Role of Root Exudates in Cadmium Accumulation of a Low-Cadmium-Accumulating Tobacco Line (Nicotiana tabacum L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Experimental Design
2.3. Cd Treatment and Root Exudate Collection
2.4. Root Exudate Analysis
2.5. Biomass and Cd Concentrations of Tobacco Lines
2.6. Statistical Analysis
3. Results
3.1. Plant Biomass
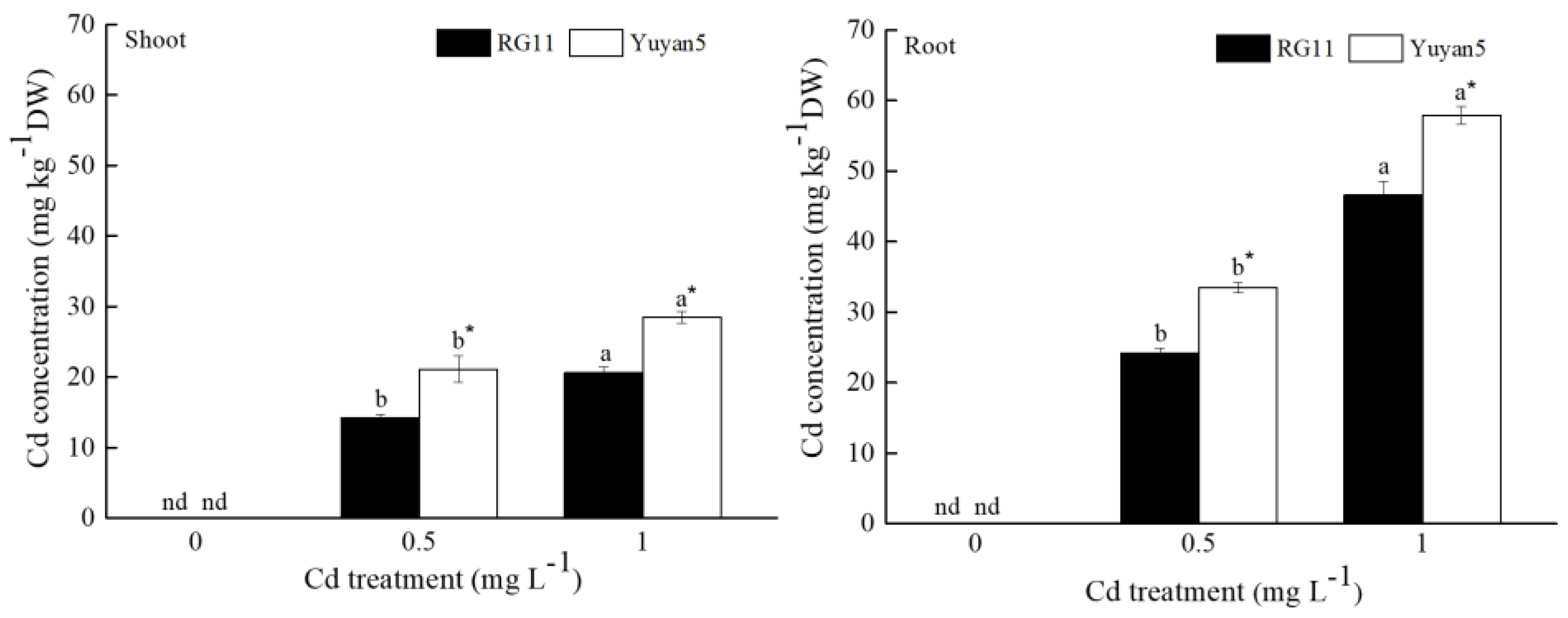
3.2. Cd Concentration
3.3. Organic Acid in Root Exudates
3.4. Amino Acid in Root Exudates
4. Discussion
4.1. Response of Plants to Cd
4.2. The Effect of Amino Acids on Cd Accumulation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Q.; Li, Z.; Lu, X.; Duan, Q.; Huang, L.; Bi, J. A review of soil heavy metal pollution from industrial and agricultural regions in China: Pollution and risk assessment. Sci. Total Environ. 2018, 642, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Cheng, H.; Tao, S. The challenges and solutions for cadmium-contaminated rice in China: A critical review. Environ. Int. 2016, 92, 515–532. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Saleem, M.H.; Alsafran, M.; Jabri, H.A.; Mehwish, H.J.; Rizwan, M.; Nawaz, M.; Ali, S.; Usman, K. Response of cauliflower (Brassica oleracea L.) to nitric oxide application under cadmium stress. Ecotoxicol. Environ. Saf. 2022, 243, 113969. [Google Scholar] [CrossRef] [PubMed]
- Altaf, M.A.; Shahid, R.; Ren, M.X.; Altaf, M.M.; Naz, S.; Khan, L.U.; Lal, L.K.; Tiwari, R.K.; Shakoor, A. Melatonin mitigates cadmium toxicity by promoting root architecture and mineral homeostasis of tomato genotypes. J. Soil Sci. Plant Nutr. 2022, 22, 1112–1128. [Google Scholar] [CrossRef]
- Alves, L.R.; Monteiro, C.C.; Carvalho, R.F.; Riberio, P.C.; Tezotto, T.; Azevedo, A.; Gratão, P.L. Cadmium stress related to root-to-shoot communication depends on ethylene and auxin in tomato plants. Environ. Exp. Bot. 2017, 134, 102–115. [Google Scholar] [CrossRef]
- Feng, J.; Jia, W.; Lv, S.; Bao, H.; Miao, F.; Zhang, X.; Wang, J.; Li, H.; Li, D.; Zhu, C.; et al. Comparative transcriptome combined with morpho-physiological analyses revealed key factors for differential cadmium accumulation in two contrasting sweet sorghum genotypes. Plant Biotechnol. J. 2017, 16, 558–571. [Google Scholar] [CrossRef]
- Xin, J.; Dai, H.; Huang, B. Assessing the roles of roots and shoots in the accumulation of cadmium in two sweet potato cultivars using split-root and reciprocal grafting systems. Plant Soil 2017, 412, 413–424. [Google Scholar] [CrossRef]
- Zhou, H.; Zhu, W.; Yang, W.T.; Gu, J.F.; Gao, J.X.; Chen, L.W.; Du, W.Q.; Zhang, P.; Peng, P.Q.; Liao, B.H. Cadmium uptake, accumulation, and remobilization in iron plaque and rice tissues at different growth stages. Ecotoxicol. Environ. Saf. 2018, 152, 91–97. [Google Scholar] [CrossRef]
