Y-27632 Impairs Angiogenesis on Extra-Embryonic Vasculature in Post-Gastrulation Chick Embryos
Abstract
1. Introduction
2. Materials and Methods
2.1. Measurements of EEM Formation
2.2. Fractal Analysis
2.3. Western Blot
2.4. Real-Time Quantitative Reverse Transcription Polymerase Chain Reaction (RT-PCR)
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Statistical Analysis
3. Results
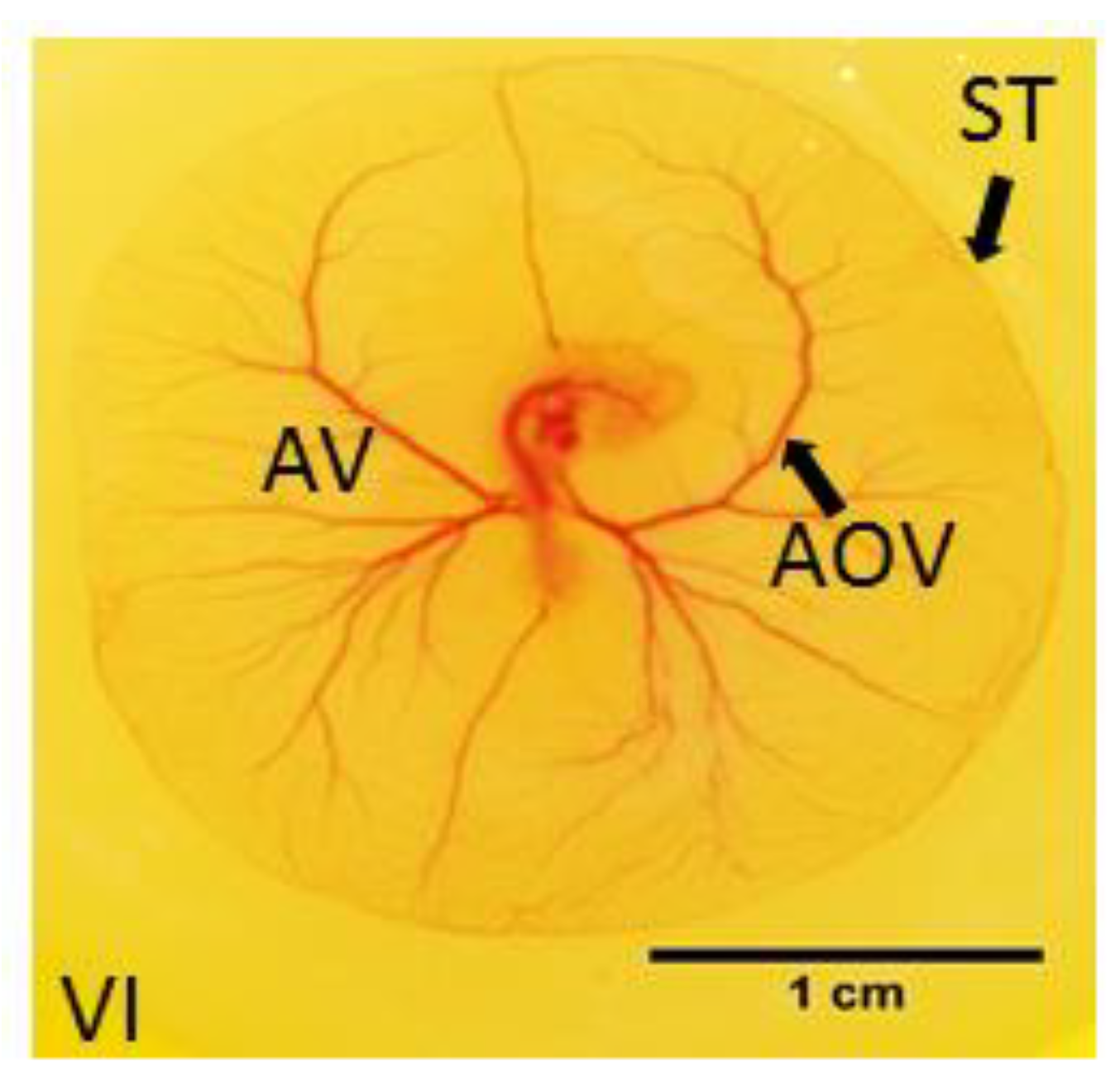
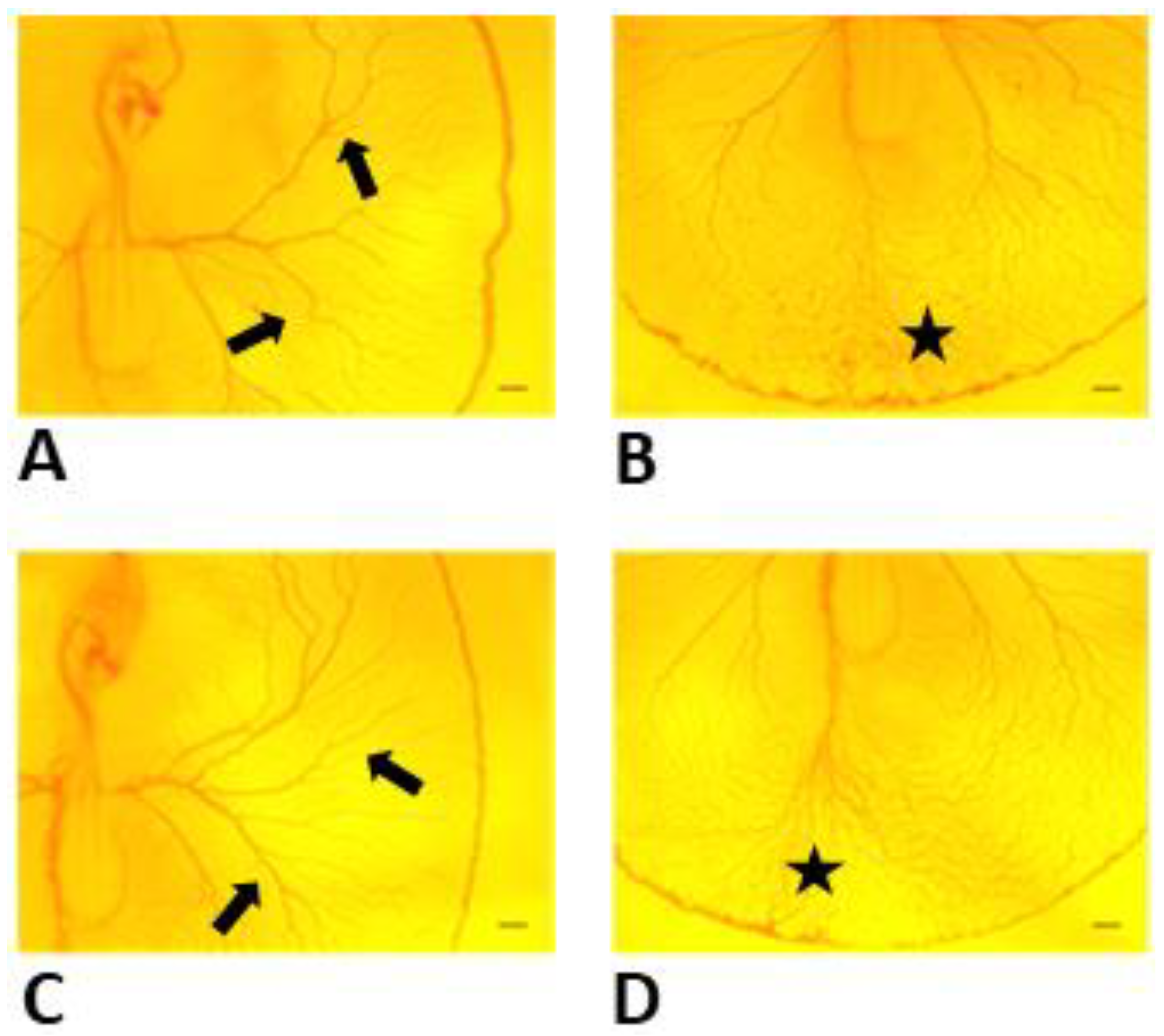
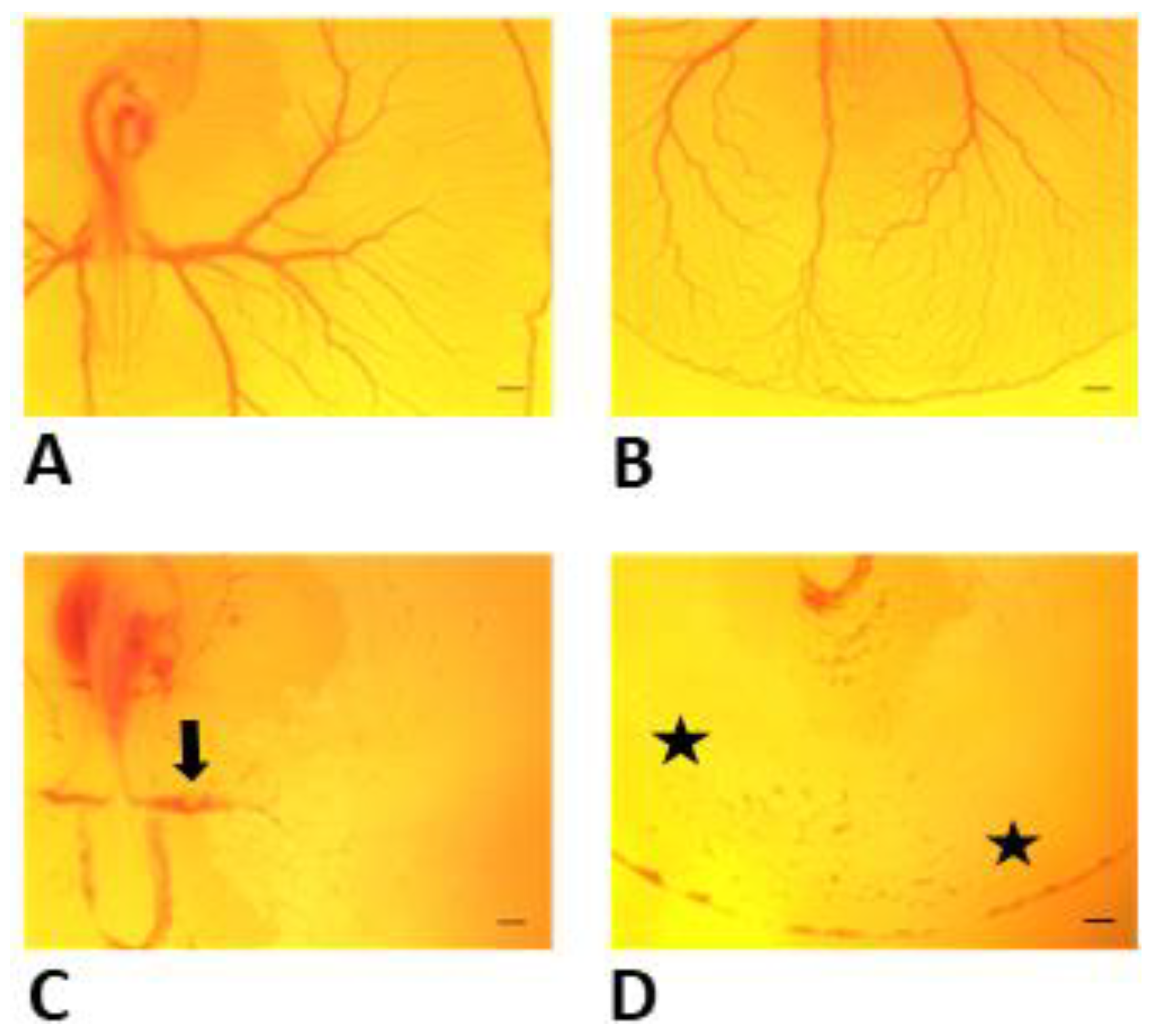
3.1. Effects of Y-27632 on Angiogenic Development
3.2. Measurements of EEM Formation
3.3. Fractal Analysis
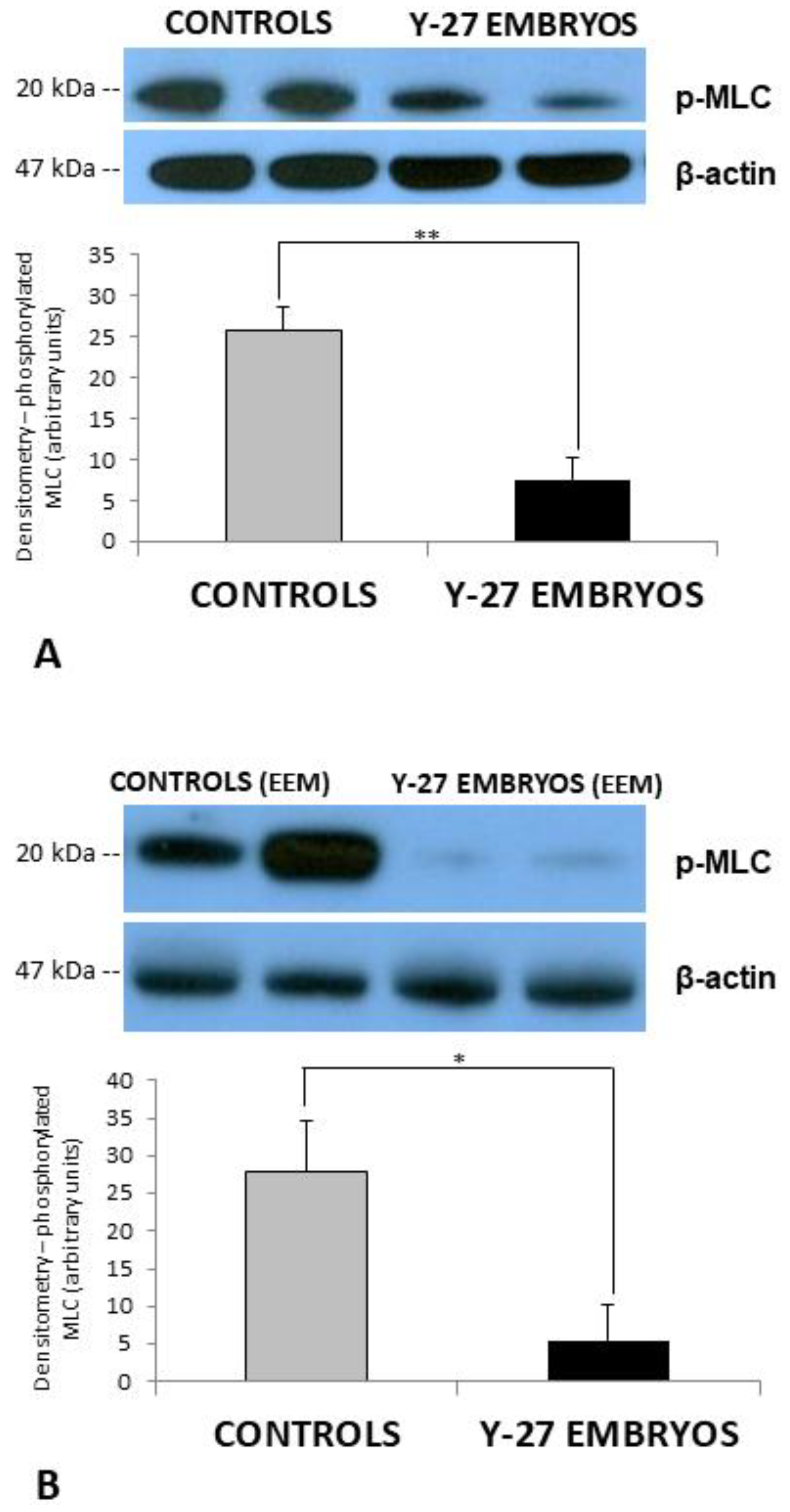
3.4. Western Blot
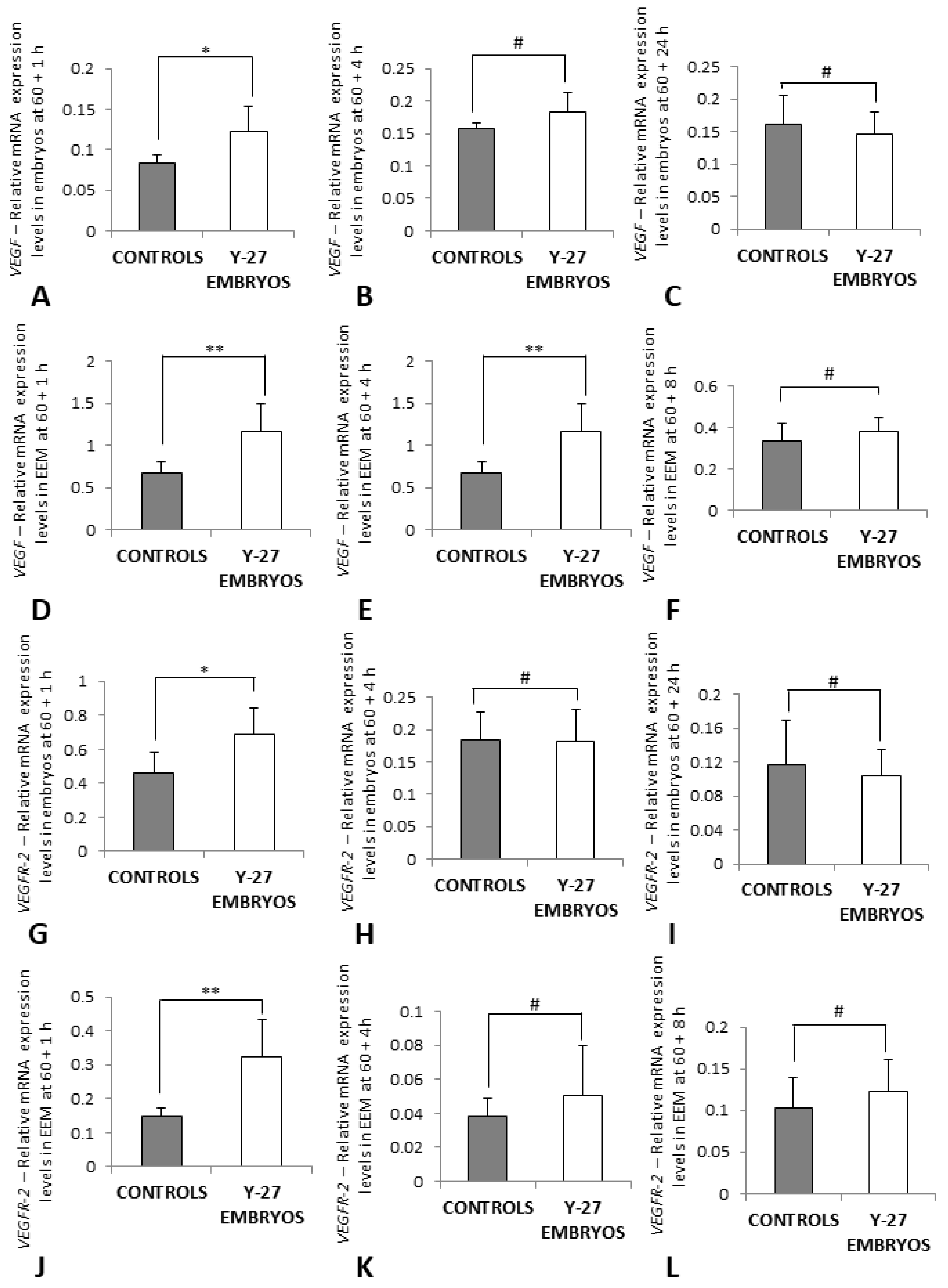
3.5. RT-PCR of VEGF and VEGFR-2
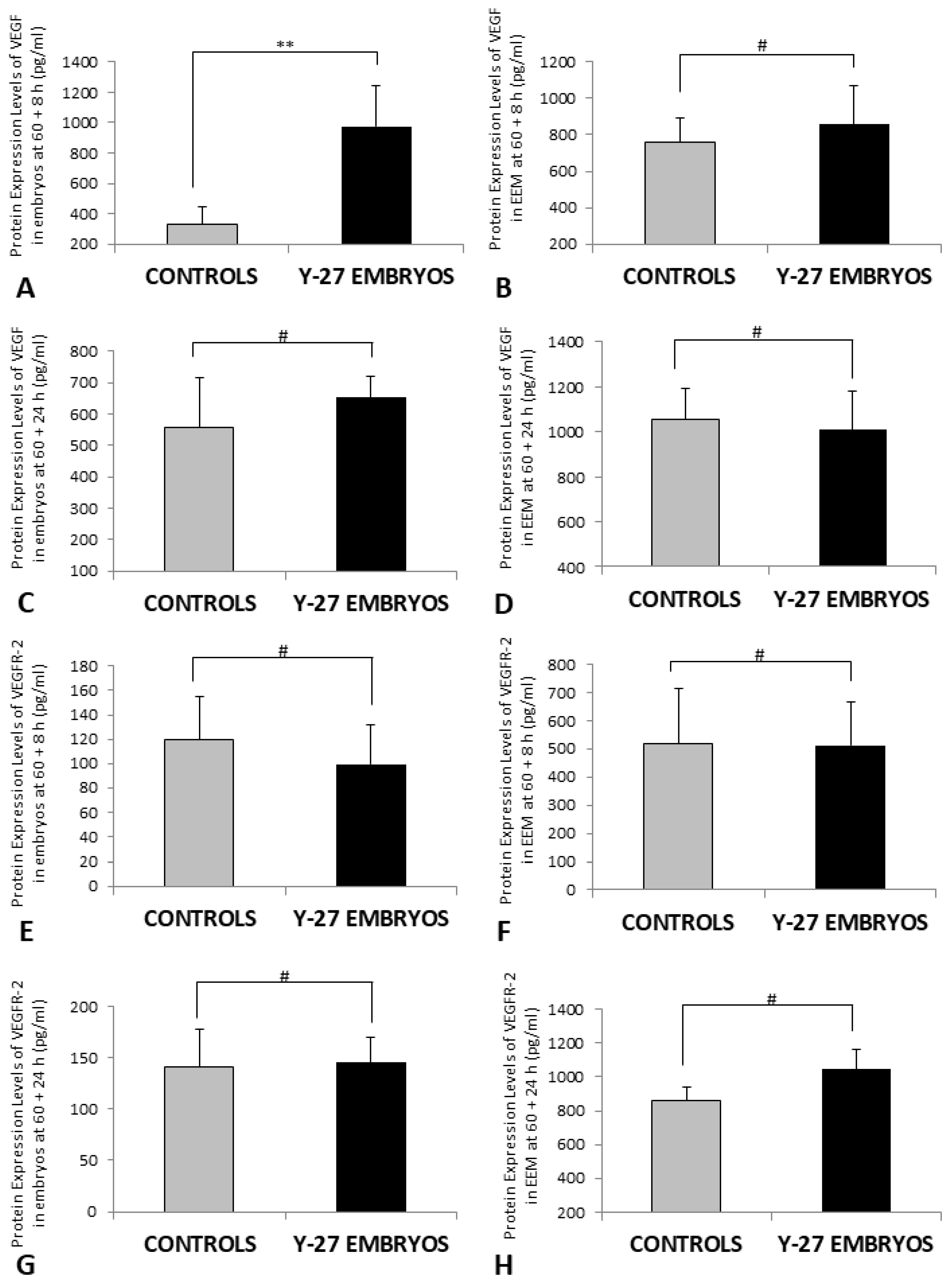
3.6. Protein Expression of VEGF and VEGFR-2 (ELISA)
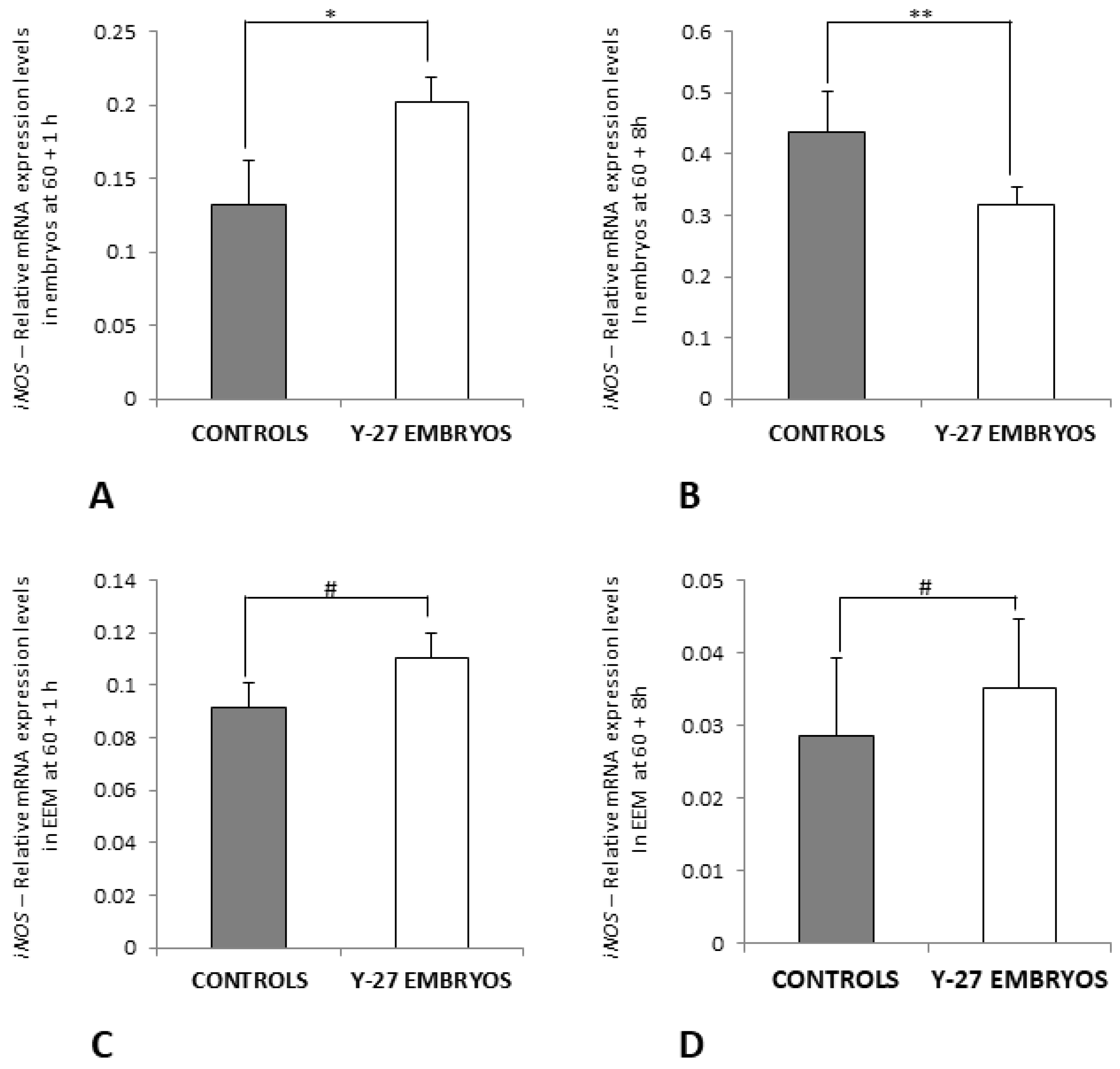
3.7. RT-PCR of Ang-2 and iNOS
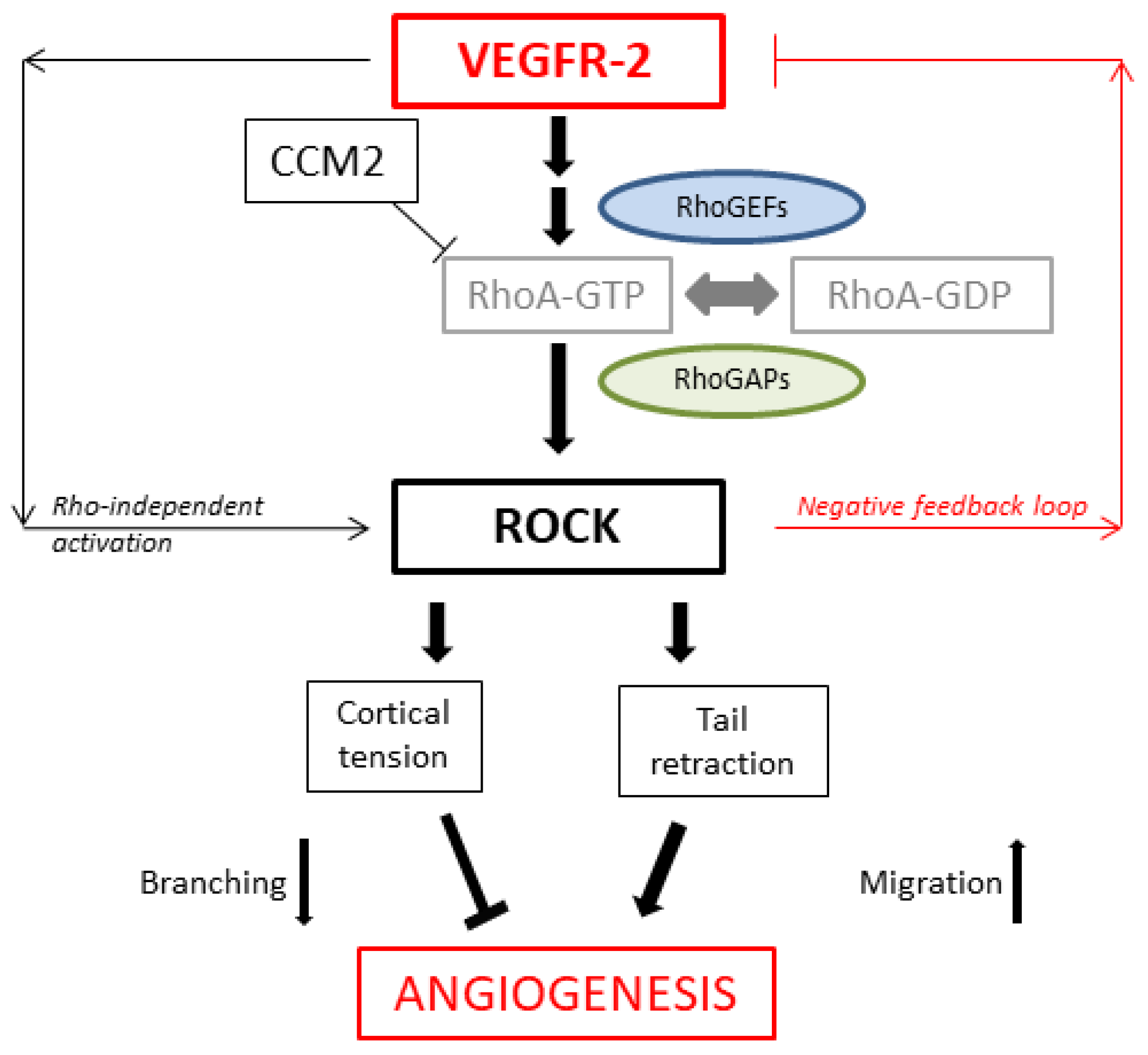
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feldkamp, M.L.; Carey, J.C.; Sadler, T.W. Development of gastroschisis: Review of hypotheses, a novel hypothesis, and implications for research. Am. J. Med. Genet. A 2007, 143A, 639–652. [Google Scholar] [CrossRef] [PubMed]
- Williams, T. Animal models of ventral body wall closure defects: A personal perspective on gastroschisis. Am. J. Med. Genet. C Semin. Med. Genet. 2008, 148C, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Pechriggl, E.; Blumer, M.; Tubbs, R.S.; Olewnik, L.; Konschake, M.; Fortelny, R.; Stofferin, H.; Honis, H.R.; Quinones, S.; Maranillo, E.; et al. Embryology of the Abdominal Wall and Associated Malformations—A Review. Front. Surg. 2022, 9, 891896. [Google Scholar] [CrossRef]
- Revels, J.W.; Wang, S.S.; Nasrullah, A.; Revzin, M.; Iyer, R.S.; Deutsch, G.; Katz, D.S.; Moshiri, M. An Algorithmic Approach to Complex Fetal Abdominal Wall Defects. AJR Am. J. Roentgenol. 2020, 214, 218–231. [Google Scholar] [CrossRef]
- Sadler, T.W. Embryology of neural tube development. Am. J. Med. Genet. C Semin. Med. Genet. 2005, 135C, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Sadler, T.W. The embryologic origin of ventral body wall defects. Semin. Pediatr. Surg. 2010, 19, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Sadler, T.W.; Feldkamp, M.L. The embryology of body wall closure: Relevance to gastroschisis and other ventral body wall defects. Am. J. Med. Genet. C Semin. Med. Genet. 2008, 148C, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Brewer, S.; Williams, T. Finally, a sense of closure? Animal models of human ventral body wall defects. Bioessays 2004, 26, 1307–1321. [Google Scholar] [CrossRef]
