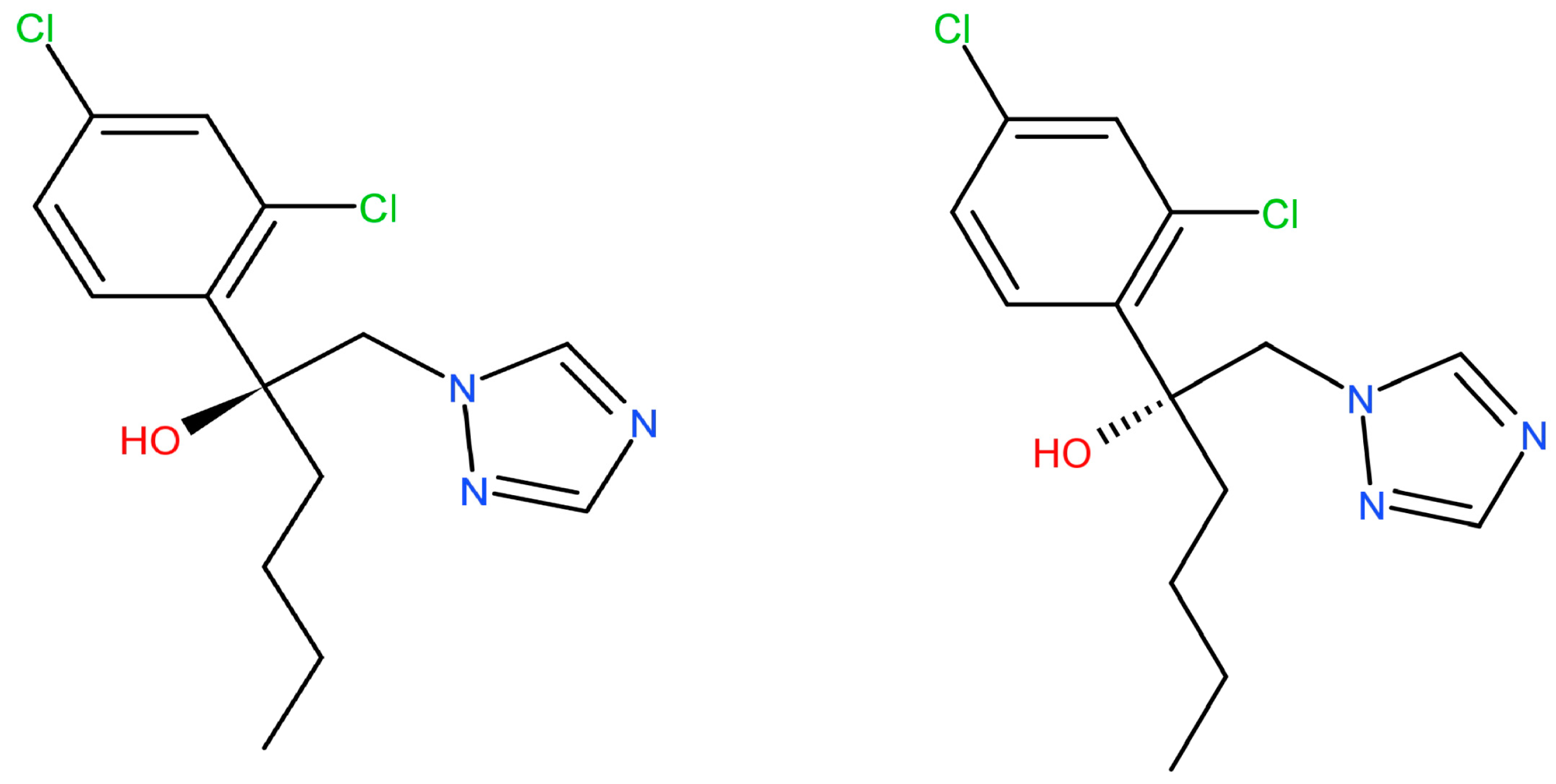
Stereoselective Toxicokinetic and Distribution Study on the Hexaconazole Enantiomers in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animal Experimental Design
2.3. Sample Preparation
2.4. UPLC–MS/MS Analysis
2.5. Method Validation
2.6. Enantioselective Toxicokinetic Study
2.7. Molecular Docking
2.8. Data Analysis
3. Results and Discussion
3.1. Method Validation
3.1.1. Selectivity
3.1.2. Recovery and Matrix Effect
3.1.3. Linearity
3.1.4. Accuracy and Precision
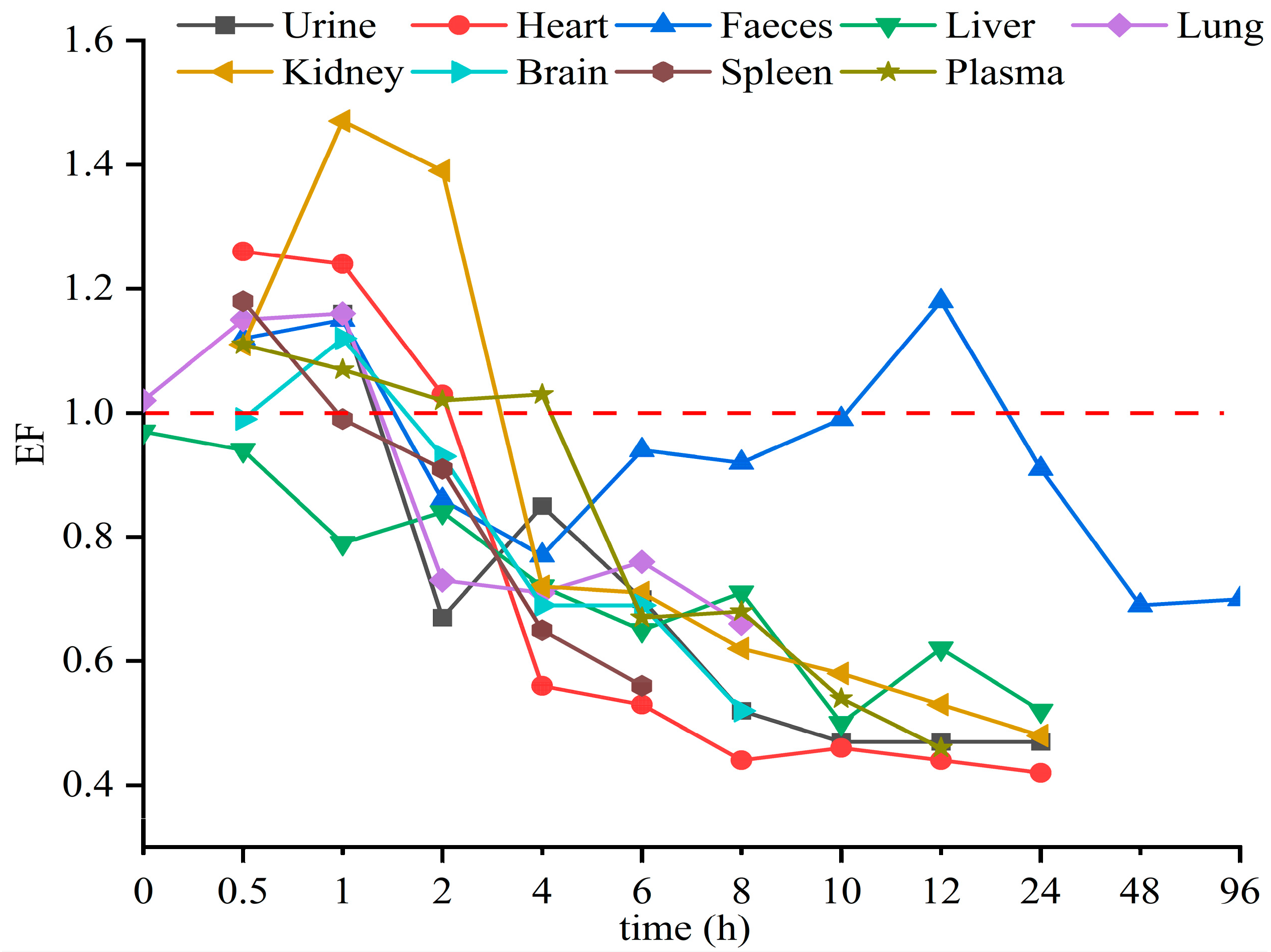
3.2. Enantioselective Distributions in Plasma
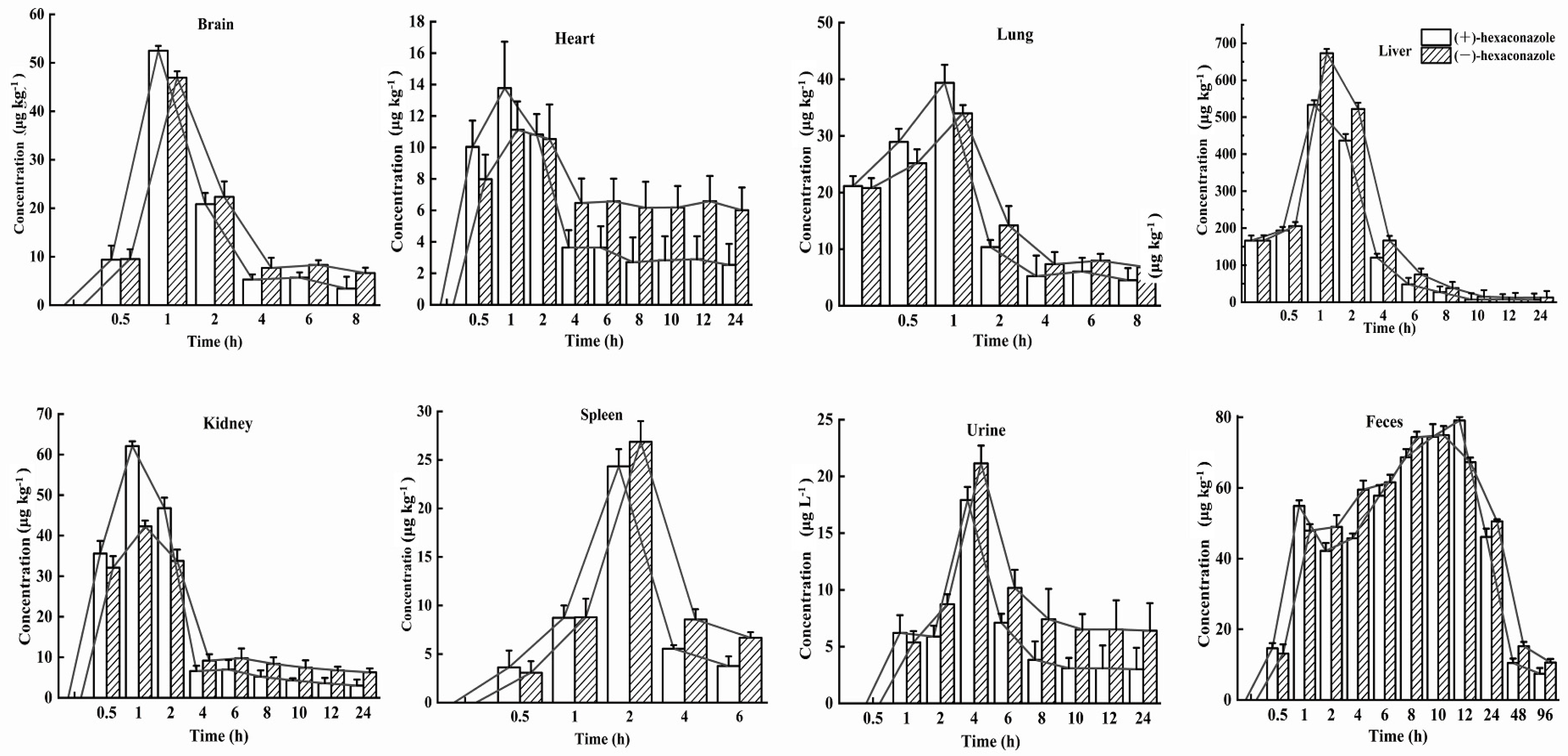
3.3. Enantioselective Distributions in Six Tissues, Urine, and Feces
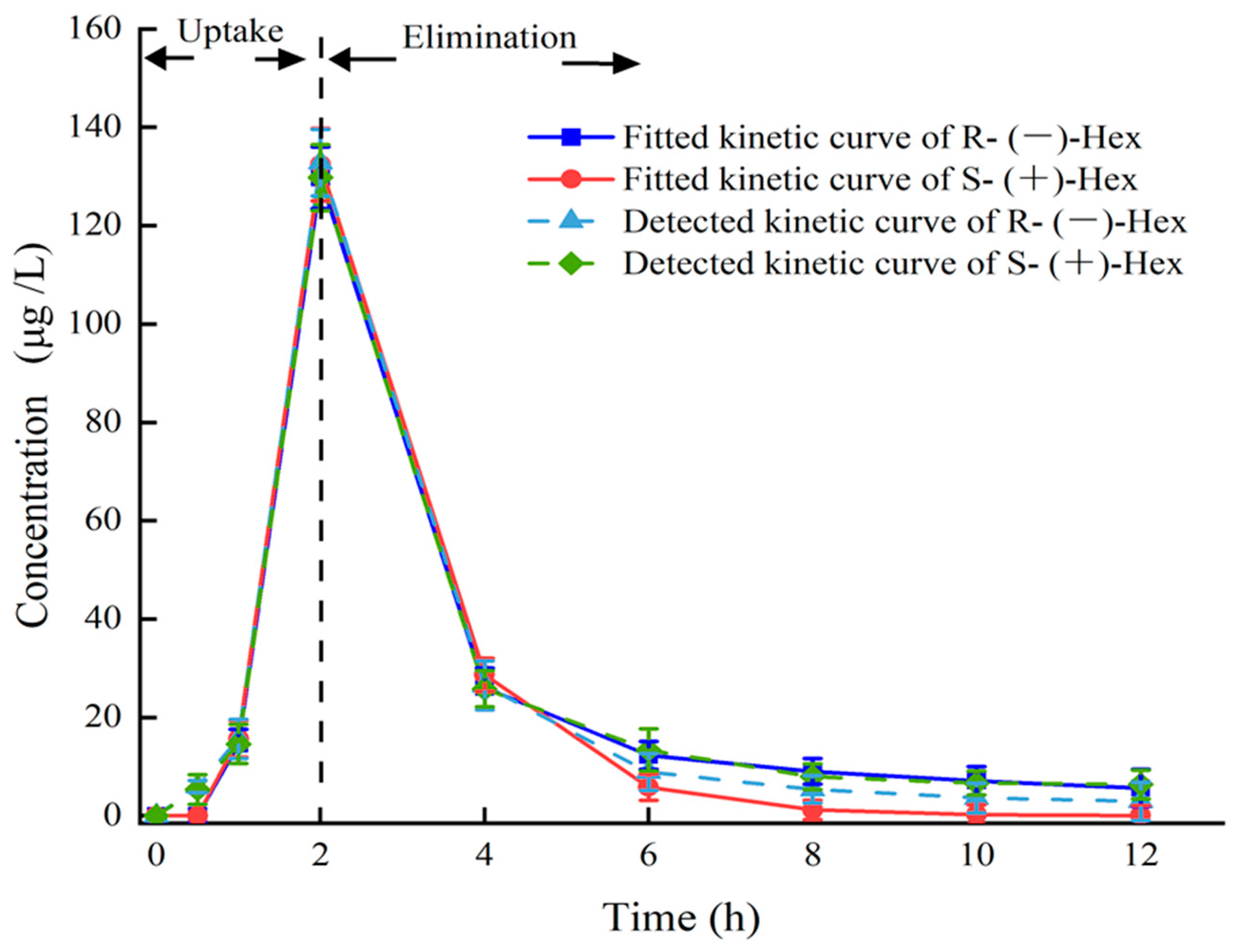
3.4. Enantioselectivity in Toxicokinetic Study
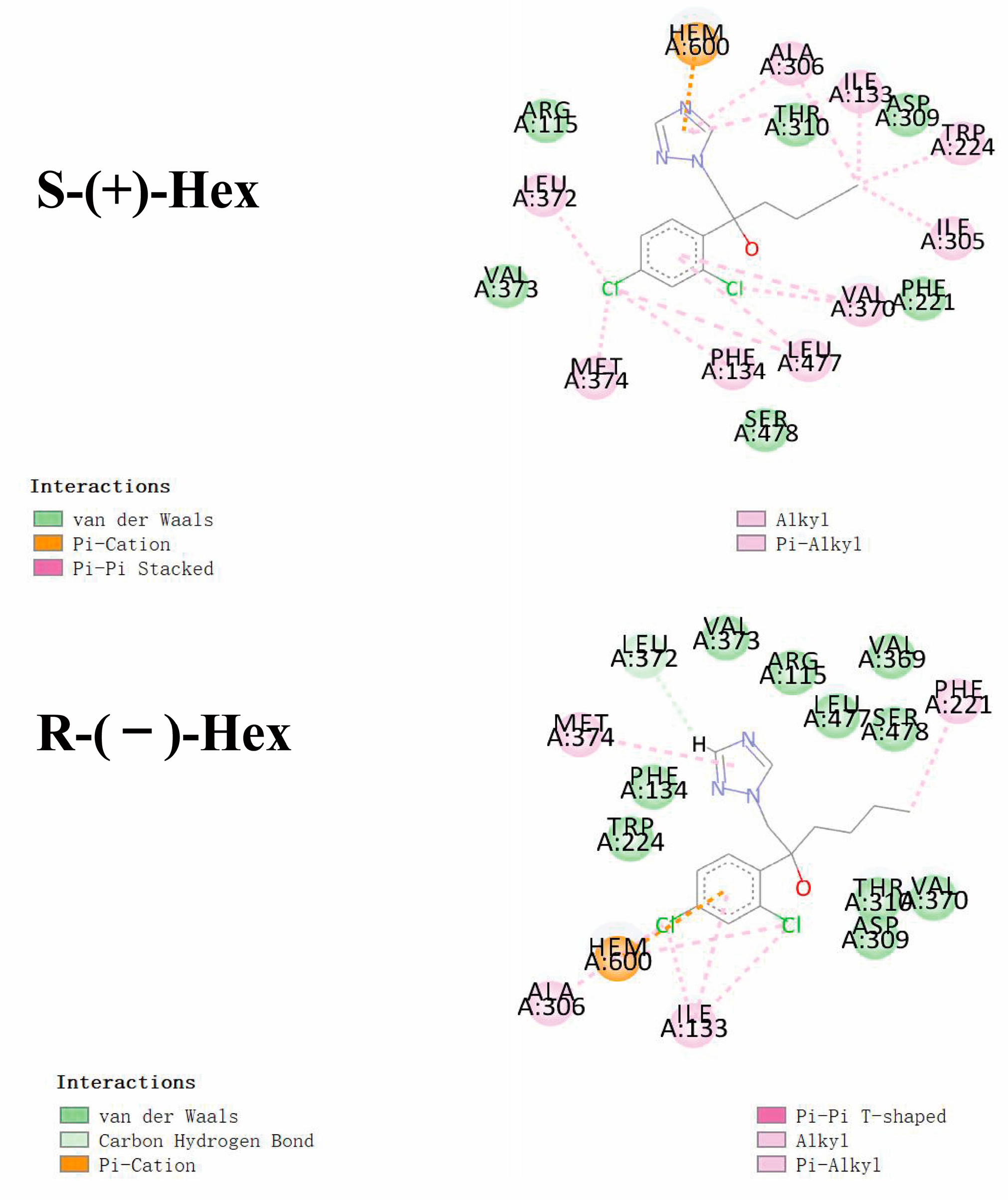
3.5. Molecular Docking of Hex to P450arom
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, L.D.; Lu, D.H.; Zhang, P.; Diao, J.L.; Zhou, Z.Q. Enantioselective toxic effects of hexaconazole enantiomers against Scenedesmus obliquus. Chirality 2012, 24, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, S.; Siddamallaiah, L.; Matadha, N.Y.; Gadigeppa, S.; Raja, D.P.; Udupi, V.R. Persistence and dissipation study of azoxystrobin, buprofezin, dinocap and hexaconazole on mango (Mangifera indica L.). Environ. Sci. Pollut. Res. Int. 2020, 27, 32820–32828. [Google Scholar] [CrossRef] [PubMed]
- Heshmati, A.; Nili-Ahmadabadi, A.; Rahimi, A.; Vahidinia, A.; Taheri, M. Dissipation behavior and risk assessment of fungicide and insecticide residues in grape under open-field, storage and washing conditions. J. Clean. Prod. 2020, 270, 12287. [Google Scholar] [CrossRef]
