TGF-β Regulates m6A RNA Methylation after PM2.5 Exposure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Ethical Approval
2.2. PM2.5 Sampling and Suspension Preparation
2.3. Cell Culture and PM2.5 Exposure
2.4. Cell Membrane Permeability Analysis
2.5. RNA Extraction and RNA m6A Determination
2.6. RT-PCR
2.7. ELISA Analysis
2.8. Use of TGF-β Inhibitors
2.9. Statistical Analysis
3. Results
3.1. TGF-β1/Smad2/3 Levels in Lungs of Mice after Real-World PM2.5 Exposure in Chambers
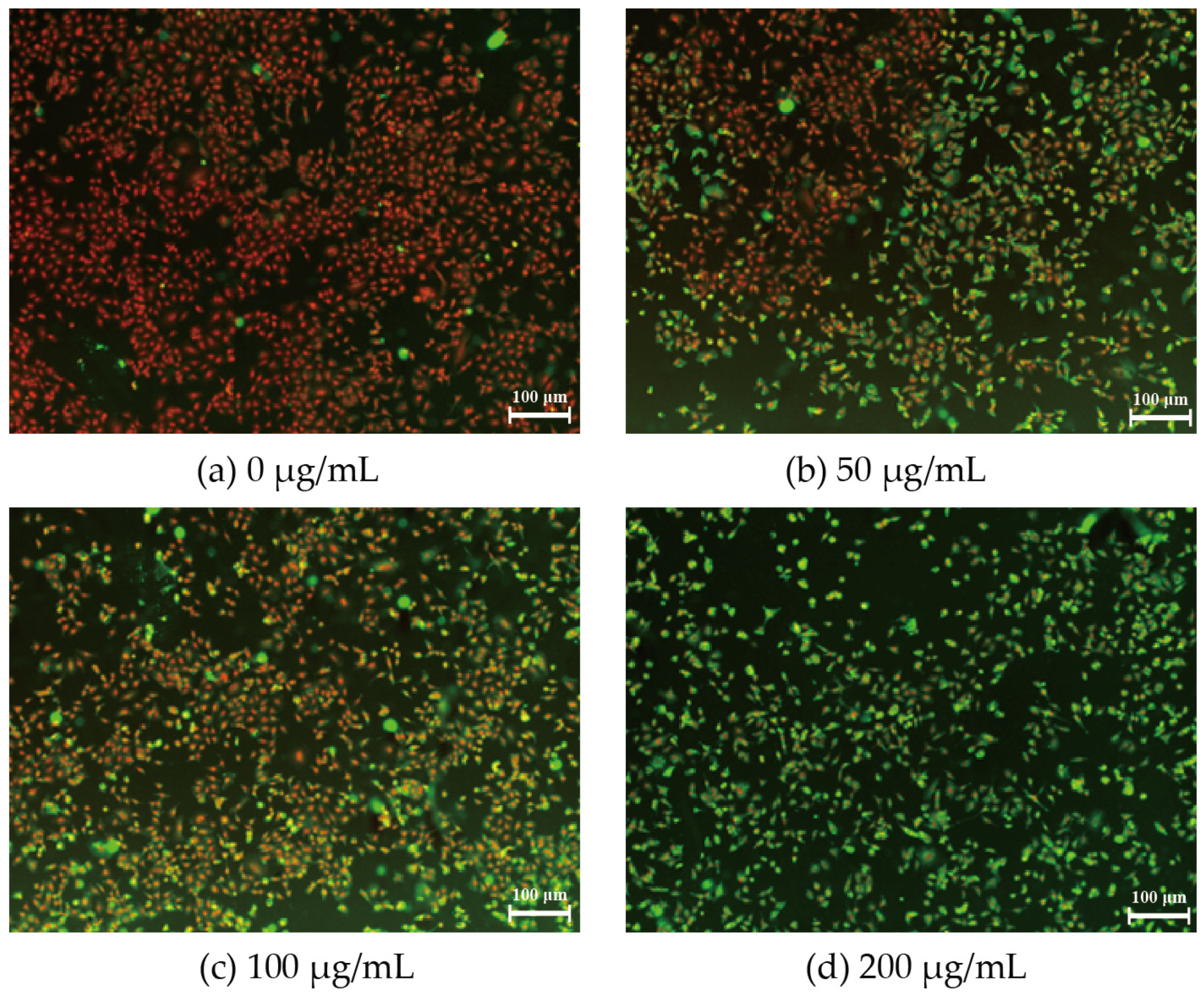
3.2. Permeability of A549 Membrane after PM2.5 Exposure
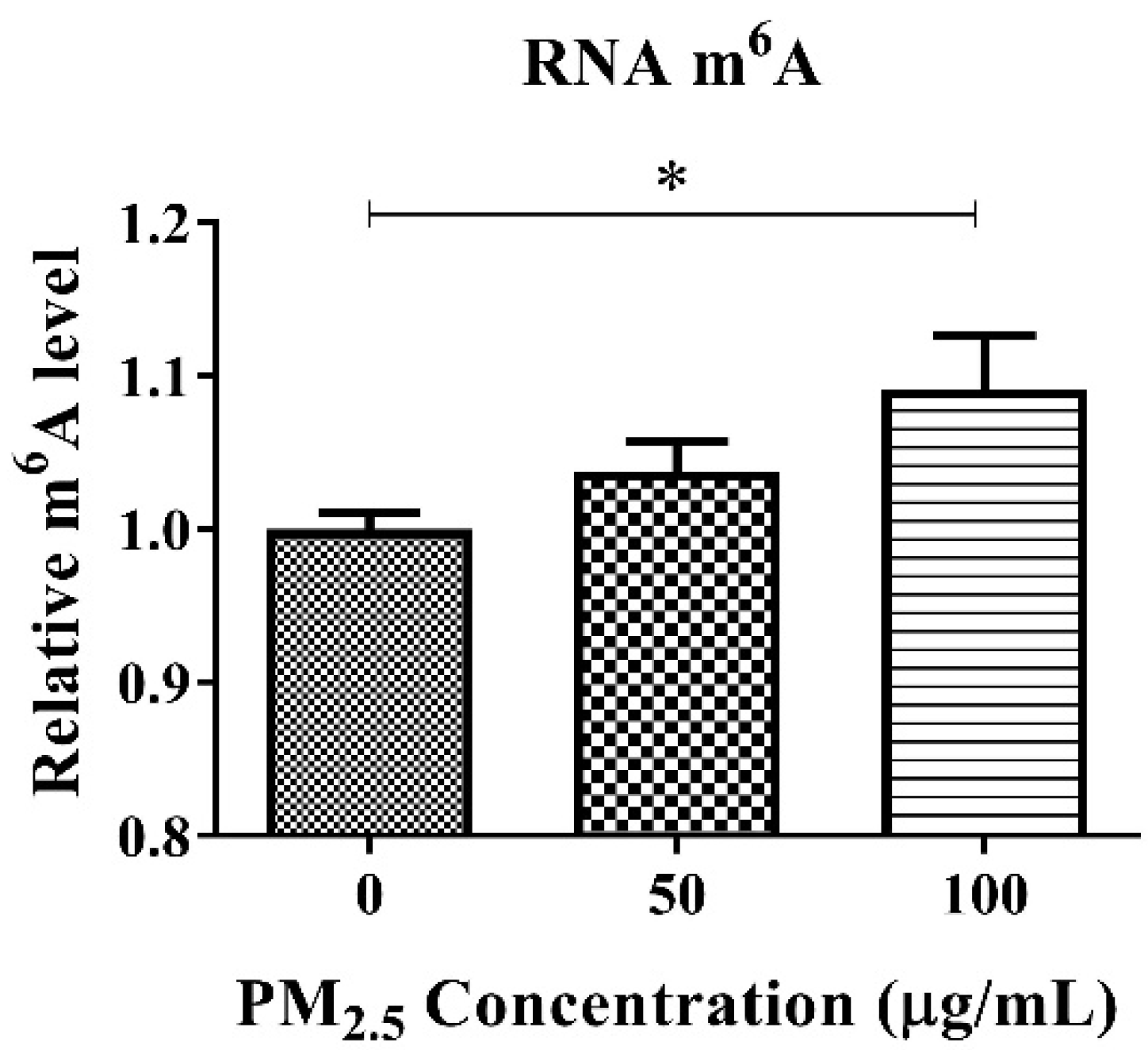
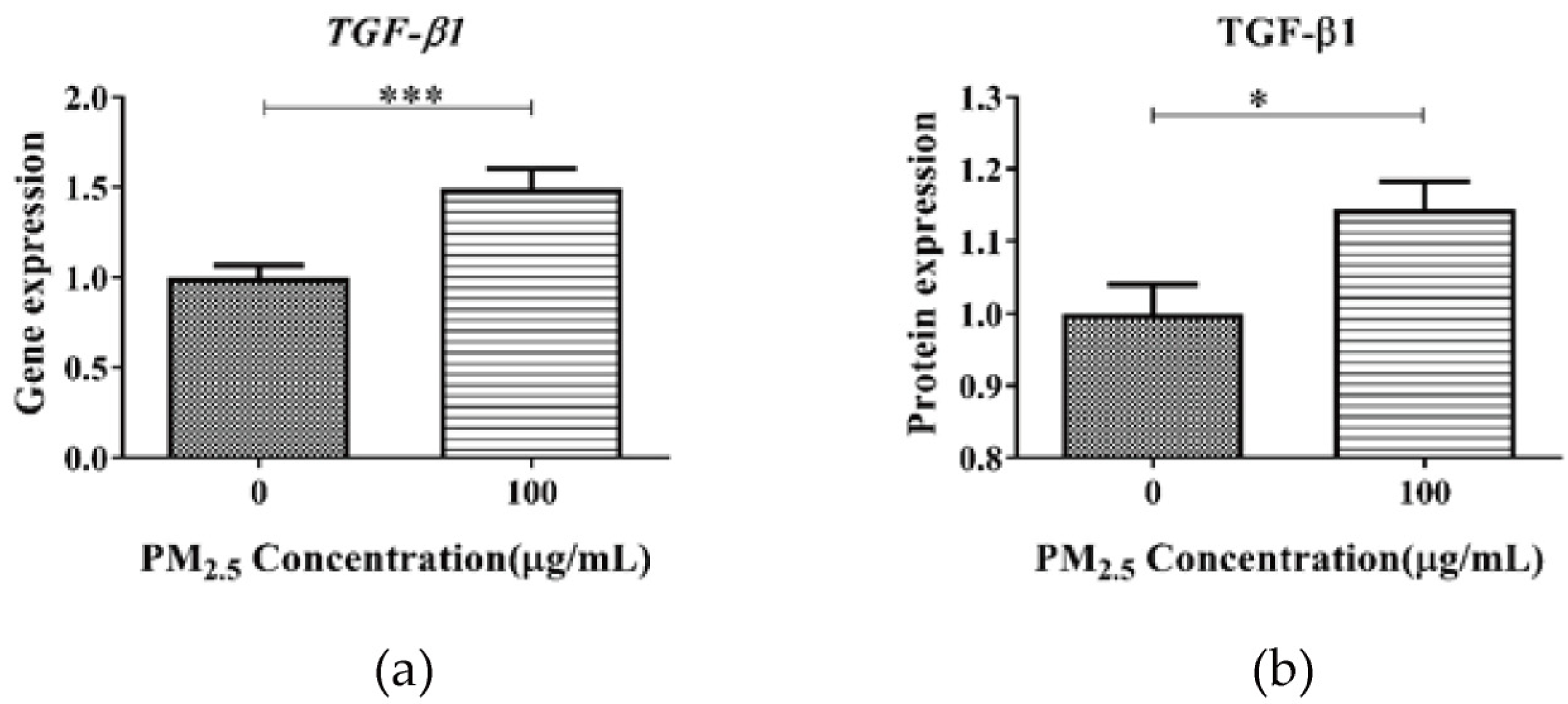
3.3. RNA m6A and TGF-β1 Levels in A549 after PM2.5 Exposure
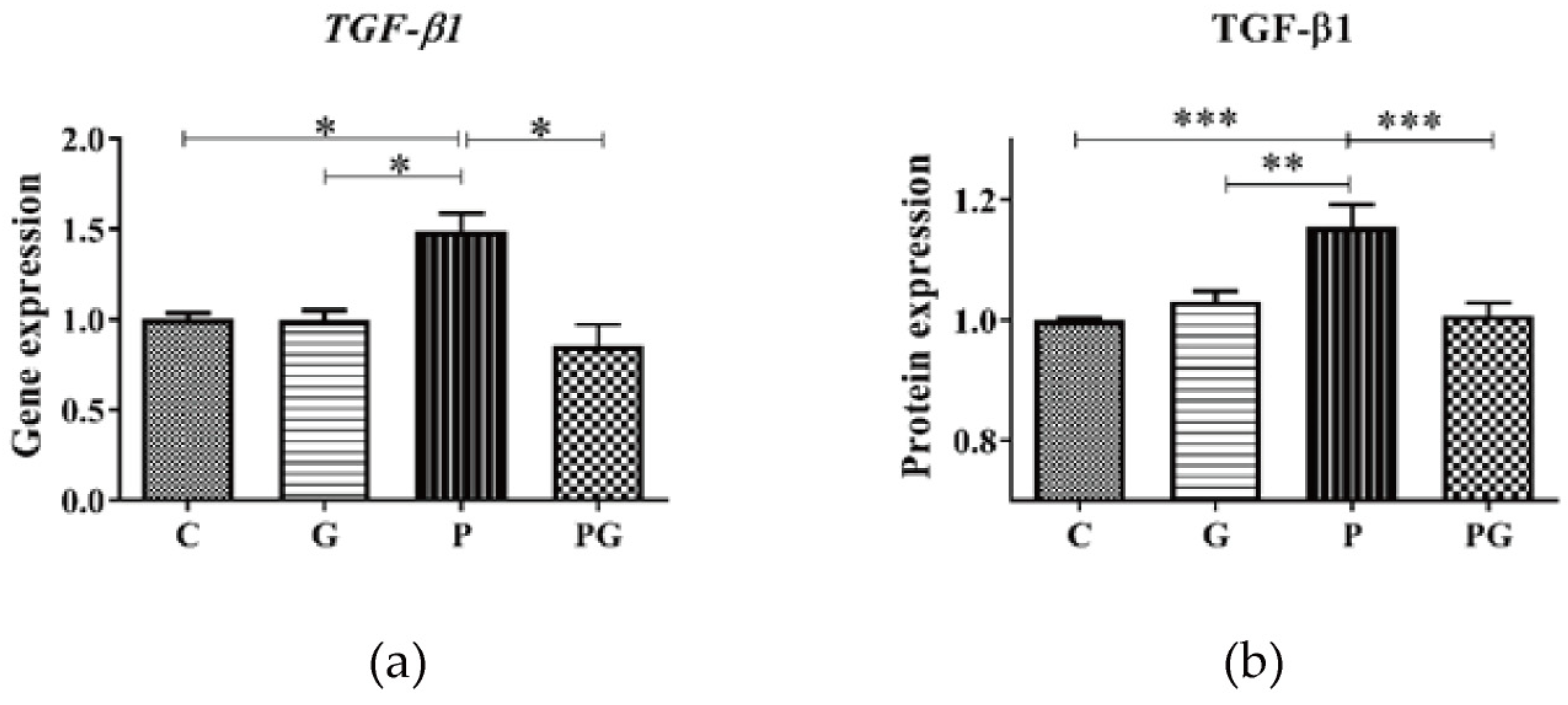
3.4. Effect of TGF-β Inhibition on m6A RNA Methylation of A549 after PM2.5 Exposure
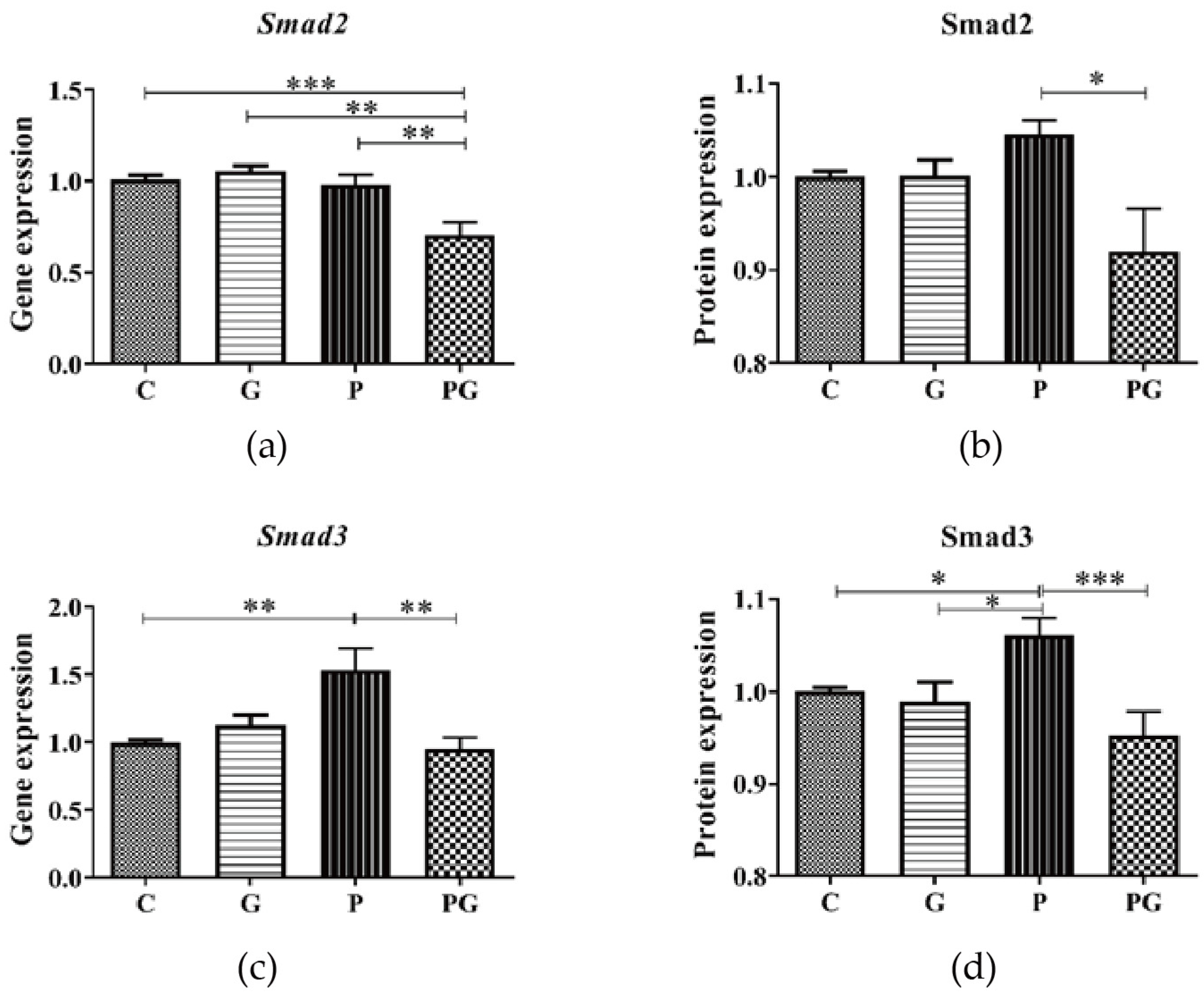
3.5. Effect of TGF-β Inhibition on the Smad2/3 in A549 after PM2.5 Exposure
3.6. Effect of TGF-β Inhibition on the RNA (de)Methyltransferase in A549 after PM2.5 Exposure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, S.; Chandra, M.; Kota, S.H. Health Effects Associated with PM2.5: A Systematic Review. Curr. Pollut. Rep. 2020, 6, 345–367. [Google Scholar] [CrossRef]
- Alemayehu, Y.A.; Asfaw, S.L.; Terfie, T.A. Exposure to urban particulate matter and its association with human health risks. Environ. Sci. Pollut. Res. Int. 2020, 27, 27491–27506. [Google Scholar] [CrossRef]
