Exploring Serum Biomarkers for Neuropathic Pain in Rat Models of Chemotherapy-Induced Peripheral Neuropathy: A Comparative Pilot Study with Oxaliplatin, Paclitaxel, Bortezomib, and Vincristine
Abstract
:1. Introduction
2. Materials and Methods
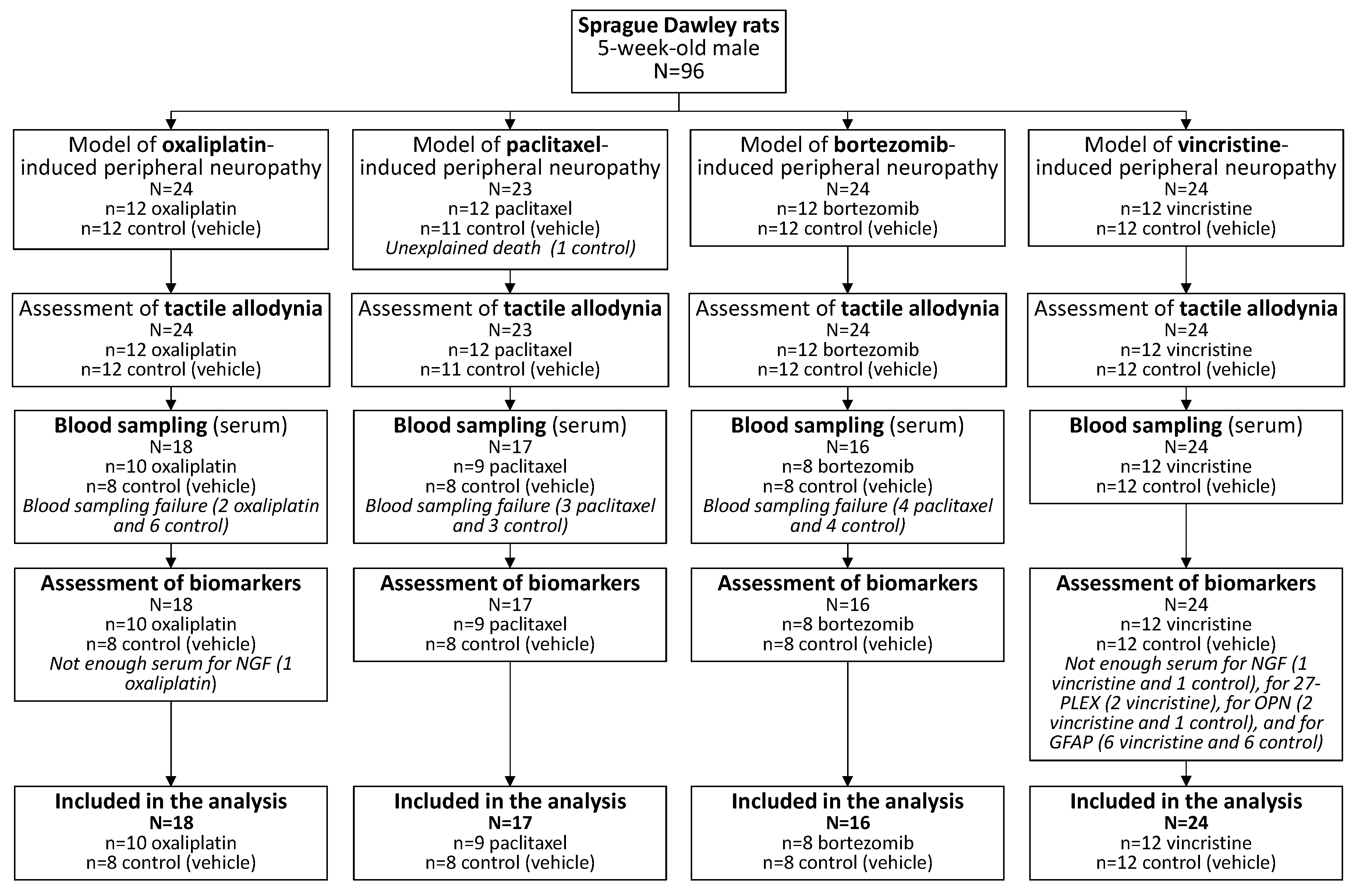
2.1. Animals
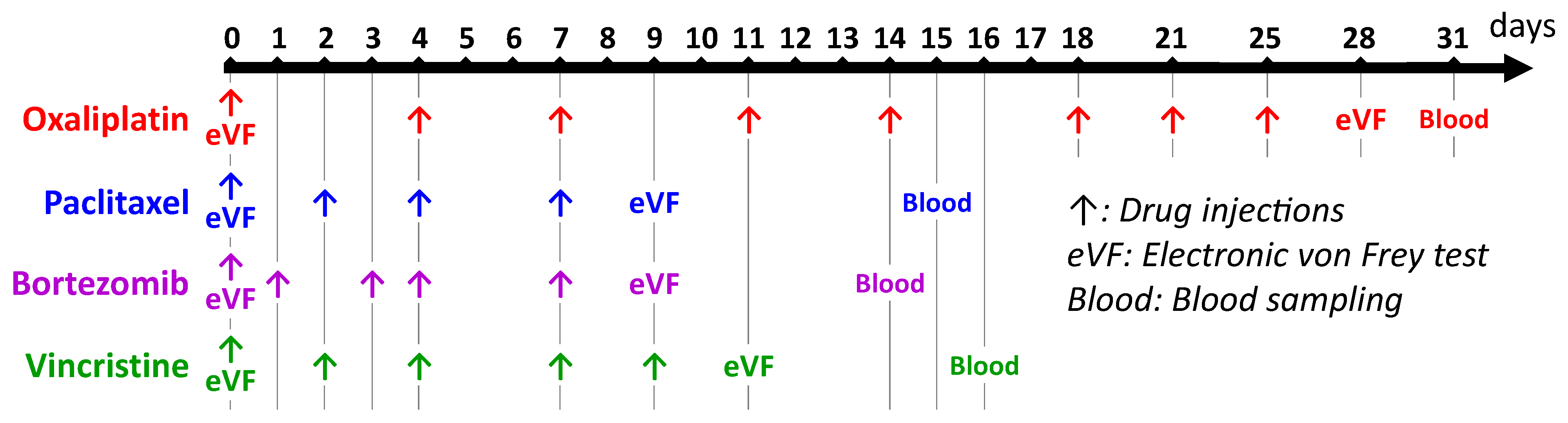
2.2. Animal Models of Chemotherapy-Induced Peripheral Neuropathy
2.3. Assessment of Nociceptive Disorders (Tactile Allodynia)
2.4. Assessments of Serum Biomarkers
2.5. Statistical Analysis
3. Results
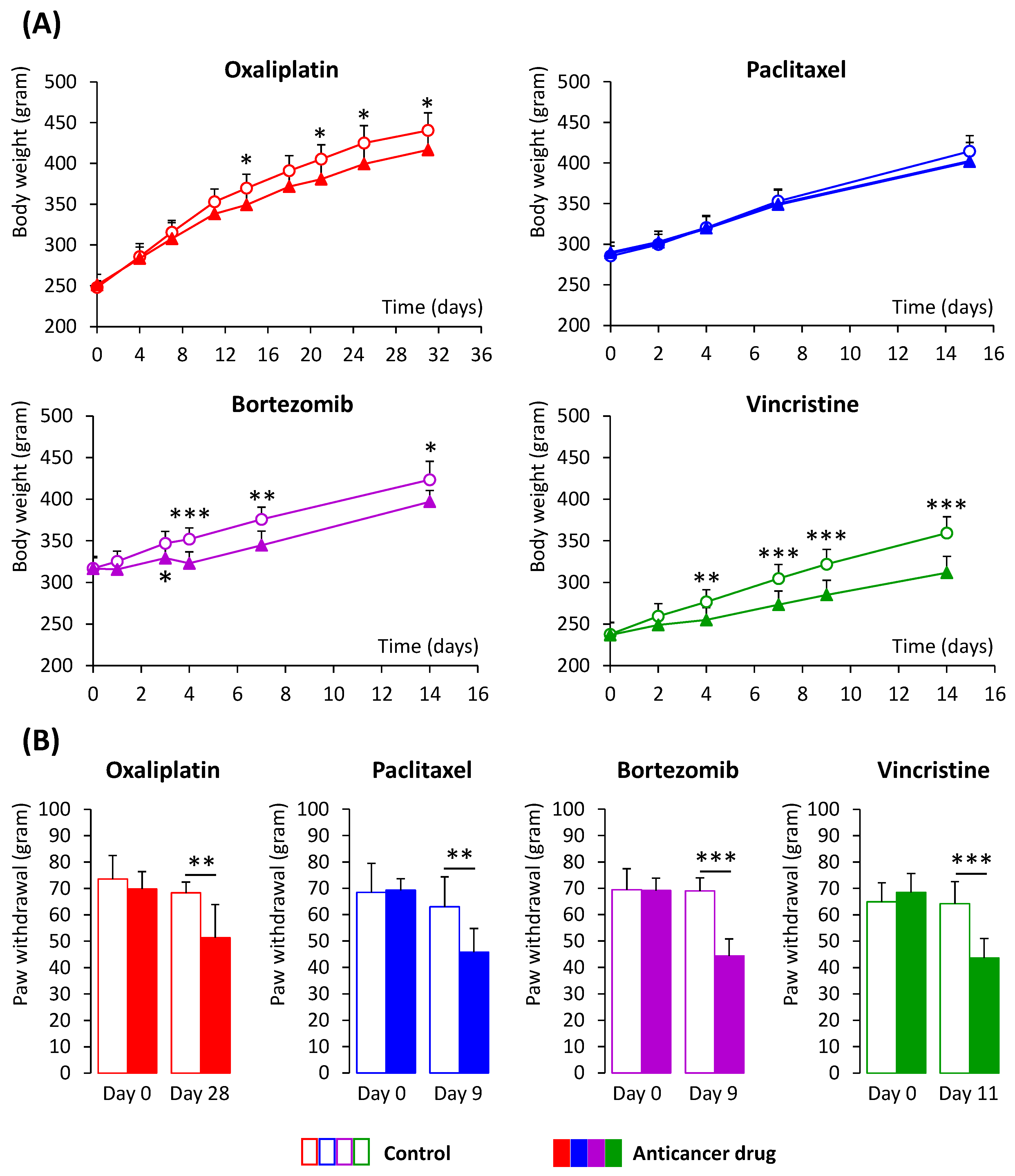
3.1. Animal Models of CIPN
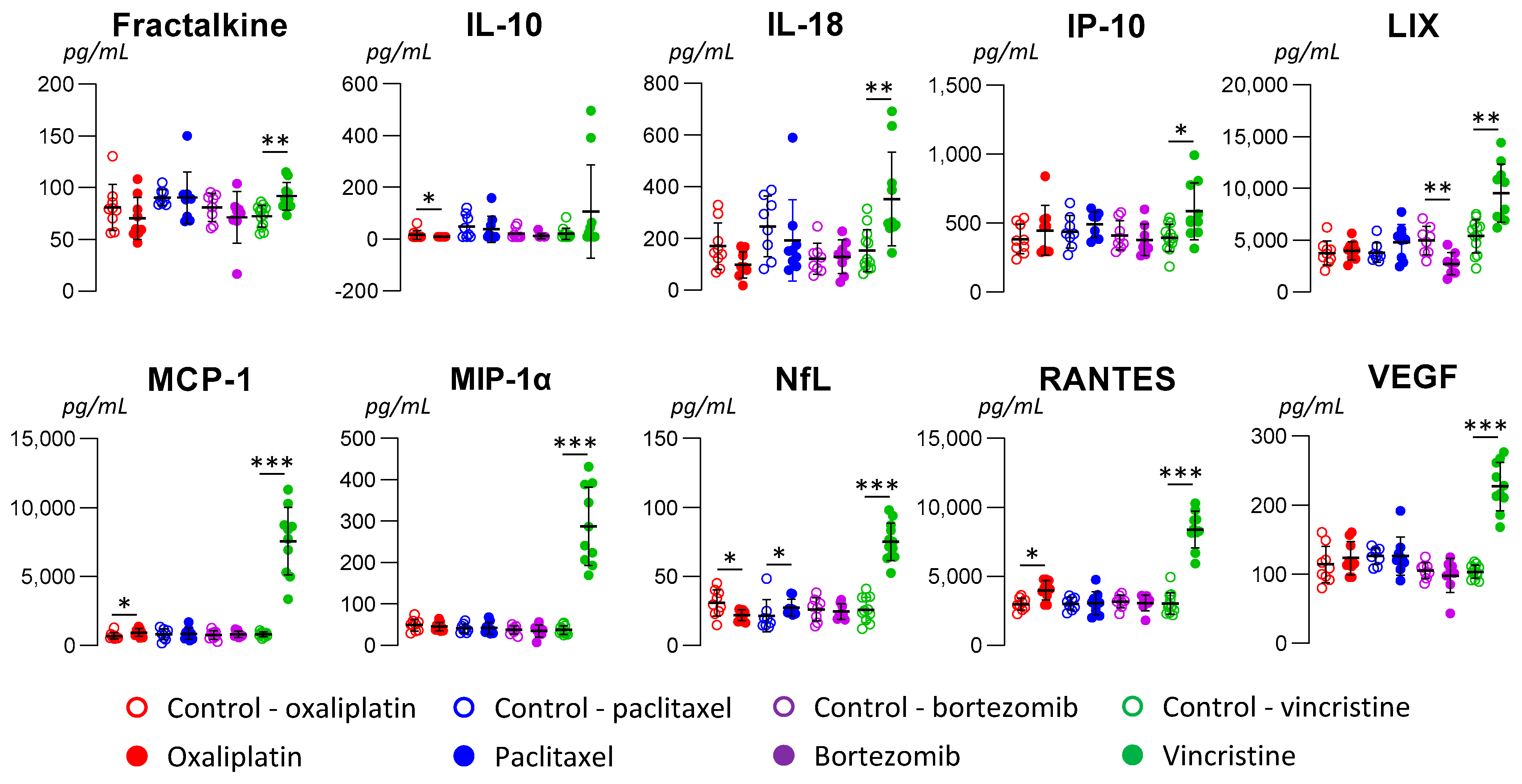
3.2. Serum Biomarkers of Neuropathic Pain
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kerckhove, N.; Collin, A.; Condé, S.; Chaleteix, C.; Pezet, D.; Balayssac, D. Long-Term Effects, Pathophysiological Mechanisms, and Risk Factors of Chemotherapy-Induced Peripheral Neuropathies: A Comprehensive Literature Review. Front. Pharmacol. 2017, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Seretny, M.; Currie, G.L.; Sena, E.S.; Ramnarine, S.; Grant, R.; MacLeod, M.R.; Colvin, L.A.; Fallon, M. Incidence, Prevalence, and Predictors of Chemotherapy-Induced Peripheral Neuropathy: A Systematic Review and Meta-Analysis. Pain 2014, 155, 2461–2470. [Google Scholar] [CrossRef] [PubMed]
- Selvy, M.; Pereira, B.; Kerckhove, N.; Gonneau, C.; Feydel, G.; Pétorin, C.; Vimal-Baguet, A.; Melnikov, S.; Kullab, S.; Hebbar, M.; et al. Long-Term Prevalence of Sensory Chemotherapy-Induced Peripheral Neuropathy for 5 Years after Adjuvant FOLFOX Chemotherapy to Treat Colorectal Cancer: A Multicenter Cross-Sectional Study. J. Clin. Med. 2020, 9, 2400. [Google Scholar] [CrossRef] [PubMed]
- Selvy, M.; Kerckhove, N.; Pereira, B.; Barreau, F.; Nguyen, D.; Busserolles, J.; Giraudet, F.; Cabrespine, A.; Chaleteix, C.; Soubrier, M.; et al. Prevalence of Chemotherapy-Induced Peripheral Neuropathy in Multiple Myeloma Patients and Its Impact on Quality of Life: A Single Center Cross-Sectional Study. Front. Pharmacol. 2021, 12, 637593. [Google Scholar] [CrossRef]
- Loprinzi, C.L.; Lacchetti, C.; Bleeker, J.; Cavaletti, G.; Chauhan, C.; Hertz, D.L.; Kelley, M.R.; Lavino, A.; Lustberg, M.B.; Paice, J.A.; et al. Prevention and Management of Chemotherapy-Induced Peripheral Neuropathy in Survivors of Adult Cancers: ASCO Guideline Update. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 3325–3348. [Google Scholar] [CrossRef] [PubMed]
- Jordan, B.; Margulies, A.; Cardoso, F.; Cavaletti, G.; Haugnes, H.S.; Jahn, P.; Le Rhun, E.; Preusser, M.; Scotté, F.; Taphoorn, M.J.B.; et al. Systemic Anticancer Therapy-Induced Peripheral and Central Neurotoxicity: ESMO-EONS-EANO Clinical Practice Guidelines for Diagnosis, Prevention, Treatment and Follow-Up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2020, 31, 1306–1319. [Google Scholar] [CrossRef]
- Dault, R.; Rousseau, M.P.; Beaudoin, A.; Frenette, M.A.; Lemay, F.; Beauchesne, M.F. Impact of Oxaliplatin-Induced Neuropathy in Patients with Colorectal Cancer: A Prospective Evaluation at a Single Institution. Curr. Oncol. Tor. Ont 2016, 23, e65–e69. [Google Scholar] [CrossRef]