- Devi, R.; Singh, R.P.; Sachan, A.K. Dissipation kinetics of hexaconazole and lambda-cyhalothrin residue in soil and potato plant. Potato Res. 2019, 62, 411–422. [Google Scholar] [CrossRef]
- Zhang, K.K.; Tang, M.M.; Zhang, J.; Li, Y.J.; Han, X.W.; Pan, S.Z.; Kong, X.X.; Li, M.C.; Chen, H.Y.; Zhang, W.; et al. Fate of hexaconazole and isoprothiolane in rice, soil and water under field conditions. Int. J. Environ. Anal. Chem. 2016, 96, 38–39. [Google Scholar] [CrossRef]
- Heshmati, A.; Mehri, F.; Mousavi Khaneghah, A. Simultaneous multi-determination of pesticide residues in black tea leaves and infusion: A risk assessment study. Environ. Sci. Pollut. Res. Int. 2021, 28, 13725–13735. [Google Scholar] [CrossRef]
- Tsukatani, H.; Tobiishi, K.; Tanaka, Y.; Sakuragi, K.; Ikeura, T.; Nakamura, M. Determination of hexaconazole in surface water samples from river and the sea by liquid chromatography-electrospray tandem mass spectrometry. Biosci. Biotech. Bioch. 2008, 72, 149–154. [Google Scholar] [CrossRef]
- Liu, T.; Fang, K.; Liu, Y.L.; Zhang, X.L.; Han, L.X.; Wang, X.G. Enantioselective residues and toxicity effects of the chiral triazole fungicide hexaconazole in earthworms (Eisenia fetida). Environ. Pollut. 2021, 270, 116269. [Google Scholar] [CrossRef]
- Jia, M.; Wang, Y.; Wang, D.Z.; Teng, M.M.; Yan, J.; Meng, Z.Y.; Li, R.S.; Yan, J.; Zhou, Z.Q.; Zhu, W.T. The effects of hexaconazole and epoxiconazole enantiomers on metabolic profile following exposure to zebrafish (Danio rerio) as well as the histopathological changes. Chemosphere 2019, 226, 520–533. [Google Scholar] [CrossRef]