- deVries, P.A. The pathogenesis of gastroschisis and omphalocele. J. Pediatr. Surg. 1980, 15, 245–251. [Google Scholar] [CrossRef]
- Ledbetter, D.J. Congenital abdominal wall defects and reconstruction in pediatric surgery: Gastroschisis and omphalocele. Surg. Clin. N. Am. 2012, 92, 713–727. [Google Scholar] [CrossRef]
- Hoyme, H.E.; Jones, M.C.; Jones, K.L. Gastroschisis: Abdominal wall disruption secondary to early gestational interruption of the omphalomesenteric artery. Semin. Perinatol. 1983, 7, 294–298. [Google Scholar] [PubMed]
- Torfs, C.P.; Katz, E.A.; Bateson, T.F.; Lam, P.K.; Curry, C.J. Maternal medications and environmental exposures as risk factors for gastroschisis. Teratology 1996, 54, 84–92. [Google Scholar] [CrossRef]
- Haddow, J.E.; Palomaki, G.E.; Holman, M.S. Young maternal age and smoking during pregnancy as risk factors for gastroschisis. Teratology 1993, 47, 225–228. [Google Scholar] [CrossRef]
- Haustein, K.O. Cigarette smoking, nicotine and pregnancy. Int. J. Clin. Pharmacol. Ther. 1999, 37, 417–427. [Google Scholar] [PubMed]
- Torfs, C.P.; Velie, E.M.; Oechsli, F.W.; Bateson, T.F.; Curry, C.J. A population-based study of gastroschisis: Demographic, pregnancy, and lifestyle risk factors. Teratology 1994, 50, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Hamburger, V.; Hamilton, H.L. A series of normal stages in the development of the chick embryo. 1951. Dev. Dyn. 1992, 195, 231–272. [Google Scholar] [CrossRef]
- Gheorghescu, A.; Thompson, J. Delayed vasculogenesis and impaired angiogenesis due to altered Ang-2 and VE-cadherin levels in the chick embryo model following exposure to cadmium. Pediatr. Surg. Int. 2016, 32, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Gheorghescu, A.K.; Tywoniuk, B.; Duess, J.; Buchete, N.V.; Thompson, J. Exposure of chick embryos to cadmium changes the extra-embryonic vascular branching pattern and alters expression of VEGF-A and VEGF-R2. Toxicol. Appl. Pharmacol. 2015, 289, 79–88. [Google Scholar] [CrossRef]
