1. Introduction
Chemotherapy-induced neurotoxicity (CIN) is a well-known complication of several very effective systemic anticancer treatments, mainly presenting as cognitive impairment (“chemo-brain”) [
1] and peripheral neuropathy (CIPN) [
2], and no effective treatment is available for either of these conditions [
3,
4]. In clinical practice, CIN occurrence and its manifestations tend to be closely monitored during chemotherapy (also because they can represent dose-limiting side effects), but their presence is generally less carefully assessed in the follow-up, despite the fact that they can potentially have a relevant impact on cancer survivors’ long-term quality of life [
5,
6,
7,
8,
9,
10]. Moreover, even less well established are the social and economic effects of CIN on patients’ lifestyles and their relationships. In fact, within this context, it is very important to recognize the importance of tertiary prevention and ensure it to people with a previous cancer diagnosis. More precise knowledge of these long-term aspects is crucial, particularly given the increasing number of cancer patients receiving effective treatments allowing them to be cured and to achieve a normal life expectancy or, at least, to gain a very long survival and active life.
Patient-reported outcome measures (PROMs) are increasingly utilized to assess the severity of CIN, particularly to evaluate CIPN [
11,
12], since they represent the most suitable and reliable method to capture the individual perception of the severity of CIN-induced events on health status. However, this patient-centered approach has not yet been extended to other domains of patients’ daily life.
In this study, we used a web-based questionnaire posted on the website of a representative oncology patients’ community to record the self-reported perception of CIN of cancer patients on their health status, but also on several different aspects of their daily life.
2. Materials and Methods
The questionnaire used in this study was created by a panel composed of physicians, experts in CIN, and “Associazione italiana malati di cancro” (Italian Association of Cancer Patients—AIMaC) members, including cancer patients.
After this initial activity, the questionnaire and the study plan were submitted to the University of Milano-Bicocca Ethics Committee for approval (granted with n. 650, 22 January 2022).
The questionnaire included questions on patients’ general demographic data, cancer-related medical history, and personal assessment of CIN’s impact on their health status, activities of daily life, working activities, and neurotoxicity management (see
Supplementary Material File S1 for the full version of the questionnaire). The questionnaire was advertised through the Aimac periodic newsletter (more than 3000 subscribers) and posted on the association website (
https://www.aimac.it/), soliciting cancer patients to anonymously complete it as had already done in previous campaigns. In order to allow the largest number of cancer patients to provide their answers, they were considered eligible if aged 18 or older, if they received any neurotoxic anticancer chemotherapy, and were able to autonomously complete the online web-based survey. The questionnaire was left online for 3 months (22 March 2022–22 June 2022) and it was introduced by a text where it was clearly explained, in lay-people language agreed with Aimac, that all the questions were exclusively related to central and/or peripheral neurotoxicity ensuing after chemotherapy, and also describing their main symptoms.
The study fulfilled the STROBE guidelines for cross-sectional studies (available at:
https://www.strobe-statement.org/ accessed on 5 January 2023), except for the following items:
- -
Item #8 was not fully applied, since the questionnaire was not validated
- -
Item #10: study size was not predefined, since the questionnaire was freely available and the number of responders was not predictable
- -
Item #12 was only partly applied, given the type of study
- -
Items #13 and #17 were not applicable
3. Results
The first and the second sections of the questionnaire described in lay-people language the main symptoms of CIPN and CIN, and specific questions were asked, thus allowing the responder to be guided and the assessor to properly check if she/he really suffered from CIPN and/or CIN.
The page with the questionnaire notice was viewed 2797 times, and the page with the questionnaire was viewed 500 times. A total of 162 patients completed the questionnaire (12.3% males, and 87.7% females, reflecting the predominance of breast cancer among the responders).
Most of the patients were aged 51–60 (38.9%), and the majority of them had a medium-to-high level of education. Regarding their cancer history, approximately half of the responders (56.8%) were in remission, with a long history of cancer (54.9% of the responders in the range 2–5 years), and 61.7% of them were off treatment. More than half of the responders had breast cancer (see
Table 1).