- Shapovalova, E.N.; Shpigun, O.A.; Nesterova, L.M.; Belov, M.Y. Determination of the optical purity of fungicides of the triazole series. J. Anal. Chem. 2004, 59, 255–259. [Google Scholar] [CrossRef]
- Li, Y.; Dong, F.S.; Liu, X.G.; Xu, J.; Chen, X.; Han, Y.; Liang, X.; Zheng, Y.Q. Studies of enantiomeric degradation of the triazole fungicide hexaconazole in tomato, cucumber, and field soil by chiral liquid chromatography-tandem mass spectrometry. Chirality. 2013, 25, 160–169. [Google Scholar] [CrossRef]
- Han, J.J.; Jiang, J.Z.; Su, H.; Sun, M.J.; Wang, P.; Liu, D.H.; Zhou, Z. Bioactivity, toxicity and dissipation of hexaconazole enantiomers. Chemosphere 2013, 93, 2523–2527. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.G.; Liu, D.H.; Wang, P.; Zhang, P.; Wang, X.R.; Zhou, Z.Q. Gender-related in vitro metabolism of hexaconazole and its enantiomers in rats. Chirality. 2013, 25, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.G.; Zhu, W.T.; Liu, D.H.; Xu, X.Y.; Zhang, P.; Zhou, Z.Q. Stereoselective degradation of tebuconazole in rat liver microsomes. Chirality 2012, 24, 67–71. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, L.; Li, D.Z.; Teng, M.M.; Zhang, R.K.; Zhou, Z.Q.; Zhu, W.T. Enantioselective bioaccumulation of hexaconazole and its toxic effects in adult zebrafish (Danio rerio). Chemosphere 2015, 138, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Bessems, J.G.M.; Geraets, L. Proper knowledge on toxicokinetics improves human hazard testing and subsequent health risk characterisation. A case study approach. Regul. Toxicol. Pharmacol. 