- Doi, T.; Puri, P.; Bannigan, J.; Thompson, J. Downregulation of ROCK-I and ROCK-II gene expression in the cadmium-induced ventral body wall defect chick model. Pediatr. Surg. Int. 2008, 24, 1297–1301. [Google Scholar] [CrossRef]
- Duess, J.W.; Puri, P.; Thompson, J. Impaired cytoskeletal arrangements and failure of ventral body wall closure in chick embryos treated with rock inhibitor (Y-27632). Pediatr. Surg. Int. 2016, 32, 45–58. [Google Scholar] [CrossRef]
- Duess, J.W.; Gosemann, J.H.; Puri, P.; Thompson, J. Teratogenesis in the chick embryo following post-gastrulation exposure to Y-27632 -effect of Y-27632 on embryonic development. Toxicol. Appl. Pharmacol. 2020, 409, 115277. [Google Scholar] [CrossRef] [PubMed]
- Duess, J.W.; Fujiwara, N.; Corcionivoschi, N.; Puri, P.; Thompson, J. ROCK inhibitor (Y-27632) disrupts somitogenesis in chick embryos. Pediatr. Surg. Int. 2013, 29, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Hoang, M.V.; Whelan, M.C.; Senger, D.R. Rho activity critically and selectively regulates endothelial cell organization during angiogenesis. Proc. Natl. Acad. Sci. USA 2004, 101, 1874–1879. [Google Scholar] [CrossRef] [PubMed]
- Moses, M.A. The regulation of neovascularization of matrix metalloproteinases and their inhibitors. Stem Cells 1997, 15, 180–189. [Google Scholar] [CrossRef]
- Eelen, G.; Treps, L.; Li, X.; Carmeliet, P. Basic and Therapeutic Aspects of Angiogenesis Updated. Circ. Res. 2020, 127, 310–329. [Google Scholar] [CrossRef] [PubMed]
- Kamijo, H.; Matsumura, Y.; Thumkeo, D.; Koike, S.; Masu, M.; Shimizu, Y.; Ishizaki, T.; Narumiya, S. Impaired vascular remodeling in the yolk sac of embryos deficient in ROCK-I and ROCK-II. Genes Cells 2011, 16, 1012–1021. [Google Scholar] [CrossRef]
- Papetti, M.; Herman, I.M. Mechanisms of normal and tumor-derived angiogenesis. Am. J. Physiol. Cell Physiol. 2002, 282, C947–C970. [Google Scholar] [CrossRef]
- Risau, W. Mechanisms of angiogenesis. Nature 1997, 386, 671–674. [Google Scholar] [CrossRef]
- Schmidt, A.; Brixius, K.; Bloch, W. Endothelial precursor cell migration during vasculogenesis. Circ. Res. 2007, 101, 125–136. [Google Scholar] [CrossRef]
- Lamalice, L.; Le Boeuf, F.; Huot, J. Endothelial cell migration during angiogenesis. Circ. Res. 2007, 100, 782–794. [Google Scholar] [CrossRef]
- Eichmann, A.; Simons, M. VEGF signaling inside vascular endothelial cells and beyond. Curr. Opin. Cell Biol. 2012, 24, 188–193. [Google Scholar] [CrossRef]
