Revisiting Genetic Influence on Mercury Exposure and Intoxication in Humans: A Scoping Review
Abstract
1. Introduction
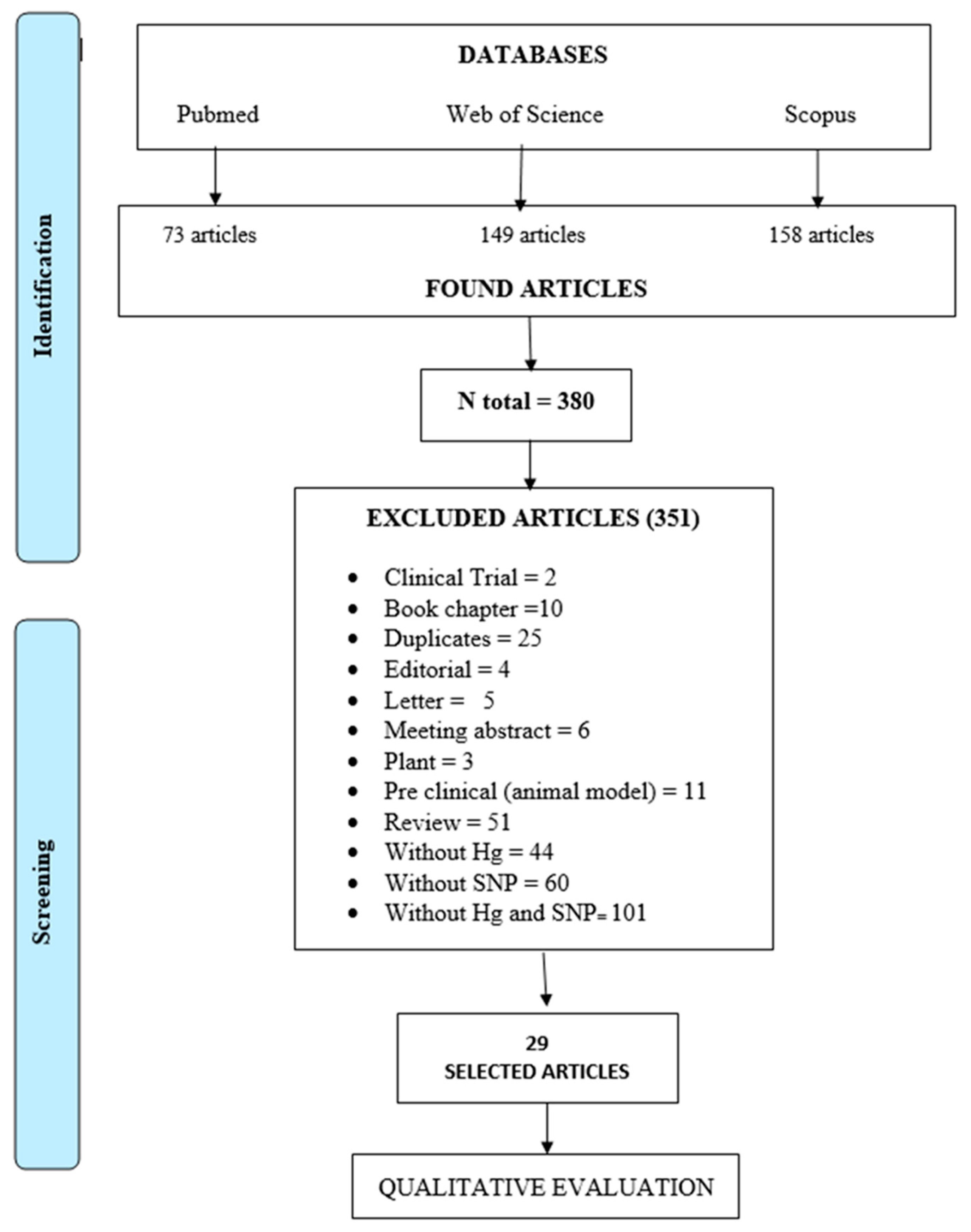
2. Materials and Methods
3. Results and Discussion
3.1. Results of the Screening Process
3.2. Origin of the Studies and Populations
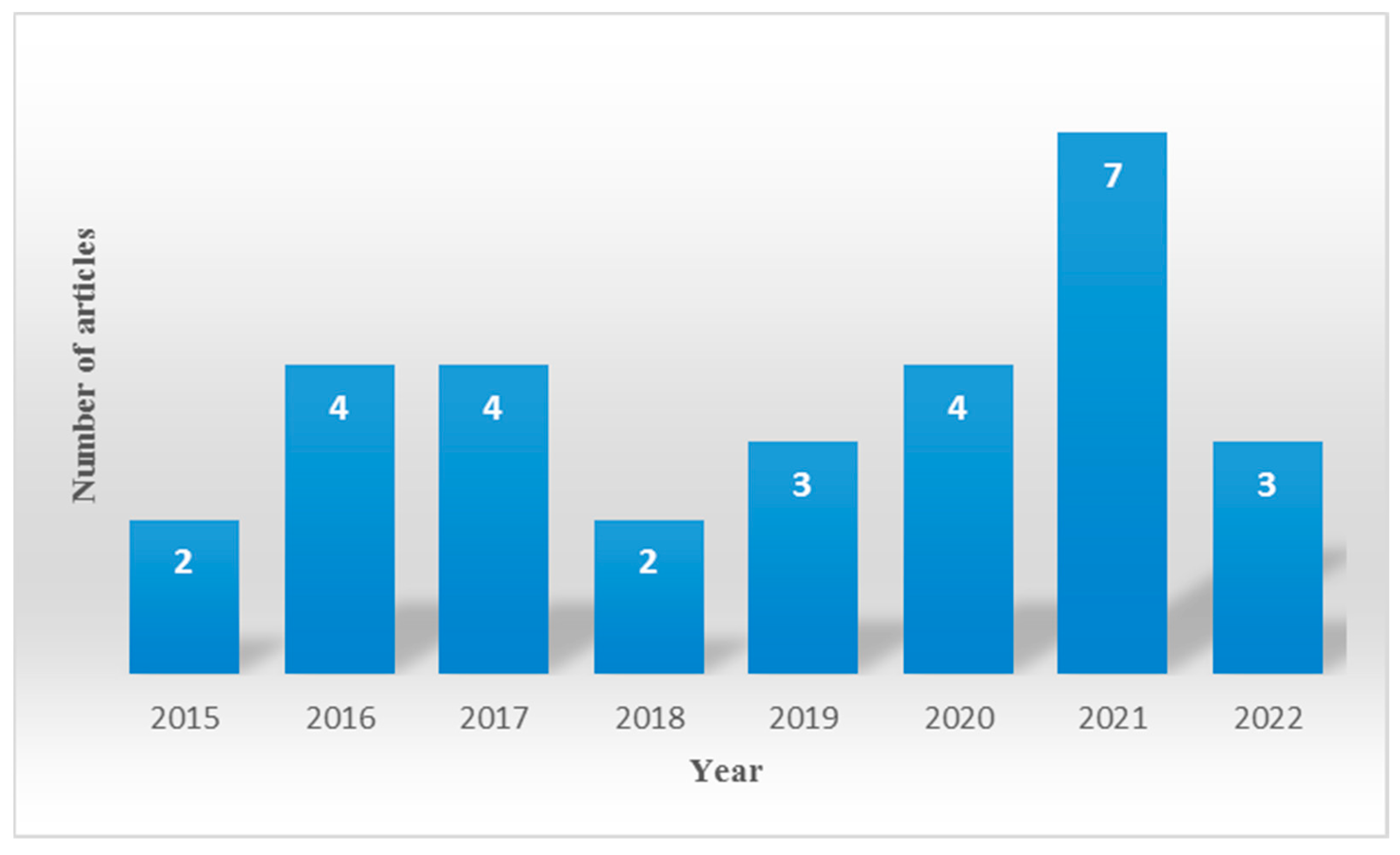
3.3. Other Features of the Studies: Year of Publication and Biomarkers
| Population | Exposure | Gene (SNPs) | Study | |||
|---|---|---|---|---|---|---|
| Main Type | N (Women, Men) | Matrix | Total Hg (Mean or Median *) | |||
| Adults | Environmental | 395 (188, 207) | Blood | 39.8 µg/L | GSTM1 (deletion), GSTT1 (deletion), GSTP1 (rs1695), GCLM (rs41303970), GCLC (rs17883901), GPX1 (rs1800668), ALAD (rs1800435), VDR (rs1544410), MDR1 (rs2032582) | [41] |
| 113 (50, 63) | 7.0 µg/L (plasma) | eNOS (rs11771443, rs1799983, VNTR 4a/4b) | [42] | |||
| 889 (498, 391) | 1.40 μg/L | PON1 (rs662) | [43] | |||
| 436 (152, 284) | 6.31 µg/L | MT1A (rs 8052394) | [44] | |||
| 200 (200, 0) | 1.8 µg/kg (erythrocytes) | MT1A (rs11640851), MT4 (rs11643815), HFE (rs1800562, rs1799945), VDR (rs1544410), ALAD (rs1800435), GSTP1 (rs1695, rs1138272), GCLC (rs17883901), GCLM (rs41303970), ABCB1 (rs2032582, rs1128503, rs2032582), ABCB11 (rs2287622, rs497692), ABCC1 (rs246221), ABCC2 (rs717620, rs2273697), ABCG2 (rs2231142), UGT2B15 (rs1902023) | [37] | |||
| 149 (149, 0) | Hair | 0.6 μg/g | GPX1 (rs1800668), GSTM1(deletion) | [45] | ||
| 823 (521, 302) | 4.84 µg/g | APOE (rs429358, rs7412) | [36] | |||
| 200 (109, 91) | 6.6 µg/g | TNF-α (rs1799964, rs1799724, rs1800629), IL6 (rs1800795), ALAD (rs1800435), GSTP1 (rs1695), VDR (rs2228570), MMP2 (rs2285053) | [46] | |||
| 2562 (1130, 1432) | Toenails | 0.066 μg/g | MTF1 (rs12751325, rs3748682), SLC7A8 (rs11624694, rs17183863), MT4 (rs11643815, rs17285449, rs7186103), MMP2 (rs17859821, rs2576550, rs11859163, rs34373154) | [39] | ||