- Chibaudel, B.; Maindrault-Goebel, F.; Lledo, G.; Mineur, L.; André, T.; Bennamoun, M.; Mabro, M.; Artru, P.; Carola, E.; Flesch, M.; et al. Can Chemotherapy Be Discontinued in Unresectable Metastatic Colorectal Cancer? The GERCOR OPTIMOX2 Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 5727–5733. [Google Scholar] [CrossRef]
- Griffith, K.A.; Merkies, I.S.J.; Hill, E.E.; Cornblath, D.R. Measures of Chemotherapy-Induced Peripheral Neuropathy: A Systematic Review of Psychometric Properties. J. Peripher. Nerv. Syst. JPNS 2010, 15, 314–325. [Google Scholar] [CrossRef]
- McCrary, J.M.; Goldstein, D.; Trinh, T.; Timmins, H.C.; Li, T.; Friedlander, M.; Bosco, A.; Harrison, M.; Maier, N.; O’Neill, S.; et al. Optimizing Clinical Screening for Chemotherapy-Induced Peripheral Neuropathy. J. Pain Symptom Manag. 2019, 58, 1023–1032. [Google Scholar] [CrossRef]
- Meregalli, C.; Fumagalli, G.; Alberti, P.; Canta, A.; Chiorazzi, A.; Monza, L.; Pozzi, E.; Carozzi, V.A.; Blennow, K.; Zetterberg, H.; et al. Neurofilament Light Chain: A Specific Serum Biomarker of Axonal Damage Severity in Rat Models of Chemotherapy-Induced Peripheral Neurotoxicity. Arch. Toxicol. 2020, 94, 2517–2522. [Google Scholar] [CrossRef] [PubMed]
- Balayssac, D.; Busserolles, J.; Broto, C.; Dalbos, C.; Prival, L.; Lamoine, S.; Richard, D.; Quintana, M.; Herbet, A.; Hilairet, S.; et al. Neurofilament Light Chain in Plasma as a Sensitive Diagnostic Biomarker of Peripheral Neurotoxicity: In Vivo Mouse Studies with Oxaliplatin and Paclitaxel—NeuroDeRisk Project. Biomed. Pharmacother. Biomedecine Pharmacother. 2023, 167, 115535. [Google Scholar] [CrossRef] [PubMed]
- Burgess, B.L.; Cho, E.; Honigberg, L. Neurofilament Light as a Predictive Biomarker of Unresolved Chemotherapy-Induced Peripheral Neuropathy in Subjects Receiving Paclitaxel and Carboplatin. Sci. Rep. 2022, 12, 15593. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Choi, M.K.; Park, N.Y.; Hyun, J.-W.; Lee, M.Y.; Kim, H.J.; Jung, S.K.; Cha, Y. Serum Neurofilament Light Chain Levels as a Biomarker of Neuroaxonal Injury and Severity of Oxaliplatin-Induced Peripheral Neuropathy. Sci. Rep. 2020, 10, 7995. [Google Scholar] [CrossRef]
- Was, H.; Borkowska, A.; Bagues, A.; Tu, L.; Liu, J.Y.H.; Lu, Z.; Rudd, J.A.; Nurgali, K.; Abalo, R. Mechanisms of Chemotherapy-Induced Neurotoxicity. Front. Pharmacol. 2022, 13, 750507. [Google Scholar] [CrossRef] [PubMed]
- Attal, N.; Bouhassira, D.; Gautron, M.; Vaillant, J.N.; Mitry, E.; Lepère, C.; Rougier, P.; Guirimand, F. Thermal Hyperalgesia as a Marker of Oxaliplatin Neurotoxicity: A Prospective Quantified Sensory Assessment Study. Pain 2009, 144, 245–252. [Google Scholar] [CrossRef]
- Boyette-Davis, J.A.; Cata, J.P.; Zhang, H.; Driver, L.C.; Wendelschafer-Crabb, G.; Kennedy, W.R.; Dougherty, P.M. Follow-up Psychophysical Studies in Bortezomib-Related Chemoneuropathy Patients. J. Pain 2011, 12, 1017–1024. [Google Scholar] [CrossRef]