3.1. Results Divided according to Their Domains
3.1.1. Health Status
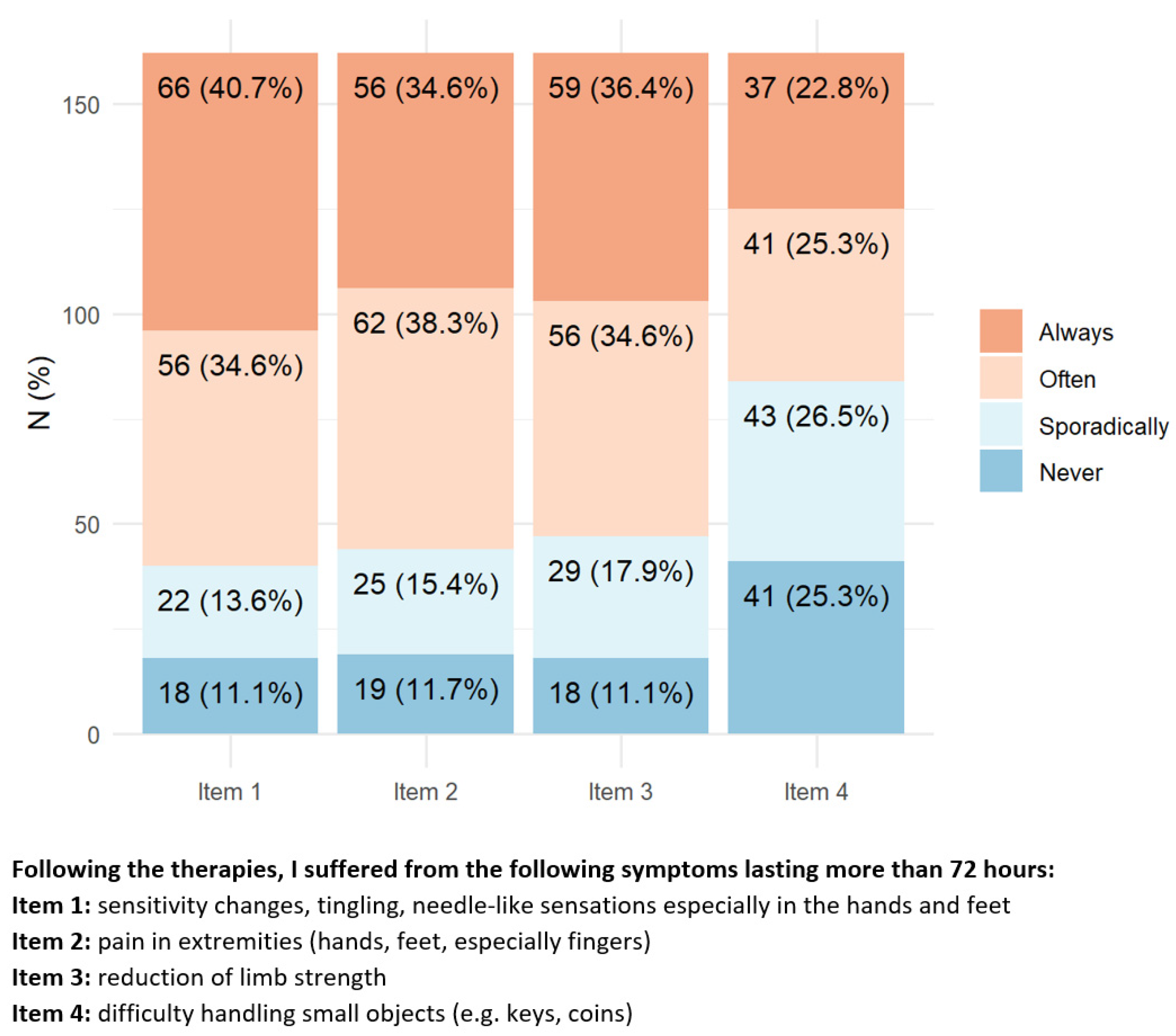
Regarding the presence of CIPN symptoms (
Figure 1), approx. 70% of responders reported having always/often alterations in sensation, distal pain, and reduced arm strength. Nearly 50% of them reported having always/often difficulty in common daily tasks such as manipulating small objects, climbing one flight of stairs, and approximately a quarter of them had problems in walking in dark rooms.