- Tao, Y.; Liu, Y.; Mi, S.; Guo, Y. Atmospheric pollution characteristics of fine particles and their effects on human health. Acta Sci. Circumstantiae 2014, 34, 6. [Google Scholar]
- Lei, F.; Liu, S.; Bao, Y.; Zhang, Y.; Ying, L.; Chao, Q.; Zhang, Z.; Xi, R.; Wang, X. Effect of Ambient PM2.5 on Migration and Invasion in Human A549 Lung Cancer Cells. Asian J. Ecotoxicol. 2018, 13, 91–98. [Google Scholar] [CrossRef]
- Robertson, S.; Miller, M.R. Ambient air pollution and thrombosis. Part. Fibre Toxicol. 2018, 15, 1. [Google Scholar] [CrossRef]
- Miller, M.R.; Newby, D.E. Air pollution and cardiovascular disease: Car sick. Cardiovasc. Res. 2019, 116, 279–294. [Google Scholar] [CrossRef]
- Meyer, K.D. m6A-mediated translation regulation. Biochim. Biophys. Acta-Gene Regul. Mech. 2019, 1862, 301–309. [Google Scholar] [CrossRef]
- Yu, J.; Chen, M.; Huang, H.; Zhu, J.; Song, H.; Zhu, J.; Park, J.; Ji, S.J. Dynamic m6A modification regulates local translation of mRNA in axons. Nucleic Acids Res. 2018, 46, 1412–1423. [Google Scholar] [CrossRef]
- Zaccara, S.; Ries, R.J.; Jaffrey, S.R. Reading, writing and erasing mRNA methylation. Nat. Rev. Mol. Cell Biol. 2019, 20, 608–624. [Google Scholar] [CrossRef]
- Chen, X.Y.; Zhang, J.; Zhu, J.S. The role of m 6 A RNA methylation in human cancer. Mol. Cancer 2019, 18, 103. [Google Scholar] [CrossRef]
- Lan, Q.; Liu, P.Y.; Bell, J.L.; Wang, J.Y.; Huttelmaier, S.; Zhang, X.D.; Zhang, L.R.; Liu, T. The Emerging Roles of RNA m6A Methylation and Demethylation as Critical Regulators of Tumorigenesis, Drug Sensitivity, and Resistance. Cancer Res. 2021, 81, 3431–3440. [Google Scholar] [CrossRef]