2013, 67, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Jang, J.H.; Cho, H.Y.; Lee, Y.B. Simultaneous determination of diethyl phthalate and its major metabolite, monoethyl phthalate, in rat plasma, urine, and various tissues collected from a toxicokinetic study by ultrahigh performance liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2019, 173, 108–119. [Google Scholar] [CrossRef]
- Jeong, S.H.; Jang, J.H.; Cho, H.Y.; Lee, Y.B. Toxicokinetics of diisobutyl phthalate and its major metabolite, monoisobutyl phthalate, in rats: UPLC-ESI-MS/MS method development for the simultaneous determination of diisobutyl phthalate and its major metabolite, monoisobutyl phthalate, in rat plasma, urine, feces, and 11 various tissues collected from a toxicokinetic study. Food Chem. Toxicol. 2020, 145, 111747. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, W.; Ju, J.X.; Wang, L.J.; Huang, R.Y.; Xu, Y.F.; Zhang, H.L.; Qi, J.L. Gender differences in acute toxicity, toxicokinetic and tissue distribution of amphotericin B liposomes in rats. Toxicol. Lett. 2021, 338, 78–84. [Google Scholar] [CrossRef]
- Kjærstad, M.B.; Taxvig, C.; Nellemann, C.; Vinggaard, A.M.; Andersen, H.R. Endocrine disrupting effects in vitro of conazole antifungals used as pesticides and pharmaceuticals. Reprod. Toxicol. 2010, 30, 573–582. [Google Scholar] [CrossRef]
- Trosken, E.R.; Fischer, K.; Volkel, W.; Lutz, W.K. Inhibition of human CYP19 by azoles used as antifungal agents and aromatase inhibitors, using a new LC-MS/MS method for the analysis of estradiol product formation. Toxicology 2006, 19, 33–40. [Google Scholar] [CrossRef]