| Environmental and occupational | 380 (142, 238) | Hair Blood Urine | 0.62 µg/g 3.75 µg/L 1.32 µg/L | GCLC (rs17883901), GLRX2 (rs912071), TXNRD2 (rs5748469), MT1B (rs7191779, rs8052334), MT1M (rs2270836), MT4 (rs11643815), SLC7A7 (rs2281677), SLC43A2 (rs4790732), DNMT1 (rs2228613), GCLC (rs17883901), GSTA4 (rs367836), GSTP1 (rs1695, rs1138272), TXNRD2 (rs5748469), MT1M (rs2270836), MT4 (rs11643815), ABCB1 (rs9282564), SLC22A8 (rs4149182), SLC43A2 (rs4790732), DNMT1 (rs2228613), GPX6 (rs6413428), SEPN1 (rs7349185), SEPN1 (rs2294228), SEPHS2 (rs1133238), SEPP1 (rs3877899), TXNRD3 (rs3108755), ATP7B (rs1801243), SLC22A6 (rs4149170), MTHFR (rs2274976), HBS1L (rs4895441) | [47] | |
| Occupational | 180 (0, 180) | Blood | 18.67 µg/L | GSTM1 (deletion) GSTT1 (deletion) | [48] | |
| 120 (0, 120) | Blood Urine | In 1987 (blood): 74.93 μg/L In 2005 (urine): 6.22 µg/L, 0.21 µg/L 18.22 µg/L | HSPA1B (rs1061581), HSP1A1 (rs1043618), HSP1AL (rs2227956) | [49] | ||
| 968 (391, 577) | Urine | 10 μg/g creatinine | ABCC2 (rs1885301, rs717620, rs2273697) | [27] | ||
| 281 (121, 160) | Blood Urine Hair | 7 µg/L 3.8 µg/g creatinine 0.8 µg/g | ABCB1 (rs1202169), ABCC2 (rs1885301), SLC22A6 (rs 4149170), SLC22A8 (rs 4149182) | [50] | ||
| 281 (121, 160) | Blood Urine Hair | 7 µg/L 3.8 µg/g creatinine 0.8 µg/g | GCLC (rs 1555903), GCLM (rs41303970), GSS (rs3761144), GSTA1 (rs3957356), GSTP1 (rs 4147581) | [29] | ||
| Mothers and children | Environmental | 1331 | Hair | 3.9 µg/g | ABCC1 (rs215088, rs1292798, rs1107529, rs246241, rs212093, rs11075290); ABCC2 (rs2273697, rs717620, rs2756103, rs7393105, rs2273697); ABCB1 (rs2032582, rs2235035, rs1027458, rs1202169, rs1202171, rs1027649, rs2032582). | [51] |
| 1688 | Hair U cord | 0.361 µg/g 0.002 µg/g | APOE (rs429358, rs7412) | [38] | ||
| 2898 | Blood Hair U cord | 18.22 μg/L 3.87 µg/g 34.48 μg/L | GCLM (rs 41303970), GCLC (rs 761142), GSTP1(rs 1695) | [52] | ||
| 562 | Blood Hair Serum Placenta U cord Cord serum | 3.54 μg/L 0.88 µg/g 0.78 μg/L 9.49 μg/kg 5.85 μg/L 0.61 μg/L | MT2A (rs 28366003) | [53] | ||
| 436 | Blood Hair Milk U cord Urine | 0.002 μg/g 0.51 µg/g 0.14 ng/g 0.003 μg/g 0.74 μg/L | APOE (rs 429358, rs7412) | [54] | ||
| 344 | Hair | 0.99 μg/g (mother) 1.02 µg/g | GCLC (rs 17883901), GCLM (rs41303970), GPX1 (rs1050450), GSTA1 (rs3957356), GSTP1 (rs1695), MT1M (rs2270836, rs9936741), MT2A (rs10636), MT4 (rs 11643815) | [55] | ||
| 946 | U cord | 35 µg/L | ABCB1 (rs2032582, rs10276499, rs1202169), ABCC1(rs11075290 e rs215088), ABCC2 (rs717620) | [56] | ||