- Tutelman, P.R.; Chambers, C.T.; Cornelissen, L.; Fernandez, C.V.; Flanders, A.; MacLeod, J.; Sherry, S.B.; Stewart, S.H.; Urquhart, R.; De Gagne, S.; et al. Long-Term Alterations in Somatosensory Functioning in Survivors of Childhood Cancer. Pain 2022, 163, 1193–1205. [Google Scholar] [CrossRef]
- Notturno, F.; Capasso, M.; DeLauretis, A.; Carpo, M.; Uncini, A. Glial Fibrillary Acidic Protein as a Marker of Axonal Damage in Chronic Neuropathies. Muscle Nerve 2009, 40, 50–54. [Google Scholar] [CrossRef]
- Pizzamiglio, C.; Ripellino, P.; Prandi, P.; Clemente, N.; Saggia, C.; Rossi, V.; Strigaro, G.; Luigi Foglio Bonda, P.; Comi, C.; Cantello, R. Nerve Conduction, Circulating Osteopontin and Taxane-Induced Neuropathy in Breast Cancer Patients. Neurophysiol. Clin. Clin. Neurophysiol. 2020, 50, 47–54. [Google Scholar] [CrossRef]
- Velasco, R.; Navarro, X.; Gil-Gil, M.; Herrando-Grabulosa, M.; Calls, A.; Bruna, J. Neuropathic Pain and Nerve Growth Factor in Chemotherapy-Induced Peripheral Neuropathy: Prospective Clinical-Pathological Study. J. Pain Symptom Manag. 2017, 54, 815–825. [Google Scholar] [CrossRef]
- Brandolini, L.; d’Angelo, M.; Antonosante, A.; Cimini, A.; Allegretti, M. Chemokine Signaling in Chemotherapy-Induced Neuropathic Pain. Int. J. Mol. Sci. 2019, 20, 2904. [Google Scholar] [CrossRef]
- Fumagalli, G.; Monza, L.; Cavaletti, G.; Rigolio, R.; Meregalli, C. Neuroinflammatory Process Involved in Different Preclinical Models of Chemotherapy-Induced Peripheral Neuropathy. Front. Immunol. 2021, 11, 626687. [Google Scholar] [CrossRef]
- Authier, N.; Balayssac, D.; Marchand, F.; Ling, B.; Zangarelli, A.; Descoeur, J.; Coudore, F.; Bourinet, E.; Eschalier, A. Animal Models of Chemotherapy-Evoked Painful Peripheral Neuropathies. Neurother. J. Am. Soc. Exp. Neurother. 2009, 6, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research. J. Pharmacol. Pharmacother. 2010, 1, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Ling, B.; Authier, N.; Balayssac, D.; Eschalier, A.; Coudore, F. Behavioral and Pharmacological Description of Oxaliplatin-Induced Painful Neuropathy in Rat. Pain 2007, 128, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Flatters, S.J.L.; Bennett, G.J. Ethosuximide Reverses Paclitaxel- and Vincristine-Induced Painful Peripheral Neuropathy. Pain 2004, 109, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Kawashiri, T.; Higuchi, H.; Tsutsumi, K.; Ushio, S.; Kaname, T.; Shirahama, M.; Egashira, N. Behavioral and Pharmacological Characteristics of Bortezomib-Induced Peripheral Neuropathy in Rats. J. Pharmacol. Sci. 2015, 129, 43–50. [Google Scholar] [CrossRef]
- Authier, N.; Gillet, J.-P.; Fialip, J.; Eschalier, A.; Coudore, F. A New Animal Model of Vincristine-Induced Nociceptive Peripheral Neuropathy. Neurotoxicology 2003, 24, 797–805. [Google Scholar] [CrossRef]
- Ferrier, J.; Marchand, F.; Balayssac, D. Assessment of Mechanical Allodynia in Rats Using the Electronic Von Frey Test. BIO-Protoc. 2016, 6, e1933. [Google Scholar] [CrossRef]