- Li, Z.; Li, N.; Guo, C.; Li, X.; Qian, Y.; Yang, Y.; Wei, Y. The global DNA and RNA methylation and their reversal in lung under different concentration exposure of ambient air particulate matter in mice. Ecotoxicol. Environ. Saf. 2019, 172, 396–402. [Google Scholar] [CrossRef]
- Gu, J.; Zhan, Y.; Zhuo, L.; Zhang, Q.; Li, G.; Li, Q.; Qi, S.; Zhu, J.; Lv, Q.; Shen, Y.; et al. Biological functions of m6A methyltransferases. Cell Biosci. 2021, 11, 177–189. [Google Scholar] [CrossRef]
- Ries, R.J.; Zaccara, S.; Klein, P.; Olarerin-George, A.; Namkoong, S.; Pickering, B.F.; Patil, D.P.; Kwak, H.; Lee, J.H.; Jaffrey, S.R. m6A enhances the phase separation potential of mRNA. Nature 2019, 571, 424–428. [Google Scholar] [CrossRef]
- Patil, D.P.; Chen, C.K.; Pickering, B.F.; Chow, A.; Jackson, C.; Guttman, M.; Jaffrey, S.R. m6A RNA methylation promotes XIST-mediated transcriptional repression. Nature 2016, 537, 369–373. [Google Scholar] [CrossRef]
- Kyung, S.Y.; Yoon, J.Y.; Kim, Y.J.; Lee, S.P.; Park, J.W.; Jeong, S.H. Asian Dust Particles Induce TGF-beta(1) via Reactive Oxygen Species in Bronchial Epithelial Cells. Tuberc. Respir. Dis. 2012, 73, 84–92. [Google Scholar] [CrossRef]
- Han, H.; Oh, E.-Y.; Lee, J.-H.; Park, J.-W.; Park, H.J. Effects of Particulate Matter 10 Inhalation on Lung Tissue RNA expression in a Murine Model. Tuberc. Respir. Dis. 2021, 84, 55–66. [Google Scholar] [CrossRef]