| 2639 | M hair U cord | NC1- Seychelles: 5.8 µg/g 39.3 μg/L NC2-Seychelles: 3.9 µg/g INMA-Spain: 11.3 μg/L PHIME-Italy: 1.0 µg/g 5.6 μg/L | CYP3A7 (rs2257401), CYP3A5 (rs776746), CYP3A4 (rs2740574) | [57] | ||
| Children | Environmental | 2172 | U cord | 2.70 μg/L | ABCA1 (rs4149268, rs3890182), TF (rs3811647), PON1 (rs662), BDNF (rs2049046), PGR (rs1042838), SOD2 (rs5746136), MT1M (rs2270836) | [58] |
| 532 | Blood | 1 µg/L | GSTP1 (rs 1695), GSTT1 (deletion), GSTM1 (deletion) | [59] | ||
| 103 | Hair | 7.0 µg/g | ALAD (rs 1800435) | [60] | ||
| 403 | 0.89 µg/g | PON1 (rs662; rs705381), BDNF (rs1519480, rs7934165, rs6265, rs12273363, rs7103411), APOA4 (rs5110) APOE (rs7412), GSTP1 (rs1695) | [28] | |||
| 412 | Hair at 9 years old U cord | Female: 1.0 μg/g 11.0 μg/L Male: 0.8 μg/g 10.7 μg/L | BDNF (rs12273363, rs7934165, rs7103411, rs1100104, rs6265, rs925946) | [61] | ||
| 466 | Urine | 1.06 μg/g creatinine (n = 238) | BDNF (rs6265, rs2883187, rs7124442) | [62] | ||
3.4. Analysis of Genetic Susceptibility to Mercury Exposure and Intoxication
3.5. Insights and Recommendations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arrifano, G.P.; Augusto-Oliveira, M.; Lopes-Araujo, A.; Santos-Sacramento, L.; Macchi, B.M.; Nascimento, J.; Crespo-Lopez, M.E. Global Human Threat: The Potential Synergism between Mercury Intoxication and COVID-19. Int. J. Environ. Res. Public Health 2023, 20, 4207. [Google Scholar] [CrossRef]
- Arrifano, G.P.; Crespo-Lopez, M.E.; Lopes-Araujo, A.; Santos-Sacramento, L.; Barthelemy, J.L.; de Nazare, C.G.L.; Freitas, L.G.R.; Augusto-Oliveira, M. Neurotoxicity and the Global Worst Pollutants: Astroglial Involvement in Arsenic, Lead, and Mercury Intoxication. Neurochem. Res. 2023, 48, 1047–1065. [Google Scholar] [CrossRef]
- Crespo-Lopez, M.E.; Augusto-Oliveira, M.; Lopes-Araújo, A.; Santos-Sacramento, L.; Yuki Takeda, P.; Macchi, B.d.M.; do Nascimento, J.L.M.; Maia, C.S.F.; Lima, R.R.; Arrifano, G.P. Mercury: What can we learn from the Amazon? Environ. Int. 2021, 146, 106223. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry. ATSDR 2022 Substance Priority List. Available online: https://www.atsdr.cdc.gov/spl/index.html (accessed on 15 September 2023).