By contrast, only a minority of responders reported difficulty in swallowing, speaking, or having visual impairment.
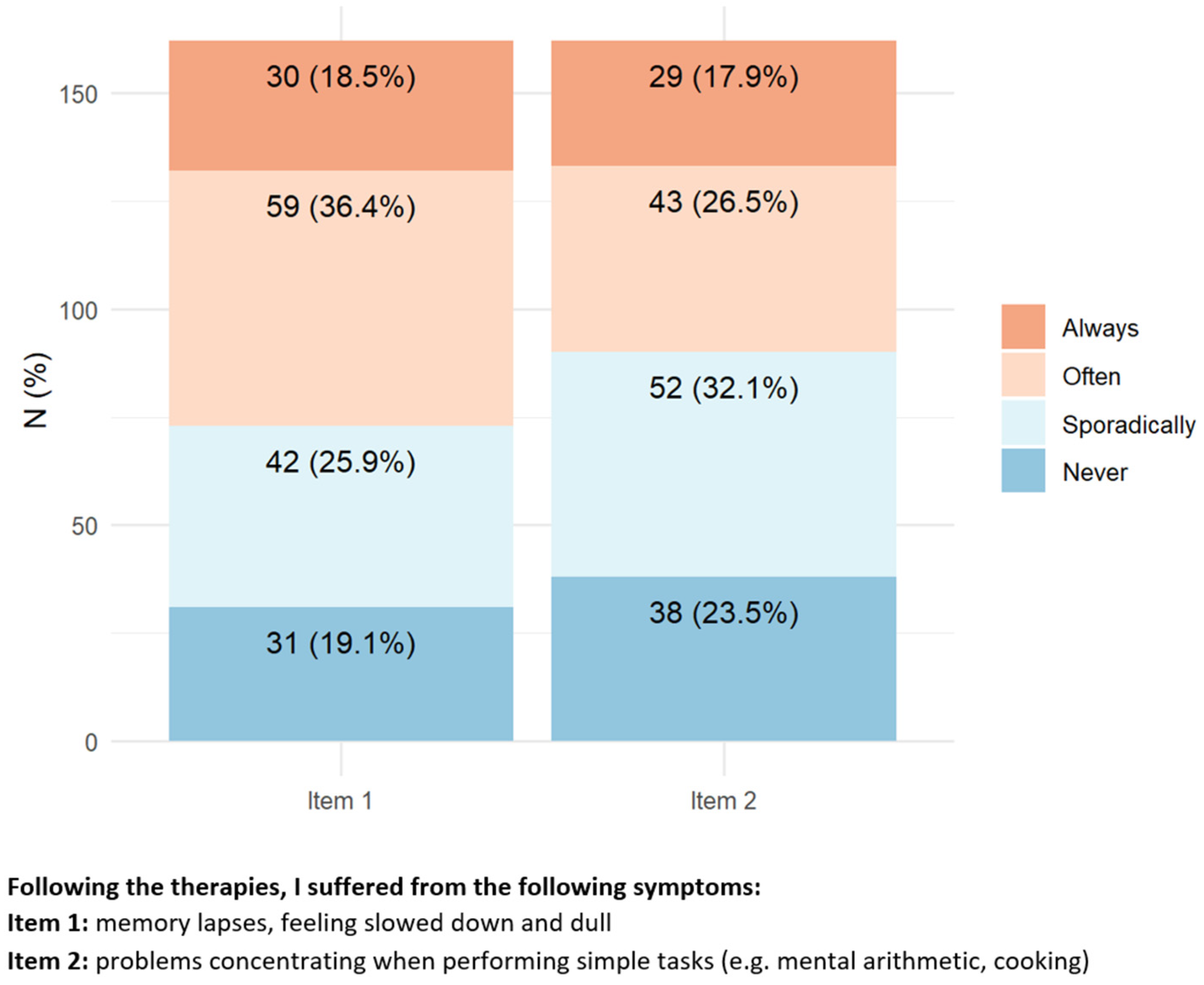
Regarding central nervous system neurotoxicity symptoms (
Figure 2), approximately 40–50% of patients reported experiencing always/often difficulty in memory or concentration while performing simple tasks (e.g., cooking, mental calculation).