- Chen, J.; Shafi, M.; Wang, Y.; Wu, J.; Ye, Z.; Liu, C.; Zhong, B.; Guo, H.; He, L.; Liu, D. Organic acid compounds in root exudation of Moso Bamboo (Phyllostachys pubescens) and its bioactivity as affected by heavy metals. Environ. Sci. Pollut. Res. 2016, 23, 20977–20984. [Google Scholar] [CrossRef]
- Luo, Q.; Sun, L.; Hu, X.; Zhou, R. The variation of root exudates from the hyperaccumulator Sedum alfredii under cadmium stress: Metabonomics analysis. PLoS ONE 2014, 9, e115581. [Google Scholar] [CrossRef]
- Vítková, M.; Komárek, M.; Tejnecký, V.; Šillerová, H. Interactions of nano-oxides with low-molecular-weight organic acids in a contaminated soil. J. Hazard. Mater. 2015, 293, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Hawrylak-Nowak, B.; Dresler, S.; Matraszek, R. Exogenous malic and acetic acids reduce cadmium phytotoxicity and enhance cadmium accumulation in roots of sunflower plants. Plant Physiol. Biochem. 2015, 94, 225–234. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, Y.; Ge, Q.; Li, Y.; Sun, J.; Zhang, Y.; Liu, X. Comparative physiological responses of Solanum nigrum and Solanum torvum to cadmium stress. New Phytol. 2012, 196, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Xin, J.; Huang, B.; Dai, H.; Mu, Y. Characterization of root morphology and root-derived low molecular weight organic acids in two sweet potato cultivars exposed to cadmium. Arch. Agron. Soil Sci. 2017, 63, 723–734. [Google Scholar] [CrossRef]
- Liu, H.; Wang, H.; Ma, Y.; Wang, H.; Shi, Y. Role of transpiration and metabolism in translocation and accumulation of cadmium in tobacco plants (Nicotiana tabacum L.). Chemosphere 2016, 144, 1960–1965. [Google Scholar] [CrossRef]
- Yu, Y.; Wan, Y.; Wang, Q.; Li, H. Effect of humic acid-based amendments with foliar application of Zn and Se on Cd accumulation in tobacco. Ecotoxicol. Environ. Saf. 2017, 138, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Huang, Y.; Li, T.; Zhang, X.; Yu, H.; Wang, Y. The characteristics of Cd accumulation in low-Cd accumulating tobacco cultivars exposed to Cd. Chin. Tob. Sci. 2017, 38, 69–76. (In Chinese) [Google Scholar]
- Zhao, M.; Li, T.; Huang, H.; Fu, H. Kinetic characteristics of cadmium uptake in roots of low cadmium accumulating tobacco lines. Chin. Tob. Sci. 2018, 39, 40–46. (In Chinese) [Google Scholar]
- Liu, D.; Li, T.; Yu, H.; Zhang, L.; Wang, Y. Evaluation of differential cadmium accumulation ability in different tobacco species. J. Agro-Environ. Sci. 2016, 35, 2067–2076. (In Chinese) [Google Scholar]