- UNEP. Global Mercury Assessment 2018; United Nations Environment Programme: Nairobi, Kenya, 2019. [Google Scholar]
- Crespo-Lopez, M.E.; Augusto-Oliveira, M.; Lopes-Araújo, A.; Santos-Sacramento, L.; Souza-Monteiro, J.R.; da Rocha, F.F.; Arrifano, G.d.P. Chapter Eight-Mercury neurotoxicity in gold miners. In Advances in Neurotoxicology; Lucchini, R.G., Aschner, M., Costa, L.G., Eds.; Academic Press: Cambridge, MA, USA, 2022; Volume 7, pp. 283–314. [Google Scholar]
- Crespo-Lopez, M.E.; Arrifano, G.P.; Augusto-Oliveira, M.; Macchi, B.M.; Lima, R.R.; do Nascimento, J.L.M.; Souza, C.B.A. Mercury in the Amazon: The danger of a single story. Ecotoxicol. Environ. Saf. 2023, 256, 114895. [Google Scholar] [CrossRef]
- Arrifano, G.P.F.; Martín-Doimeadios, R.C.R.; Jiménez-Moreno, M.; Ramírez-Mateos, V.; da Silva, N.F.S.; Souza-Monteiro, J.R.; Augusto-Oliveira, M.; Paraense, R.S.O.; Macchi, B.M.; do Nascimento, J.L.M.; et al. Large-scale projects in the amazon and human exposure to mercury: The case-study of the Tucuruí Dam. Ecotoxicol. Environ. Saf. 2018, 147, 299–305. [Google Scholar] [CrossRef]
- Arrifano, G.; Del Carmen Rodriguez Martin-Doimeadios, R.; Jimenez-Moreno, M.; Augusto-Oliveira, M.; Rogerio Souza-Monteiro, J.; Paraense, R.; Rodrigues Machado, C.; Farina, M.; Macchi, B.; do Nascimento, J.; et al. Assessing mercury intoxication in isolated/remote populations: Increased S100B mRNA in blood in exposed riverine inhabitants of the Amazon. Neurotoxicology 2018, 68, 151–158. [Google Scholar] [CrossRef]
- Kumari, P.; Chowdhury, A.; Maiti, S.K. Assessment of heavy metal in the water, sediment, and two edible fish species of Jamshedpur Urban Agglomeration, India with special emphasis on human health risk. Hum. Ecol. Risk Assess. Int. J. 2018, 24, 1477–1500. [Google Scholar] [CrossRef]
- Rashid, S.; Shah, I.A.; Supe Tulcan, R.X.; Rashid, W.; Sillanpaa, M. Contamination, exposure, and health risk assessment of Hg in Pakistan: A review. Environ. Pollut. 2022, 301, 118995. [Google Scholar] [CrossRef]
- Li, W.C.; Tse, H.F. Health risk and significance of mercury in the environment. Environ. Sci. Pollut. Res. 2015, 22, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.; Naz, A.; Maiti, S.K. Bioaccumulation of potentially toxic elements in three mangrove species and human health risk due to their ethnobotanical uses. Environ. Sci. Pollut. Res. 2021, 28, 33042–33059. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Martin-Doimeadios, R.C.; Berzas Nevado, J.J.; Guzman Bernardo, F.J.; Jimenez Moreno, M.; Arrifano, G.P.; Herculano, A.M.; do Nascimento, J.L.; Crespo-Lopez, M.E. Comparative study of mercury speciation in commercial fishes of the Brazilian Amazon. Environ. Sci. Pollut. Res. Int. 2014, 21, 7466–7479. [Google Scholar] [CrossRef]
- Machado, C.L.R.; Crespo-Lopez, M.E.; Augusto-Oliveira, M.; Arrifano, G.P.; Macchi, B.M.; Lopes-Araujo, A.; Santos-Sacramento, L.; Souza-Monteiro, J.R.; Alvarez-Leite, J.I.; Souza, C.B.A. Eating in the Amazon: Nutritional Status of the Riverine Populations and Possible Nudge Interventions. Foods 2021, 10, 1015. [Google Scholar] [CrossRef]
- Santos-Sacramento, L.; Arrifano, G.P.; Lopes-Araujo, A.; Augusto-Oliveira, M.; Albuquerque-Santos, R.; Takeda, P.Y.; Souza-Monteiro, J.R.; Macchi, B.M.; do Nascimento, J.L.M.; Lima, R.R.; et al. Human neurotoxicity of mercury in the Amazon: A scoping review with insights and critical considerations. Ecotoxicol. Environ. Saf. 2021, 208, 111686. [Google Scholar] [CrossRef]
- Lopes-Araujo, A.; Arrifano, G.P.; Macchi, B.M.; Augusto-Oliveira, M.; Santos-Sacramento, L.; Rodriguez Martin-Doimeadios, R.C.; Jimenez-Moreno, M.; Martins Filho, A.J.; Alvarez-Leite, J.I.; Oria, R.B.; et al. Hair mercury is associated with dyslipidemia and cardiovascular risk: An anthropometric, biochemical and genetic cross-sectional study of Amazonian vulnerable populations. Environ. Res. 2023, 229, 115971. [Google Scholar] [CrossRef]
- Arrifano, G.P.; Alvarez-Leite, J.I.; Macchi, B.M.; Campos, N.; Augusto-Oliveira, M.; Santos-Sacramento, L.; Lopes-Araujo, A.; Souza-Monteiro, J.R.; Alburquerque-Santos, R.; do Nascimento, J.L.M.; et al. Living in the Southern Hemisphere: Metabolic Syndrome and Its Components in Amazonian Riverine Populations. J. Clin. Med. 2021, 10, 3630. [Google Scholar] [CrossRef]
- Arrifano, G.P.F.; Alvarez-Leite, J.I.; Souza-Monteiro, J.R.; Augusto-Oliveira, M.; Paraense, R.; Macchi, B.M.; Pinto, A.; Oria, R.B.; do Nascimento, J.L.M.; Crespo-Lopez, M.E. In the Heart of the Amazon: Noncommunicable Diseases and Apolipoprotein E4 Genotype in the Riverine Population. Int. J. Environ. Res. Public Health 2018, 15, 1957. [Google Scholar] [CrossRef]
- Bakir, F.; Al-Khalidi, A.; Clarkson, T.W.; Greenwood, R. Clinical observations on treatment of alkylmercury poisoning in hospital patients. Bull. World Health Organ. 1976, 53, 87–92. [Google Scholar]
- Nierenberg, D.W.; Nordgren, R.E.; Chang, M.B.; Siegler, R.W.; Blayney, M.B.; Hochberg, F.; Toribara, T.Y.; Cernichiari, E.; Clarkson, T. Delayed cerebellar disease and death after accidental exposure to dimethylmercury. N. Engl. J. Med. 1998, 338, 1672–1676. [Google Scholar] [CrossRef] [PubMed]