- Ferrara, N.; Gerber, H.P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Koch, S.; Claesson-Welsh, L. Signal transduction by vascular endothelial growth factor receptors. Cold Spring Harb. Perspect. Med. 2012, 2, a006502. [Google Scholar] [CrossRef] [PubMed]
- Koch, S.; Tugues, S.; Li, X.; Gualandi, L.; Claesson-Welsh, L. Signal transduction by vascular endothelial growth factor receptors. Biochem. J. 2011, 437, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Cebe-Suarez, S.; Zehnder-Fjallman, A.; Ballmer-Hofer, K. The role of VEGF receptors in angiogenesis; complex partnerships. Cell. Mol. Life Sci. 2006, 63, 601–615. [Google Scholar] [CrossRef]
- Bryan, B.A.; D’Amore, P.A. What tangled webs they weave: Rho-GTPase control of angiogenesis. Cell. Mol. Life Sci. 2007, 64, 2053–2065. [Google Scholar] [CrossRef]
- Park, H.J.; Kong, D.; Iruela-Arispe, L.; Begley, U.; Tang, D.; Galper, J.B. 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors interfere with angiogenesis by inhibiting the geranylgeranylation of RhoA. Circ. Res. 2002, 91, 143–150. [Google Scholar] [CrossRef] [PubMed]
- van Nieuw Amerongen, G.P.; van Hinsbergh, V.W. Role of ROCK I/II in vascular branching. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H903–H905. [Google Scholar] [CrossRef]
- Varon, C.; Basoni, C.; Reuzeau, E.; Moreau, V.; Kramer, I.J.; Genot, E. TGFbeta1-induced aortic endothelial morphogenesis requires signaling by small GTPases Rac1 and RhoA. Exp. Cell Res. 2006, 312, 3604–3619. [Google Scholar] [CrossRef]
- Liu, J.; Wada, Y.; Katsura, M.; Tozawa, H.; Erwin, N.; Kapron, C.M.; Bao, G.; Liu, J. Rho-Associated Coiled-Coil Kinase (ROCK) in Molecular Regulation of Angiogenesis. Theranostics 2018, 8, 6053–6069. [Google Scholar] [CrossRef]
- Urade, R.; Chiu, Y.H.; Chiu, C.C.; Wu, C.Y. Small GTPases and Their Regulators: A Leading Road toward Blood Vessel Development in Zebrafish. Int. J. Mol. Sci. 2022, 23, 4991. [Google Scholar] [CrossRef] [PubMed]
- Riento, K.; Ridley, A.J. Rocks: Multifunctional kinases in cell behaviour. Nat. Rev. Mol. Cell Biol. 2003, 4, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.W.; Olson, M.F. LIM kinases: Function, regulation and association with human disease. J. Mol. Med. (Berl.) 2007, 85, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Montalvo, J.; Spencer, C.; Hackathorn, A.; Masterjohn, K.; Perkins, A.; Doty, C.; Arumugam, A.; Ongusaha, P.P.; Lakshmanaswamy, R.; Liao, J.K.; et al. ROCK1 & 2 perform overlapping and unique roles in angiogenesis and angiosarcoma tumor progression. Curr. Mol. Med. 2013, 13, 205–219. [Google Scholar]