- Scotton, C.J.; Krupiczojc, M.A.; Königshoff, M.; Mercer, P.F.; Lee, Y.G.; Kaminski, N.; Morser, J.; Post, J.M.; Maher, T.M.; Nicholson, A.G.; et al. Increased local expression of coagulation factor X contributes to the fibrotic response in human and murine lung injury. J. Clin. Investig. 2009, 119, 2550–2563. [Google Scholar] [CrossRef]
- Bertero, A.; Brown, S.; Madrigal, P.; Osnato, A.; Ortmann, D.; Yiangou, L.; Kadiwala, J.; Hubner, N.C.; de Los Mozos, I.R.; Sadee, C.; et al. The SMAD2/3 interactome reveals that TGFbeta controls m6A mRNA methylation in pluripotency. Nature 2018, 555, 256–259. [Google Scholar] [CrossRef]
- Sánchez-Pérez, Y.; Chirino, Y.I.; Osornio-Vargas, Á.R.; Morales-Bárcenas, R.; Gutiérrez-Ruíz, C.; Vázquez-López, I.; García-Cuellar, C.M. DNA damage response of A549 cells treated with particulate matter (PM 10) of urban air pollutants. Cancer Lett. 2009, 278, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Schiliro, T.; Alessandria, L.; Degan, R.; Traversi, D.; Gilli, G. Chemical characterisation and cytotoxic effects in A549 cells of urban-air PM10 collected in Torino, Italy. Environ. Toxicol. Pharmacol. 2010, 29, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.; Movia, D.; Smith, R.J.; Hanlon, D.; Lebre, F.; Lavelle, E.C.; Byrne, H.J.; Coleman, J.N.; Volkov, Y.; McIntyre, J. Industrial grade 2D molybdenum disulphide (MoS2): An in vitro exploration of the impact on cellular uptake, cytotoxicity, and inflammation. 2D Materials 2017, 4, 025065. [Google Scholar] [CrossRef]