- Tao, Q.; Hou, D.; Yang, X.; Li, T. Oxalate secretion from the root apex of Sedum alfredii contributes to hyperaccumulation of Cd. Plant Soil 2016, 398, 139–152. [Google Scholar] [CrossRef]
- Zhan, F.; Qin, L.; Guo, X.; Tan, J.; Liu, L.; Zu, Y.; Li, Y. Cadmium and lead accumulation and low-molecular-weight organic acids secreted by roots in an intercropping of a cadmium accumulator Sonchus asper L. with Vicia faba L. RSC Adv. 2016, 6, 33240–33248. [Google Scholar] [CrossRef]
- Chen, Y.T.; Wang, Y.; Yeh, K.C. Role of root exudates in metal acquisition and tolerance. Curr. Opin. Plant Biol. 2017, 39, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Montiel-Rozas, M.; Madejón, E.; Madejón, P. Effect of heavy metals and organic matter on root exudates (low molecular weight organic acids) of herbaceous species: An assessment in sand and soil conditions under different levels of contamination. Environ. Pollut. 2016, 216, 273–281. [Google Scholar] [CrossRef]
- Dresler, S.; Hanaka, A.; Bednarek, W.; Maksymiec, W. Accumulation of low-molecular-weight organic acids in roots and leaf segments of Zea mays plants treated with cadmium and copper. Acta Physiol. Plant. 2014, 36, 1565–1575. [Google Scholar] [CrossRef]
- Guo, H.; Feng, X.; Hong, C.; Chen, H.; Zeng, F.; Zheng, B.; Jiang, D. Malate secretion from the root system is an important reason for higher resistance of Miscanthus sacchariflorus to cadmium. Physiol. Plant. 2017, 159, 340–353. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Cao, X.; Tan, C.; Deng, Y.; Cai, R.; Peng, X.; Bai, J. Analysis of the effect of cadmium stress on root exudates of Sedum plumbizincicola based on metabolomics. Ecotoxicol. Environ. Saf. 2020, 205, 111152. [Google Scholar] [CrossRef]
- Javed, M.T.; Akram, M.S.; Tanwir, K.; Chaudhary, H.J.; Ali, Q.; Stoltz, E.; Lindberg, S. Cadmium spiked soil modulates root organic acids exudation and ionic contents of two differentially Cd tolerant maize (Zea mays L.) cultivars. Ecotoxicol. Environ. Saf. 2017, 141, 216–225. [Google Scholar] [CrossRef]
- Li, T.; Tao, Q.; Liang, C.; Shohag, M.J.I.; Yang, X.; Sparks, D.L. Complexation with dissolved organic matter and mobility control of heavy metals in the rhizosphere of hyperaccumulator Sedum alfredii. Environ. Pollut. 2013, 182, 248–255. [Google Scholar] [CrossRef]
- Xin, J.; Huang, B.; Dai, H.; Zhou, W.; Yi, Y.; Peng, L. Roles of rhizosphere and root-derived organic acids in Cd accumulation by two hot pepper cultivars. Environ. Sci. Pollut. Res. 2015, 22, 6254–6261. [Google Scholar] [CrossRef]