- Selvy, M.; Mattévi, C.; Dalbos, C.; Aissouni, Y.; Chapuy, E.; Martin, P.-Y.; Collin, A.; Richard, D.; Dumontet, C.; Busserolles, J.; et al. Analgesic and Preventive Effects of Donepezil in Animal Models of Chemotherapy-Induced Peripheral Neuropathy: Involvement of Spinal Muscarinic Acetylcholine M2 Receptors. Biomed. Pharmacother. 2022, 149, 112915. [Google Scholar] [CrossRef] [PubMed]
- Ferrier, J.; Bayet-Robert, M.; Dalmann, R.; Guerrab, A.E.; Aissouni, Y.; Graveron-Demilly, D.; Chalus, M.; Pinguet, J.; Eschalier, A.; Richard, D.; et al. Cholinergic Neurotransmission in the Posterior Insular Cortex Is Altered in Preclinical Models of Neuropathic Pain: Key Role of Muscarinic M2 Receptors in Donepezil-Induced Antinociception. J. Neurosci. 2015, 35, 16418–16430. [Google Scholar] [CrossRef] [PubMed]
- Keizer, R.J.; Jansen, R.S.; Rosing, H.; Thijssen, B.; Beijnen, J.H.; Schellens, J.H.M.; Huitema, A.D.R. Incorporation of Concentration Data below the Limit of Quantification in Population Pharmacokinetic Analyses. Pharmacol. Res. Perspect. 2015, 3, e00131. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Hillsdale, N.J., Ed.; Lawrence Erlbaum Associates: Mahwah, NJ, USA, 1988; ISBN 978-0-8058-0283-2. [Google Scholar]
- Gadgil, S.; Ergün, M.; van den Heuvel, S.A.; van der Wal, S.E.; Scheffer, G.J.; Hooijmans, C.R. A Systematic Summary and Comparison of Animal Models for Chemotherapy Induced (Peripheral) Neuropathy (CIPN). PLoS ONE 2019, 14, e0221787. [Google Scholar] [CrossRef]
- Gordon, B.A. Neurofilaments in Disease: What Do We Know? Curr. Opin. Neurobiol. 2020, 61, 105–115. [Google Scholar] [CrossRef]
- Meregalli, C.; Fumagalli, G.; Alberti, P.; Canta, A.; Carozzi, V.A.; Chiorazzi, A.; Monza, L.; Pozzi, E.; Sandelius, Å.; Blennow, K.; et al. Neurofilament Light Chain as Disease Biomarker in a Rodent Model of Chemotherapy Induced Peripheral Neuropathy. Exp. Neurol. 2018, 307, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, E.; Micheli, L.; Rajagopalan, R.; Ciampi, C.; Branca, J.J.V.; Pacini, A.; Leandri, M.; Rajagopalan, P.; Ghelardini, C.; Di Cesare Mannelli, L. Broad-Spectrum Neuroprotection Exerted by DDD-028 in a Mouse Model of Chemotherapy-Induced Neuropathy. Pain 2023, 164, 2581–2595. [Google Scholar] [CrossRef]
- Huehnchen, P.; Schinke, C.; Bangemann, N.; Dordevic, A.D.; Kern, J.; Maierhof, S.K.; Hew, L.; Nolte, L.; Körtvelyessy, P.; Göpfert, J.C.; et al. Neurofilament Proteins as a Potential Biomarker in Chemotherapy-Induced Polyneuropathy. JCI Insight 2022, 7, e154395. [Google Scholar] [CrossRef]
- Karteri, S.; Bruna, J.; Argyriou, A.A.; Mariotto, S.; Velasco, R.; Alemany, M.; Kalofonou, F.; Alberti, P.; Dinoto, A.; Velissaris, D.; et al. Prospectively Assessing Serum Neurofilament Light Chain Levels as a Biomarker of Paclitaxel-Induced Peripheral Neurotoxicity in Breast Cancer Patients. J. Peripher. Nerv. Syst. JPNS 2022, 27, 166–174. [Google Scholar] [CrossRef]
- Kim, S.-H.; Kim, K.H.; Hyun, J.-W.; Kim, J.H.; Seo, S.-S.; Kim, H.J.; Park, S.-Y.; Lim, M.C. Blood Neurofilament Light Chain as a Biomarker for Monitoring and Predicting Paclitaxel-Induced Peripheral Neuropathy in Patients with Gynecological Cancers. Front. Oncol. 2022, 12, 942960. [Google Scholar] [CrossRef]