3.1.2. Personal and Social Impact
According to the questionnaire answers, only 4.3% of the responders needed assistance with eating, dressing, and personal hygiene. However, the majority of responders had to change their habits (e.g., they reported difficulties in performing common activities requiring physical fitness such as carrying a heavy shopping bag and being unable to walk for a relatively long distance easily achievable before chemotherapy), 57.4% reported feeling the need to sit or rest in bed several hours during the day, and 71% reported sexual problems ensuing after chemotherapy, without any relevant association with the tumor site (
Figure 3). Moreover, 26.5% of responders reported being unable even to walk for a short distance (e.g., taking the dog for a walk, or going to buy the newspaper).
3.1.3. Working Impact
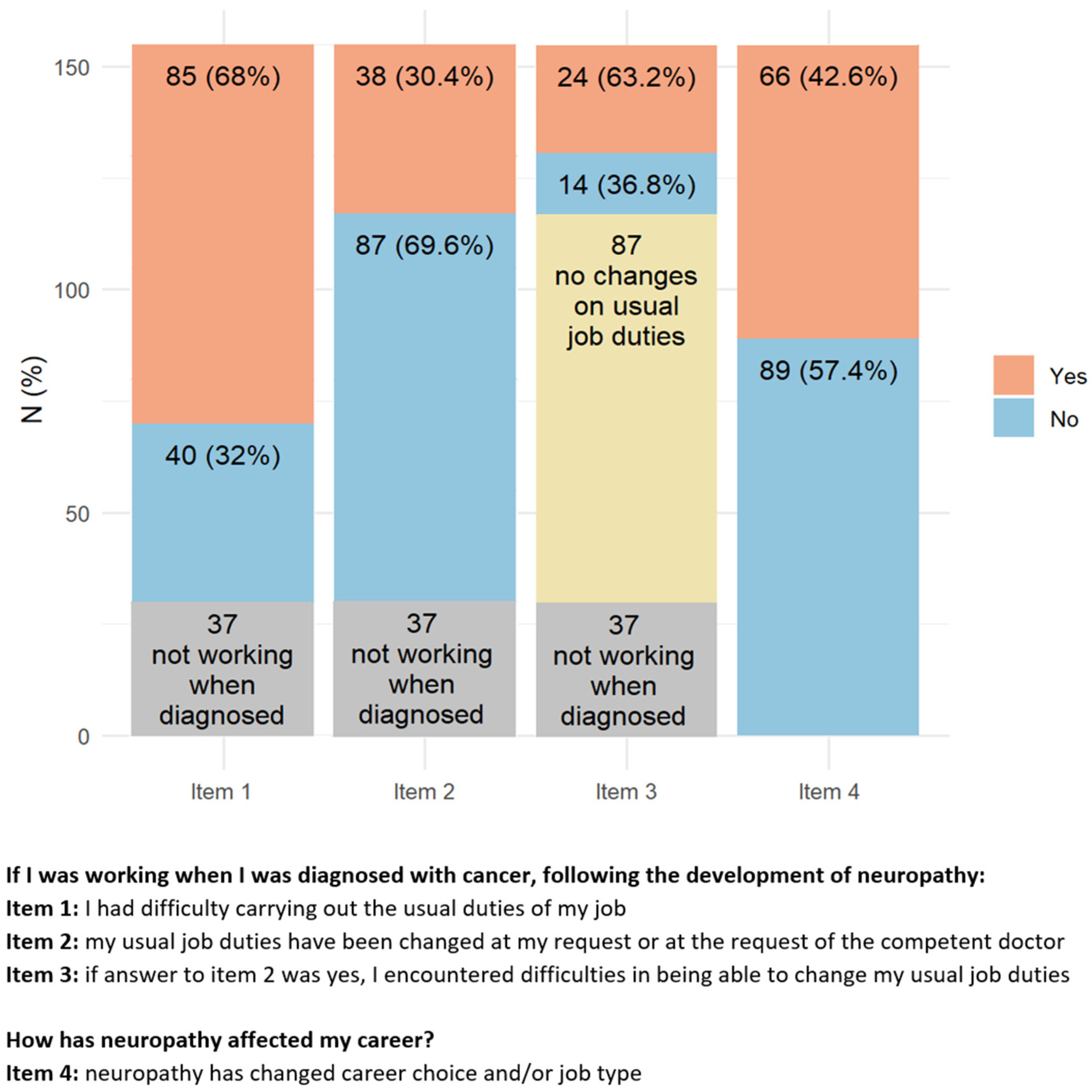
Most of the responders (77.2%) were active workers at the moment of cancer diagnosis (
Figure 4). After cancer treatment, 68% of them reported having difficulties due to CIPN symptoms in performing their usual working duties, 30.4% had to change their working activity, and for 63.2% of them, this change was troublesome. Despite this not being the case for the majority of responders, 42.6% of them modified their working plans, 28.4% felt that CIPN presence hampered the development of their career, 35.5% had to move from full to part-time, and 25.6% reported hostile behaviors due to their health conditions. A minority of responders (12.9%) attributed work loss to the presence of CIN.