- Sun, H.; Breslin, J.W.; Zhu, J.; Yuan, S.Y.; Wu, M.H. Rho and ROCK signaling in VEGF-induced microvascular endothelial hyperpermeability. Microcirculation 2006, 13, 237–247. [Google Scholar] [CrossRef]
- Dugan, J.D., Jr.; Lawton, M.T.; Glaser, B.; Brem, H. A new technique for explantation and in vitro cultivation of chicken embryos. Anat. Rec. 1991, 229, 125–128. [Google Scholar] [CrossRef]
- Parsons-Wingerter, P.; Elliott, K.E.; Clark, J.I.; Farr, A.G. Fibroblast growth factor-2 selectively stimulates angiogenesis of small vessels in arterial tree. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1250–1256. [Google Scholar] [CrossRef]
- Masters, B.R. Fractal analysis of the vascular tree in the human retina. Annu. Rev. Biomed. Eng. 2004, 6, 427–452. [Google Scholar] [CrossRef]
- Stosic, T.; Stosic, B.D. Multifractal analysis of human retinal vessels. IEEE Trans. Med. Imaging 2006, 25, 1101–1107. [Google Scholar] [CrossRef]
- Liew, G.; Mitchell, P.; Rochtchina, E.; Wong, T.Y.; Hsu, W.; Lee, M.L.; Wainwright, A.; Wang, J.J. Fractal analysis of retinal microvasculature and coronary heart disease mortality. Eur. Heart J. 2011, 32, 422–429. [Google Scholar] [CrossRef]
- Lammer, E.J.; Iovannisci, D.M.; Tom, L.; Schultz, K.; Shaw, G.M. Gastroschisis: A gene-environment model involving the VEGF-NOS3 pathway. Am. J. Med. Genet. C Semin. Med. Genet. 2008, 148C, 213–218. [Google Scholar] [CrossRef]
- Khan, F.A.; Raymond, S.L.; Hashmi, A.; Islam, S. Anatomy and embryology of abdominal wall defects. Semin. Pediatr. Surg. 2022, 31, 151230. [Google Scholar] [CrossRef] [PubMed]
- Bryan, B.A.; Dennstedt, E.; Mitchell, D.C.; Walshe, T.E.; Noma, K.; Loureiro, R.; Saint-Geniez, M.; Campaigniac, J.-P.; Liao, J.K. D’Amore, P.A. RhoA/ROCK signaling is essential for multiple aspects of VEGF-mediated angiogenesis. FASEB J. 2010, 24, 3186–3195. [Google Scholar] [CrossRef] [PubMed]
- Olsson, A.K.; Dimberg, A.; Kreuger, J.; Claesson-Welsh, L. VEGF receptor signalling—In control of vascular function. Nat. Rev. Mol. Cell Biol. 2006, 7, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Mao, K.; Liu, Z.; Dinh-Xuan, A.T. The role of the RhoA/Rho kinase pathway in angiogenesis and its potential value in prostate cancer (Review). Oncol. Lett. 2014, 8, 1907–1911. [Google Scholar] [CrossRef]