- Lacouture, M.E.; Morris, J.C.; Lawrence, D.P.; Tan, A.R.; Olencki, T.E.; Shapiro, G.I.; Dezube, B.J.; Berzofsky, J.A.; Hsu, F.J.; Guitart, J. Cutaneous keratoacanthomas/squamous cell carcinomas associated with neutralization of transforming growth factor β by the monoclonal antibody fresolimumab (GC1008). Cancer Immunol. Immunother. 2015, 64, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Real, A.D.; Santurtun, A.; Teresa Zarrabeitia, M. Epigenetic related changes on air quality. Environ. Res 2021, 197, 111155. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.S.; Roundtree, I.A.; He, C. Post-transcriptional gene regulation by mRNA modifications. Nat. Rev. Mol. Cell Biol. 2017, 18, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Pan, T. N6-methyl-adenosine modification in messenger and long non-coding RNA. Trends Biochem. Sci. 2013, 38, 204–209. [Google Scholar] [CrossRef]
- Slobodin, B.; Han, R.; Calderone, V.; Vrielink, J.A.F.O.; Loayza-Puch, F.; Elkon, R.; Agami, R. Transcription Impacts the Efficiency of mRNA Translation via Co-transcriptional N6-adenosine Methylation. Cell 2017, 169, 326–337.e312. [Google Scholar] [CrossRef]
- Li, Y.; Kang, X.; Zhou, Z.; Pan, L.; Chen, H.; Liang, X.; Chu, J.; Dong, S.; Liu, C.; Yu, S.; et al. The m6A methyltransferase Mettl3 deficiency attenuates hepatic stellate cell activation and liver fibrosis. Mol. Ther. 2022, 30, 3714–3728. [Google Scholar] [CrossRef]