- Mortensen, C.; Steffensen, K.D.; Simonsen, E.; Herskind, K.; Madsen, J.S.; Olsen, D.A.; Iversen, D.B.; Bergmann, T.K.; Pottegård, A.; Stage, T.B. Neurofilament Light Chain as a Biomarker of Axonal Damage in Sensory Neurons and Paclitaxel-Induced Peripheral Neuropathy in Patients with Ovarian Cancer. Pain 2022, 164, 1502–1511. [Google Scholar] [CrossRef] [PubMed]
- Velasco, R.; Argyriou, A.A.; Marco, C.; Mariotto, S.; Stradella, A.; Hernández, J.; Pernas, S.; Ferrari, S.; Bruna, J. Serum Neurofilament Levels Correlate with Electrodiagnostic Evidence of Axonal Loss in Paclitaxel-Induced Peripheral Neurotoxicity. J. Neurol. 2023, 270, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Cebulla, N.; Schirmer, D.; Runau, E.; Flamm, L.; Gommersbach, S.; Stengel, H.; Zhou, X.; Einsele, H.; Reinhold, A.-K.; Rogalla von Bieberstein, B.; et al. Neurofilament Light Chain Levels Indicate Acute Axonal Damage under Bortezomib Treatment. J. Neurol. 2023, 270, 2997–3007. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Xu, J.; Hou, T.; Zhu, J.; Jiang, Y.; Sun, L.; Huang, C.; Sun, L.; Liu, S. Multiplex Assessment of Serum Chemokines CCL2, CCL5, CXCL1, CXCL10, and CXCL13 Following Traumatic Brain Injury. Inflammation 2023, 46, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Anshita, D.; Ravichandiran, V. MCP-1: Function, Regulation, and Involvement in Disease. Int. Immunopharmacol. 2021, 101, 107598. [Google Scholar] [CrossRef]
- Ang, D.C.; Moore, M.N.; Hilligoss, J.; Tabbey, R. MCP-1 and IL-8 as Pain Biomarkers in Fibromyalgia: A Pilot Study. Pain Med. Malden Mass 2011, 12, 1154–1161. [Google Scholar] [CrossRef]
- García-Fernández, P.; Höfflin, K.; Rausch, A.; Strommer, K.; Neumann, A.; Cebulla, N.; Reinhold, A.-K.; Rittner, H.; Üçeyler, N.; Sommer, C. Systemic Inflammatory Markers in Patients with Polyneuropathies. Front. Immunol. 2023, 14, 1067714. [Google Scholar] [CrossRef]
- Appay, V.; Rowland-Jones, S.L. RANTES: A Versatile and Controversial Chemokine. Trends Immunol. 2001, 22, 83–87. [Google Scholar] [CrossRef]
- Lakritz, J.R.; Robinson, J.A.; Polydefkis, M.J.; Miller, A.D.; Burdo, T.H. Loss of Intraepidermal Nerve Fiber Density during SIV Peripheral Neuropathy Is Mediated by Monocyte Activation and Elevated Monocyte Chemotactic Proteins. J. Neuroinflamm. 2015, 12, 237. [Google Scholar] [CrossRef]
- Liu, T.-W.; Chen, C.-M.; Chang, K.-H. Biomarker of Neuroinflammation in Parkinson’s Disease. Int. J. Mol. Sci. 2022, 23, 4148. [Google Scholar] [CrossRef]
- Geisler, S. Vincristine- and Bortezomib-Induced Neuropathies—From Bedside to Bench and Back. Exp. Neurol. 2021, 336, 113519. [Google Scholar] [CrossRef]
- Starobova, H.; Vetter, I. Pathophysiology of Chemotherapy-Induced Peripheral Neuropathy. Front. Mol. Neurosci. 2017, 10, 174. [Google Scholar] [CrossRef]
- Pollard, K.J.; Bolon, B.; Moore, M.J. Comparative Analysis of Chemotherapy-Induced Peripheral Neuropathy in Bioengineered Sensory Nerve Tissue Distinguishes Mechanistic Differences in Early-Stage Vincristine-, Cisplatin-, and Paclitaxel-Induced Nerve Damage. Toxicol. Sci. Off. J. Soc. Toxicol. 2021, 180, 76–88. [Google Scholar] [CrossRef]