- Brittain, H.K.; Scott, R.; Thomas, E. The rise of the genome and personalised medicine. Clin. Med. 2017, 17, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Kamps, R.; Brandão, R.D.; Bosch, B.J.; Paulussen, A.D.; Xanthoulea, S.; Blok, M.J.; Romano, A. Next-Generation Sequencing in Oncology: Genetic Diagnosis, Risk Prediction and Cancer Classification. Int. J. Mol. Sci. 2017, 18, 308. [Google Scholar] [CrossRef] [PubMed]
- Llop, S.; Ballester, F.; Broberg, K. Effect of Gene-Mercury Interactions on Mercury Toxicokinetics and Neurotoxicity. Curr. Environ. Health Rep. 2015, 2, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Joneidi, Z.; Mortazavi, Y.; Memari, F.; Roointan, A.; Chahardouli, B.; Rostami, S. The impact of genetic variation on metabolism of heavy metals: Genetic predisposition? Biomed. Pharmacother. 2019, 113, 108642. [Google Scholar] [CrossRef] [PubMed]
- Arrifano, G.P.F.; de Oliveira, M.A.; Souza-Monteiro, J.R.; Paraense, R.O.; Ribeiro-Dos-Santos, A.; Vieira, J.; Silva, A.; Macchi, B.M.; do Nascimento, J.L.M.; Burbano, R.M.R.; et al. Role for apolipoprotein E in neurodegeneration and mercury intoxication. Front. Biosci. Elite Ed. 2018, 10, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Kolbinger, V.; Engström, K.; Berger, U.; Bose-O’Reilly, S. Polymorphisms in potential mercury transporter ABCC2 and neurotoxic symptoms in populations exposed to mercury vapor from goldmining. Environ. Res. 2019, 176, 108512. [Google Scholar] [CrossRef] [PubMed]
- Lozano, M.; Murcia, M.; Soler-Blasco, R.; González, L.; Iriarte, G.; Rebagliato, M.; Lopez-Espinosa, M.J.; Esplugues, A.; Ballester, F.; Llop, S. Exposure to mercury among 9-year-old children and neurobehavioural function. Environ. Int. 2021, 146, 106173. [Google Scholar] [CrossRef] [PubMed]
- Medina Pérez, O.M.; Flórez-Vargas, O.; Rincón Cruz, G.; Rondón González, F.; Rocha Muñoz, L.; Sánchez Rodríguez, L.H. Glutathione-related genetic polymorphisms are associated with mercury retention and nephrotoxicity in gold-mining settings of a Colombian population. Sci. Rep. 2021, 11, 8716. [Google Scholar] [CrossRef] [PubMed]
- Andreoli, V.; Sprovieri, F. Genetic Aspects of Susceptibility to Mercury Toxicity: An Overview. Int. J. Environ. Res. Public Health 2017, 14, 93. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Galvis, S.R. The Amazon Biome in the Face of Mercury Contamination: An Overview of Mercury Trade, Science, and Policy in the Amazonian Countries. WWF Gaia Amaz. 2020, 1–168. Available online: https://wwfint.awsassets.panda.org/downloads/reporte_eng_2.pdf (accessed on 15 September 2023).
- Augusto-Oliveira, M.; Arrifano, G.P.; Lopes-Araujo, A.; Santos-Sacramento, L.; Lima, R.R.; Lamers, M.L.; Le Blond, J.; Crespo-Lopez, M.E. Salivary biomarkers and neuropsychological outcomes: A non-invasive approach to investigate pollutants-associated neurotoxicity and its effects on cognition in vulnerable populations. Environ. Res. 2021, 200, 111432. [Google Scholar] [CrossRef]
- Parra, F.C.; Amado, R.C.; Lambertucci, J.R.; Rocha, J.; Antunes, C.M.; Pena, S.D. Color and genomic ancestry in Brazilians. Proc. Natl. Acad. Sci. USA 2003, 100, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Lins, T.C.; Vieira, R.G.; Abreu, B.S.; Grattapaglia, D.; Pereira, R.W. Genetic composition of Brazilian population samples based on a set of twenty-eight ancestry informative SNPs. Am. J. Hum. Biol. Off. J. Hum. Biol. Counc. 2010, 22, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Arrifano, G.; Martin-Doimeadios, R.; Jimenez-Moreno, M.; Fernandez-Trujillo, S.; Augusto-Oliveira, M.; Souza-Monteiro, J.; Macchi, B.; Alvarez-Leite, J.; do Nascimento, J.; Amador, M.; et al. Genetic Susceptibility to Neurodegeneration in Amazon: Apolipoprotein E Genotyping in Vulnerable Populations Exposed to Mercury. Front. Genet. 2018, 9, 285. [Google Scholar] [CrossRef] [PubMed]
- Gundacker, C.; Graf-Rohrmeister, K.; Gencik, M.; Hengstschläger, M.; Holoman, K.; Rosa, P.; Kroismayr, R.; Offenthaler, I.; Plichta, V.; Reischer, T.; et al. Gene Variants Determine Placental Transfer of Perfluoroalkyl Substances (PFAS), Mercury (Hg) and Lead (Pb), and Birth Outcome: Findings From the UmMuKi Bratislava-Vienna Study. Front. Genet. 2021, 12, 664946. [Google Scholar] [CrossRef]
- Snoj Tratnik, J.; Falnoga, I.; Trdin, A.; Mazej, D.; Fajon, V.; Miklavcic, A.; Kobal, A.B.; Osredkar, J.; Sesek Briski, A.; Krsnik, M.; et al. Prenatal mercury exposure, neurodevelopment and apolipoprotein E genetic polymorphism. Environ. Res. 2017, 152, 375–385. [Google Scholar] [CrossRef]
- Creed, J.H.; Peeri, N.C.; Anic, G.M.; Thompson, R.C.; Olson, J.J.; LaRocca, R.V.; Chowdhary, S.A.; Brockman, J.D.; Gerke, T.A.; Nabors, L.B.; et al. Methylmercury exposure, genetic variation in metabolic enzymes, and the risk of glioma. Sci. Rep. 2019, 9, 10861. [Google Scholar] [CrossRef]
- W.H.O. Guidance for Identifying Populations at Risk from Mercury Exposure. Available online: http://www.who.int/foodsafety/publications/risk-mercury-exposure/en/ (accessed on 17 September 2021).