- Shamsollahi, H.R.; Jahanbin, B.; Rafieian, S.; Yunesian, M.J.E.S.; Research, P. Particulates induced lung inflammation and its consequences in the development of restrictive and obstructive lung diseases: A systematic review. Environ. Sci. Pollut. Res. 2021, 28, 25035–25050. [Google Scholar] [CrossRef]
- Falcon-Rodriguez, C.I.; Osornio-Vargas, A.R.; Sada-Ovalle, I.; Segura-Medina, P. Aeroparticles, Composition, and Lung Diseases. Front. Immunol. 2016, 7, 3. [Google Scholar] [CrossRef]
- Cayir, A.; Barrow, T.M.; Guo, L.; Byun, H.M. Exposure to environmental toxicants reduces global N6-methyladenosine RNA methylation and alters expression of RNA methylation modulator genes. Environ. Res 2019, 175, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Li, Z.; Liao, Z.; Gao, S.; Hua, L.; Ye, X.; Wang, Y.; Jiang, S.; Wang, N.; Zhou, D.; et al. PM2.5 induced pulmonary fibrosis in vivo and in vitro. Ecotoxicol. Environ. Saf. 2019, 171, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, M.; Derynck, R.; Miyazono, K. TGF-beta and the TGF-beta Family: Context-Dependent Roles in Cell and Tissue Physiology. Cold Spring Harb. Perspect. Biol. 2016, 8, a021873. [Google Scholar] [CrossRef] [PubMed]
- Aschner, Y.; Downey, G.P. Transforming Growth Factor-beta: Master Regulator of the Respiratory System in Health and Disease. Am. J. Respir. Cell Mol. Biol. 2016, 54, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Derynck, R.; Budi, E.H. Specificity, versatility, and control of TGF-β family signaling. Sci. Signal. 2019, 12, eaav5183. [Google Scholar] [CrossRef]