- Crowell, S.R.; Henderson, W.M.; Fisher, J.W.; Kenneke, J.F. Gender and species differences in triadimefon metabolism by rodent hepatic microsomes. Toxicol. Lett. 2010, 193, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Huang, L.; Tang, S.H.; Li, Z.; Ye, Q.F. Enantioselective absorption and transformation of a novel chiral neonicotinoid [(14)C]-cycloxaprid in rats. Environ. Pollut. 2016, 213, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Goetz, A.K.; Dix, D.J. Mode of action for reproductive and hepatic toxicity inferred from a genomic study of triazole antifungals. Toxicol. Sci. 2009, 110, 449–462. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, W.T.; Qiu, J.; Wang, X.R.; Zhang, P.; Yan, J.; Zhou, Z.Q. Monitoring tryptophan metabolism after exposure to hexaconazole and the enantioselective metabolism of hexaconazole in rat hepatocytes in vitro. J. Hazard. Mater. 2015, 295, 9–16. [Google Scholar] [CrossRef]
- Meng, Z.Y.; Liu, L.; Xi, Y.X.; Jia, M.; Yan, S.; Tian, S.N.; Sun, W.; Zhu, W.T.; Li, X.F.; Zhou, Z.Q. Different effects of exposure to penconazole and its enantiomers on hepatic glycolipid metabolism of male mice. Environ. Pollut. 2020, 257, 113555. [Google Scholar] [CrossRef]
- Othmène, Y.B.; Hamdi, H.; Amara, I.; Abid-Essefi, S. Tebuconazole induced oxidative stress and histopathological alterations in adult rat heart. Pestic. Biochem. Physiol. 2020, 170, 104671. [Google Scholar] [CrossRef]
- Shearer, J.J.; Sandler, D.P.; Andreotti, G.; Murata, K.; Shrestha, S.; Parks, C.G.; Liu, D.; Alavanja, M.C.; Landgren, O.; Beane Freeman, L.E.; et al. Pesticide use and kidney function among farmers in the biomarkers of exposure and effect in agriculture study. Environ. Res. 2021, 199, 111276. [Google Scholar] [CrossRef] [PubMed]