- van Nieuw Amerongen, G.P.; Koolwijk, P.; Versteilen, A.; van Hinsbergh, V.W. Involvement of RhoA/Rho kinase signaling in VEGF-induced endothelial cell migration and angiogenesis in vitro. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 211–217. [Google Scholar] [CrossRef]
- Shalaby, F.; Ho, J.; Stanford, W.L.; Fischer, K.D.; Schuh, A.C.; Schwartz, L.; Bernstein, A.; Rossant, J. A requirement for Flk1 in primitive and definitive hematopoiesis and vasculogenesis. Cell 1997, 89, 981–990. [Google Scholar] [CrossRef]
- Shalaby, F.; Rossant, J.; Yamaguchi, T.P.; Gertsenstein, M.; Wu, X.F.; Breitman, M.L.; Schuh, A.C. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature 1995, 376, 62–66. [Google Scholar] [CrossRef]
- Carmeliet, P.; Ferreira, V.; Breier, G.; Pollefeyt, S.; Kieckens, L.; Gertsenstein, M.; Fahrig, M.; Vandenhoeck, A.; Harpal, K.; Eberhardt, C.; et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 1996, 380, 435–439. [Google Scholar] [CrossRef]
- Ferrara, N.; Carver-Moore, K.; Chen, H.; Dowd, M.; Lu, L.; O’Shea, K.S.; Powell-Braxton, L.; Hillan, K.J.; Moore, M.W. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 1996, 380, 439–442. [Google Scholar] [CrossRef]
- Eckenstaler, R.; Hauke, M.; Benndorf, R.A. A current overview of RhoA, RhoB, and RhoC functions in vascular biology and pathology. Biochem. Pharmacol. 2022, 206, 115321. [Google Scholar] [CrossRef]
- Combedazou, A.; Gayral, S.; Colombie, N.; Fougerat, A.; Laffargue, M.; Ramel, D. Small GTPases orchestrate cell-cell communication during collective cell movement. Small GTPases 2020, 11, 103–112. [Google Scholar] [CrossRef]
- Uchida, S.; Watanabe, G.; Shimada, Y.; Maeda, M.; Kawabe, A.; Mori, A.; Arii, S.; Uehata, M.; Kishimoto, T.; Oikawa, T.; et al. The suppression of small GTPase rho signal transduction pathway inhibits angiogenesis in vitro and in vivo. Biochem. Biophys. Res. Commun. 2000, 269, 633–640. [Google Scholar] [CrossRef]
- Kroll, J.; Epting, D.; Kern, K.; Dietz, C.T.; Feng, Y.; Hammes, H.P.; Wieland, T.; Augustin, H.G. Inhibition of Rho-dependent kinases ROCK I/II activates VEGF-driven retinal neovascularization and sprouting angiogenesis. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H893–H899. [Google Scholar] [CrossRef]