- Budi, E.H.; Duan, D.; Derynck, R. Transforming Growth Factor-beta Receptors and Smads: Regulatory Complexity and Functional Versatility. Trends Cell Biol. 2017, 27, 658–672. [Google Scholar] [CrossRef]
- Zheng, R.; Tao, L.; Jian, H.; Chang, Y.; Cheng, Y.; Feng, Y.; Zhang, H. NLRP3 inflammasome activation and lung fibrosis caused by airborne fine particulate matter. Ecotoxicol. Environ. Saf. 2018, 163, 612–619. [Google Scholar] [CrossRef]
- Dysart, M.M.; Galvis, B.R.; Russell, A.G.; Barker, T.H. Environmental particulate (PM2.5) augments stiffness-induced alveolar epithelial cell mechanoactivation of transforming growth factor beta. PLoS ONE 2014, 9, e106821. [Google Scholar] [CrossRef]
- Singh, N.; Arora, N. Diesel exhaust exposure in mice induces pulmonary fibrosis by TGF-beta/Smad3 signaling pathway. Sci. Total Environ. 2021, 807, 150623. [Google Scholar] [CrossRef]
- Yin, H.; Chen, L.; Piao, S.; Wang, Y.; Zhange, L.; Lin, Y.; Tang, X.; Zhang, H.; Zhang, H.; Wang, X. M6A RNA methylation-mediated RMRP stability renders proliferation and progression of non-small cell lung cancer through regulating TGFBR1/SMAD2/SMAD3 pathway. Cell Death Differ. 2023, 30, 605–617. [Google Scholar] [CrossRef]




| Time | Temperature (°C) | Humidity (%) |
|---|---|---|
| November 2019 | 1~9 | 20~46 |
| December 2019 | −3~4 | 54~86 |
| January 2020 | −9~2 | 39~53 |
| February 2020 | −6~5 | 53~86 |
| Gene | Sequence of Primer (5′→3′) | |
|---|---|---|
| Forward | Reverse | |