- Barcelos, G.R.; Souza, M.F.; Oliveira, A.A.; Lengert, A.; Oliveira, M.T.; Camargo, R.B.; Grotto, D.; Valentini, J.; Garcia, S.C.; Braga, G.U.; et al. Effects of genetic polymorphisms on antioxidant status and concentrations of the metals in the blood of riverside Amazonian communities co-exposed to Hg and Pb. Environ. Res. 2015, 138, 224–232. [Google Scholar] [CrossRef]
- Barcelos, G.R.; De Marco, K.C.; de Rezende, V.B.; Braga, G.; Antunes, L.M.; Tanus-Santos, J.E.; Barbosa, F., Jr. Genetic Effects of eNOS Polymorphisms on Biomarkers Related to Cardiovascular Status in a Population Coexposed to Methylmercury and Lead. Arch. Environ. Contam. Toxicol. 2015, 69, 173–180. [Google Scholar] [CrossRef]
- Almeida Lopes, A.C.B.; Urbano, M.R.; Souza-Nogueira, A.; Oliveira-Paula, G.H.; Michelin, A.P.; Carvalho, M.F.H.; Camargo, A.E.I.; Peixe, T.S.; Cabrera, M.A.S.; Paoliello, M.M.B. Association of lead, cadmium and mercury with paraoxonase 1 activity and malondialdehyde in a general population in Southern Brazil. Environ. Res. 2017, 156, 674–682. [Google Scholar] [CrossRef]
- Sirivarasai, J.; Chaisungnern, K.; Panpunuan, P.; Chanprasertyothin, S.; Chansirikanjana, S.; Sritara, P. Role of MT1A Polymorphism and Environmental Mercury Exposure on the Montreal Cognitive Assessment (MoCA). Neuropsychiatr. Dis. Treat. 2021, 17, 2429–2439. [Google Scholar] [CrossRef]
- Rocha, A.V.; Rita Cardoso, B.; Zavarize, B.; Almondes, K.; Bordon, I.; Hare, D.J.; Teixeira Favaro, D.I.; Franciscato Cozzolino, S.M. GPX1 Pro198Leu polymorphism and GSTM1 deletion do not affect selenium and mercury status in mildly exposed Amazonian women in an urban population. Sci. Total Environ. 2016, 571, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Basta, P.C.; Viana, P.V.S.; Vasconcellos, A.C.S.; Périssé, A.R.S.; Hofer, C.B.; Paiva, N.S.; Kempton, J.W.; Ciampi de Andrade, D.; Oliveira, R.A.A.; Achatz, R.W.; et al. Mercury Exposure in Munduruku Indigenous Communities from Brazilian Amazon: Methodological Background and an Overview of the Principal Results. Int. J. Environ. Res. Public Health 2021, 18, 9222. [Google Scholar] [CrossRef] [PubMed]
- Parajuli, R.P.; Goodrich, J.M.; Chou, H.N.; Gruninger, S.E.; Dolinoy, D.C.; Franzblau, A.; Basu, N. Genetic polymorphisms are associated with hair, blood, and urine mercury levels in the American Dental Association (ADA) study participants. Environ. Res. 2016, 149, 247–258. [Google Scholar] [CrossRef]
- Ilyinskikh, N.N.; Ilyinskikh, E.N.; Ilyinskikh, I.N.; Gvozdareva, O.V. Gene Polymorphisms, Mercury Concentration, and Cytogenetic Damage in the Inhabitants of the Mercury Ore Minining and Recycling Area. Hum. Ecol. 2016, 23, 17–23. [Google Scholar] [CrossRef]
- Chernyak, Y.I.; Merinova, A.P. HSP70 (HSPA1) polymorphisms in former workers with chronic mercury vapor exposure. Int. J. Occup. Med. Environ. Health 2017, 30, 77–85. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sánchez Rodríguez, L.H.; Medina Pérez, O.M.; Rondón González, F.; Rincón Cruz, G.; Rocha Muñoz, L.; Flórez-Vargas, O. Genetic Polymorphisms in Multispecific Transporters Mitigate Mercury Nephrotoxicity in an Artisanal and Small-Scale Gold Mining Community in Colombia. Toxicol. Sci. Off. J. Soc. Toxicol. 2020, 178, 338–346. [Google Scholar] [CrossRef]
- Engström, K.; Love, T.M.; Watson, G.E.; Zareba, G.; Yeates, A.; Wahlberg, K.; Alhamdow, A.; Thurston, S.W.; Mulhern, M.; McSorley, E.M.; et al. Polymorphisms in ATP-binding cassette transporters associated with maternal methylmercury disposition and infant neurodevelopment in mother-infant pairs in the Seychelles Child Development Study. Environ. Int. 2016, 94, 224–229. [Google Scholar] [CrossRef]
- Wahlberg, K.; Love, T.M.; Pineda, D.; Engström, K.; Watson, G.E.; Thurston, S.W.; Yeates, A.J.; Mulhern, M.S.; McSorley, E.M.; Strain, J.J.; et al. Maternal polymorphisms in glutathione-related genes are associated with maternal mercury concentrations and early child neurodevelopment in a population with a fish-rich diet. Environ. Int. 2018, 115, 142–149. [Google Scholar] [CrossRef]
- Sekovanić, A.; Piasek, M.; Orct, T.; Sulimanec Grgec, A.; Matek Sarić, M.; Stasenko, S.; Jurasović, J. Mercury Exposure Assessment in Mother-Infant Pairs from Continental and Coastal Croatia. Biomolecules 2020, 10, 821. [Google Scholar] [CrossRef]
- Trdin, A.; Snoj Tratnik, J.; Stajnko, A.; Marc, J.; Mazej, D.; Sešek Briški, A.; Kastelec, D.; Prpić, I.; Petrović, O.; Špirić, Z.; et al. Trace elements and APOE polymorphisms in pregnant women and their new-borns. Environ. Int. 2020, 143, 105626. [Google Scholar] [CrossRef]
- Chan, P.H.Y.; Chan, K.Y.Y.; Schooling, C.M.; Hui, L.L.; Chan, M.H.M.; Li, A.M.; Cheung, R.C.K.; Lam, H.S. Association between genetic variations in GSH-related and MT genes and low-dose methylmercury exposure in children and women of childbearing age: A pilot study. Environ. Res. 2020, 187, 109703. [Google Scholar] [CrossRef] [PubMed]