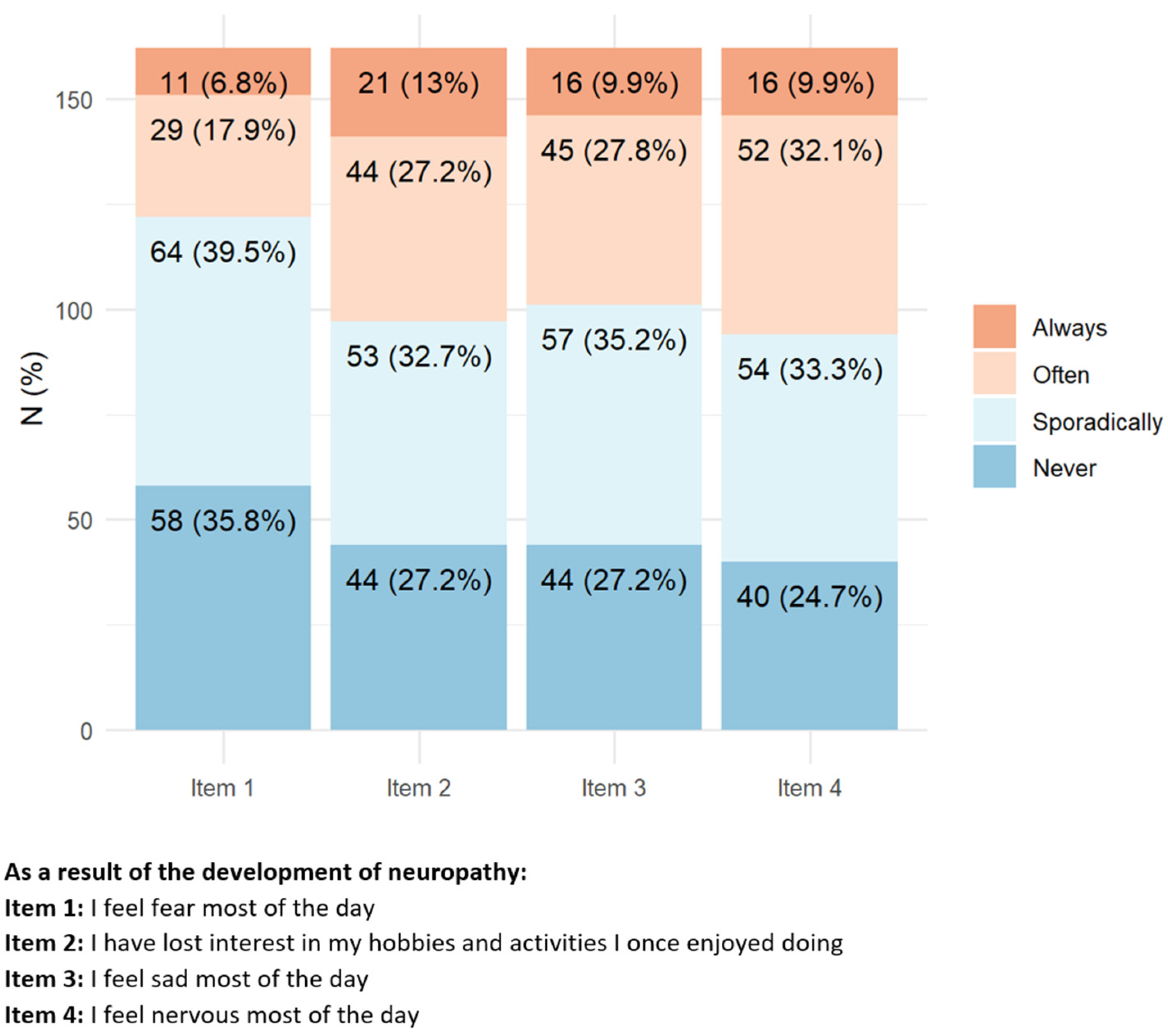
3.1.4. Psychological Impact
More than 80% of the responders reported having a very positive attitude toward their ability to deal with cancer (
Figure 5). However, several responders reported suffering always/often from mood changes (mainly irritability, sadness, fear, and loss of interest in hobbies). A smaller proportion of the responders (20–30%) felt always/often to be socially distant from their family/relatives, to feel loneliness, and, overall, were “angry” for most of the day.
3.1.5. Neurotoxicity Management
Once asked regarding their opinion on the management of their CIPN, only 42.6% of the responders reported that they received useful and detailed information; among them, 63.9% were informed by the hospital medical team, while only 9.3% of the responders were assisted by their general practitioner, despite most of them being no longer under hospital-based follow-up. Regarding CIPN treatment, 44.4% of the responders received some drug, and only approximately 20% were prescribed physical rehabilitation or alternative therapies (in most cases acupuncture, gymnastic yoga, or non-prescription substances).
Figure 5.
Items with the highest percentage of positive answers indicating a relevant psychological impact of peripheral neurotoxicity.
Figure 5.
Items with the highest percentage of positive answers indicating a relevant psychological impact of peripheral neurotoxicity.
4. Discussion
This exploratory study was designed to detect largely under-assessed features of long-term (i.e., persisting after treatment withdrawal) chemotherapy neurotoxic side effects [
5,
6,
13,
14], and the importance of its results are closely linked to the impact of these side effects on several relevant aspects of cancer patients’ daily life.
The responders feedback obtained by the questionnaire was in line with previous campaigns organized by Aimac (e.g., a questionnaire investigating the impact of the SARS-CoV-2 pandemic on cancer patients left online in the period April 2020–April 2021 received 540 answers), thus confirming the interest of the association affiliates on the topic of neurotoxicity.
The remarkable impact of CIN on the individual health of cancer survivors is confirmed by the answers collected in the study, and these data are in line with previous studies [
15,
16]. Remarkably, most of the responders had a breast cancer diagnosis and a tumor treated with drugs (namely taxanes) reported a high incidence of CIN [
15].
However, what emerges very clearly from the study is that the impact of CIN on personal, social, and working activities is also very high. A similar effect was also observed when the psychological impact is assessed. Moreover, there is evidence suggesting that the management of CIPN perceived by the responders is suboptimal, a result partially due to a lack of effective drugs, but also lack of appropriate advice from healthcare providers.