| C57 BL/6J mice | ||
| TGF-β1 | AGCTGCGCTTGCAGAGATTA | AGCCCTGTATTCCGTCTCCT |
| Smad2 | AGGATGATGGGGACGGGAAT | AGCCCGGTAAATCTACCCAGAA |
| Smad3 | AGGAGAAGTGGTGCGAGAAG | CCATCCAGTGACCTGGGGAT |
| β-actin | CACTGTCGAGTCGCGTCC | TCATCCATGGCGAACTGGTG |
| A549 cell | ||
| TGF-β1 | CAAGTGGACATCAACGGGTTC | TCCGTGGAGCTGAAGCAATAG |
| Smad2 | ACCAAGCACTTGCTCTGAAA | ACGACCATCAAGAGACCTGG |
| Smad3 | TAATTTATTGCCGCCGCTCG | ATCCAGGGACTCAAACGTGG |
| METTL3 | GTGATCGTAGCTGAGGTTCGT | GGGTTGCACATTGTGTGGTC |
| METTL14 | GGACCTTGGAAGAGTGTGTTT | GATCCCCATGAGGCAGTGTT |
| WTAP | GCTTCTGCCTGGAGAGGATT | GTGTACTTGCCCTCCAAAGC |
| ALKBH5 | TGACTGTGCTCAGTGGATATG | TGACAGGCGATCTGAAGCAT |
| FTO | GAGCGCGAAGCTAAGAAACTG | TGGGGGTCAGATAAGGGAGC |
| β-actin | GCCGCCAGCTCACCAT | TCGTCGCCCACATAGGAATC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, T.; Liu, B.; Wei, Y.; Li, Z. TGF-β Regulates m6A RNA Methylation after PM2.5 Exposure. Toxics 2023, 11, 1026. https://doi.org/10.3390/toxics11121026
Wu T, Liu B, Wei Y, Li Z. TGF-β Regulates m6A RNA Methylation after PM2.5 Exposure. Toxics. 2023; 11(12):1026. https://doi.org/10.3390/toxics11121026
Chicago/Turabian StyleWu, Tingting, Bingqian Liu, Yongjie Wei, and Zhigang Li. 2023. "TGF-β Regulates m6A RNA Methylation after PM2.5 Exposure" Toxics 11, no. 12: 1026. https://doi.org/10.3390/toxics11121026
APA StyleWu, T., Liu, B., Wei, Y., & Li, Z. (2023). TGF-β Regulates m6A RNA Methylation after PM2.5 Exposure. Toxics, 11(12), 1026. https://doi.org/10.3390/toxics11121026