- Sun, W.; Hao, Y.; Li, R.; Hiu Ting Ho, I.; Wu, S.; Li, N.; Ba, X.; Wang, J.; Xiong, D.; Jiang, C.; et al. Comparative Transcriptome of Dorsal Root Ganglia Reveals Distinct Etiologies of Paclitaxel- and Oxaliplatin-Induced Peripheral Neuropathy in Rats. Neuroscience 2023, 516, 1–14. [Google Scholar] [CrossRef]
- Pachman, D.R.; Qin, R.; Seisler, D.; Smith, E.M.L.; Kaggal, S.; Novotny, P.; Ruddy, K.J.; Lafky, J.M.; Ta, L.E.; Beutler, A.S.; et al. Comparison of Oxaliplatin and Paclitaxel-Induced Neuropathy (Alliance A151505). Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2016, 24, 5059–5068. [Google Scholar] [CrossRef] [PubMed]
- Authier, N.; Gillet, J.P.; Fialip, J.; Eschalier, A.; Coudore, F. Assessment of Neurotoxicity Following Repeated Cremophor/Ethanol Injections in Rats. Neurotox. Res. 2001, 3, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Kerckhove, N.; Selvy, M.; Lambert, C.; Gonneau, C.; Feydel, G.; Pétorin, C.; Vimal-Baguet, A.; Melnikov, S.; Kullab, S.; Hebbar, M.; et al. Colorectal Cancer Survivors Suffering From Sensory Chemotherapy-Induced Peripheral Neuropathy Are Not a Homogenous Group: Secondary Analysis of Patients’ Profiles with Oxaliplatin-Induced Peripheral Neuropathy. Front. Pharmacol. 2021, 12, 744085. [Google Scholar] [CrossRef]
- Turner, P.V.; Pang, D.S.; Lofgren, J.L. A Review of Pain Assessment Methods in Laboratory Rodents. Comp. Med. 2019, 69, 451–467. [Google Scholar] [CrossRef] [PubMed]
- Marmiroli, P.; Nicolini, G.; Miloso, M.; Scuteri, A.; Cavaletti, G. The Fundamental Role of Morphology in Experimental Neurotoxicology: The Example of Chemotherapy-Induced Peripheral Neurotoxicity. Ital. J. Anat. Embryol. Arch. Ital. Anat. Ed Embriologia 2012, 117, 75–97. [Google Scholar]
- Boehmerle, W.; Huehnchen, P.; Peruzzaro, S.; Balkaya, M.; Endres, M. Electrophysiological, Behavioral and Histological Characterization of Paclitaxel, Cisplatin, Vincristine and Bortezomib-Induced Neuropathy in C57Bl/6 Mice. Sci. Rep. 2014, 4, 6370. [Google Scholar] [CrossRef]
- Selvy, M.; Pereira, B.; Kerckhove, N.; Busserolles, J.; Farsi, F.; Guastella, V.; Merle, P.; Pezet, D.; Balayssac, D. Prevention, Diagnosis and Management of Chemotherapy-Induced Peripheral Neuropathy: A Cross-Sectional Study of French Oncologists’ Professional Practices. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2021, 29, 4033–4043. [Google Scholar] [CrossRef] [PubMed]
- Sardo, S.; Varrassi, G.; Scartozzi, M.; Pace, M.C.; Schweiger, V.; Tamburin, S.; Musu, M.; Finco, G. Exploring Outcome Priorities and Real-Life Management of Chemotherapy-Induced Peripheral Neurotoxicity: A Survey of the Italian Association for the Study of Pain Members. J. Pain Res. 2023, 16, 3227–3238. [Google Scholar] [CrossRef] [PubMed]
- Cavaletti, G.; Pizzamiglio, C.; Man, A.; Engber, T.M.; Comi, C.; Wilbraham, D. Studies to Assess the Utility of Serum Neurofilament Light Chain as a Biomarker in Chemotherapy-Induced Peripheral Neuropathy. Cancers 2023, 15, 4216. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, D.; Park, S.B.; Li, T.; Timmins, H.C.; Trinh, T.; Au, K.; Battaglini, E.; Wyld, D.; Henderson, R.D.; Grimison, P.; et al. Hemoglobin, Body Mass Index, and Age as Risk Factors for Paclitaxel- and Oxaliplatin-Induced Peripheral Neuropathy. JAMA Netw. Open 2021, 4, e2036695. [Google Scholar] [CrossRef] [PubMed]