- Fan, H.; Wang, X.; Zhou, W. Low molecular weight organic acids in rhizosphere and their effects on cadmium accumulation in two cultivars of amaranth (Amaranthus mangostanus L.). Sci. Agric. Sin. 2007, 40, 2727–2733. (In Chinese) [Google Scholar]
- Grąz, M.; Jarosz-Wilkołazka, A.; Pawlikowska-Pawlęga, B. Abortiporus biennis tolerance to insoluble metal oxides: Oxalate secretion, oxalate oxidase activity, and mycelial morphology. Biometals 2009, 22, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zheng, C.; Hu, Y.T.; Jiang, T.; Liu, Y.; Dong, N.; Yang, J.; Zheng, S. Cadmium-induced oxalate secretion from root apex is associated with cadmium exclusion and resistance in Lycopersicon esulentum. Plant Cell Environ. 2011, 34, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Weiss, D.J.; Weng, B.; Liu, J.; Liu, H.; Yan, C. The short-term effect of cadmium on low molecular weight organic acid and amino acid exudation from mangrove (Kandelia obovata (S., L.) Yong) roots. Environ. Sci. Pollut. Res. 2013, 20, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, É.C.; Marthiellen, R.L.; Kreusch, M.G.; Pereira, D.T.; Costa, J.B.; Simioni, C.; Ouriques, L.C.; Steiner, N.; Chow, F.; Floh, E.S.H. Profiles of carotenoids and amino acids and total phenolic compounds of the red alga Pterocladiella capillacea exposed to cadmium and different salinities. J. Appl. Phycol. 2016, 28, 1955–1963. [Google Scholar] [CrossRef]
- Zhu, G.; Xiao, H.; Guo, Q.; Zhang, Z.; Zhao, J.; Yang, D. Effects of cadmium stress on growth and amino acid metabolism in two Compositae plants. Ecotoxicol. Environ. Saf. 2018, 158, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Qiang, W.Y.; Chen, T.; Tang, H.G.; Feng, H.Y.; An, L.Z.; Wang, X.L. Effect of cadmium and enhanced UV-B radiation on soybean root excretion. Chin. J. Plant Ecol. 2003, 27, 293. [Google Scholar]
- Jien, S.; Lin, Y. Proteins in xylem exudates from rapeseed plants (Brassica napus L.) play a crucial role in cadmium phytoremediation. CLEAN-Soil Air Water 2018, 46, 1700164. [Google Scholar] [CrossRef]
- Shi, J.; Wu, B.; Yuan, X.; Chen, X.; Chen, Y.; Hu, T. An X-ray absorption spectroscopy investigation of speciation and biotransformation of copper in Elsholtzia splendens. Plant Soil 2008, 302, 163–174. [Google Scholar] [CrossRef]
- Huang, G.; Guo, G.; Yao, S.; Zhang, N.; Hu, H. Organic acids, amino acids compositions in the root exudates and Cu-accumulation in castor (Ricinus communis L.) under Cu stress. Int. J. Phytoremediat. 2016, 18, 33–40. [Google Scholar] [CrossRef]
- Khodamoradi, K.; Khoshgoftarmanesh, A.H.; Maibody, S.A.M.M. Root uptake and xylem transport of cadmium in wheat and triticale as affected by exogenous amino acids. Crop Pasture Sci. 2017, 68, 415–420. [Google Scholar] [CrossRef]