- Yin, L.; Morishige, K.; Takahashi, T.; Hashimoto, K.; Ogata, S.; Tsutsumi, S.; Takata, K.; Ohta, T.; Kawagoe, J.; Takahashi, K.; et al. Fasudil inhibits vascular endothelial growth factor-induced angiogenesis in vitro and in vivo. Mol. Cancer Ther. 2007, 6, 1517–1525. [Google Scholar] [CrossRef]
- Yadgary, L.; Kedar, O.; Adepeju, O.; Uni, Z. Changes in yolk sac membrane absorptive area and fat digestion during chick embryonic development. Poult. Sci. 2013, 92, 1634–1640. [Google Scholar] [CrossRef] [PubMed]
- Yadgary, L.; Yair, R.; Uni, Z. The chick embryo yolk sac membrane expresses nutrient transporter and digestive enzyme genes. Poult. Sci. 2011, 90, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Minko, K.; Bollerot, K.; Drevon, C.; Hallais, M.F.; Jaffredo, T. From mesoderm to blood islands: Patterns of key molecules during yolk sac erythropoiesis. Gene Expr. Patterns 2003, 3, 261–272. [Google Scholar] [CrossRef]
- Hyvelin, J.M.; Howell, K.; Nichol, A.; Costello, C.M.; Preston, R.J.; McLoughlin, P. Inhibition of Rho-kinase attenuates hypoxia-induced angiogenesis in the pulmonary circulation. Circ. Res. 2005, 97, 185–191. [Google Scholar] [CrossRef]
- Felcht, M.; Luck, R.; Schering, A.; Seidel, P.; Srivastava, K.; Hu, J.; Bartol, A.; Kienast, Y.; Vettel, C.; Loos, E.K.; et al. Angiopoietin-2 differentially regulates angiogenesis through TIE2 and integrin signaling. J. Clin. Investig. 2012, 122, 1991–2005. [Google Scholar] [CrossRef] [PubMed]
- Lee, O.H.; Fueyo, J.; Xu, J.; Yung, W.K.; Lemoine, M.G.; Lang, F.F.; Bekele, B.N.; Zhou, X.; Alonso, M.A.; Aldape, K.D.; et al. Sustained angiopoietin-2 expression disrupts vessel formation and inhibits glioma growth. Neoplasia 2006, 8, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Maisonpierre, P.C.; Suri, C.; Jones, P.F.; Bartunkova, S.; Wiegand, S.J.; Radziejewski, C.; Compton, D.; McClain, J.; Aldrich, T.H.; Papadopoulos, N.; et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 1997, 277, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.; Yancopoulos, G.D. The Angiopoietins: Yin and Yang in Angiogenesis. In Vascular Growth Factors and Angiogenesis; Claesson-Welsh, L., Ed.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 173–185. [Google Scholar]