- Noshy, P.A.; Elhady, M.A.; Khalaf, A.A.A.; Kamel, M.M.; Hassanen, E.I. Ameliorative effect of carvacrol against propiconazole-induced neurobehavioral toxicity in rats. Neurotoxicology 2018, 67, 141–149. [Google Scholar] [CrossRef]
- Sanchez, C.L.; Souders, C.L.; Pena-Delgado, C.J.; Nguyen, K.T.; Kroyter, N.; Ahmadie, N.E.; Aristizabal-Henao, J.J.; Bowden, J.A.; Martyniuk, C.J. Neurotoxicity assessment of triazole fungicides on mitochondrial oxidative respiration and lipids in differentiated human SH-SY5Y neuroblastoma cells. Neurotoxicology 2020, 80, 76–86. [Google Scholar] [CrossRef]
- Othmène, Y.B.; Hamdi, H.; Salem, I.B.; Annabi, E.; Amara, I.; Neffati, F.; Najjar, M.F.; Abid-Essefi, S. Oxidative stress, DNA damage and apoptosis induced by tebuconazole in the kidney of male Wistar rat. Chem. Biol. Interact. 2020, 330, 109114. [Google Scholar] [CrossRef] [PubMed]
- Bielska, L.; Hale, S.E.; Skulcova, L. A review on the stereospecific fate and effects of chiral conazole fungicides. Sci. Total. Environ. 2021, 750, 141600. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.T.; Qiu, J.; Dang, Z.H.; Lv, C.G.; Jia, G.F.; Li, L.; Zhou, Z.Q. Stereoselective degradation kinetics of tebuconazole in rabbits. Chirality 2007, 19, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Groppelli, S.; Pennati, R.; De Bernardi, F.; Menegola, E.; Giavini, E.; Sotgia, C. Teratogenic effects of two antifungal triazoles, triadimefon and triadimenol, on Xenopus laevis development: Craniofacial defects. Aquat. Toxicol. 