Pathol. 2011, 39, 850–860. [Google Scholar] [CrossRef]
- Frazier, K.S.; Seely, J.C.; Hard, G.C.; Betton, G.; Burnett, R.; Nakatsuji, S.; Nishikawa, A.; Durchfeld-Meyer, B.; Bube, A. Proliferative and nonproliferative lesions of the rat and mouse urinary system. Toxicol. Pathol. 2012, 40, 14S–86S. [Google Scholar] [CrossRef]
- Mubagwa, K. Cardiac effects and toxicity of chloroquine: A short update. Int. J. Antimicrob. Agents 2020, 56, 106057. [Google Scholar] [CrossRef]
- Wiwanitkit, V. Antimalarial drug and renal toxicity. J. Nephropharmacol. 2016, 5, 11–12. [Google Scholar] [PubMed]
- Campos, S.B.; Rouch, L.H.; Seguro, A.C. Effects of sodium artesunate, a new antimalarial drug, on renal function. Kidney Int. 2001, 59, 1044–1051. [Google Scholar] [CrossRef] [PubMed]


| Gene | Primers | |
|---|---|---|
| 18S | F | 5′GCCGCTAGAGGTGAAATTCTTG3′ |
| R | 5′GAAAACATTCTTGGCAAATGCTT3′ | |
| Bax | F | 5′AGAACCATCATGGGCTGGAC3′ |
| R | 5′AGATGGTCACTGTCTGCCATGT3′ | |
| Bnip3 | F | 5′CAGAGCGGGGAGGAGAAC3′ |
| R | 5′GAAGCTGGAACGCTGCTC3′ | |
| Parameters | Normal Range # | Control | 200 mg/kg of PQ |
|---|---|---|---|
| WBC (106/µL) | 3.51–4.95 | 4.92 ± 7.37 | 5.07 ± 5.09 ↑ |
| RBC (106/µL) | 8.64–9.90 | 9.01 ± 0.27 | 7.56 ± 0.77 * ↓ |
| HGB (g/dL) | 16.76–18.80 | 17.15 ± 0.07 | 15.65 ± 1.62 ↓ |
| HCT (%) | 52.65–60.25 | 54.55 ± 0.49 | 47.45 ± 5.02 * ↓ |
| MCV (fl) | 59.18–62.68 | 63.55 ± 3.18 | 62.75 ± 0.21 |
| MCH (pg) | 18.69–20.71 | 20.20 ± 0.84 | 20.70 ± 0.00 |
| MCHC (g/dL) | 31.12–31.88 | 31.75 ± 0.02 | 22.95 ± 0.07 * ↓ |
| PLT (103/µL) | 667.62–941.38 | 1032.50 ± 132.22 ↑ | 941.50 ± 94.04 |
| RDW (%) | 15.85–19.73 | 15.00 ± 0.56 | 12.45 ± 0.21 * ↓ |
| Neutrophils (%) | 4.75–18.71 | 13.70 ± 10.32 | 21.50 ± 7.77 ↑ |
| Lymphocytes (%) | 72.85–86.91 | 80.20 ± 4.52 | 74.00 ± 11.31 |
| Eosinophils (%) | 0.84–1.50 | 0.05 ± 0.07 | 0.50 ± 0.70 |
| Basophils (%) | 0.00–0.73 | 0.35 ± 0.49 | 0.00 ± 0.00 |
| Monocytes (%) | 5.38–8.36 | 5.70 ± 5.23 | 4.00 ± 4.24 |
| Blood urea nitrogen (mg/dL) | 18.22–23.24 | 21.10 ± 0.98 | 28.10 ± 16.70 ↑ |
| Creatinine (mg/dL) | 0.63–0.71 | 0.16 ± 0.01 | 0.63 ± 0.00 * |
| SGPT (U/L) | 43.03–63.33 | 36.84 ± 6.84 | 593.60 ± 124.87 ** ↑ |
| SGOT (U/L) | 81.77–105.69 | 30.55 ± 2.05 | 55.3 ± 10.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabiablok, A.; Hanboonkunupakarn, B.; Tuentam, K.; Fongsodsri, K.; Kanjanapruthipong, T.; Ampawong, S. High-Dose Primaquine Induces Proximal Tubular Degeneration and Ventricular Cardiomyopathy Linked to Host Cells Mitochondrial Dysregulation. Toxics 2023, 11, 146. https://doi.org/10.3390/toxics11020146
Rabiablok A, Hanboonkunupakarn B, Tuentam K, Fongsodsri K, Kanjanapruthipong T, Ampawong S. High-Dose Primaquine Induces Proximal Tubular Degeneration and Ventricular Cardiomyopathy Linked to Host Cells Mitochondrial Dysregulation. Toxics. 2023; 11(2):146. https://doi.org/10.3390/toxics11020146
Chicago/Turabian StyleRabiablok, Atthasit, Borimas Hanboonkunupakarn, Khwanchanok Tuentam, Kamonpan Fongsodsri, Tapanee Kanjanapruthipong, and Sumate Ampawong. 2023. "High-Dose Primaquine Induces Proximal Tubular Degeneration and Ventricular Cardiomyopathy Linked to Host Cells Mitochondrial Dysregulation" Toxics 11, no. 2: 146. https://doi.org/10.3390/toxics11020146
APA StyleRabiablok, A., Hanboonkunupakarn, B., Tuentam, K., Fongsodsri, K., Kanjanapruthipong, T., & Ampawong, S. (2023). High-Dose Primaquine Induces Proximal Tubular Degeneration and Ventricular Cardiomyopathy Linked to Host Cells Mitochondrial Dysregulation. Toxics, 11(2), 146. https://doi.org/10.3390/toxics11020146