- Love, T.M.; Wahlberg, K.; Pineda, D.; Watson, G.E.; Zareba, G.; Thurston, S.W.; Davidson, P.W.; Shamlaye, C.F.; Myers, G.J.; Rand, M.; et al. Contribution of child ABC-transporter genetics to prenatal MeHg exposure and neurodevelopment. Neurotoxicology 2022, 91, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Llop, S.; Tran, V.; Ballester, F.; Barbone, F.; Sofianou-Katsoulis, A.; Sunyer, J.; Engström, K.; Alhamdow, A.; Love, T.M.; Watson, G.E.; et al. CYP3A genes and the association between prenatal methylmercury exposure and neurodevelopment. Environ. Int. 2017, 105, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Julvez, J.; Davey Smith, G.; Ring, S.; Grandjean, P. A Birth Cohort Study on the Genetic Modification of the Association of Prenatal Methylmercury With Child Cognitive Development. Am. J. Epidemiol. 2019, 188, 1784–1793. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, M.H.; Samms-Vaughan, M.; Saroukhani, S.; Bressler, J.; Hessabi, M.; Grove, M.L.; Shakspeare-Pellington, S.; Loveland, K.A.; Beecher, C.; McLaughlin, W. Associations of Metabolic Genes (GSTT1, GSTP1, GSTM1) and Blood Mercury Concentrations Differ in Jamaican Children with and without Autism Spectrum Disorder. Int. J. Environ. Res. Public Health 2021, 18, 1377. [Google Scholar] [CrossRef]
- Perini, J.A.; Silva, M.C.; Vasconcellos, A.C.S.; Viana, P.V.S.; Lima, M.O.; Jesus, I.M.; Kempton, J.W.; Oliveira, R.A.A.; Hacon, S.S.; Basta, P.C. Genetic Polymorphism of Delta Aminolevulinic Acid Dehydratase (ALAD) Gene and Symptoms of Chronic Mercury Exposure in Munduruku Indigenous Children within the Brazilian Amazon. Int. J. Environ. Res. Public Health 2021, 18, 8746. [Google Scholar] [CrossRef]
- Sarzo, B.; Ballester, F.; Soler-Blasco, R.; Lopez-Espinosa, M.J.; Lozano, M.; Iriarte, G.; Beneito, A.; Riutort-Mayol, G.; Murcia, M.; Llop, S. Pre and postnatal exposure to mercury and sexual development in 9-year-old children in Spain: The role of brain-derived neurotrophic factor. Environ. Res. 2022, 213, 113620. [Google Scholar] [CrossRef]
- Zhu, K.; Liu, Q.; Xie, X.; Jiang, Q.; Feng, Y.; Xiao, P.; Wu, X.; Song, R. The combined effect between BDNF genetic polymorphisms and exposure to metals on the risk of Chinese dyslexia. Environ. Pollut. 2022, 308, 119640. [Google Scholar] [CrossRef]
- Lv, H.; Zhen, C.; Liu, J.; Yang, P.; Hu, L.; Shang, P. Unraveling the Potential Role of Glutathione in Multiple Forms of Cell Death in Cancer Therapy. Oxidative Med. Cell. Longev. 2019, 2019, 3150145. [Google Scholar] [CrossRef]
- Karam, R.A.; Pasha, H.F.; El-Shal, A.S.; Rahman, H.M.; Gad, D.M. Impact of glutathione-S-transferase gene polymorphisms on enzyme activity, lung function and bronchial asthma susceptibility in Egyptian children. Gene 2012, 497, 314–319. [Google Scholar] [CrossRef]
- Mandal, R.K.; Mittal, R.D. Glutathione S-Transferase P1 313 (A > G) Ile105Val Polymorphism Contributes to Cancer Susceptibility in Indian Population: A Meta-analysis of 39 Case-Control Studies. Indian J. Clin. Biochem. IJCB 2020, 35, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Lopez, M.E.; Augusto-Oliveira, M.; Lopes-Araujo, A.; Santos-Sacramento, L.; Barthelemy, J.L.; Aschner, M.; Lima, R.R.; Macchi, B.M.; do Nascimento, J.L.M.; Arrifano, G.P. Translational relevance for in vitro/in vivo models: A novel approach to mercury dosing. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2022, 166, 113210. [Google Scholar] [CrossRef] [PubMed]
- Engstrom, K.S.; Wennberg, M.; Stromberg, U.; Bergdahl, I.A.; Hallmans, G.; Jansson, J.H.; Lundh, T.; Norberg, M.; Rentschler, G.; Vessby, B.; et al. Evaluation of the impact of genetic polymorphisms in glutathione-related genes on the association between methylmercury or n-3 polyunsaturated long chain fatty acids and risk of myocardial infarction: A case-control study. Environ. Health A Glob. Access Sci. Source 2011, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Llop, S.; Engström, K.; Ballester, F.; Franforte, E.; Alhamdow, A.; Pisa, F.; Tratnik, J.S.; Mazej, D.; Murcia, M.; Rebagliato, M.; et al. Polymorphisms in ABC transporter genes and concentrations of mercury in newborns—Evidence from two Mediterranean birth cohorts. PLoS ONE 2014, 9, e97172. [Google Scholar] [CrossRef] [PubMed]
- Babula, P.; Masarik, M.; Adam, V.; Eckschlager, T.; Stiborova, M.; Trnkova, L.; Skutkova, H.; Provaznik, I.; Hubalek, J.; Kizek, R. Mammalian metallothioneins: Properties and functions. Met. Integr. Biometal Sci. 2012, 4, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.F.; Lowe, M.; Chan, H.M. Mercury exposure, cardiovascular disease, and mortality: A systematic review and dose-response meta-analysis. Environ. Res. 2021, 193, 110538. [Google Scholar] [CrossRef]
- Arrifano, G.P.; Augusto-Oliveira, M.; Souza-Monteiro, J.R.; Macchi, B.M.; Lima, R.R.; Sunol, C.; do Nascimento, J.L.M.; Crespo-Lopez, M.E. Revisiting Astrocytic Roles in Methylmercury Intoxication. Mol. Neurobiol. 2021, 58, 4293–4308. [Google Scholar] [CrossRef]
- Hu, X.F.; Singh, K.; Chan, H.M. Mercury Exposure, Blood Pressure, and Hypertension: A Systematic Review and Dose-response Meta-analysis. Environ. Health Perspect. 2018, 126, 076002. [Google Scholar] [CrossRef]

| Main Exposure | Matrix | Study | Main Significant Associations |
|---|---|---|---|
| Occupational | Blood Urine | [49] | Individuals showing CC-HSPA1A (+190G/C) and GG-HSPA1B (+1267A/G), alone or in combination, have a high predicted risk of developing chronic mercury poisoning. |
| Urine | [27] | The SNPs ABCC2 (rs1885301, rs2273697) may differently modulate the individual performance of exposed individuals in neurological tests depending on ancestral background: in African populations, A allele carriers (rs1885301) showed significantly worse performance on the pencil tapping test; in African and Asian populations: A-allele carriers (rs2273697) showed a significantly better performance than GG carriers on the pencil tapping test. | |