2005, 73, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.X.; Gao, B.B.; He, Z.Z.; Li, L.S.; Shi, H.Y.; Wang, M.H. Enantioselective metabolism of four chiral triazole fungicides in rat liver microsomes. Chemosphere 2019, 224, 77–84. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Zhang, J.; Zhao, X.J.; Gao, B.B.; He, Z.Z.; Li, L.S.; Shi, H.Y.; Wang, M.H. Stereoselective uptake and metabolism of prothioconazole caused oxidative stress in zebrafish (Danio rerio). J. Hazard. Mater. 2020, 396, 122756. [Google Scholar] [CrossRef]
| Samples | Enantiomers | Calibration Curves | R2 | LOD (μg L−1) | LOQ (μg L−1) | Recoveryrate (%) | RSDs (%) |
|---|---|---|---|---|---|---|---|
| Plasma | S-(+)-Hex | y = 5951x − 64555 | 0.9956 | 0.20 | 0.70 | 90.3 | 3.45 |
| R-(−)-Hex | y = 5889x − 58921 | 0.9945 | 0.20 | 0.70 | 89.2 | 4.56 | |
| Urine | S-(+)-Hex | y = 6323x − 45432 | 0.9957 | 0.10 | 0.60 | 91.4 | 7.58 |
| R-(−)-Hex | y = 6454x − 34324 | 0.9945 | 0.10 | 0.60 | 90.3 | 4.54 | |
| Feces | S-(+)-Hex | y = 6345x − 54354 | 0.9912 | 0.10 | 0.60 | 101.3 | 6.41 |
| R-(−)-Hex | y = 6534x − 87645 | 0.9945 | 0.10 | 0.60 | 104.2 | 8.46 | |
| Heart | S-(+)-Hex | y = 5645x − 79521 | 0.9972 | 0.10 | 0.50 | 94.6 | 3.56 |
| R-(−)-Hex | y = 5956x − 74413 | 0.9986 | 0.10 | 0.50 | 88.7 | 5.79 | |
| Liver | S-(+)-Hex | y = 6721x − 56843 | 0.9945 | 0.10 | 0.50 | 96.3 | 6.75 |
| R-(−)-Hex | y = 6942x − 52485 | 0.9978 | 0.10 | 0.50 | 98.5 | 4.56 | |
| Lung | S-(+)-Hex | y = 7842x − 45214 | 0.9996 | 0.10 | 0.50 | 89.2 | 7.87 |
| R-(−)-Hex | y = 7254x − 49511 | 0.9986 | 0.10 | 0.50 | 88.8 | 9.46 | |
| Kidney | S-(+)-Hex | y = 5952x − 22457 | 0.9982 | 0.10 | 0.60 | 101.4 | 3.43 |
| R-(−)-Hex | y = 5142x − 55621 | 0.9945 | 0.10 | 0.60 | 103.5 | 2.51 | |