- Warncke, U.O.; Toma, W.; Meade, J.A.; Park, A.J.; Thompson, D.C.; Caillaud, M.; Bigbee, J.W.; Bryant, C.D.; Damaj, M.I. Impact of Dose, Sex, and Strain on Oxaliplatin-Induced Peripheral Neuropathy in Mice. Front. Pain Res. 2021, 2, 683168. [Google Scholar] [CrossRef]
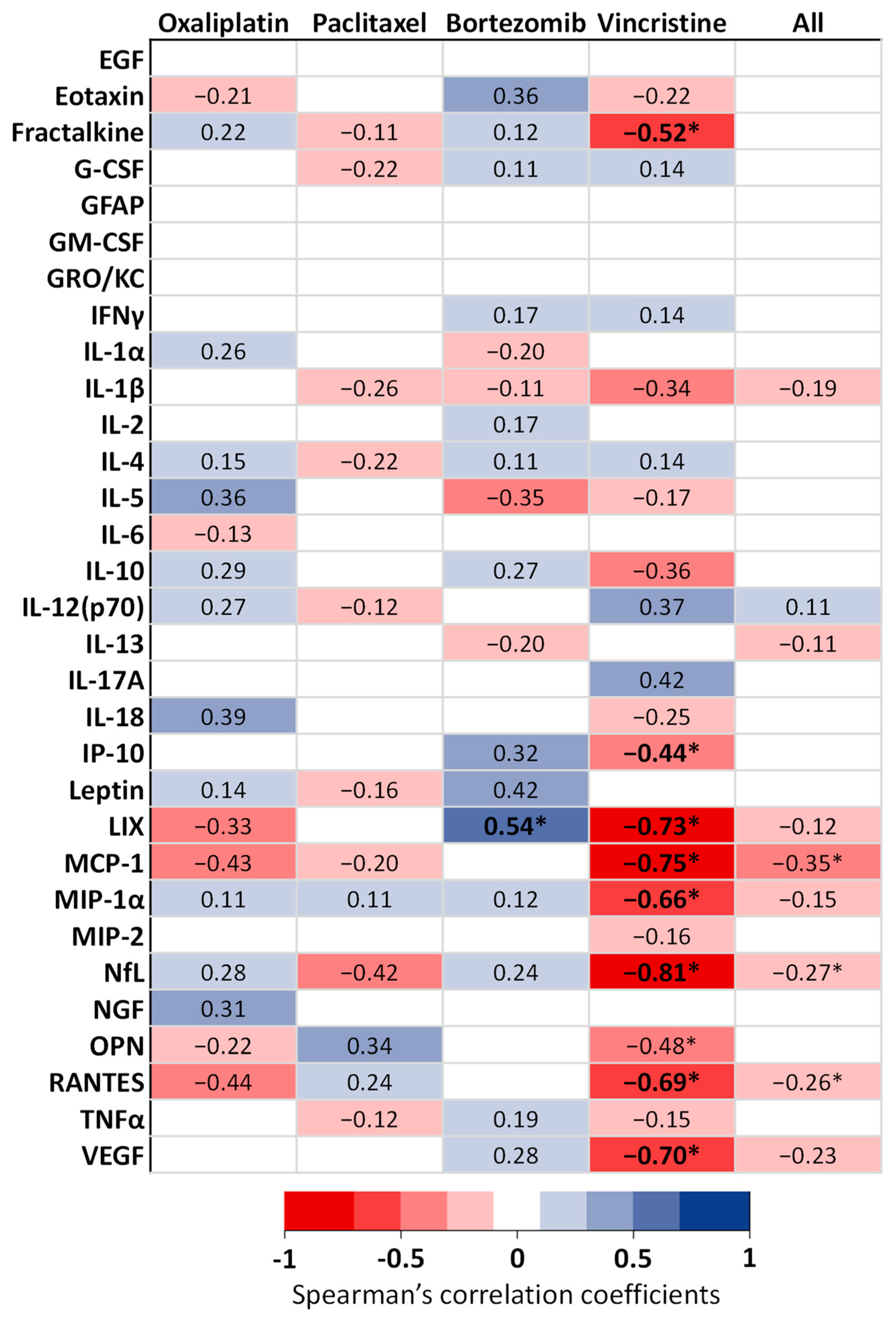
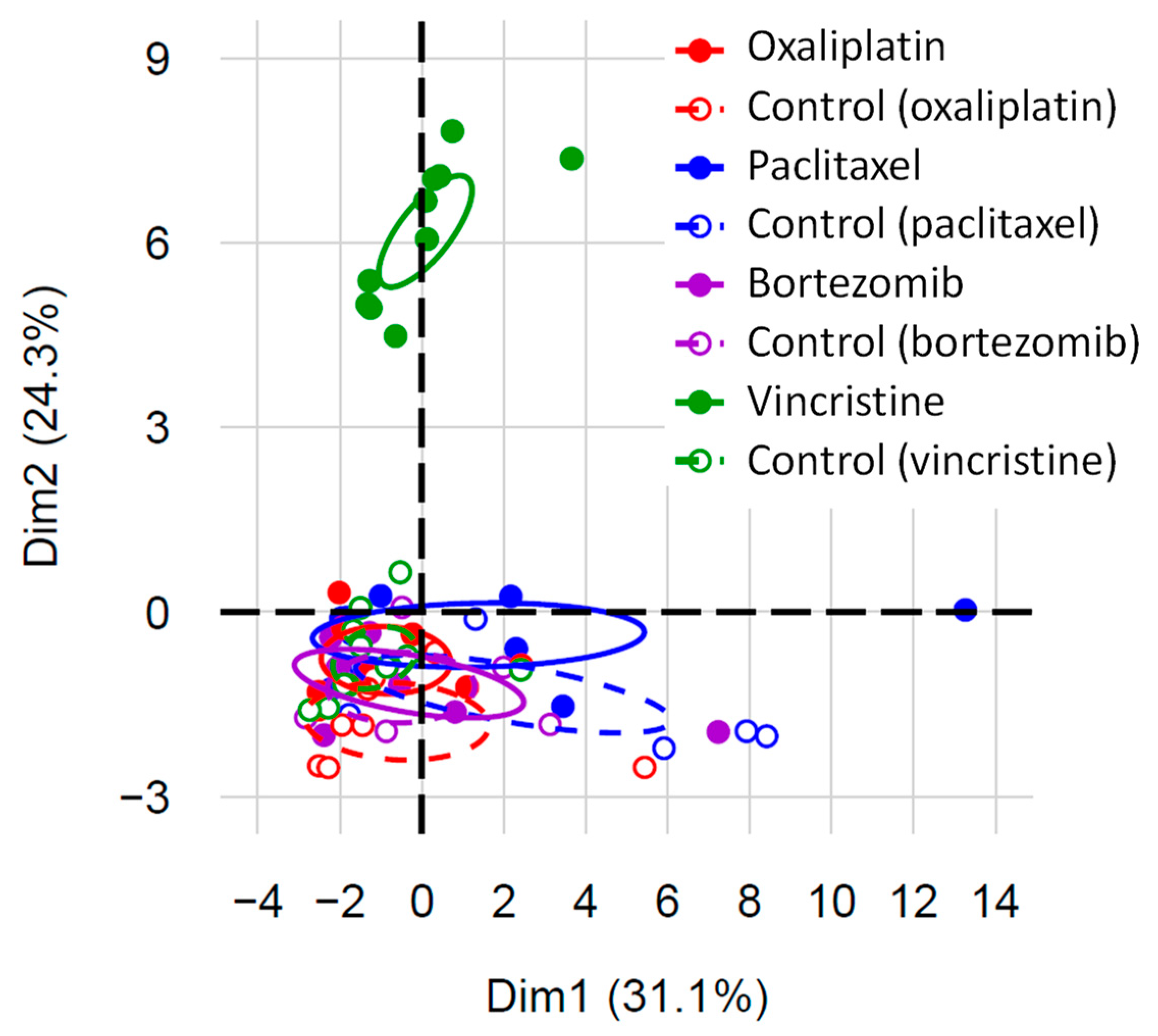
| Biomarkers (pg/mL) | Oxaliplatin | Paclitaxel | Bortezomib | Vincristine | Comparison of Controls | ||||
|---|---|---|---|---|---|---|---|---|---|
| Controls (n = 8) | Treated (n = 10) | Controls (n = 8) | Treated (n = 9) | Controls (n = 8) | Treated (n = 8) | Controls (n = 12) | Treated (n = 12) | ||
| EGF | 0.9 ± 0.0 # | 0.9 ± 0.0 # | 0.9 ± 0.0 # | 1.3 ± 1.1 | 0.9 ± 0.0 # | 0.9 ± 0.0 # | 0.9 ± 0.0 # | 0.9 ± 0.0 # | NA |
| Eotaxin | 8.1 ± 6.7 | 8.3 ± 6.3 | 18.9 ± 13.7 | 17.1 ± 18.4 | 11.5 ± 7.7 | 6.8 ± 6.2 | 7.6 ± 5.9 | 14.4 ± 12.7 | NS |
| Fractalkine | 81.3 ± 23.6 | 71.0 ± 19.2 | 90.5 ± 7.6 | 90.3 ± 24.8 | 81.0 ± 13.6 | 71.3 ± 24.8 | 72.5 ± 10.6 | 91.8 ± 13.3 ** | § |
| G-CSF | 6.7 ± 4.4 | 6.0 ± 4.7 | 21.7 ± 25.0 | 17.9 ± 30.7 | 6.2 ± 3.3 | 9.0 ± 12.2 | 6.5 ± 6.8 | 5.3 ± 2.5 | NS |
| GFAP | 15.6 ± 0.0 # | 15.6 ± 0.0 # | 15.6 ± 0.0 # | 15.6 ± 0.0 # | 15.6 ± 0.0 # | 15.6 ± 0.0 # | 15.6 ± 0.0 # | 15.6 ± 0.0 # | NA |
| GM-CSF | 10.0 ± 0.0 # | 10.0 ± 0.0 # | 10.0 ± 0.0 # | 10.0 ± 0.0 # | 10.0 ± 0.0 # | 10.0 ± 0.0 # | 10.0 ± 0.0 # | 10.0 ± 0.0 # | NA |
| GRO/KC | 58.2 ± 0.0 # | 58.2 ± 0.0 # | 58.2 ± 0.0 # | 58.2 ± 0.0 # | 58.2 ± 0.0 # | 58.2 ± 0.0 # | 58.2 ± 0.0 # | 58.2 ± 0.0 # | NA |
| IFNγ | 33 ± 64 | 44 ± 74 | 206 ± 284 | 186 ± 374 | 70 ± 101 | 57 ± 96 | 37 ± 92 | 29 ± 58 | NS |
| IL-1α | 49 ± 25 | 40 ± 0.0 # | 85 ± 82 | 93 ± 158 | 40 ± 0 # | 62 ± 64 | 40 ± 0 # | 40 ± 0 # | § |
| IL-1β | 20 ± 15 | 14 ± 9 | 47 ± 35 | 55 ± 38 | 22 ± 17 | 26 ± 15 | 34 ± 25 | 179 ± 305 | NS |
| IL-2 | 49 ± 73 | 34 ± 51 | 114 ± 95 | 89 ± 139 | 53 ± 46 | 39 ± 49 | 20 ± 22 | 22 ± 18 | NS |
| IL-4 | 35.8 ± 40.3 | 19.7 ± 4.9 | 57.7 ± 58.3 | 60.4 ± 98.4 | 26.6 ± 17.3 | 33.8 ± 38.2 | 22.0 ± 14.0 | 18.7 ± 2.4 | NS |
| IL-5 | 122 ± 70 | 110 ± 64 | 212 ± 109 | 159 ± 145 | 113 ± 74 | 149 ± 78 | 113 ± 49 | 119 ± 37 | NS |
| IL-6 | 282 ± 7 | 305 ± 80 | 452 ± 314 | 478 ± 593 | 280 ± 0 # | 280 ± 0 # | 280 ± 0 # | 280 ± 0 # | § |
| IL-10 | 17 ± 19 | 8 ± 0.0 #* | 48 ± 46 | 38 ± 51 | 21 ± 21 | 12 ± 10 | 21 ± 22 | 106 ± 181 | NS |
| IL-12(p70) | 203 ± 171 | 122 ± 104 | 304 ± 262 | 351 ± 438 | 219 ± 159 | 272 ± 308 | 152 ± 93 | 92 ± 77 | NS |
| IL-13 | 17.5 ± 0.0 # | 17.5 ± 0.0 # | 35.9 ± 27.6 | 31.9 ± 39.2 | 17.5 ± 0.0 # | 26.3 ± 24.7 | 17.5 ± 0.0 # | 17.5 ± 0.0 # | § |
| IL-17A | 17.7 ± 17.8 | 15.0 ± 10.5 | 57.5 ± 41.7 | 49.9 ± 70.2 | 22.7 ± 22.4 | 54.5 ± 92.2 | 16.6 ± 16.3 | 11.5 ± 12.1 | § |
| IL-18 | 150 ± 71 | 121 ± 87 | 247 ± 117 | 192 ± 157 | 122 ± 60 | 129 ± 65 | 153 ± 81 | 353 ± 181 ** | NS |