- Fukumura, D.; Gohongi, T.; Kadambi, A.; Izumi, Y.; Ang, J.; Yun, C.O.; Buerk, D.G.; Huang, P.L.; Jain, R.K. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc. Natl. Acad. Sci. USA 2001, 98, 2604–2609. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.F.; Ling, Y.; Gou, W.Y.; Zhang, H.; Wu, C.X. Identification of chicken eNOS gene and differential expression in highland versus lowland chicken breeds. Poult. Sci. 2012, 91, 2275–2281. [Google Scholar] [CrossRef]
- Kroll, J.; Waltenberger, J. VEGF-A induces expression of eNOS and iNOS in endothelial cells via VEGF receptor-2 (KDR). Biochem. Biophys. Res. Commun. 1998, 252, 743–746. [Google Scholar] [CrossRef]




| Procedure | Time Point |
|---|---|
| Treatment | at 60 h incubation |
| Gross Morphology of extra-embryonic vasculature | 60 + 8 h, +24 h, +48 h, +4 d |
| Measurements of EEM | 60 + 24 h |
| Fractal analysis | 60 + 4 d |
| Western Blot | 60 + 8 h |
| Real-Time qRT-PCR | 60 + 1 h, +4 h, +8 h/+24 h |
| ELISA | 60 + 8 h, +24 h |
| Gene | Sequence (5′-3′) |
|---|---|
| GAPDH | |
| Forward | cctctctggcaaagtccaag |
| Reverse | ggtcacgctcctggaagata |
| VEGF | |
| Forward | caattgagaccctggtggac |
| Reverse | catcagaggcacacaggatg |
| VEGFR-2 | |
| Forward | gacagtggcatggtgttcag |
| Reverse | gtgcagtttccttcctggag |
| Ang-2 | |
| Forward | ttgaggaggttggacagttc |
| Reverse | gcttcatttccttcccagtc |
| iNOS | |
| Forward | agtggtatgctctgcctgct |
| Reverse | ccagtcccattcttcttcc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duess, J.W.; Gosemann, J.-H.; Kaskova Gheorghescu, A.; Puri, P.; Thompson, J. Y-27632 Impairs Angiogenesis on Extra-Embryonic Vasculature in Post-Gastrulation Chick Embryos. Toxics 2023, 11, 134. https://doi.org/10.3390/toxics11020134
Duess JW, Gosemann J-H, Kaskova Gheorghescu A, Puri P, Thompson J. Y-27632 Impairs Angiogenesis on Extra-Embryonic Vasculature in Post-Gastrulation Chick Embryos. Toxics. 2023; 11(2):134. https://doi.org/10.3390/toxics11020134
Chicago/Turabian StyleDuess, Johannes W., Jan-Hendrik Gosemann, Anna Kaskova Gheorghescu, Prem Puri, and Jennifer Thompson. 2023. "Y-27632 Impairs Angiogenesis on Extra-Embryonic Vasculature in Post-Gastrulation Chick Embryos" Toxics 11, no. 2: 134. https://doi.org/10.3390/toxics11020134
APA StyleDuess, J. W., Gosemann, J.-H., Kaskova Gheorghescu, A., Puri, P., & Thompson, J. (2023). Y-27632 Impairs Angiogenesis on Extra-Embryonic Vasculature in Post-Gastrulation Chick Embryos. Toxics, 11(2), 134. https://doi.org/10.3390/toxics11020134