| Blood Urine Hair | [50] | The G allele carriers for SLC22A8 (rs4149182) and the T allele carriers for ABCB1 (rs1202169) had an increased urinary clearance rate for mercury. The A allele carriers for SLC22A6 (rs4149170) and the C allele carriers for ABCB1 (rs1202169) showed abnormal levels of estimated glomerular filtration rate and beta-2-microglobulin. | |
| Blood Urine Hair | [29] | The T allele carriers for GCLM (rs41303970) were associated with higher urinary clearance rate of mercury. The C allele carriers for GCLC (rs1555903) were associated with lower levels of beta-2-microglobulin in the exposed group. An interaction between GSTA1 C allele (rs3957356) and GSS G allele (rs3761144) was associated with higher urinary levels of mercury in the exposed group. | |
| Occupational and environmental | Hair Blood Urine | [47] | Multivariate analyses with Bonferroni corrections showed that heterozygotes and minor homozygotes of MT1M rs2270836, MT4 rs11643815 and GCLC rs138528239 accumulated more mercury in high consumers of fish. However, heterozygotes and minor homozygotes of ATP7B rs1061472 and rs732774 and BDNF rs6265 accumulated less mercury in high consumers of fish. |
| Environmental | Blood | [41] | GSTM1 deletion, ALAD1/2 (rs1800435) and A allele carriers for VDR (rs1544410) had higher Hg concentrations in the blood. |
| [44] | G allele carriers for MT1A (rs8052394) in the third tertile of blood mercury showed significantly lower total and attention score. | ||
| [37] | GSTT1 deletion was associated with reduced placental transfer of mercury. | ||
| [59] | The SNP GSTP1 (rs1695, A allele) was associated with high concentrations of blood mercury. | ||
| Hair | [51] | The SNPs ABCC1 (rs11075290, T allele; rs212093, G allele; and rs215088, G allele), ABCC2 (rs717620, T allele) and ABCB1 (rs10276499, T allele; rs1202169, C allele; and rs2032582, T allele) were associated with increased mercury concentration in maternal hair. The SNP ABCC1 (rs11075290, C allele) was associated with poorer performance in childhood neurodevelopment. | |
| [36] | In individuals showing ≥10 µg/g of total mercury in hair, E4 allele carriers for APOE (rs429358, rs7412) had higher levels than E2 allele carriers. | ||
| [55] | The SNPs GCLC-129 (rs17883901, A allele), GPX1-198 (rs1050450, T allele) and MT1M (rs9936741, C allele) were associated with significantly lower hair mercury levels in mothers. The SNPs GSTP1 (rs1695, G allele) and MT1M (rs2270836, T allele) were associated with higher maternal hair mercury concentrations. | ||
| [28] | With an increase in mercury levels, children carrying the GSTP1 rs1695 GA or GG alleles scored worse on problems such as anxiety, depression and somatic complaints than children with the AA genotype. The presence of the E4 allele for APOE (rs7412) was associated with signs such as anxiety, depression, somatic complaints, and social, thought and attention problems in exposed children. | ||
| U cord | [56] | The SNP ABCC1 (rs11075290, T allele) was associated with decreased umbilical cord mercury concentrations. | |
| Hair U cord | [38] | E4 allele carriers for APOE (rs429358, rs7412) were associated with decreased cognitive performance in children. | |
| [61] | In girls, the BDNF SNPs rs7934165 GA, rs7103411 TT, rs11030104 AA and rs6265 CC were associated with lower estradiol levels with increasing cord blood mercury concentrations. In boys, the BDNF SNPs rs6265 CC and rs11030104 AA genotypes were associated with higher testosterone levels with increasing cord blood mercury concentrations. | ||
| Blood Hair Milk U cord Urine | [54] | E4 allele carrier mothers had significantly higher mean levels of (methyl)mercury in peripheral venous blood, cord blood and hair. | |
| Blood Hair U cord | [52] | The SNP GCLC (rs761142, T allele) polymorphism and the combination of GCLC (rs761142, TT) and GCLM (rs41303970, CC) were associated with increased maternal hair mercury concentrations. Additionally, maternal GSTP1 (rs1695, G allele) was associated with a poorer neurodevelopmental performance in children. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crespo-Lopez, M.E.; Barthelemy, J.L.; Lopes-Araújo, A.; Santos-Sacramento, L.; Leal-Nazaré, C.G.; Soares-Silva, I.; Macchi, B.M.; do Nascimento, J.L.M.; Arrifano, G.d.P.; Augusto-Oliveira, M. Revisiting Genetic Influence on Mercury Exposure and Intoxication in Humans: A Scoping Review. Toxics 2023, 11, 967. https://doi.org/10.3390/toxics11120967
Crespo-Lopez ME, Barthelemy JL, Lopes-Araújo A, Santos-Sacramento L, Leal-Nazaré CG, Soares-Silva I, Macchi BM, do Nascimento JLM, Arrifano GdP, Augusto-Oliveira M. Revisiting Genetic Influence on Mercury Exposure and Intoxication in Humans: A Scoping Review. Toxics. 2023; 11(12):967. https://doi.org/10.3390/toxics11120967
Chicago/Turabian StyleCrespo-Lopez, Maria Elena, Jean Ludger Barthelemy, Amanda Lopes-Araújo, Leticia Santos-Sacramento, Caio Gustavo Leal-Nazaré, Isabela Soares-Silva, Barbarella M. Macchi, José Luiz M. do Nascimento, Gabriela de Paula Arrifano, and Marcus Augusto-Oliveira. 2023. "Revisiting Genetic Influence on Mercury Exposure and Intoxication in Humans: A Scoping Review" Toxics 11, no. 12: 967. https://doi.org/10.3390/toxics11120967
APA StyleCrespo-Lopez, M. E., Barthelemy, J. L., Lopes-Araújo, A., Santos-Sacramento, L., Leal-Nazaré, C. G., Soares-Silva, I., Macchi, B. M., do Nascimento, J. L. M., Arrifano, G. d. P., & Augusto-Oliveira, M. (2023). Revisiting Genetic Influence on Mercury Exposure and Intoxication in Humans: A Scoping Review. Toxics, 11(12), 967. https://doi.org/10.3390/toxics11120967