The study has some weaknesses, mainly represented by the relatively low number of patients who completed the questionnaire, the unbalanced mix of cancers, the absence of specific assessments to rule out possible confounding factors (e.g., depression), and a possible selection bias inducing mainly those patients with the most relevant impact of CIN on their lifestyle to complete the questionnaire. The need to better understand the link between specific treatments and side effects, based on different chemotherapies specific per cancer organs, as well as re-habilitation costs for the National Health System, also remains. Moreover, definite results on the socio-economic impact of anticancer chemotherapy neurotoxicity should be achieved through more formal, case–control trials, and a multi-national approach would also be advisable, since, for instance, different levels of social support in different countries might produce variable levels of impact on cancer patients. However, the study’s major strengths are the involvement of patients in questionnaire development and the novelty of the results, since this is the first study specifically investigating the socio-economic impact of anticancer chemotherapy neurotoxicity using a self-reported assessment method.
5. Conclusions
Our study should not be considered as the final answer to the unsettled issue of the socio-economic impact of anticancer chemotherapy-related neurotoxicity, but it is rather an exploratory study, strongly highlighting the need for a more formal and structured analysis. In fact, only the healthcare costs and work loss burden of patients with CIPN has been formally investigated, showing that average healthcare costs were USD 17,344 higher for CIPN cases than their non-CIPN controls, with outpatient costs being the highest component [
17].
Our study based on the self-assessment of the impact of CIN on cancer survivors’ lifestyles provides initial evidence of the relevance of the issue and spotlights the need for larger and more structured investigation of these long-term side effects of anticancer chemotherapy to gain more precise knowledge of the real burden of limitations associated with the use of neurotoxic drugs.
Moreover, the study perfectly fits into the 7th Recommendation of the Report of the Mission Board for Cancer “Conquering Cancer: Mission Possible”, which aims at developing an EU-wide research program and policy support to improve the quality of life of cancer patients and survivors, family members and carers.
Author Contributions
Conceptualization, G.C., A.D., G.T. and G.M.; methodology, G.C., D.P.B. and A.D.; formal analysis, D.P.B.; data curation, A.P.; writing—original draft preparation, G.C.; writing—review and editing, G.C., L.D.C. and F.D.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the University of Milano-Bicocca Ethics Committee (approval n. 650, 22 January 2022).
Informed Consent Statement
Informed consent was not required since there was not the need for collecting/storing data allowing identification of the participants.
Data Availability Statement
Acknowledgments
The collaboration of the cancer patients completing the questionnaire is gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mounier, N.M.; Abdel-Maged, A.E.; Wahdan, S.A.; Gad, A.M.; Azab, S.S. Chemotherapy-induced cognitive impairment (CICI): An overview of etiology and pathogenesis. Life Sci. 2020, 258, 118071. [Google Scholar] [CrossRef] [PubMed]