- Maeda, H.; Yoo, H.; Dudareva, N. Prephenate aminotransferase directs plant phenylalanine biosynthesis via arogenate. Nat. Chem. Biol. 2011, 7, 19–21. [Google Scholar] [CrossRef] [PubMed]
- She, W.; Zhu, S.; Jie, Y.; Xing, H.; Cui, G. Expression profiling of cadmium response genes in ramie (Boehmeria nivea L.) root. Bull. Environ. Contam. Toxicol. 2015, 94, 453–459. [Google Scholar] [CrossRef] [PubMed]

| Cd Treatment (mg L−1) | Line | Organic Acids Concentration (mg 24 h−1 g−1 FW) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxalic Acid | Tartaric Acid | Formic Acid | Malic Acid | Lactic Acid | Acetic Acid | Maleic Acid | Propionic Acid | Succinic Acid | Total | ||
| 0 | RG11 | 8.82 b * | nd | 0.15 b | nd | 0.97 c | 0.67 b | 0.04 b | 0.25 b | 2.30 c | 13.20 c |
| 0.5 | 10.84 b | 5.07 b | 0.72 b | 1.59 b | 9.01 b | 0.83 b | 0.15 a | 0.43 b | 8.42 b | 37.06 b | |
| 1 | 20.69 a * | 9.75 a | 2.03 a | 7.29 a | 16.88 a | 2.71 a * | 0.39 a | 1.35 a | 14.05 a | 75.14 a | |
| 0 | Yuyan5 | 4.79 b | nd | 0.66 b * | nd | nd | 0.43 b | 0.16 b * | nd | 18.62 c * | 24.66 c * |
| 0.5 | 9.68 a | 7.48 b * | 4.60 a * | 7.70 b * | 16.66 b * | 0.98 ab | 0.26 b | nd | 26.28 b * | 73.64 b * | |
| 1 | 12.69 a | 14.81 a * | 5.73 a * | 13.17 a * | 22.41 a * | 1.38 a | 0.57 a | nd | 55.79 a * | 126.55 a * | |
| Line | Model | Parameter | Equation | r2 | F | n |
|---|---|---|---|---|---|---|
| RG11 | 1 | Propionic acid | y = −19.17 + 13.38x1 | 0.906 | 0.000 | 9 |
| 2 | Propionic acid | y = 17.19 + 20.40x1 − 7.01x2 | 0.954 | 0.029 | 9 | |
| Oxalic acid | ||||||
| 3 | Propionic acid | y = 45.15 + 20.16x1 − 11.86x2 + 57.95x3 | 0.985 | 0.014 | 9 | |
| Oxalic acid | ||||||
| Succinic acid | ||||||
| Yuyan5 | 1 | Lactic acid | y = −0.83 + 7.36x1 | 0.975 | 0.000 | 9 |
| Line | Independent Variable | r | Path Coefficient | Contribution | Indirect Path Coefficient | |||
|---|---|---|---|---|---|---|---|---|
| x1 | x2 | x3 | Total | |||||
| RG11 | x1 (Propionic acid) | 0.958 | 1.443 | 1.383 | - | −0.849 | 0.364 | −0.485 |
| x2 (Oxalic acid) | 0.792 | −0.926 | −0.734 | 1.323 | - | 0.395 | 1.718 | |
| x3 (Succinic acid) | 0.803 | 0.426 | 0.342 | 1.236 | −0.859 | - | 0.377 | |
| Yuyan5 | x1 (Lactic acid) | 0.989 | 0.989 | 0.978 | - | - | - | - |
| Cd Treatment (mg L−1) | Line | Amino Acids Concentration (mg 24 h−1 g−1 FW) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Asp | Thr | Ser | Glu | Gly | Ala | Cys | Val | Met | Ile | Leu | Tyr | Phe | Lys | His | Pro | Total | ||
| 0 | RG11 | nd | 0.03 b | Nd | 0.04 c | 0.23 c * | 0.05 c | nd | 0.20 c | 0.08 c | 0.10 c | 0.07 c | 0.24 c | 0.16 c | 0.23 c | 0.15 c | 0.05 b | 1.61 c |