| IP-10 | 391 ± 113 | 435 ± 175 | 438 ± 118 | 488 ± 95 | 410 ± 107 | 378 ± 115 | 393 ± 99 | 585 ± 208 * | NS |
| Leptin | 5964 ± 2515 | 5094 ± 2872 | 7720 ± 3797 | 8257 ± 2664 | 6602 ± 3301 | 4473 ± 1069 | 9962 ± 6639 | 7736 ± 5571 | NS |
| LIX | 3476 ± 768 | 4192 ± 1104 | 3808 ± 950 | 4833 ± 1679 | 4967 ± 1371 | 2722 ± 1081 ** | 5386 ± 1630 | 9536 ± 2792 ** | § |
| MCP-1 | 633 ± 111 | 979 ± 294 * | 819 ± 399 | 857 ± 415 | 768 ± 321 | 819 ± 222 | 819 ± 167 | 7575 ± 2457 *** | NS |
| MIP-1α | 50 ± 15 | 46 ± 10 | 42 ± 11 | 42 ± 14 | 38 ± 10 | 35 ± 14 | 37 ± 10 | 288 ± 95 *** | NS |
| MIP-2 | 23.9 ± 0.0 # | 23.9 ± 0.0 # | 24.2 ± 0.7 | 28.1 ± 9.5 | 23.9 ± 0.0 # | 23.9 ± 0.0 # | 23.9 ± 0.0 # | 24.5 ± 1.9 | NS |
| NfL | 31.8 ± 9.6 | 22.0 ± 3.6 * | 21.5 ± 11.5 | 27.5 ± 5.9 * | 26.1 ± 8.3 | 24.6 ± 5.6 | 25.7 ± 9.0 | 75.0 ± 13.5 *** | NS |
| NGF | 6.8 ± 4.2 | 5.3 ± 0.0 # | 6.3 ± 3.0 | 5.4 ± 0.2 | 5.3 ± 0.0 # | 5.3 ± 0.0 # | 5.3 ± 0.0 # | 5.3 ± 0.0 # | NS |
| OPN | 0.8 ± 0.1 | 0.9 ± 0.2 | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.8 ± 0.1 | 0.8 ± 0.2 | 0.8 ± 0.1 | 0.9 ± 0.2 * | NS |
| RANTES | 3008 ± 468 | 3887 ± 763 * | 3016± 462 | 3060 ± 839 | 3181 ± 472 | 3061 ± 572 | 3036 ± 778 | 8394 ± 1318 *** | NS |
| TNFα | 4.8 ± 3.7 | 6.0 ± 4.3 | 10.7 ± 9.3 | 12.0 ± 13.9 | 6.4 ± 3.9 | 6.1 ± 4.2 | 5.8 ± 2.3 | 6.1 ± 4.0 | NS |
| VEGF | 108 ± 21 | 127 ± 26 | 126 ± 12 | 127 ± 28 | 105 ± 12 | 98 ± 25 | 104 ± 10 | 227 ± 35 *** | § |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balayssac, D.; Durif, J.; Lambert, C.; Dalbos, C.; Chapuy, E.; Etienne, M.; Demiot, C.; Busserolles, J.; Martin, V.; Sapin, V. Exploring Serum Biomarkers for Neuropathic Pain in Rat Models of Chemotherapy-Induced Peripheral Neuropathy: A Comparative Pilot Study with Oxaliplatin, Paclitaxel, Bortezomib, and Vincristine. Toxics 2023, 11, 1004. https://doi.org/10.3390/toxics11121004
Balayssac D, Durif J, Lambert C, Dalbos C, Chapuy E, Etienne M, Demiot C, Busserolles J, Martin V, Sapin V. Exploring Serum Biomarkers for Neuropathic Pain in Rat Models of Chemotherapy-Induced Peripheral Neuropathy: A Comparative Pilot Study with Oxaliplatin, Paclitaxel, Bortezomib, and Vincristine. Toxics. 2023; 11(12):1004. https://doi.org/10.3390/toxics11121004
Chicago/Turabian StyleBalayssac, David, Julie Durif, Céline Lambert, Cristelle Dalbos, Eric Chapuy, Monique Etienne, Claire Demiot, Jérôme Busserolles, Vincent Martin, and Vincent Sapin. 2023. "Exploring Serum Biomarkers for Neuropathic Pain in Rat Models of Chemotherapy-Induced Peripheral Neuropathy: A Comparative Pilot Study with Oxaliplatin, Paclitaxel, Bortezomib, and Vincristine" Toxics 11, no. 12: 1004. https://doi.org/10.3390/toxics11121004
APA StyleBalayssac, D., Durif, J., Lambert, C., Dalbos, C., Chapuy, E., Etienne, M., Demiot, C., Busserolles, J., Martin, V., & Sapin, V. (2023). Exploring Serum Biomarkers for Neuropathic Pain in Rat Models of Chemotherapy-Induced Peripheral Neuropathy: A Comparative Pilot Study with Oxaliplatin, Paclitaxel, Bortezomib, and Vincristine. Toxics, 11(12), 1004. https://doi.org/10.3390/toxics11121004