- Alberti, P.; Cavaletti, G.; Cornblath, D.R. Toxic neuropathies: Chemotherapy Induced Peripheral Neurotoxicity. Curr. Opin. Neurol. 2019, 32, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Cavaletti, G.; Marmiroli, P. Pharmacotherapy options for managing chemotherapy-induced peripheral neurotoxicity. Expert Opin. Pharmacother. 2018, 19, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Jordan, B.; Margulies, A.; Cardoso, F.; Cavaletti, G.; Haugnes, H.S.; Jahn, P.; Le Rhun, E.; Preusser, M.; Scotté, F.; Taphoorn, M.J.B.; et al. Systemic anticancer therapy-induced peripheral and central neurotoxicity: ESMO-EONS-EANO Clinical Practice Guidelines for diagnosis, prevention, treatment and follow-up. Ann. Oncol. 2020, 31, 1306–1319. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, W.; Kim, J.H.; Woo, M.K.; Baek, J.Y.; Kim, S.Y.; Chung, S.H.; Kim, H.J. A prospective study of chronic oxaliplatin-induced neuropathy in patients with colon cancer: Long-Term Outcomes and Predictors of Severe Oxaliplatin-Induced Neuropathy. J. Clin. Neurol. 2018, 14, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Hoffman, E.M.; Mauermann, M.L.; Loprinzi, C.L.; Windebank, A.J.; Klein, C.J.; Staff, N.P. Incidence and disease burden of chemotherapy-induced peripheral neuropathy in a population-based cohort. J. Neurol. Neurosurg. Psychiatry 2018, 89, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Fung, C.; Sesso, H.D.; Williams, A.M.; Kerns, S.L.; Monahan, P.; Abu Zaid, M.; Feldman, D.R.; Hamilton, R.J.; Vaughn, D.J.; Beard, C.J.; et al. Multi-institutional assessment of adverse health outcomes among North American testicular cancer survivors after modern cisplatin-based chemotherapy. J. Clin. Oncol. 2017, 35, 1211–1222. [Google Scholar] [CrossRef] [PubMed]
- Kerckhove, N.; Collin, A.; Condé, S.; Chaleteix, C.; Pezet, D.; Balayssac, D. Long-Term Effects, Pathophysiological mechanisms, and risk factors of chemotherapy-induced peripheral neuropathies: A comprehensive literature review. Front. Pharmacol. 2017, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Argyriou, A.A.; Assimakopoulos, K.; Iconomou, G.; Giannakopoulou, F.; Kalofonos, H.P. Either called “chemobrain” or “chemofog”, the long-term chemotherapy-induced cognitive decline in cancer survivors is real. J. Pain Symptom Manag. 2011, 41, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Marín, A.P.; Sánchez, A.R.; Arranz, E.E.; Auñón, P.Z.; Barón, M.G. Adjuvant chemotherapy for breast cancer and cognitive impairment. South Med. J. 2009, 102, 929–934. [Google Scholar] [CrossRef] [PubMed]
- McCrary, J.M.; Goldstein, D.; Boyle, F.; Cox, K.; Grimison, P.; Kiernan, M.C.; Krishnan, A.V.; Lewis, C.R.; Webber, K.; Baron-Hay, S.; et al. Optimal clinical assessment strategies for chemotherapy-induced peripheral neuropathy (CIPN): A systematic review and Delphi survey. Support. Care Cancer 2017, 25, 3485–3493. [Google Scholar] [CrossRef] [PubMed]
- Gewandter, J.S.; Gibbons, C.H.; Campagnolo, M.; Lee, J.; Chaudari, J.; Ward, N.; Burke, L.; Cavaletti, G.; Herrmann, D.N.; McArthur, J.C.; et al. Clinician-rated measures for distal symmetrical axonal polyneuropathy: ACTTION systematic review. Neurology 2019, 93, 346–360. [Google Scholar] [CrossRef] [PubMed]
- Tofthagen, C.S.; Cheville, A.L.; Loprinzi, C.L. The Physical Consequences of Chemotherapy-Induced Peripheral Neuropathy. Curr. Oncol. Rep. 2020, 22, 50. [Google Scholar] [CrossRef] [PubMed]
- Kenzik, K.M. Health care use during cancer survivorship: Review of 5 years of evidence. Cancer 2019, 125, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Q. Neurologic complications of cancer therapies. Curr. Neurol. Neurosci. Rep. 2021, 21, 66. [Google Scholar] [CrossRef] [PubMed]
- Országhová, Z.; Mego, M.; Chovanec, M. Long-term cognitive dysfunction in cancer survivors. Front. Mol. Biosci. 2021, 8, 770413. [Google Scholar] [CrossRef] [PubMed]
- Pike, C.T.; Birnbaum, H.G.; Muehlenbein, C.E.; Pohl, G.M.; Natale, R.B. Healthcare costs and workloss burden of patients with chemotherapy-associated peripheral neuropathy in breast, ovarian, head and neck, and nonsmall cell lung cancer. Chemother. Res. Pract. 2012, 2012, 913848. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).