| 0.5 | 0.02 b | 0.02 b | 0.01 b | 0.69 b | 0.74 b * | 0.27 b | 0.07 b | 0.67 b | 0.14 b * | 1.17 b | 1.24 b | 0.77 b | 1.52 b | 1.41 b | 1.03 b | 0.17 a | 10.51 b | |
| 1 | 0.20 a * | 0.06 a | 0.05 a | 1.35 a | 1.10 a * | 0.35 a | 0.18 a | 0.99 a | 0.48 a * | 1.41 a | 1.74 a | 1.11 a | 1.68 a * | 2.37 a | 1.81 a | 0.18 a | 15.71 a | |
| 0 | Yuyan5 | nd | 0.02 c | 0.04 c | 0.04 c | 0.02 b | 0.05 c | 0.01 b | 0.16 c | nd | 0.12 b | 0.39 c * | 0.25 c | 0.15 b | 0.21 c | 0.86 c * | 0.06 b | 2.40 c * |
| 0.5 | nd | 0.04 b * | 0.08 a * | 0.54 b | 0.18 a | 0.34 b | 0.10 b | 1.38 b * | 0.08 b | 1.58 a * | 2.30 b * | 0.70 b | 1.38 a | 1.97 b * | 3.95 b * | 0.39 a * | 14.44 b * | |
| 1 | 0.04 | 0.09 a | 0.06 b | 3.74 a * | 0.19 a | 0.42 a | 1.44 a * | 1.80 a * | 0.15 a | 1.58 a | 2.76 a * | 1.12 a | 1.25 a | 3.02 a * | 4.78 a * | 0.39 a * | 22.08 a * | |
| Line | Model | Parameter | Equation | r2 | F | n |
|---|---|---|---|---|---|---|
| RG11 | 1 | Lys | y = −8.83 + 57.64x1 | 0.977 | 0.000 | 9 |
| 2 | Lys | y = −13.76 + 38.44x1 + 34.13x2 | 0.987 | 0.047 | 9 | |
| Phe | ||||||
| Yuyan5 | 1 | Val | y = −17.86 + 101.33x1 | 0.991 | 0.000 | 9 |
| 2 | Val | y = −24.00 + 81.02x1 + 41.58x2 | 0.997 | 0.012 | 9 | |
| Tyr |
| Line | Independent Variable | r | Path Coefficient | Contribution | Indirect Path Coefficient | ||
|---|---|---|---|---|---|---|---|
| x1 | x2 | Total | |||||
| RG11 | x1 (Lys) | 0.990 | 0.660 | 0.653 | - | 0.330 | 0.330 |
| x2 (Phe) | 0.976 | 0.345 | 0.337 | 0.631 | - | 0.631 | |
| Yuyan5 | x1 (Val) | 0.996 | 0.796 | 0.793 | - | 0.200 | 0.200 |
| x2 (Tyr) | 0.962 | 0.212 | 0.204 | 0.750 | - | 0.750 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.; Lu, R.; Zhan, J.; He, J.; Wang, Y.; Li, T. Role of Root Exudates in Cadmium Accumulation of a Low-Cadmium-Accumulating Tobacco Line (Nicotiana tabacum L.). Toxics 2023, 11, 141. https://doi.org/10.3390/toxics11020141
Huang H, Lu R, Zhan J, He J, Wang Y, Li T. Role of Root Exudates in Cadmium Accumulation of a Low-Cadmium-Accumulating Tobacco Line (Nicotiana tabacum L.). Toxics. 2023; 11(2):141. https://doi.org/10.3390/toxics11020141
Chicago/Turabian StyleHuang, Huagang, Runze Lu, Juan Zhan, Jinsong He, Yong Wang, and Tingxuan Li. 2023. "Role of Root Exudates in Cadmium Accumulation of a Low-Cadmium-Accumulating Tobacco Line (Nicotiana tabacum L.)" Toxics 11, no. 2: 141. https://doi.org/10.3390/toxics11020141
APA StyleHuang, H., Lu, R., Zhan, J., He, J., Wang, Y., & Li, T. (2023). Role of Root Exudates in Cadmium Accumulation of a Low-Cadmium-Accumulating Tobacco Line (Nicotiana tabacum L.). Toxics, 11(2), 141. https://doi.org/10.3390/toxics11020141