| Brain | S-(+)-Hex | y = 4158x − 75121 | 0.9998 | 0.10 | 0.50 | 91.3 | 5.49 |
| R-(−)-Hex | y = 4682x − 84520 | 0.9976 | 0.10 | 0.50 | 94.6 | 7.59 | |
| Spleen | S-(+)-Hex | y = 8614x − 42152 | 0.9946 | 0.10 | 0.50 | 98.3 | 9.45 |
| R-(−)-Hex | y = 8145x − 46212 | 0.9978 | 0.10 | 0.50 | 99.5 | 8.68 |
| Pharmacokinetic Parameters | Parameter Values | Unit | |
|---|---|---|---|
| S-(+)-Hex | R-(−)-Hex | ||
| AUC0–12 | 304.41 ± 20.12 | 321.18 ± 19.11 | µg/L × h |
| AUC0-∞ | 314.38 ± 12.11 | 339.99 ± 11.12 | µg/L × h |
| t1/2 | 3.07 ± 1.13 | 3.17 ± 0.78 | h |
| Tmax | 2.00 ± 0.28 | 2.00 ± 0.55 | h |
| Vd | 2.82 ± 1.12 | 2.69 ± 0.91 | L/kg |
| CL | 0.64 ± 0.09 | 0.59 ± 0.05 | L/h/kg |
| Cmax | 132.78 ± 13.14 | 129.73 ± 10.12 | µg/L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, G.; Pang, J.; Sun, D.; Zhang, Q. Stereoselective Toxicokinetic and Distribution Study on the Hexaconazole Enantiomers in Mice. Toxics 2023, 11, 145. https://doi.org/10.3390/toxics11020145
Luo G, Pang J, Sun D, Zhang Q. Stereoselective Toxicokinetic and Distribution Study on the Hexaconazole Enantiomers in Mice. Toxics. 2023; 11(2):145. https://doi.org/10.3390/toxics11020145
Chicago/Turabian StyleLuo, Guofei, Junxiao Pang, Dali Sun, and Qinghai Zhang. 2023. "Stereoselective Toxicokinetic and Distribution Study on the Hexaconazole Enantiomers in Mice" Toxics 11, no. 2: 145. https://doi.org/10.3390/toxics11020145
APA StyleLuo, G., Pang, J., Sun, D., & Zhang, Q. (2023). Stereoselective Toxicokinetic and Distribution Study on the Hexaconazole Enantiomers in Mice. Toxics, 11(2), 145. https://doi.org/10.3390/toxics11020145





